Abstract
Purpose
We intended to determine whether dexrazoxane (DZR) is cardioprotective during administration of adjuvant anthracycline-based chemotherapy followed by a 1-year trastuzumab treatment.
Methods
The medical records of 228 patients who underwent surgical resection and received adjuvant chemotherapy with trastuzumab for human epidermal growth factor receptor type 2 (HER2)-positive breast cancer between January 2010 and December 2014 were reviewed. Approximately 25% of patients received DZR prior to each administration of doxorubicin during doxorubicin with cyclophosphamide (AC) chemotherapy. DZR was not administered during the 1-year trastuzumab maintenance period. Rates of cardiac events (reduction in left ventricular ejection fraction [LVEF] by 10% or more; reduction in absolute LVEF to <45%) and cardiac event-free duration (CFD) were examined. The trastuzumab interruption rate was also assessed.
Results
Twelve percent of patients experienced a cardiac event. Repeated-measures analysis of variance for ejection fraction revealed a significant main effect of time, and a significant group (DZR)×time interaction. The group treated with adjuvant chemotherapy and DZR experienced significantly lower frequencies of cardiac events than the adjuvant chemotherapy only group. In multivariate analysis, DZR administration was associated with significantly fewer cardiac events. Moreover, DZR administration was an independent good prognostic factor for CFD. Only one patient (2.3%) experienced early interruption of trastuzumab in the adjuvant chemotherapy with DZR group due to cardiac toxicity, whereas 10 patients (7.6%) experienced a trastuzumab stop event in the adjuvant chemotherapy only group.
Conclusion
DZR is cardioprotective in HER2-positive breast cancer patients who received adjuvant chemotherapy with trastuzumab. A large cohort randomized trial is needed to determine if DZR has an effect on trastuzumab interruption and completion of 12-month trastuzumab. Because cardiac toxicity has a significant negative effect on trastuzumab maintenance and quality of life, DZR administration could be considered concomitantly with anthracycline-based adjuvant chemotherapy with trastuzumab.
Keywords: Adjuvant chemotherapy, Breast neoplasms, Cardiotoxicity, Dexrazoxane, Trastuzumab
INTRODUCTION
Approximately 20% of patients with breast cancer have human epidermal growth factor receptor type 2 (HER2)-positive tumors; prior to the introduction of anti-HER2 therapy, these malignancies were associated with a worse prognosis [1]. The addition of trastuzumab to standard adjuvant chemotherapy in patients with HER2-positive early-stage breast cancer significantly improves the survival rates [2,3]. Based on these results, trastuzumab has become the standard of care for the treatment of HER2-positive early-stage breast cancer in the adjuvant setting. Although generally well tolerated, an important issue of concern regarding trastuzumab is cardiac toxicity, which can lead to treatment discontinuation, thus depriving patients of therapeutic benefits [4,5]. In particular, trastuzumab-induced cardiac toxicity often develops when combined with chemotherapy such as doxorubicin and epirubicin, agents with documented dose-related cardiac toxicity that can lead to chronic heart failure, reduced quality of life, and even death [6,7,8].
Dexrazoxane (DZR) is an inhibitor of topoisomerase IIb and an intracellular iron chelator. This latter property prevents complex formation between metal ions and anthracycline, thereby reducing anthracycline-dependent reactive oxygen species generation and cardiotoxicity [9]. Many clinical studies have suggested that DZR could significantly reduce the cardiac toxicity associated with anthracycline-based chemotherapy [10]. However, it is unclear whether the cardioprotective effects of DZR are maintained during anthracycline-based adjuvant chemotherapy followed by a 1-year trastuzumab treatment. Furthermore, although DZR is frequently considered in a metastatic setting in which higher cumulative doses of anthracycline are used, it is often omitted in the adjuvant setting in which the cumulative doses of anthracycline are comparatively low. However, the risk of cardiac toxicity in HER2-positive patients is high, because both trastuzumab and anthracycline-based chemotherapy are administered. Indeed, in clinical fieldwork, we encountered several cases in which the scheduled 1-year trastuzumab treatments were discontinued due to cardiac toxicity. Therefore, prevention of adjuvant-driven cardiac toxicity is important during the treatment of HER2-positive patients. Considering this, we evaluated whether DZR was cardioprotective in HER2-positive early-stage breast cancer patients receiving anthracycline-based adjuvant chemotherapy followed by a 1-year trastuzumab treatment.
METHODS
Study population
The present study's retrospective design was approved by the Institutional Review Board (KC16RISI0103) at the Seoul St. Mary's Hospital. We reviewed the medical records of 228 patients who underwent surgical resection and received adjuvant chemotherapy for HER2-positive breast cancer at the Seoul St. Mary's Hospital between January 2010 and December 2014. All diagnoses were pathologically confirmed via a surgical specimen from the primary tumor. We excluded 37 patients who had other neoplasms, kidney or heart transplantation, dialysis, heart failure, chronic pulmonary disease, hypertrophic cardiomyopathy, valvular heart disease, and/or aortic aneurysm and low cardiac function (left ventricular ejection fraction [LVEF], <55%). Sixteen patients who received neoadjuvant chemotherapy or prior anthracycline-based chemotherapy for other causes were excluded. Baseline characteristics were assessed at the chemotherapy start time, and the comorbidities of interest were smoking, hypertension (blood pressure ≥140/90 mm Hg, or those individuals taking hypertension medication), diabetes mellitus (DM; fasting glucose ≥126 mg/dL or taking DM medication), hyperlipidemia (low-density lipoprotein >160 mg/dL or taking hyperlipidemia medication), and being overweight (body mass index >25). Patients were classified as “never-smokers” (those with a lifetime exposure of 100 cigarettes or less) and “ever-smokers” (those with a lifetime exposure of more than 100 cigarettes) based on the guidelines of the U.S. Centers for Disease Control and Prevention [11].
Study design and echocardiographic evaluation
All patients received anthracycline-based adjuvant chemotherapy (four cycles of doxorubicin/cyclophosphamide [AC] or four cycles of AC followed by four cycles of docetaxel [AC+D]) followed by a 1-year of trastuzumab maintenance (8 mg/kg/day 1 followed by 6 mg/kg every 21 days). Anthracycline-based adjuvant chemotherapy followed by a 1-year course of trastuzumab was defined as “ADJ.” All adjuvant chemotherapy treatments were initiated within 2 months after surgery. DZR was administered to patients who wanted to receive DZR for insurance reasons. DZR was infused intravenously over approximately 15 minutes at a 10:1 dose ratio, 30 minutes prior to each administration of doxorubicin during AC chemotherapy. DZR was not administered during the 1-year trastuzumab maintenance period. Hormone therapy including treatment with antiestrogens (e.g., tamoxifen) and aromatase inhibitors was allowed in hormone receptor-positive patients.
An evaluation of LVEF through transthoracic echocardiography (TTE) was performed before the first cycle of AC or AC+D and the first cycle of trastuzumab. Follow-up evaluation of LVEF was performed every 4±1 months during trastuzumab maintenance. Follow-up TTE was performed 6 months after the conclusion of trastuzumab maintenance.
The primary efficacy parameter was the incidence of cardiac events. A cardiac event was defined as: a reduction in LVEF by 10% absolute percentage points or more as measured by TTE; reduction in absolute LVEF (measured by TTE) to <45%. The secondary efficacy parameter was the trastuzumab interruption rate.
Statistical analysis
The significance of the correlations between the clinicopathologic factors (age, hypertension, DM, overweight status, hyperlipidemia, smoking, stage, operation type, hormone receptor, prior radiotherapy, adjuvant chemotherapy regimen, and baseline low LVEF) and DZR administration was analyzed using the chi-square test. An LVEF change depending on DZR administration was assessed by repeated-measures analysis of variance (ANOVA). The significance of correlations between cardiac events and DZR was analyzed using Fisher exact test. Univariate binary logistic regression was used to estimate the probability of cardiac events based on DZR and potential cardiac risk factors (age, hypertension, DM, overweight, hyperlipidemia, smoking, radiation, aggressive chemotherapy, and low baseline LVEF). Multivariate binary logistic regression with significant potential risk factors (p-value <0.2 in univariate analysis) was used to verify the cardioprotective effects of DZR use on cardiac events.
The cardiac event-free duration (CFD) was calculated from the date on which anthracycline-based adjuvant chemotherapy was started until the date of the cardiac event. Overall survival (OS) and relapse-free survival (RFS) were calculated from the date on which anthracycline-based adjuvant chemotherapy was started until the date of death or date of disease relapse, respectively. For the survival analyses, patients who were alive, in a disease-free state, and free of cardiac events causing trastuzumab withdrawal from the date of last contact were censored. Univariate analyses for CFD were performed using the Kaplan-Meier method with the log-rank test. Multivariate analyses were performed using Cox regression models for CFD with significant potential risk factors (p-value < 0.2 in univariate analysis) to verify the impact of DZR. All statistical analyses were performed using SPSS software version 22 (IBM Corp., Armonk, USA), and a two-sided p-value of <0.05 was considered statistically significant.
RESULTS
A total of 175 female patients were included in our analyses, and their characteristics are listed in Table 1. The median patient age was 53 years (range, 22–70 years). The median follow-up for the survival analysis was 32.3 months (range, 18.1–73.8 months). Twenty-one patients (12.0%) experienced a cardiac event. A total of 38 (21.7%) and 29 patients (16.6%) had hypertension and DM, respectively. The frequency of overweight status or hyperlipidemia was 25.1% (44/175) and 22.9% (40/175), respectively. A total of 20 patients (11.4%) were ever-smokers. The pathology was stage I in 32 patients (18.3%), stage II in 117 patients (66.9%), and stage III in 26 patients (14.9%). The mean baseline LVEF was 59.76% (range, 55%–77%). Cumulative doxorubicin dose prior to start of trastuzumab was 240 mg/m2 in all patients. A total of 44 patients (25.1%) were allocated to the “adjuvant anthracycline-based chemotherapy with DZR followed by trastuzumab” (ADJ with DZR) group, whereas 131 (74.9%) were categorized in to the “only adjuvant anthracycline-based chemotherapy followed by trastuzumab” (ADJ) group.
Table 1. Baseline characteristics.
| Characteristic | Total (n = 175) No. (%) |
Only ADJ (n = 131) No (%) |
ADJ with DZR (n = 44) No. (%) |
p-value |
|---|---|---|---|---|
| Age (yr)* | 53 (22–70) | 53 (22–70) | 53 (31–64) | 0.889 |
| ≥ 55 | 74 (42.3) | 55 (42.0) | 19 (43.2) | |
| < 55 | 101 (57.7) | 76 (58.0) | 25 (56.8) | |
| Hypertension | 0.541 | |||
| Yes | 38 (21.7) | 27 (20.6) | 11 (25.0) | |
| No | 137 (78.3) | 104 (79.4) | 33 (75.0) | |
| DM | 0.204 | |||
| Yes | 29 (16.6) | 19 (14.5) | 10 (22.7) | |
| No | 146 (83.4) | 112 (85.5) | 34 (77.3) | |
| Overweight | 0.707 | |||
| Yes | 44 (25.1) | 32 (24.4) | 12 (27.3) | |
| No | 131 (74.9) | 99 (75.6) | 32 (72.7) | |
| Hyperlipidemia | 0.981 | |||
| Yes | 40 (22.9) | 30 (22.9) | 10 (22.7) | |
| No | 135 (77.1) | 101 (77.1) | 34 (77.3) | |
| Smoking† | 0.411‡ | |||
| Ever-smoker | 20 (11.4) | 17 (13.0) | 3 (6.8) | |
| Nonsmoker | 155 (88.6) | 114 (87.0) | 41 (93.2) | |
| Pathologic stage | 0.963 | |||
| I | 32 (18.3) | 24 (18.3) | 8 (18.2) | |
| II | 117 (66.9) | 87 (66.4) | 30 (68.2) | |
| III | 26 (14.9) | 20 (15.3) | 6 (13.6) | |
| Operation type | 0.365 | |||
| BCS with SLNB | 42 (24.0) | 34 (26.0) | 8 (18.2) | |
| BCS with ALND | 56 (32.0) | 41 (31.3) | 15 (34.1) | |
| SM with SLNB | 24 (13.7) | 20 (15.3) | 4 (9.1) | |
| MRM | 53 (30.3) | 36 (27.5) | 17 (38.6) | |
| Hormone receptor | 0.316 | |||
| Negative | 96 (54.9) | 69 (52.7) | 27 (61.4) | |
| Positive | 79 (45.1) | 62 (47.3) | 17 (38.6) | |
| Radiation before trastuzumab | 0.334 | |||
| Yes | 108 (61.7) | 78 (59.5) | 30 (68.2) | |
| No | 67 (38.3) | 53 (40.5) | 14 (31.8) | |
| Regimen | 0.104 | |||
| AC followed by trastuzumab | 74 (42.3) | 60 (45.8) | 14 (31.8) | |
| AC+docetaxel followed by trastuzumab | 101 (57.7) | 71 (54.2) | 30 (68.2) | |
| LVEF (%)§ | 59.76 ± 3.48 | 59.85 ± 3.66 | 59.48 ± 2.89 | 0.055 |
| ≥ 60 | 70 (40.0) | 47 (35.9) | 23 (52.3) | |
| < 60 | 105 (60.0) | 84 (64.1) | 21 (47.7) |
ADJ=anthracycline-based adjuvant chemotherapy followed by trastuzumab; DZR=dexrazoxane; DM=diabetes mellitus; BCS=breast-conserving surgery; SLNB=sentinel lymph node biopsy; ALND=axillary lymph node dissection; SM=simple mastectomy; MRM =modified radical mastectomy; AC = doxorubicin with cyclophosphamide; LVEF=left ventricular ejection fraction.
*Median (range); †Smoking was classified as nonsmokers (those with a lifetime exposure of 100 cigarettes or less) and ever-smokers (those with a lifetime exposure of more than 100 cigarettes); ‡Fisher exact test; §Mean±SD.
Cardioprotective effect of dexrazoxane
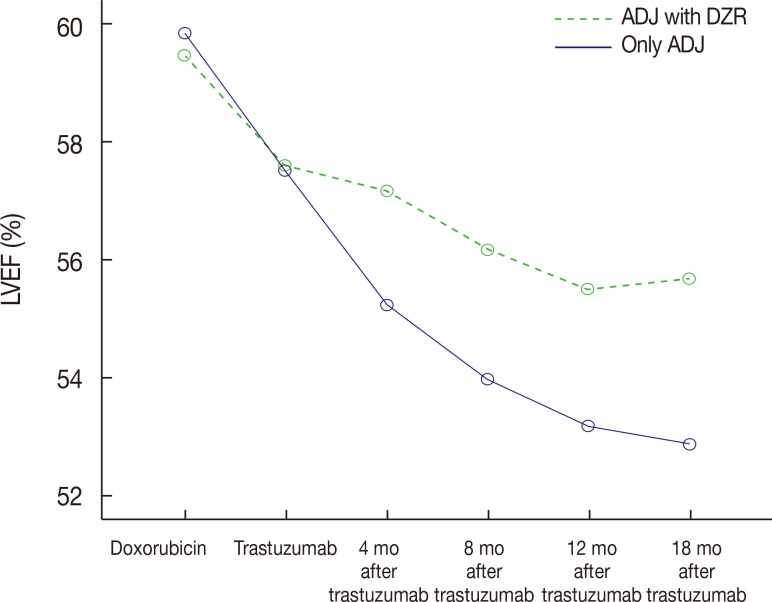
The repeated-measures ANOVA for LVEF (Table 2) revealed a significant main effect of time (F=83.659, p<0.001) and a significant group (DZR)×time interaction (F=8.122, p < 0.001). Figure 1 shows the changes in mean LVEF over time. The mean baseline LVEF was 59.9%±3.7% and 59.5%±2.9% in the ADJ only group and the ADJ with DZR group. However, the ADJ only group had a lower LVEF than did the ADJ with DZR group at 4 months (55.2%±5.3% vs. 57.2%±4.4%), 8 months (54.0%±6.1% vs. 56.2% ±3.3%), 12 months (53.2%±6.6% vs. 55.5%±3.3%), and 18 months (52.9%±6.5% vs. 55.7%±3.6%) after beginning trastuzumab treatment.
Table 2. Left ventricular ejection fraction change analysis by repeated measures ANOVA.
| Group | LVEF (%) | ANOVA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-AC chemotherapy (SD) | T start (SD) | 4 mo (SD) | 8 mo (SD) | 12 mo (SD) | 18 mo (SD) | Group | Time | Group×time | ||||
| F* | p-value | F* | p-value | F* | p-value | |||||||
| No DZR | 59.9 (3.7) | 57.5 (4.2) | 55.2 (5.3) | 54.0 (6.1) | 53.2 (6.6) | 52.9 (6.5) | 3.748 | 0.054 | 83.659 | <0.001 | 8.122 | <0.001 |
| DZR | 59.5 (2.9) | 57.6 (3.9) | 57.2 (4.4) | 56.2 (3.3) | 55.5 (3.3) | 55.7 (3.6) | ||||||
ANOVA=analysis of variance; LVEF=left ventricular ejection fraction; AC=doxorubicin with cyclophosphamide; SD=standard deviation; T=trastuzumab; DZR=dexrazoxane.
*F for group, time and group×time interaction.
Figure 1. Changes in mean left ventricular ejection fraction (LVEF) depending on dexrazoxane (DZR) administration over time. Only ADJ group showed significant decrease of LVEF than ADJ with DZR group (group×time interaction, F=8.122, p<0.001). F for group, time and group×time interaction.
ADJ=anthracycline-based adjuvant chemotherapy followed by trastuzumab.
The associations between cardiac events and DZR were evaluated. A significantly lower incidence of cardiac events (p=0.029) occurred in the ADJ with DZR group compared to the ADJ only group. To determine whether DZR administration correlated with the efficacy of cardioprotection, we analyzed the causal relationships between DZR administration and cardiac events. Univariate analysis revealed that DZR administration to patients with anthracycline-based adjuvant chemotherapy was associated with lower rates of cardiac events (odds ratio [OR], 0.13; 95% confidence interval [CI], 0.02–0.99; p=0.049). In multivariate analysis using a binary logistic regression model, DZR administration was a factor associated with significantly lower frequencies of cardiac events (OR, 0.01; 95% CI, 0.01–0.89; p=0.039). No other clinicopathologic factors were associated with significant changes in the frequency of cardiac events (Table 3).
Table 3. Risk factors for cardiac event.
| Cardiac event | ||||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age (yr) | - | - | - | |||
| < 55 | 1 | |||||
| ≥ 55 | 1.278 | 0.512–3.190 | 0.599 | |||
| Hypertension | ||||||
| No | 1 | 1 | ||||
| Yes | 1.984 | 0.738–5.334 | 0.175 | 2.498 | 0.852–7.329 | 0.095 |
| DM | - | - | - | |||
| No | 1 | |||||
| Yes | 1.693 | 0.567–5.057 | 0.346 | |||
| Overweight | - | - | - | |||
| No | 1 | |||||
| Yes | 0.459 | 0.129–1.641 | 0.231 | |||
| Hyperlipidemia | - | - | - | |||
| No | 1 | |||||
| Yes | 0.771 | 0.244–2.439 | 0.658 | |||
| Smoking | - | - | - | |||
| No | 1 | |||||
| Yes | 1.343 | 0.358–5.038 | 0.662 | |||
| Radiation | ||||||
| No | 1 | 1 | ||||
| Yes | 2.157 | 0.751–6.191 | 0.153 | 2.832 | 0.932–8.599 | 0.066 |
| Regimen | ||||||
| AC+T | 1 | 1 | ||||
| AC+T with docetaxel | 1.977 | 0.728–5.367 | 0.181 | 2.499 | 0.864–6.942 | 0.092 |
| Baseline LVEF | ||||||
| ≥ 60% | 1 | 1 | ||||
| < 60% | 2.337 | 0.815–6.704 | 0.114 | 2.075 | 0.691–6.235 | 0.193 |
| Dexrazoxane use | ||||||
| No | 1 | 1 | ||||
| Yes | 0.129 | 0.017–0.992 | 0.049 | 0.012 | 0.014–0.891 | 0.039 |
OR=odds ratio; CI=confidence interval; DM=diabetes mellitus; AC+T=doxorubicin with cyclophosphamide+trastuzumab; LVEF=left ventricular ejection fraction.
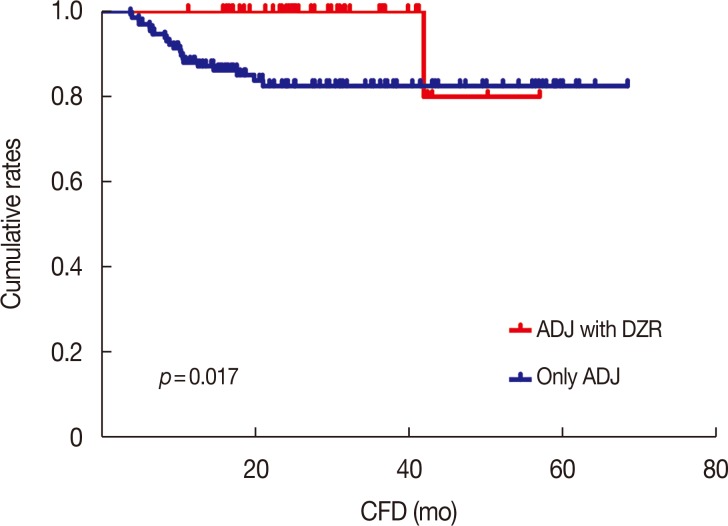
The CFD was analyzed based on whether DZR was administered. Kaplan-Meier analysis with the log-rank test showed that ADJ with DZR prolonged the CFD compared with only ADJ (p=0.017) (Figure 2). Multivariate analysis using a Cox regression model was also performed (Table 4). DZR administration was an independent factor of good prognosis for CFD (hazard ratio [HR], 0.12; 95% CI, 0.02–0.90; p=0.040). None of the other clinicopathologic factors was significantly associated with CFD. Finally, multivariate analysis revealed that neither OS (p=0.941) nor RFS (p=0.808) were significantly prolonged by DZR treatment.
Figure 2. Kaplan-Meier analysis with the log-rank test showed that ADJ with dexrazoxane (DZR) group showed remarkable prolonged cardiac event-free duration (CFD) (p=0.017) compared with only ADJ group. A cumulative 3-year cardiac event free rates were 100% and 87.1% in ADJ with DZR and only ADJ group, respectively.
ADJ=anthracycline-based adjuvant chemotherapy followed by trastuzumab.
Table 4. Univariate and multivariate analysis for cardiac event-free duration.
| Cardiac event-free duration | ||||||
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (yr) | - | - | - | |||
| < 55 | 1 | |||||
| ≥ 55 | 1.350 | 0.571–3.192 | 0.494 | |||
| Hypertension | ||||||
| No | 1 | 1 | ||||
| Yes | 1.938 | 0.780–4.813 | 0.154 | 1.961 | 0.787–4.887 | 0.148 |
| DM | - | - | - | |||
| No | 1 | |||||
| Yes | 1.751 | 0.641–4.781 | 0.275 | |||
| Overweight | - | - | - | |||
| No | 1 | |||||
| Yes | 0.536 | 0.156–1.836 | 0.321 | |||
| Hyperlipidemia | - | - | - | |||
| No | 1 | |||||
| Yes | 0.807 | 0.271–2.401 | 0.700 | |||
| Smoking | - | - | - | |||
| No | 1 | |||||
| Yes | 1.423 | 0.419–4.832 | 0.572 | |||
| Radiation | - | - | - | |||
| No | 1 | |||||
| Yes | 1.906 | 0.698–5.204 | 0.208 | |||
| Regimen | ||||||
| AC+T | 1 | 1 | ||||
| AC+T with docetaxel | 2.048 | 0.794–5.283 | 0.138 | 2.524 | 0.973–6.547 | 0.057 |
| Baseline LVEF | ||||||
| ≥ 60% | 1 | 1 | ||||
| < 60% | 2.147 | 0.786–5.865 | 0.136 | 1.838 | 0.665–5.077 | 0.241 |
| Dexrazoxane use | ||||||
| No | 1 | 1 | ||||
| Yes | 0.128 | 0.017–0.953 | 0.045 | 0.119 | 0.016–0.904 | 0.040 |
HR=hazard ratio; CI=confidence interval; DM=diabetes mellitus; AC+T=doxorubicin with cyclophosphamide+trastuzumab; LVEF=left ventricular ejection fraction.
Trastuzumab interruption and dexrazoxane
The mean duration of trastuzumab treatment was 11.5 months in the ADJ with DZR group and 11.0 months in the ADJ only group. Only 2 patients (4.5%) experienced early interruption of trastuzumab in the ADJ with DZR group, one due to cardiac toxicities (2.3%) and the other due to a personal reason. No patient was administered trastuzumab for a period shorter than 6 months in the ADJ with DZR group. In the ADJ only group, 14 patients (10.6%) experienced a trastuzumab stop event. Reasons for the interruption of trastuzumab included cardiac toxicities in 10 (7.6%) and other causes in four patients (3.1%). Five patients were administered trastuzumab for a period shorter than 6 months (four due to cardiac toxicity and one due to another reason) in the ADJ only group. DZR administration was not associated with the trastuzumab early interruption rate due to cardiotoxicities (p=0.295).
DISCUSSION
Our study was designed to determine whether DZR exerted cardioprotective effects during adjuvant anthracycline-based chemotherapy followed by a 1-year trastuzumab treatment, and to determine whether this altered the trastuzumab withdrawal rates.
Anthracyclines such as doxorubicin have been applied effectively in the treatment of breast cancer [12]. Randomized controlled studies show that administration of DZR with anthracyclines significantly reduces cardiac toxicity [13,14]. Specifically, Lopez et al. [13] showed that DZR significantly protected against epirubicin-induced cardiac toxicity without limiting the drug's antitumor capacity. Swain et al. [14] also showed that DZR had significant cardioprotective effects in patients treated with doxorubicin. However, few studies have analyzed the effects of DZR in HER2-positive breast cancer patients treated with anthracyclines and trastuzumab in an adjuvant setting. It is important that DZR was not administered in many cases, since in the adjuvant setting, the cumulative dose of anthracyclines is typically less than the known maximum safe cumulative dose (doxorubicin dose of 450–550 mg/m2; epirubicin dose of 900–1,100 mg/m2) [6]. However, since HER2-positive patients are usually also treated with trastuzumab as a part of the current standard adjuvant therapy, we propose a preventive strategy to ameliorate cardiac toxicity specifically in HER2-positive patients. To our knowledge, this is the first study to demonstrate cardioprotection in HER2-positive patients treated with anthracycline-based adjuvant chemotherapy followed by a 1-year trastuzumab administration. Here, we show for the first time that DZR treatment significantly reduces cardiac toxicity and the frequency of cardiac events associated with anthracycline-based chemotherapy followed by a 1-year trastuzumab treatment.
These findings have direct and significant implications for clinical oncology practice. First, the addition of DZR will reduce the frequency of cardiac toxicity and delay timing of cardiac toxicity onset, which are potent risk factors of trastuzumab interruption in the adjuvant setting. Moreover, our results showed that only one of 44 patients (2.3%) experienced a trastuzumab interruption event in the ADJ with DZR group, whereas 10 of 131 (7.6%) patients in the ADJ only group experienced a trastuzumab interruption due to cardiac toxicity. However, these results should be interpreted very carefully because of their statistical significance. However, because cardiac toxicity is a well-known crucial side effect in patients with breast cancer who receive anthracycline and trastuzumab, it is clear that increased cardiac events and early presence of cardiac toxicity might induce more trastuzumab interruption. Therefore, prevention of cardiac toxicity might increase the probability of continuing trastuzumab for an entire 12-month period. This is important, because this is currently the goal of standard trastuzumab adjuvant treatment [15]. Pivot et al. [16] failed to show that 6 months of treatment with trastuzumab was noninferior to 12 months of trastuzumab in an adjuvant setting, and thus proposed that 12 months of adjuvant trastuzumab should remain the standard of care despite the increased frequency of cardiac toxicity. Thus, discontinuing or reducing the period of trastuzumab treatment is unlikely to have any clinical benefits in terms of reducing disease recurrence and survival. The molecular mechanisms explaining the beneficial effects of DZR administration at the time of anthracycline chemotherapy on subsequent trastuzumab treatment are unclear. DZR can potentially counteract anthracycline-induced cardiotoxicity, or perhaps exert a cardioprotective effect against trastuzumab itself. It is well known that DZR can prevent the formation of anthracycline-iron complexes and the subsequent generation of free radicals that lead to oxidative damage in the cardiac tissue of breast cancer patients undergoing anthracycline-based chemotherapy [17]. However, whether DZR has a similar effect on trastuzumab or anthracycline/trastuzumab treatment regimens is unknown. A preclinical animal study showed that DZR protects against cardiotoxicity induced by anthracycline combined with trastuzumab [18]. Apparently, this is because DZR robustly induces calpain-2, which inhibits cardiomyocyte apoptosis. However, more investigation is required definitively to elucidate the underlying molecular mechanism of the DZR protective effect. Despite this lack of mechanistic insight, we believe that the administration of DZR could effectively reduce cardiotoxicity, which might increase the duration of trastuzumab exposure; this will enhance its antitumor activities and improve survival outcomes. Although DZR did not reduce the frequency of trastuzumab interruption and increase trastuzumab exposure time in the present study, this might be attributable to the small sample size. In support of this, a large cohort randomized trial is needed to determine if DZR has an effect on trastuzumab interruption and completion of 12-month trastuzumab treatment, which might have an impact on RFS and OS.
Second, DZR treatment will likely improve patient quality of life. The ultimate aim of adjuvant chemotherapy for early breast cancer patients is not to provide palliative care, but to cure them. Because patients can live for many years after cure, maintaining quality of life is essential. Furthermore, because many breast cancer patients who experience recurrence after adjuvant chemotherapy receive palliative chemotherapy that can affect cardiac function, prevention of adjuvant-induced cardiac toxicity is crucial for maintaining patients' quality of life. Anthracyclines can induce irreversible cardiac toxicity, which can be further aggravated by subsequent trastuzumab addition [19]. Furthermore, several meta-analyses showed that anthracycline-based regimens with trastuzumab are associated with more severe cardiac toxicity than non-anthracycline- based regimens with trastuzumab in HER2-positive breast cancer patients [7,8]. Although breast cancer recurrence might not be observed after surgery and adjuvant treatment, severe cardiac toxicity (such as that leading to congestive heart failure) can reduce quality of life. Previous studies of early breast cancer showed that various factors (including old age, history of cardiac disease, hypertension, diabetes, dyslipidemia, being overweight, or smoking) render individuals more vulnerable to severe cardiotoxicity during chemotherapy [8,20,21,22,23]. Although due to the small number of patients we did not perform a subgroup analysis considering these factors, we suggest that DZR administration to HER2-positive patients might be beneficial for improving the quality of life of patients who are at an increased risk of cardiotoxicity. Meanwhile, other chemotherapy regimens that do not use anthracycline should be discussed in high cardiac risk patients who have a lower possibility of tumor recurrence. Tolaney et al. [24] suggested that because patients with small, node-negative, HER2-positive breast cancer have a lower possibility of recurrence than those with more advanced disease, they could be treated with the non-anthracycline adjuvant chemotherapy regimen of paclitaxel and trastuzumab. Because our results showed that ADJ without DZR is associated with significant cardiac toxicity, we suggest that clinicians should consider non-anthracycline-based chemotherapy with trastuzumab, particularly when the risk/benefit ratio regarding the possibility of recurrence and quality of life are considered.
The present study has several limitations. First, although we evaluated LVEF 6 months after the completion of trastuzumab maintenance, we were not able regularly to evaluate this at later time points. In women with early breast cancer, the frequency of severe cardiac toxicity increases in the first 2 years (2.4% and 3.1%) after initiation of trastuzumab treatment, and levels off in the third year (2.9%) after the treatment is stopped [7]. In addition, a previous report indicates that monitoring cardiac function is important in HER2-positive breast cancer [25]. Furthermore, our data also showed that one patient in the DZR group experienced a cardiac event after 40 months, although it is not clear that this cardiac event is due to a late adverse effect of chemotherapy. We therefore suggest that the need for careful cardiac function monitoring after treatment, for at least an additional 2 to 3 years. Further studies will be required to confirm this last point.
A second limitation is that we could not examine adverse event due to DZR. In general, it is well known that DZR is well tolerated and safe for breast cancer patients [17]. However, this point should be considered in a future study.
A third limitation is that the number of patients recruited in this study was small. Although the addition of DZR showed a statistical difference for CFD between the two groups, the CFD curves cross over at a point somewhere after 40 months of treatment. This finding is ascribable to the relatively smaller sample size in the ADJ with DZR group compared to that of the ADJ only group, which results in an abrupt reversal of the CFD curves, although only one patient in the ADJ with DZR group had a cardiac event. In addition, our result did not show that DZR reduces the frequency of trastuzumab interruption. Therefore, a larger sample size will be required to provide the appropriate statistical power to confirm our findings. Fourth, all clinical analyses in this study were performed retrospectively; a prospective study would be necessary.
In conclusion, we show that DZR has cardioprotective effects in HER2-positive breast cancer patients in an adjuvant setting. Because cardiac toxicity is a very important cause of discontinuing trastuzumab in patients, we suggest that prevention of cardiac toxicity might facilitate the completion of 12-month trastuzumab treatments, which has a significant prognostic benefit. We therefore suggest that DZR administration could be considered during anthracycline-based adjuvant chemotherapy in HER2-positive patients who will subsequently receive a 1-year adjuvant trastuzumab treatment.
Footnotes
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 3.Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE, Jr, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayres LR, de Almeida Campos MS, de Oliveira Gozzo T, Martinez EZ, Ungari AQ, de Andrade JM, et al. Trastuzumab induced cardiotoxicity in HER2 positive breast cancer patients attended in a tertiary hospital. Int J Clin Pharm. 2015;37:365–372. doi: 10.1007/s11096-015-0070-y. [DOI] [PubMed] [Google Scholar]
- 5.Xue J, Jiang Z, Qi F, Lv S, Zhang S, Wang T, et al. Risk of trastuzumab-related cardiotoxicity in early breast cancer patients: a prospective observational study. J Breast Cancer. 2014;17:363–369. doi: 10.4048/jbc.2014.17.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 7.Mantarro S, Rossi M, Bonifazi M, D'Amico R, Blandizzi C, La Vecchia C, et al. Risk of severe cardiotoxicity following treatment with trastuzumab: a meta-analysis of randomized and cohort studies of 29,000 women with breast cancer. Intern Emerg Med. 2016;11:123–140. doi: 10.1007/s11739-015-1362-x. [DOI] [PubMed] [Google Scholar]
- 8.Bonifazi M, Franchi M, Rossi M, Moja L, Zambelli A, Zambon A, et al. Trastuzumab-related cardiotoxicity in early breast cancer: a cohort study. Oncologist. 2013;18:795–801. doi: 10.1634/theoncologist.2013-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351:145–153. doi: 10.1056/NEJMoa035153. [DOI] [PubMed] [Google Scholar]
- 10.Speyer J, Wasserheit C. Strategies for reduction of anthracycline cardiac toxicity. Semin Oncol. 1998;25:525–537. [PubMed] [Google Scholar]
- 11.Schoenborn CA, Adams PE. Health behaviors of adults: United States, 2005-2007. Vital Health Stat 10. 2010;(245):1–132. [PubMed] [Google Scholar]
- 12.O'Shaughnessy J, Twelves C, Aapro M. Treatment for anthracycline-pretreated metastatic breast cancer. Oncologist. 2002;7(Suppl 6):4–12. doi: 10.1634/theoncologist.7-suppl_6-4. [DOI] [PubMed] [Google Scholar]
- 13.Lopez M, Vici P, Di Lauro K, Conti F, Paoletti G, Ferraironi A, et al. Randomized prospective clinical trial of high-dose epirubicin and dexrazoxane in patients with advanced breast cancer and soft tissue sarcomas. J Clin Oncol. 1998;16:86–92. doi: 10.1200/JCO.1998.16.1.86. [DOI] [PubMed] [Google Scholar]
- 14.Swain SM, Whaley FS, Gerber MC, Weisberg S, York M, Spicer D, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol. 1997;15:1318–1332. doi: 10.1200/JCO.1997.15.4.1318. [DOI] [PubMed] [Google Scholar]
- 15.Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. NCCN guidelines insights breast cancer, version 1.2016. J Natl Compr Canc Netw. 2015;13:1475–1485. doi: 10.6004/jnccn.2015.0176. [DOI] [PubMed] [Google Scholar]
- 16.Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, et al. 6 Months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14:741–748. doi: 10.1016/S1470-2045(13)70225-0. [DOI] [PubMed] [Google Scholar]
- 17.Marty M, Espié M, Llombart A, Monnier A, Rapoport BL, Stahalova V, et al. Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapy. Ann Oncol. 2006;17:614–622. doi: 10.1093/annonc/mdj134. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Meng T, Liu J, Zhang X, Zhang J. Cardiac protective effects of dexrazoxane on animal cardiotoxicity model induced by anthracycline combined with trastuzumab is associated with upregulation of calpain-2. Medicine (Baltimore) 2015;94:e445. doi: 10.1097/MD.0000000000000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albini A, Cesana E, Donatelli F, Cammarota R, Bucci EO, Baravelli M, et al. Cardio-oncology in targeting the HER receptor family: the puzzle of different cardiotoxicities of HER2 inhibitors. Future Cardiol. 2011;7:693–704. doi: 10.2217/fca.11.54. [DOI] [PubMed] [Google Scholar]
- 20.Tan-Chiu E, Yothers G, Romond E, Geyer CE, Jr, Ewer M, Keefe D, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23:7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 21.Cha C, Ahn SG, Lee HM, Lee HW, Lee SA, Jeong J. Assessment of adjuvant trastuzumab-associated cardiac toxicity in Korean patients with breast cancer: a single-center analysis. Oncology. 2013;85:228–234. doi: 10.1159/000354836. [DOI] [PubMed] [Google Scholar]
- 22.Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26:1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caussa L, Kirova YM, Gault N, Pierga JY, Savignoni A, Campana F, et al. The acute skin and heart toxicity of a concurrent association of trastuzumab and locoregional breast radiotherapy including internal mammary chain: a single-institution study. Eur J Cancer. 2011;47:65–73. doi: 10.1016/j.ejca.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372:134–141. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung HW, Chan AL. Trastuzumab-induced cardiotoxicity in elderly women with HER-2-positive breast cancer: a meta-analysis of real-world data. Expert Opin Drug Saf. 2015;14:1661–1671. doi: 10.1517/14740338.2015.1089231. [DOI] [PubMed] [Google Scholar]