Abstract
Purpose
Considering the distinctive biology of triple-negative breast cancer (TNBC), this study aimed to identify TNBC-specific prognostic factors and determine the prognostic value of the Nottingham Prognostic Index (NPI) and its variant indices.
Methods
A total of 233 patients with newly diagnosed stage I to III TNBC from 2003 to 2012 were reviewed. We retrospectively analyzed the patients' demographics, clinicopathologic parameters, treatment, and survival outcomes. The NPI was calculated as follows: tumor size (cm)×0.2+node status+Scarff-Bloom-Richardson (SBR) grade. The modified NPI (MNPI) was obtained by adding the modified SBR grade rather than the SBR grade.
Results
The median follow-up was 67.8 months. Five-year disease-free survival (DFS) and overall survival (OS) were 81.4% and 89.9%, respectively. Multivariate analyses showed that the MNPI was the most significant and common prognostic factor of DFS (p=0.001) and OS (p=0.019). Young age (≤35 years) was also correlated with poor DFS (p=0.006). A recursive partitioning for establishing the prognostic model for DFS was performed based on the results of multivariate analysis. Patients with a low MNPI (≤6.5) were stratified into the low-risk group (p<0.001), and patients with a high MNPI (>6.5) were subdivided into the intermediate (>35 years) and high-risk (≤35 years) groups. Age was not a prognostic factor in patients with a low MNPI, whereas in patients with a high MNPI, it was the second key factor in subdividing patients according to prognosis (p=0.023).
Conclusion
The MNPI could be used to stratify patients with stage I to III TNBC according to prognosis. It was the most important prognosticator for both DFS and OS. The prognostic significance of young age for DFS differed by MNPI.
Keywords: Age factors, Prognosis, Triple negative breast neoplasms
INTRODUCTION
Triple-negative breast cancer (TNBC), an intrinsic subtype of breast cancer, is defined as a tumor that does not express the estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2) [1]. Despite racial disparity, the overall incidence of TNBC is estimated at 15% to 20% of all diagnosed invasive breast cancers [2,3]. TNBC has distinctive characteristics and natural history; it is usually associated with African-American ethnicity, young age at diagnosis, advanced disease, and poor outcome [2,4]. Metastasis is characterized by an early peak of recurrence and a high incidence of visceral metastasis, particularly to the lungs and brain [1,2].
The Nottingham Prognostic Index (NPI), a value composed of tumor size, lymph node (LN) status, and histologic grade, was suggested for discriminating patients according to prognosis in the adjuvant setting of primary operable breast cancer [5]. Use of the NPI has been subsequently validated in long-term follow-up data [6,7] and extensive multicenter studies from the Danish Breast Cancer Cooperative Group [8]. After preoperative systemic therapy (PST) was widely accepted in clinical settings, it has been shown that residual tumor burden in the breast and/or LNs, as defined by the NPI, is an independent prognostic factor. However, Chollet et al. [9] showed that the modified breast grading index (MBGI), which scores histologic grade using a French modified Scarff-Bloom-Richardson (MSBR) grading system, rather than the traditional Scarff-Bloom-Richardson (SBR) system, has a higher prognostic influence than the conventional NPI after induction chemotherapy. Although some studies have reported that the attenuated relationship between tumor size or stage and probability of survival was observed in TNBC [1,2], a smaller-scale study showed that the NPI seems to be able to stratify and predict the prognosis of patients with TNBC in the adjuvant setting [10].
Considering the distinctive tumor biology of TNBC, there is a need to identify TNBC-specific prognostic factors. In addition, the NPI and its modified indicators have not yet been evaluated in patients with TNBC in a clinical practice setting including PST. Therefore, we sought to evaluate the prognostic influence of various clinicopathologic factors including the NPI and other indices.
METHODS
Patient identification
A total of 233 patients newly diagnosed with stage I to III TNBC at Seoul National University Bundang Hospital from March 2003 to December 2012 were reviewed. To identify the patients with TNBC, we reviewed initial histopathologic parameters, including ER, PR, and HER2 status. TNBC was defined as the subtype showing no expression of ER, PR, or HER2 according to the 2013 St. Gallen Consensus. HER2 negativity was defined as a negative or 1+ score for c-erbB-2 by immunohistochemistry, or no amplification of HER2 by fluorescence in situ hybridization. After obtaining approval from our Institutional Review Board (B-1505/298-116), we retrospectively reviewed the patients' medical charts to collect data on demographics, clinicopathologic parameters, treatment, and survival outcomes.
All patients were staged according to the American Joint Committee on Cancer staging system, seventh edition. For the analysis, initial clinical stage was used for patients treated with PST, and pathologic stage was used for patients who were not treated with PST. Baseline Ki-67 and cyclooxygenase 2 (COX-2) were recorded based on the results of initial immunohistochemistry. COX-2 was considered positive with a staining score of 3+, as previously described [11]. Pathologic factors, including histology, histologic grade, extracapsular extension (ECE), lymphovascular invasion (LVI), and multiplicity, were based on the pathologic report of the curative surgical specimen. Node ratio (NR) was defined as the ratio of positive to excised nodes. The NPI was calculated as follows [6]: tumor size (cm)×0.2+node status (1, node negative; 2, 1–3 positive LNs; 3, ≥4 positive LNs)+SBR grade (1, grade I; 2, grade II; 3, grade III). The modified NPI (MNPI) was obtained by adding the MSBR grade [12] instead of the SBR grade. The breast grading index (BGI) and MBGI were also calculated by the summation of tumor size (cm)×0.2 and SBR or MSBR grade, respectively [9].
Treatment
PST was administered to 57 patients (24.5%). The most common regimen was doxorubicin and cyclophosphamide (40.4%), followed by docetaxel and doxorubicin (31.6%). Breast conserving surgery was performed in 150 patients (64.4%). Sentinel LN biopsy alone and LN dissection were performed in 118 patients (50.6%) and 115 patients (49.4%), respectively. Adjuvant chemotherapy was administered to 187 patients (80.3%), and the fluorouracil, doxorubicin, and cyclophosphamide regimen was the most common treatment (29.9%). Radiotherapy was provided to 180 patients (77.3%) to the whole breast or chest wall (median dose, 50.4 Gy/28 fx). When required, a median boost of 9 Gy was administered.
Clinical endpoint and statistical analyses
Disease-free survival (DFS) was defined as the duration from the date of initiating treatment to the first failure or last follow-up. Overall survival (OS) was calculated from the date of initiating any treatment to the date of death from any cause or the last follow-up. Survival data were collected through inquiries to the Resident Registration of the Ministry of Security and Public Administration of the Republic of Korea. In terms of treatment failure, locoregional failure (LRF) was defined as a failure occurring in the ipsilateral breast/chest wall or the ipsilateral regional LNs (including the axillary, supra/infraclavicular, and internal mammary LNs), while distant failure (DF) was defined as any failure that did not qualify as LRF, including contralateral breast events. Locoregional failure-free survival (LRFS) and distant metastasis-free survival (DMFS) were defined as the duration from the date of initiating treatment to the date of last follow-up or failure (LFR and DF, respectively).
The actuarial survival curves were estimated using the Kaplan-Meier method, and the effects of each variable on survival were evaluated by log-rank test. For multivariate analysis, we fitted a Cox regression model with the forward stepwise selection method, as entering the variables confirmed that the assumption of proportional hazards was met. A conditional inference tree was used to estimate a regression relationship by binary recursive partitioning. Statistical analyses were performed using STATA version 13 (Stata Corp., College Station, USA) and R program version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria). A p-value below 0.05 was considered statistically significant.
RESULTS
Patient and tumor characteristics
Patient and tumor characteristics are summarized in Tables 1 and 2. The median patient age at diagnosis was 48 years (range, 20–89 years). The most common tumor histology was infiltrating ductal carcinoma (83.3%), with metaplastic carcinoma as the second most common histology (8.6%). Of 57 patients who received PST, the pathologic complete response (pCR) rate was 26.3%. The median number of harvested LNs was 9, and this increased to 20 in patients with an NR >0.2 (8.6%). The median NPI and MNPI were 4.44 (range, 2.60–7.30) and 6.38 (range, 3.04–9.30), respectively. Immunostaining of Ki-67 was performed in all, but three, patients. The median value of baseline Ki-67 was 40%. COX-2 expression was available in 112 patients, and 23.2% patients were positive for COX-2.
Table 1. Patient characteristics.
| Characteristic | No. (%) |
|---|---|
| Age at diagnosis (yr)* | 48 (20–89) |
| Menopause | |
| Premenopause | 138 (60.3) |
| Postmenopause | 91 (39.7) |
| Initial T stage† | |
| T1 | 91 (39.1) |
| T2 | 110 (47.2) |
| T3 | 24 (10.3) |
| T4 | 8 (3.4) |
| Initial N stage† | |
| N0 | 147 (63.1) |
| N1 | 52 (22.3) |
| N2 | 16 (6.9) |
| N3 | 18 (7.7) |
| Surgery type of breast | |
| BCS | 150 (64.4) |
| Mastectomy | 83 (35.6) |
| Lymph node resection | |
| SLNB only | 118 (50.6) |
| ALND | 115 (49.4) |
| No. of excised lymph node‡ | 9.0 ± 11.9 |
| Nodal ratio‡ | 5.7 ± 15.9 |
| Neoadjuvant chemotherapy | |
| Yes | 57 (24.5) |
| No | 176 (75.5) |
| Adjuvant chemotherapy | |
| Yes | 187 (80.3) |
| No | 17 (7.3) |
| Radiotherapy | |
| Yes | 180 (77.3) |
| No | 53 (22.7) |
BCS=breast-conserving surgery; SLNB=sentinel lymph node biopsy; ALND=axillary lymph node dissection.
*Median (range); †Clinical staging system was applied to patients with neoadjuvant chemotherapy and pathologic staging system was used for patients treated by upfront radical surgery; ‡Mean±SD.
Table 2. Tumor characteristics.
| Characteristic | No. (%) |
|---|---|
| Histology | |
| Infiltrating ductal carcinoma | 194 (83.3) |
| Others | 39 (16.7) |
| Histologic grade | |
| I/II | 43 (21.1) |
| III | 161 (78.9) |
| Histologic grade (MSBR) | |
| II/III | 39 (19.0) |
| IV | 42 (20.5) |
| V | 124 (60.5) |
| Extracapsular extension | |
| Negative | 197 (87.6) |
| Positive | 28 (12.0) |
| Unknown | 8 (3.4) |
| Lymphovascular invasion | |
| Negative | 147 (63.1) |
| Positive | 62 (26.6) |
| Unknown | 24 (10.3) |
| Multiplicity | |
| Negative | 200 (85.8) |
| Positive | 33 (14.2) |
| NPI* | 4.44 (2.60–7.30) |
| MNPI* | 6.38 (3.04–9.30) |
| BGI* | 3.38 (1.60–4.72) |
| MBGI* | 5.30 (2.04–6.72) |
| Baseline Ki-67 (%) | |
| ≤ 20 | 58 (25.2) |
| > 20 | 172 (74.8) |
| Baseline COX-2 | |
| Negative | 86 (76.8) |
| Positive | 26 (23.2) |
MSBR=modified Scarff-Bloom-Richardson grade; NPI=Nottingham Prognostic Index; MNPI=modified NPI; BGI=breast grading index; MBGI=modified BGI; COX-2=cyclooxygenase 2.
*Median (range).
Survival outcomes and patterns of failure
The median follow-up for all patients was 67.8 months (range, 0.7–147.7 months). Five-year DFS and OS were 81.4% and 89.9%, respectively. During the follow-up period, 45 patients experienced failure (crude failure rate, 19.3%). DF occurred in 38 patients, and the lung was the most common site (47.4%) of the first DF. Of 38 patients with DF, 18 experienced both DF and LRF. Isolated LRF occurred in seven patients. Five-year DMFS and LRFS were 85.2% and 88.6%, respectively.
Univariate analysis
Patients less than 35 years of age at diagnosis had a significantly shorter DFS (p=0.002). Both LRF and DF occurred more frequently in this group. However, this did not translate to compromised OS. Initial T stage and N stage affected both DFS and DMFS. Histologic grade according to the SBR system was not a significant prognostic factor for any endpoints. However, DFS marginally decreased in patients with MSBR grade 5 (p=0.069). With respect to failure type, a high MSBR grade was significantly associated with decreased DMFS (5-year, 91.1% vs. 80.3%, p=0.029), but not LRFS (5-year, 89.5% vs. 86.5%, p=0.431). ECE and tumor multiplicity both influenced DFS, mainly contributing to a significant decrease in DMFS. The presence of LVI affected both DFS and OS. Of the four indices for predicting prognosis, a high NPI or MNPI was well correlated with poor DFS and OS, while neither BGI nor MBGI were significant prognostic factors. Adjuvant chemotherapy or radiotherapy was not associated with any endpoint. Neither baseline Ki-67 nor COX-2 contributed to survival outcomes. Table 3 summarizes the key results of univariate analysis for each endpoint.
Table 3. Univariate analysis for each survival outcomes.
| Characteristic | No. (%) | 5-Year DFS (%) | p-value* | 5-Year OS (%) | p-value* | 5-Year LRFS (%) | p-value* | 5-Year DMFS (%) | p-value* | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | ≤ 35 | 28 (12.0) | 74.8 | 0.002 | 85.2 | 0.253 | 74.2 | 0.006 | 73.7 | 0.003 |
| > 35 | 205 (88.0) | 83.9 | 90.5 | 90.6 | 86.7 | |||||
| T stage | 1 | 91 (39.1) | 89.4 | 0.026 | 95.2 | 0.063 | 93.0 | 0.100 | 92.9 | 0.013 |
| 2–4 | 142 (60.9) | 76.4 | 86.5 | 85.8 | 80.2 | |||||
| N stage | 0–1 | 199 (85.4) | 83.9 | 0.004 | 91.8 | 0.038 | 89.8 | 0.151 | 87.3 | 0.002 |
| 2–3 | 34 (14.6) | 67.5 | 78.6 | 81.5 | 73.1 | |||||
| Nodal ratio (%) | ≤ 20 | 213 (91.4) | 84.4 | < 0.001 | 92.9 | < 0.001 | 90.5 | 0.001 | 87.6 | < 0.001 |
| > 20 | 20 (8.6) | 55.0 | 59.2 | 67.4 | 60.0 | |||||
| Histologic grade | I/II | 43 (21.1) | 80.2 | 0.897 | 87.3 | 0.673 | 84.8 | 0.689 | 85.7 | 0.553 |
| III | 161 (78.9) | 81.8 | 89.3 | 88.3 | 84.3 | |||||
| ECE | Negative | 197 (87.6) | 84.3 | 0.021 | 91.1 | 0.138 | 89.2 | 0.513 | 87.2 | 0.017 |
| Positive | 28 (12.4) | 67.7 | 78.2 | 84.8 | 71.3 | |||||
| LVI | Negative | 147 (70.3) | 85.6 | 0.003 | 94.0 | < 0.001 | 92.8 | < 0.001 | 87.7 | 0.008 |
| Positive | 62 (29.7) | 70.0 | 76.3 | 76.6 | 75.1 | |||||
| Multiplicity | Negative | 200 (85.8) | 84.1 | 0.010 | 91.4 | 0.127 | 89.9 | 0.102 | 86.9 | 0.011 |
| Positive | 33 (14.2) | 67.4 | 80.2 | 80.7 | 74.2 | |||||
| NPI | ≤ 4.6 | 120 (58.8) | 90.0 | < 0.001 | 96.3 | < 0.001 | 92.0 | 0.021 | 92.9 | < 0.001 |
| > 4.6 | 84 (41.2) | 69.5 | 78.9 | 81.5 | 73.0 | |||||
| MNPI | ≤ 6.5 | 136 (66.3) | 88.9 | < 0.001 | 95.1 | < 0.001 | 91.4 | 0.016 | 92.2 | < 0.001 |
| > 6.5 | 69 (33.7) | 67.2 | 77.3 | 80.5 | 70.0 | |||||
| Baseline Ki-67 (%) | ≤ 20 | 58 (25.2) | 82.3 | 0.582 | 90.8 | 0.629 | 89.4 | 0.861 | 84.1 | 0.758 |
| > 20 | 172 (74.8) | 80.9 | 89.4 | 88.1 | 85.3 | |||||
| Baseline COX-2 | Negative | 86 (76.8) | 78.0 | 0.946 | 89.2 | 0.091 | 86.5 | 0.668 | 83.1 | 0.854 |
| Positive | 26 (23.2) | 80.6 | 79.7 | 84.3 | 84.3 | |||||
DFS=disease-free survival; OS=overall survival; LRFS=locoregional recurrence-free survival; DMFS=distant metastasis-free survival; ECE=extracapsular extension; LVI=lymphovascular invasion; NPI=Nottingham Prognostic Index; MNPI=modified NPI; COX-2=cyclooxygenase 2.
*p-value by log-rank test.
Multivariate analysis
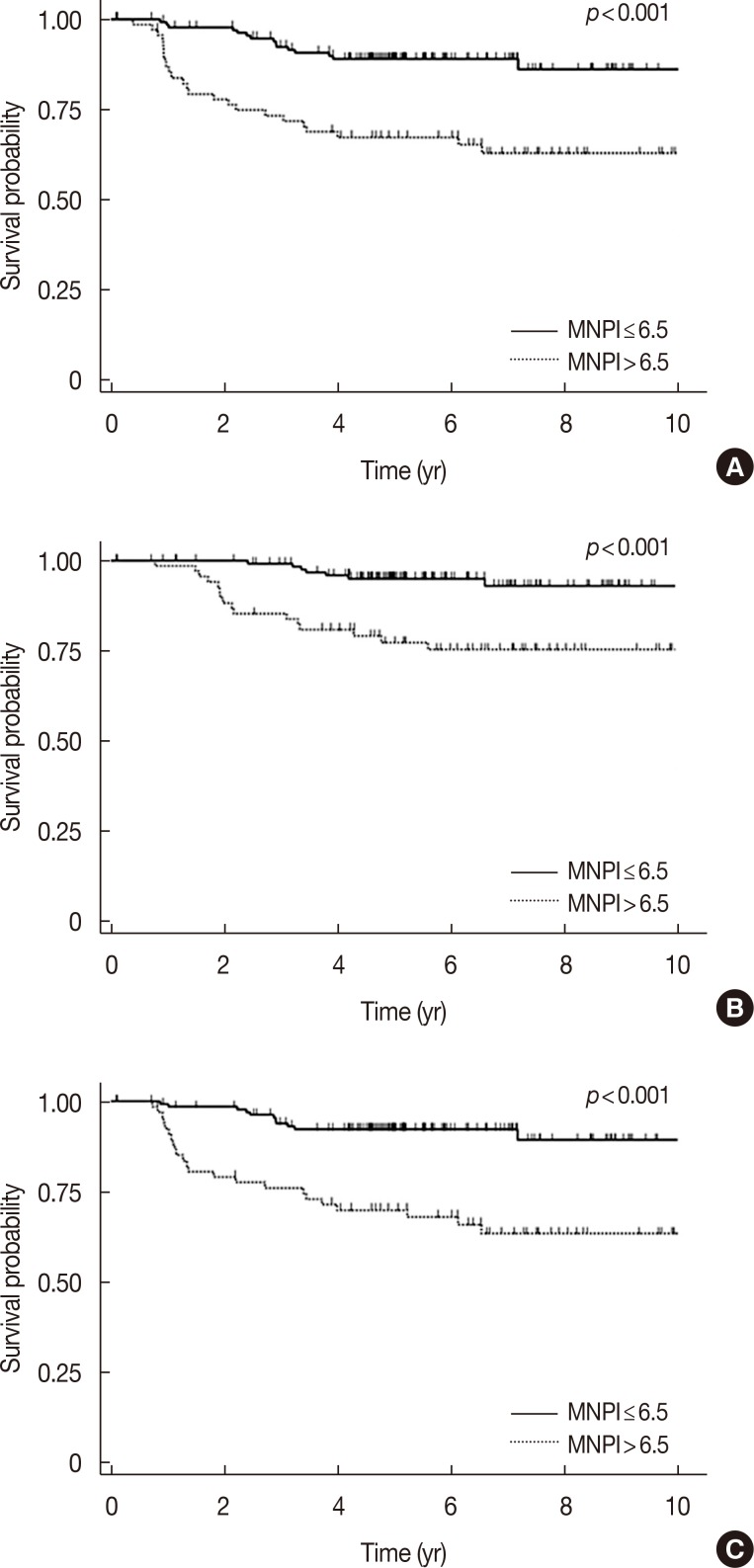
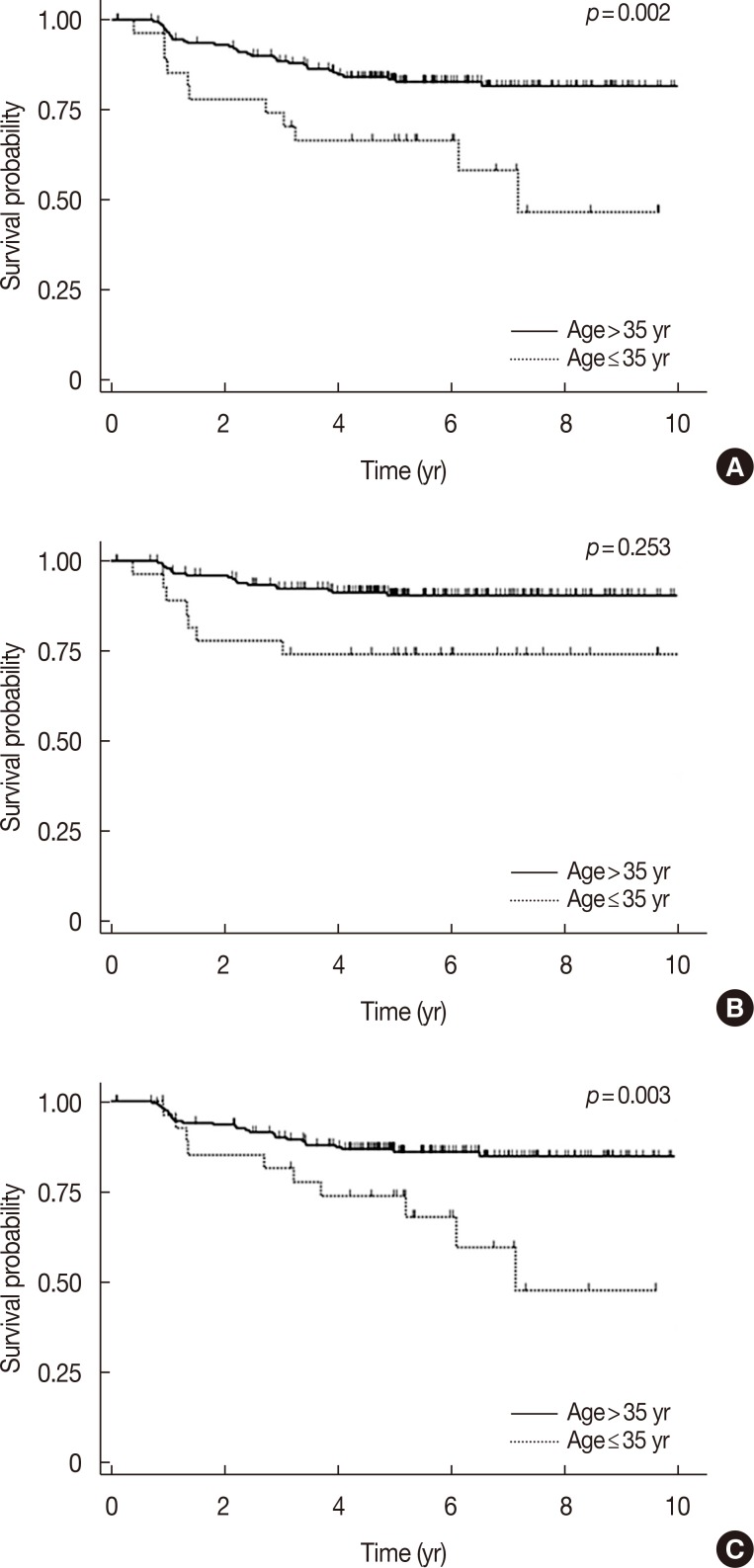
After the identification of significant variables in univariate analyses, we performed multivariate analyses to adjust for interaction between factors. As shown in Table 4, multivariate analysis showed that the MNPI was the most significant and common prognostic factor of DFS (p=0.001) and OS (p=0.019). Patients with high MNPIs had a threefold increased risk of failure or death (hazard ratio [HR] for DFS, 3.0; for OS, 2.98). In detailed analysis with respect to failure type, the MNPI retained its significance for DMFS (p=0.002, HR 3.37). Figure 1 shows survival according to MNPI. Young age (≤35 years) also correlated with poor DFS (p=0.006) (Figure 2). All other variables considered, the statistical significance on DFS of pathologic tumor size, LN status, ECE, multiplicity, LVI, and NPI disappeared.
Table 4. Multivariate analysis.
| Characteristic | HR (95% CI) | p-value* | |
|---|---|---|---|
| DFS | |||
| MNPI | ≤ 6.5 | 1 | 0.001 |
| > 6.5 | 3.00 (1.58–6.13) | ||
| Age (yr) | ≤ 35 | 1 | 0.006 |
| > 35 | 0.36 (0.17–0.75) | ||
| OS | |||
| MNPI | ≤ 6.5 | 1 | 0.019 |
| > 6.5 | 2.98 (1.20–7.42) | ||
| Nodal ratio (%) | ≤ 20 | 1 | 0.038 |
| > 20 | 2.75 (1.06–7.14) | ||
| LRFS | |||
| LVI | Negative | 1 | 0.001 |
| Positive | 3.90 (1.73–8.80) | ||
| Age (yr) | ≤ 35 | 1 | 0.005 |
| > 35 | 0.28 (0.12–0.68) | ||
| DMFS | |||
| MNPI | ≤ 6.5 | 1 | 0.002 |
| > 6.5 | 3.37 (1.56–7.28) | ||
| Age (yr) | ≤ 35 | 1 | 0.015 |
| > 35 | 0.38 (0.18–0.83) | ||
| Nodal ratio (%) | ≤ 20 | 1 | 0.035 |
| > 20 | 2.48 (1.07–5.77) |
HR=hazard ratio; CI=confidence interval; DFS=disease-free survival; MNPI =modified Nottingham prognostic index; OS =overall survival; LRFS=locoregional recurrence-free survival; LVI=lymphovascular invasion; DMFS=distant metastasis-free survival.
*p-value by Cox-regression forward conditional stepwise method.
Figure 1. Survival outcomes according to modified Nottingham Prognostic Index (MNPI) scores. (A) Disease-free survival, (B) overall survival, and (C) distant metastasis-free survival.
Figure 2. Survival outcomes according to age at diagnosis. (A) Disease-free survival, (B) overall survival, and (C) distant metastasis-free survival.
Prognostic model for DFS
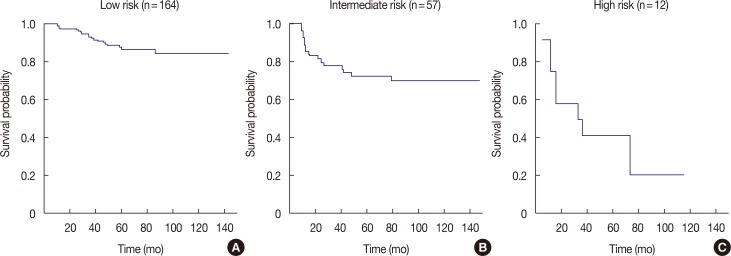
Based on the results of multivariate analysis, we performed a recursive partitioning for establishing a prognostic model for DFS. As shown in Figure 3, the patients were divided into three risk groups. Patients with a low MNPI (≤6.5) were stratified into the low-risk group (p<0.001). Patients with a high MNPI (>6.5) were subdivided according to age into two groups: intermediate (>35 years) and high-risk (≤35 years). Age was not an important factor in patients with a low MNPI, whereas young age was the second key factor in subdividing patients with a high MNPI according to prognosis (p=0.023). The 5-year DFS of patients with high risk (high MNPI and age ≤35 years) was estimated as 41.7%, in contrast with patients with intermediate risk (high MNPI and age >35 years; 72.7%).
Figure 3. Estimated disease-free survival by recursive partitioning analysis. (A) Low risk group; modified Nottingham Prognostic index (MNPI) ≤6.5, (B) intermediate risk group; MNPI >6.5 and age >35 years, and (C) high risk group; MNPI >6.5 and age ≤35 years.
For the validation of the impact of age on DFS, multivariate analysis of subgroup according to MNPI was performed. This also demonstrated that young age was a significant factor in the high MNPI group (p=0.034, HR, 2.65), while its significance disappeared in the low MNPI group.
DISCUSSION
This study identified the prognostic value of the MNPI in TNBC. A high MNPI was found to contribute to decreased DFS and OS. Patients less than 35 years of age had decreased DFS, though age was not correlated with decreased OS. Interestingly, however, the influence of age varied between the risk groups according to MNPI. In patients with a low MNPI, survival outcome was not affected by age, but DFS was significantly decreased in patients less than 35 years of age with a high MNPI.
To the best of our knowledge, no previous study has evaluated the prognostic value of the MNPI in TNBC cases including a non-PST group, although the NPI has been validated in a large-scale study without a PST group [6,7,8]. In a study of 168 patients who did not receive PST, Albergaria et al. [10] showed that the NPI is able to predict prognosis in TNBC patients. In this study, the MNPI, including MSBR grade, was not analyzed. The first study evaluating the MNPI and other NPI-related indices showed that MBGI was the only prognosticator for DFS in multivariate analysis [9]. However, a subsequent study including 710 patients demonstrated a high prognostic significance of the MSBR and MNPI [13]. Unlike our study, these studies were conducted only with patients, regardless of intrinsic subtype, who had been treated with PST.
The reason why the MNPI is more closely related to prognosis than the NPI can be ascribed to differences in the histologic grade system. Use of MSBR to score the histologic grade is based on nuclear pleomorphism and mitosis without considering tubular formation. Thus, it allows the grading of all tumors, including non-invasive ductal carcinoma, unlike SBR. Also, unlike SBR's three groups, MSBR categorizes tumors into five groups [12]. In this study, most of the patients were SBR grade 3 (78.92%). However, when applying MSBR, the patients with SBR grade 3 were classified into two categories (MSBR grade 4, 23.6%; MSBR grade 5, 76.4%), and patients with SBR grade 2 were separated into MSBR grades 1 to 4 (Supplementary Figure 1, available online). This resulted in high prognostic influence outcomes (MSBR vs. SBR: DFS, p=0.07 vs. p=0.90; DMFS, p=0.03 vs. p=0.55). These findings are consistent with those reported in earlier studies [12,13].
It is well known that TNBC is associated with young age at diagnosis [2,14,15]. However, the prognostic value of young age in TNBC remains controversial. Lee et al. [14] showed that, although age had more influence on survival, age <35 years was not a prognosticator in TNBC. Fayaz et al. [15] also reported that young age did not correlate with other prognostic factors, such as histologic grade, T stage, N stage, LVI, and Ki-67 positivity, and did not negatively impact any survival outcomes of TNBC patients. Meanwhile, our study showed that young age was significantly associated with decreased DFS, but not OS. Ovcaricek et al. [16] observed that patients younger than 65 years had a higher risk of relapse, as compared to older patients; however, as with our results, younger age did not result in decreased OS in multivariate analysis, despite the discrepancy in cutoff value. Taken together, it remains unclear whether younger patients are at a higher risk of death from TNBC than older patients. However, an impact of young age on recurrence could not be completely ruled out. As shown in this study, the prognostic significance of young age might be enhanced in the high-risk subgroup. This inconsistency between studies may be because previous studies did not analyze the prognostic impact of young age according to other risk factors, such as the MNPI. Thus, further investigation is warranted.
TNBC has a high propensity for DF, despite an LRF rate similar to the luminal subtypes [17]. Therefore, it is important to identify the patients at risk of distant metastasis. The shortened DFS observed in patients with a high MNPI and young age seems to stem mainly from a high rate of DF in our study. In addition to high MNPI and age, an NR >0.2 was also significantly associated not only with poor DMFS, but also with poor OS.
The number of positive axillary LNs has been considered to be the most important prognostic factor in breast cancer [11,13,18]. Thus, N stage is determined solely by the number of positive LNs. However, the possibility of underestimating the LN status by incomplete axillary LN dissection has been repeatedly raised, since a more extensive axillary LN dissection could increase the chances of finding positive LNs [18]. Furthermore, the growing use of sentinel LN biopsy leads to a less extensive axillary evaluation. It has been suggested that NR could adjust the extent of dissection.
NR as a prognosticator of breast cancer has been validated in our previous studies, as well as large-scale studies [11,18]. Meta-analysis also showed that NR significantly correlates with OS, DFS, and breast cancer-specific survival [19]. However, in most studies of TNBC patients, the prognostic significance of nodal status was evaluated by the number of positive LNs, not NR. These studies demonstrated that patients with fewer positive LNs had significantly more favorable DFS and OS [20,21]. Recently, Solak et al. [22] reported a clearer prognostic separation for TNBC using NR as compared to using pN staging. These results are consistent with our findings, but the prognostic value of NR in TNBC requires further validation in a large study.
In addition to the abovementioned traditional factors, there have recently been attempts to identify biomolecular markers for more individualized prediction. Ki-67, which reflects the proliferation rate of various malignant tumors, has been suggested as a prognostic or predictive factor in breast cancer [23,24]. In our study, the median Ki-67 value was 40%. This was similar to a previous report showing that TNBC had a higher Ki-67 index (median, 50%) than other breast cancer subtypes [24]. The study by Nishimura et al. [24] that included 356 patients with TNBC revealed that tumor size and nodal status, but not Ki-67 (cutoff value, 20%), significantly affected DFS in multivariate analysis. They also showed that the higher Ki-67 index was closely related with the following clinicopathologic factors in their whole cohort (including 2,638 breast cancer patients): young age, large tumor size, positive LNs, a high nuclear grade, negative for ER/PR, p53 overexpression and positive for HER2. Similarly, in our study, despite no association between prognosis and Ki-67, the Ki-67 index was significantly higher in patients of young age (p=0.021) and with a higher MNPI (p=0.020). However, high Ki-67 expression was not correlated with various survival outcomes. Keam et al. [25] reported that TNBCs with high Ki-67 (≥10%) expression were associated with a higher pCR rate than TNBCs with low Ki-67 expression, and high Ki-67 was correlated with poor DFS and OS, paradoxically, in 105 TNBC patients treated with PST. Several recent retrospective studies have consistently suggested that Ki-67 may be a prognosticator in TNBC patients, as well as in patients with luminal type A breast cancer [20,26]. However, this needs further study to examine whether the Ki-67 index can be used to subdivide TNBC.
COX-2 is a key enzyme for the production of prostaglandins, and elevated prostaglandins can enhance angiogenesis, cell proliferation and tumor cell invasion. In breast cancer, COX-2 has been observed to be expressed more frequently in TNBC than in other subtypes [27]. However, there are few studies addressing the relationship between COX-2 expression and prognosis in TNBC patients. Kim et al. [28] reported that COX-2 expression translated into shortened relapse-free survival in ER negative breast cancer, but they did not report any association with TNBC. Chikman et al. [29] showed that TNBC patients with COX-2 expression had significantly decreased DFS. However, the study consisted of a small number of TNBC patients (67 patients). In this study, we found no relation between COX-2 expression and survival outcome. It is still difficult to draw a clinical relevance of COX-2 expression in TNBC, considering the scarcity of data.
Some studies have reported that the relationship between stage and survival outcomes is not clear in TNBC, unlike other intrinsic subtypes [1,2]. Nevertheless, many researchers have observed that tumor size or LN status have a significant association with DFS or OS in TNBC patients [14,16,20,21]. Thus, the MNPI, which is calculated with input from tumor size, LN stage, and modified histologic grade, appears to be theoretically appropriate to predict the prognosis of patients with TNBC, and our study revealed that the MNPI is the most significant prognosticator for DFS, DMFS, and OS. With the increasing use of PST, the prognostic value of the MNPI based on surgical specimens after PST has already been determined [9,13] and our subgroup analysis yielded identical results (DFS and OS, p<0.001 for both in the PST group). Therefore, the MNPI could be applied to predict prognosis in the PST group. Various tools for stratifying patients according to prognosis, in particular, those including molecular markers, have been developed [30]. These tools could help us consider an individual patient's biological features. However, the advantage of the MNPI is that it does not require additional efforts or costs.
This study is the first to evaluate the prognostic impact of the MNPI on DFS, DMFS, and OS. In addition, our recursive partitioning revealed that young age (≤35 years) is an important prognosticator of DFS in patients with a high MNPI. To the best of our knowledge, we are the first to demonstrate that the prognostic impact of young age could change according to risk group. This study has several limitations related to it being a retrospective study. Furthermore, our study includes a relatively small number of patients, and the population was heterogeneous in terms of stage, pathologic features, and treatment modality. In particular, the mixed cohort of patients in our study with regards to PST suggests that the effects of prognosticators be confounded. Thus, the results should be interpreted with caution. Additionally, the follow-up duration was not sufficiently long, considering the disease course of breast cancer. Therefore, caution should be exercised when drawing definite conclusions from our results. However, to minimize the selection bias, we tried to include all consecutive patients with TNBC. This increased the heterogeneity of the patients, but could have had an impact on the realistic clinical setting.
In conclusion, the MNPI that contains information on tumor size, LN status, and tumor grade according to MSBR can be used to stratify patients with stage I to III TNBC according to prognosis. It was the most important prognosticator for DFS, DMFS, and OS in patients with TNBC. The prognostic meaning of young age for DFS (especially with respect to DMFS) varied according to MNPI in our recursive partitioning analysis.
ACKNOWLEDGMENTS
Sung-Won Kim currently works at Daerim St. Mary's Hospital, Seoul, Korea.
Footnotes
This work was supported by Seoul National University Bundang Hospital Research Fund (grant number: 11-2011-014) and Cancer Control Program from Korean Ministry of Health & Welfare (grant number: 0820010).
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
SUPPLEMENTARY MATERIAL
Distribution of tumor histologic grades according to the grading system.
MSBR=modified Scarff-Bloom-Richardson; SBR=Scarff-Bloom-Richardson.
References
- 1.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 2.Criscitiello C, Azim HA, Jr, Schouten PC, Linn SC, Sotiriou C. Understanding the biology of triple-negative breast cancer. Ann Oncol. 2012;23(Suppl 6):vi13–vi18. doi: 10.1093/annonc/mds188. [DOI] [PubMed] [Google Scholar]
- 3.Brewster AM, Chavez-MacGregor M, Brown P. Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. Lancet Oncol. 2014;15:e625–e634. doi: 10.1016/S1470-2045(14)70364-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gogia A, Raina V, Deo SV, Shukla NK, Mohanti BK. Triple-negative breast cancer: an institutional analysis. Indian J Cancer. 2014;51:163–166. doi: 10.4103/0019-509X.138275. [DOI] [PubMed] [Google Scholar]
- 5.Haybittle JL, Blamey RW, Elston CW, Johnson J, Doyle PJ, Campbell FC, et al. A prognostic index in primary breast cancer. Br J Cancer. 1982;45:361–366. doi: 10.1038/bjc.1982.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galea MH, Blamey RW, Elston CE, Ellis IO. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat. 1992;22:207–219. doi: 10.1007/BF01840834. [DOI] [PubMed] [Google Scholar]
- 7.Blamey RW, Ellis IO, Pinder SE, Lee AH, Macmillan RD, Morgan DA, et al. Survival of invasive breast cancer according to the Nottingham Prognostic Index in cases diagnosed in 1990-1999. Eur J Cancer. 2007;43:1548–1555. doi: 10.1016/j.ejca.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Balslev I, Axelsson CK, Zedeler K, Rasmussen BB, Carstensen B, Mouridsen HT. The Nottingham Prognostic Index applied to 9,149 patients from the studies of the Danish Breast Cancer Cooperative Group (DBCG) Breast Cancer Res Treat. 1994;32:281–290. doi: 10.1007/BF00666005. [DOI] [PubMed] [Google Scholar]
- 9.Chollet P, Amat S, Belembaogo E, Curé H, de Latour M, Dauplat J, et al. Is Nottingham Prognostic Index useful after induction chemotherapy in operable breast cancer? Br J Cancer. 2003;89:1185–1191. doi: 10.1038/sj.bjc.6601258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albergaria A, Ricardo S, Milanezi F, Carneiro V, Amendoeira I, Vieira D, et al. Nottingham Prognostic Index in triple-negative breast cancer: a reliable prognostic tool? BMC Cancer. 2011;11:299. doi: 10.1186/1471-2407-11-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koo TR, Eom KY, Kang EY, Kim YJ, Kim SW, Kim JH, et al. Prognostic value of the nodal ratio and Ki-67 expression in breast cancer patients treated with postmastectomy radiotherapy. J Breast Cancer. 2013;16:274–284. doi: 10.4048/jbc.2013.16.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Doussal V, Tubiana-Hulin M, Friedman S, Hacene K, Spyratos F, Brunet M. Prognostic value of histologic grade nuclear components of Scarff-Bloom-Richardson (SBR): an improved score modification based on a multivariate analysis of 1262 invasive ductal breast carcinomas. Cancer. 1989;64:1914–1921. doi: 10.1002/1097-0142(19891101)64:9<1914::aid-cncr2820640926>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 13.Abrial SC, Penault-Llorca F, Delva R, Bougnoux P, Leduc B, Mouret-Reynier MA, et al. High prognostic significance of residual disease after neoadjuvant chemotherapy: a retrospective study in 710 patients with operable breast cancer. Breast Cancer Res Treat. 2005;94:255–263. doi: 10.1007/s10549-005-9008-8. [DOI] [PubMed] [Google Scholar]
- 14.Lee JA, Kim KI, Bae JW, Jung YH, An H, Lee ES, et al. Triple negative breast cancer in Korea-distinct biology with different impact of prognostic factors on survival. Breast Cancer Res Treat. 2010;123:177–187. doi: 10.1007/s10549-010-0998-5. [DOI] [PubMed] [Google Scholar]
- 15.Fayaz MS, Demian GA, El-Sherify M, Abuzalouf S, George T, Samir S, et al. Is young age a poor prognostic factor in triple-negative breast cancer patients? Analysis of 363 patients from single institution registry. J Clin Oncol. 2013;31(5 Suppl):e12023. [Google Scholar]
- 16.Ovcaricek T, Frkovic SG, Matos E, Mozina B, Borstnar S. Triple negative breast cancer: prognostic factors and survival. Radiol Oncol. 2011;45:46–52. doi: 10.2478/v10019-010-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson SJ, Provenzano E, Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur J Cancer. 2009;45(Suppl 1):27–40. doi: 10.1016/S0959-8049(09)70013-9. [DOI] [PubMed] [Google Scholar]
- 18.Dings PJ, Elferink MA, Strobbe LJ, de Wilt JH. The prognostic value of lymph node ratio in node-positive breast cancer: a Dutch nationwide population-based study. Ann Surg Oncol. 2013;20:2607–2614. doi: 10.1245/s10434-013-2932-7. [DOI] [PubMed] [Google Scholar]
- 19.Liu D, Chen Y, Deng M, Xie G, Wang J, Zhang L, et al. Lymph node ratio and breast cancer prognosis: a meta-analysis. Breast Cancer. 2014;21:1–9. doi: 10.1007/s12282-013-0497-8. [DOI] [PubMed] [Google Scholar]
- 20.Huang L, Liu Z, Chen S, Liu Y, Shao Z. A prognostic model for triple-negative breast cancer patients based on node status, cathepsin-D and Ki-67 index. PLoS One. 2013;8:e83081. doi: 10.1371/journal.pone.0083081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asaga S, Kinoshita T, Hojo T, Suzuki J, Jimbo K, Tsuda H. Prognostic factors for triple-negative breast cancer patients receiving preoperative systemic chemotherapy. Clin Breast Cancer. 2013;13:40–46. doi: 10.1016/j.clbc.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Solak M, Turkoz FP, Keskin O, Aksoy S, Babacan T, Sarici F, et al. The lymph node ratio as an independent prognostic factor for non-metastatic node-positive breast cancer recurrence and mortality. J BUON. 2015;20:737–745. [PubMed] [Google Scholar]
- 23.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174–183. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura R, Osako T, Okumura Y, Hayashi M, Toyozumi Y, Arima N. Ki-67 as a prognostic marker according to breast cancer subtype and a predictor of recurrence time in primary breast cancer. Exp Ther Med. 2010;1:747–754. doi: 10.3892/etm.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keam B, Im SA, Lee KH, Han SW, Oh DY, Kim JH, et al. Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res. 2011;13:R22. doi: 10.1186/bcr2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Han X, Liu Y, Liu G, Dong G. Ki67 as a predictor of poor prognosis in patients with triple-negative breast cancer. Oncol Lett. 2015;9:149–152. doi: 10.3892/ol.2014.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosalpuria K. COX-2 expression in operable triple negative breast cancer: a hospital based cross-sectional study [dissertation] [Houston, USA]: The University of Texas School of Public Health; 2010. [Google Scholar]
- 28.Kim HS, Moon HG, Han W, Yom CK, Kim WH, Kim JH, et al. COX2 overexpression is a prognostic marker for Stage III breast cancer. Breast Cancer Res Treat. 2012;132:51–59. doi: 10.1007/s10549-011-1521-3. [DOI] [PubMed] [Google Scholar]
- 29.Chikman B, Vasyanovich S, Lavy R, Habler L, Tolstov G, Kapiev A, et al. COX2 expression in high-grade breast cancer: evidence for prognostic significance in the subset of triple-negative breast cancer patients. Med Oncol. 2014;31:989. doi: 10.1007/s12032-014-0989-1. [DOI] [PubMed] [Google Scholar]
- 30.Rakha EA, Soria D, Green AR, Lemetre C, Powe DG, Nolan CC, et al. Nottingham Prognostic Index plus (NPI+): a modern clinical decision making tool in breast cancer. Br J Cancer. 2014;110:1688–1697. doi: 10.1038/bjc.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of tumor histologic grades according to the grading system.
MSBR=modified Scarff-Bloom-Richardson; SBR=Scarff-Bloom-Richardson.