Abstract
Background
Interactions between the liver, the gut, and the immune system are critical components of alcoholic liver disease (ALD). The aim of this study was to explore the associations between alcohol-induced liver injury, endotoxemia, and inflammation at admission and over time during abstinence, as well as to examine the sex-related differences in these parameters in alcohol-dependent individuals admitted to an alcohol treatment program.
Methods
A cohort of 48 otherwise healthy participants with alcohol use disorder, but no clinical signs of alcoholic liver injury (34 males (M)/14 females (F)) admitted to an alcohol detoxification program, was stratified into two groups based on baseline plasma alanine aminotransferase (ALT) levels (as a marker of liver injury). Group 1 (ALT < 40 U/L, 7M/8F) and Group 2 (ALT ≥ 40 U/L, 27M/6F) were identified. Plasma biomarkers of liver damage, endotoxemia and inflammation were examined at baseline, day 8 and day 15 of the admission. The drinking history was also evaluated.
Results
Sixty-nine percent of patients had elevated ALT and other markers of liver damage, including aspartate aminotransferase and cytokeratin 18 (CK18 M65 and M30) at baseline, indicating the presence of mild ALD. Elevated CK18 M65:M30 ratio suggested a greater contribution of necrotic rather than apoptotic hepatocyte cell death in the liver injury observed in these individuals. Females showed greater elevations of liver injury markers compared to males, although they had fewer drinks per day and shorter lifetime duration of heavy drinking. Liver injury was associated with systemic inflammation, specifically, elevated plasma tumor necrosis factor-alpha levels. Compared to patients without liver injury, patients with mild ALD had greater endotoxemia (increased serum lipopolysaccharide (LPS) levels), which decreased with abstinence and this decrease preceded the drop in CK18 M65 levels.
Conclusions
The study documented the association of mild alcohol-induced liver injury and endotoxemia, which improved with two weeks of abstinence, in a subset of individuals admitted to an alcohol detoxification program.
Keywords: Alcohol-dependent subjects, mild alcoholic liver disease, endotoxemia
INTRODUCTION
Alcohol consumption is an important social, economic, and clinical problem. The 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions III revealed that the twelve-month and lifetime prevalence of alcohol use disorders was 13.9% and 29.1%, respectively, among US adults aged 18 and older (Grant et al., 2015). The prevalence of these two parameters was generally higher for males (17.6% and 36.0%, respectively) compared to females (10.4% and 22.7%, respectively). Heavy alcohol consumption results in a wide range of multi-organ pathology, including alcoholic liver disease (ALD), a major cause of alcohol-related morbidity and mortality in the United States and worldwide. The clinical spectrum of ALD ranges from reversible fatty liver to alcoholic hepatitis, fibrosis and cirrhosis, which may progress even further to hepatocellular carcinoma. It has been demonstrated that the development and the severity of ALD are closely associated with the amount and duration of alcohol consumption. Daily alcohol consumption exceeding 40-80 g/day for males and 20-40 g/day for females for 10 years or more will likely lead to ALD (Becker et al., 1996). The advanced stages of ALD develop only in a subset of long-term heavy drinkers, with females thought to be at a higher risk (Becker et al., 1996; Bellentani et al., 1997; Schwartz and Reinus, 2012). Approximately 10-35% of chronic heavy drinkers will develop alcoholic hepatitis, and alcoholic cirrhosis will occur in approximately 10-20% of long-term heavy drinkers (D'Amico et al., 2006; Rehm et al., 2013; Wakim-Fleming and Mullen, 2005). Sex (Eagon, 2010; Frezza et al., 1990), genetic polymorphisms (Anstee et al., 2015), epigenetic changes (e.g., DNA methylation and various histone modifications (Page et al., 2015)), environmental factors (e.g., smoking, dietary habits), and drinking pattern (Askgaard et al., 2015) are among other well known risk factors for ALD development.
It has been postulated for more than half a century that bacteria-derived endotoxins, specifically lipopolysaccharides (LPS), play critical roles in liver injury, including ALD (Broitman et al., 1964; Nolan, 2010). Indeed, alcohol-induced endotoxemia is well documented in patients with different stages of ALD (Bode et al., 1987; Fujimoto et al., 2000; Fukui et al., 1991; Keshavarzian et al., 1999; Parlesak et al., 2000; Schafer et al., 1997; Urbaschek et al., 2001). In some studies, patients with severe ALD (e.g., alcoholic cirrhosis) showed higher LPS levels compared to subjects with mild ALD (Fukui, 2005; Schafer et al., 1997), and in patients with ALD, elevated levels of endotoxin correlated with acute excessive alcohol ingestion (Urbaschek et al., 2001). It has been shown, that LPS levels were positively correlated with increased intestinal permeability (Parlesak et al., 2000), and alcohol-dependent patients with liver damage showed greater gut barrier “leakiness” compared to alcoholics without ALD (Keshavarzian et al., 1999). One of the major effects of LPS is stimulation of hepatic Kupffer cells to increase production of pro-inflammatory cytokines and chemokines, including tumor necrosis factor-alpha (TNF-α), which plays critical roles in alcohol-induced liver injury (McClain et al., 1998). Significant positive correlations were observed between LPS and TNF-α in patients with ALD, specifically alcoholic cirrhosis (Hanck et al., 1998). Endotoxemia and alterations in the intestinal barrier integrity after chronic or acute ethanol administration have also been confirmed in numerous experimental animal models of ALD (Kirpich et al., 2012; Lippai et al., 2014). It is noteworthy that endotoxemia and the Kupffer cell pro-inflammatory response may be different in males and females due to hormonal differences, and females have been postulated to be more susceptible to ALD (Becker et al., 1996).
Although significantly increased circulating levels of bacteria-derived endotoxins were found in patients with different stages of ALD (Bode et al., 1987; Fukui et al., 1991; Keshavarzian et al., 1999; Parlesak et al., 2000), studies examining endotoxemia in early human ALD are limited. The sex differences in endotoxemia in patients with ALD also have not been well defined. The aim of this study was to evaluate the associations between alcohol-induced liver injury, endotoxemia and inflammation at admission and over time during abstinence, as well as to examine the sex-related differences in these parameters in otherwise healthy alcohol-dependent individuals admitted to an alcohol treatment program. We hypothesized that compared to alcohol-dependent individuals without liver injury, the alcohol-dependent individuals with mild ALD would have greater endotoxemia which would decrease with abstinence. We also postulated that females with mild ALD would have higher levels of endotoxemia and biomarkers of liver injury compared to males. To test our hypotheses, we assessed plasma concentrations of bacteria-derived products, including LPS and flagellin, as well as lipopolysaccharide binding protein (LBP) and serum-soluble innate microbial receptor sCD14 (indirect markers of LPS translocation from the gut lumen to the systemic circulation). We also measured the plasma levels of TNF-α and PAI-1, critical pro-inflammatory cytokines that play important roles in ALD development and progression (Beier and Arteel, 2012; McClain et al., 1998). Given that the serum ALT and AST levels (the classical markers of hepatocyte damage) do not always correlate with the severity of liver injury, other recently proposed sensitive markers of liver damage, namely cytokeratin 18 (CK18 M65 and CK18 M30) (Cave et al., 2011; Cusi et al., 2014; Feldstein et al., 2009) and liver fatty acid-binding protein (L-FABP) (Pelsers et al., 2005; Pelsers et al., 2002) were also analyzed. Of note, the serum whole CK18 protein (CK18 M65, which measures total cell death) and the caspase-cleaved fragment (CK18 M30, surrogate marker of apoptotic cell death) provide important information regarding the type of hepatocyte cell death. Finally, the associations between markers of endotoxemia, systemic inflammation and liver injury were examined.
MATERIAL AND METHODS
Subjects and Clinical Assessment
The present study is one of the arms of a larger clinical investigation (NCT#00106106) conducted at the National Institute of Alcohol Abuse and Alcoholism (NIAAA) of the National Institute of Health (NIH). The study included 48 male and female alcohol-dependent participants (aged 21 – 65). Patients were admitted to the NIAAA inpatient unit in the NIH Clinical Center for the alcohol withdrawal program. Prior to admission, patients underwent a telephone screening to eliminate subjects with significant medical issues, including advanced liver disease. The study recruited patients with a diagnosis of alcohol dependence according to Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV), based on the alcohol dependence module of the Structured Clinical Interview (SCID-I); and alcohol withdrawal, based on either: (a) clinically manifested significant alcohol withdrawal symptoms, as demonstrated by Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA-Ar) scores of 9 or above, with or without detectable blood alcohol concentrations (BAC); or (b) in the absence of withdrawal, if they exhibited current intoxication above 0.1 g/dl BAC, self-reported history of continuous alcohol use >1 month, and self-reported previous episodes of significantly distressful alcohol withdrawal symptoms. Alcohol consumption during the preceding 3 months was evaluated using Time-Line Follow-Back (TLFB; (Sobell et al., 2003)). The exclusion criteria for the study included the presence of severe psychiatric and/or somatic illnesses, including advanced lung disease, unstable cardiovascular disease (decompensation, as demonstrated through chest X-ray, pathological electrocardiogram), and/or renal failure (creatinine clearance < 30 ml/min). Other exclusion criteria were: presence of HIV, pregnancy or ongoing breastfeeding; pronounced anxiety provoked by enclosed spaces; and/or positive urine screen for any illicit drug. Importantly, no patient had clinically evident alcoholic hepatitis.
All patients received standard clinical inpatient care for alcohol detoxification, including a complete work-up consisting of history, physical examination including neurological status, and laboratory tests; standard thiamine supplementation; nursing; nutrition; multidisciplinary discharge planning; and any necessary referrals for treatment of concomitant morbidity (Umhau et al., 2010). In addition, they received medical management in compliance with the “Human Subjects Protection” protocol with NIH.
Blood Biochemical Measurements and Enzyme Linked Immunosorbent Assays (ELISAs)
Standard blood biochemical parameters, including liver function tests such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST), were measured at the NIH clinical laboratory. This clinical blood biochemistry data set was used to stratify subjects into two groups on the basis of ALT levels at the admission. We used plasma ALT levels < 40 U/L as within the normal reference range, and plasma ALT levels ≥ 40 U/L were considered to be elevated indicating the presence of liver injury. Patients were divided into two groups, namely Group 1 (alcohol-dependent subjects with normal ALT levels; no liver injury) and Group 2 (alcohol-dependent subjects with elevated ALT levels; mild ALD).
Plasma samples were stored in the NIH bio-repository and were later retrieved for analysis. We performed analysis of variables in samples obtained at the onset (baseline, T1), day 8 (T2) and day 15 (T3) of the alcohol withdrawal program. Plasma cytokeratin 18 whole protein (CK18 M65 - indicator for overall cell death) and caspase-cleaved fragment (CK18 M30 - indicator for apoptotic cell death) were measured by ELISA (Peviva-VLVbio, Nacka, Sweden) according to the manufacturer’s instructions. Abnormal CK18 was defined as either CK18 M65 ≥ 300 U/L, or CK18 M30 ≥ 200 U/L on the basis of prior studies (Cave et al., 2011; Cave et al., 2010; Feldstein et al., 2009). Plasma flagellin (MyBioSource, San Diego, CA), soluble cluster of differentiation 14 (sCD14, R&D System, Minneapolis, MN), lipopolysaccharide binding protein (LBP, Hycult Biotech, Plymouth Meeting, PA), and liver and intestinal fatty acid binding proteins (L-FABP and I-FABP, Hycult Biotech, Plymouth Meeting, PA) were measured by ELISAs according to the manufacturers’ instructions.
Plasma Cytokine Level Assessment
Plasma pro-inflammatory cytokines, including TNF-α, interleukin 1β, interleukin 6 and interleukin 8 (IL-1β, IL-6 and IL-8), plasminogen activator inhibitor-1 (PAI-1), and monocyte chemoattractant protein-1 (MCP-1) were determined by multi-analyte chemiluminescent detection using Mulliplex kits (Millipore, Billerica, MA) on the Luminex (Luminex, Austin, TX) platform according to manufacturers’ instructions.
Plasma Lipopolysaccharide Measurement
Plasma lipopolysacharide (LPS) levels were measured using the Kinetic Chromogenic Limulus Amebocyte Lysate Assay (Lonza, Walkersville, MD) according to the manufacturer’s instructions.
Statistical Analysis
Patients were stratified into two groups on the basis of initial plasma ALT levels: “no injury” and “injury” groups (Group 1 and Group 2, respectively). ALT < 40 U/L (for Group 1) or ≥ 40 U/L (for Group 2) were the cut-off points. Demographics, drinking history and laboratory variables were then compared between these two groups. The total patient cohort as well as Group 1 and Group 2 were sub-categorized by sex. Sex of the participants was used as a co-factor for between group analyses. Statistical significance between two groups was determined by Student’s t-test (Fig. 1A-C). Multiple group comparisons were performed using One-way analysis of variance (ANOVA) followed by the Tukey’s or Sidak’s multiple-comparison post hoc tests. One-way ANOVA of repeated measures was performed to evaluate significant differences between variables at baseline, day 8, and day 15 of the alcohol withdrawal program. The correlation between various subject characteristics (e.g., drinking history, laboratory variables of liver injury, systemic inflammation and endotoxemia) were assessed using Pearson correlation analysis. LPS was used an independent variable and ALT was considered as a dependent variable (Fig.4B). A p value ≤ 0.05 was considered statistically significant. The data are expressed as means ± standard error of the mean (SEM). Statistical analyses were performed using GraphPad Prism version 6.05 for Windows (GraphPad Software, Inc., La Jolla, CA).
RESULTS
Patient Cohort Demographic Characteristics and Drinking History Profile
The study population demographics and alcohol consumption history are summarized in Table 1. Forty-eight alcohol-dependent subjects admitted to the detoxification program were included in this study. There were 34 male (71%) and 14 female (29%) subjects. The patient mean age was 43.2 ± 1.5 years, which was similar for males and females. The mean body mass index (BMI) was 26.8 ± 0.8 for the entire cohort, and did not differ significantly between males and females. While the mean alcohol consumption (measured as average drinks per drinking day) was similar between the males and females, the mean duration of alcohol consumption (measured as lifetime of heavy drinking, years) was significantly higher in males compared to females (17.7 ± 1.7 vs 10.5 ± 1.8, p <0.05). Of note, 15% of males and 50% of females reported fewer than 10 years of drinking history; 47% of males and 36% of females had 10-20 years of heavy drinking, and 38% of males and 14% of females reported more than 20 years of heavy alcohol consumption.
Table 1.
Distribution of Demographic and Drinking History Variables of the Alcohol-dependent Individuals Stratified by ALT Levels
| Whole Patient Cohort | Group 1 (ALT < 40) | Group 2 (ALT ≥ 40) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study Data | All Subjects |
Male | Female | All Subjects |
Male | Female | All Subjects |
Male | Female |
| Demographic Variables | |||||||||
| Number of subjects, n | 48 | 34 | 14 | 15 | 7 | 8 | 33 | 27 | 6 |
| Age, years | 43.2±1.5 | 43.3±1.6 | 42.9±3.2 | 40.4±2.9 | 38.9±3.8 | 41.4±1.8 | 44.5±1.7 | 44.4±1.8 | 44.5±4.6 |
| BMI | 26.8±0.8 | 26.5±0.7 | 27.5±2.0 | 28.4±2.0 | 29.0±1.9 | 27.9±3.6 | 25.9±0.6 | 25.7±0.7 | 26.8±1.1 |
| Drinking History Variables | |||||||||
| Average drinks per day | 15.03±0.9 | 15.5±0.9 | 13.9±2.0 | 16.8±1.9 | 16.8±2.6 | 16.7±3.1 | 14.3±0.9 | 15.1±1.0 | 10.5±1.9 |
| Years of heavy drinking | 15.5±1.4 | 17.7±1.7 | 10.5±1.8* | 10.9±1.6 | 12.3±2.8 | 9.7±1.7 | 17.6±1.8 & | 18.9±1.9 | 11.5±3.6 |
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index;
p < 0.05 male vs female;
p < 0.05 Group 1 vs Group 2.
ALT and AST Levels were Increased in a Subset of Alcohol-dependent Patients
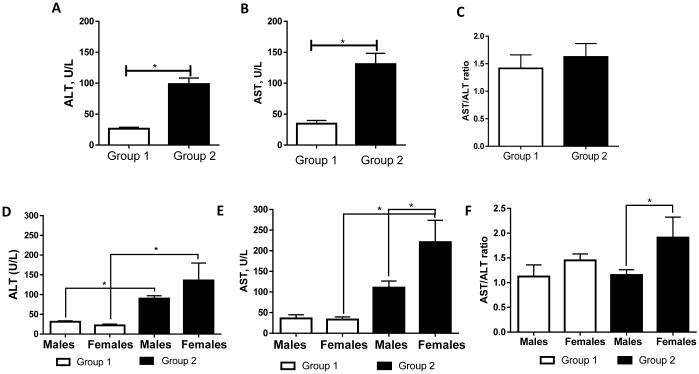
Patients with clinically evident alcoholic hepatitis were excluded, while subjects with mildly increased liver enzymes without clinically overt liver injury were included in this study. Thus, 31% (15 of 48) of individuals had initial plasma ALT levels < 40 U/L (Group 1, mean ALT levels were 26.47 ± 2.28 U/L) which was considered to be in the normal range for ALT. Liver injury, as assessed by admission ALT levels ≥ 40 U/L, was observed in 69% (33 of 48) of individuals with the mean ALT value of 98.55 ± 9.82 U/L (Group 2; Fig. 1A). As expected, significant differences between Group 1 and Group 2 were also observed for several other markers of liver injury, including plasma AST levels (34.53 ± 5.18 vs 130.7 ± 17.49 U/L, p < 0.05; Fig. 1B). AST/ALT ratio was not different between Group 1 and Group 2 (Fig.1C). Analysis of the sex differences in Group 2 revealed higher ALT and AST levels, and AST/ALT ratio in females compared to males (Fig. 1D-E).
Fig. 1.
Plasma ALT and AST levels in a cohort of alcohol-dependent individuals at the beginning of alcohol detoxification program. (A) Based on initial ALT levels, patients were divided into two groups: Group 1 (ALT < 40 U/L, n=15) and Group 2 (ALT ≥ 40 U/L, n=33). (B) AST levels in Group 1 and Group 2 paralleled ALT levels. (C) The AST/ALT ratio. (D-F) Sex differences between Groups 1 and 2 in ALT, AST levels, and AST/ALT ratio. The data are expressed as means ± SEM, * p < 0.05. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
The demographic and alcohol consumption history comparisons between Groups 1 and 2 are presented in Table 1. While Group 1 had a nearly equal number of males and females, males were predominant in Group 2 (81% males). Although not significantly so, subjects of Group 1 were younger; and they had a slightly higher BMI compared to Group 2. The mean age and BMI did not differ between males and females in either group. While the mean daily alcohol consumption was similar between Group 1 and Group 2 subjects, the lifetime duration of heavy drinking was significantly higher in Group 2 (17.6 ± 1.8 vs 10.9 ± 1.6, p <0.05). Interestingly, females in Group 2 had fewer drinks per drinking day compared to Group 1, while lifetime duration of heavy drinking was similar. In contrast, males in Group 2 had similar drinks per drinking day but longer lifetime of heavy drinking compared to Group 1. Among the Group 1 and Group 2 subjects, 36% and 22% respectively, reported less than 10 years of drinking history, while 57% and 38%, respectively, had 10-20 years of heavy drinking. Less than 7% in Group 1 vs 40% in Group 2 reported more than 20 years of heavy alcohol consumption.
Evaluation of Cytokeratin 18 (CK18 M65 and CK18 M30) and Liver Fatty Acid-Binding Protein (L-FABP) as Markers of Liver Injury
The initial analysis of classical markers of liver injury revealed a cohort of patients with elevated ALT and AST levels, suggesting the presence of mild alcohol-mediated liver injury in these individuals, but without overt clinical alcoholic hepatitis. To confirm this observation and further to examine liver injury in these patients, we evaluated two additional markers of liver injury, namely CK18 (M30 and M65) and liver fatty acid-binding protein (L-FABP). The serum whole CK18 protein (CK18 M65, which measures total cell death) and the caspase-cleaved fragment (CK18 M30, surrogate marker of apoptotic cell death) have been proposed as clinically useful biomarkers for liver injury, including nonalcoholic and toxicant-associated steatohepatitis (Cave et al., 2011; Cusi et al., 2014; Feldstein et al., 2009). We utilized these tests because AST and ALT do not correlate (especially not in a linear fashion) with severity of liver injury or indicate the type of cell death. Thus, new biomarkers that accurately reflect the full spectrum of severity of liver cell injury and type of cell death are needed.
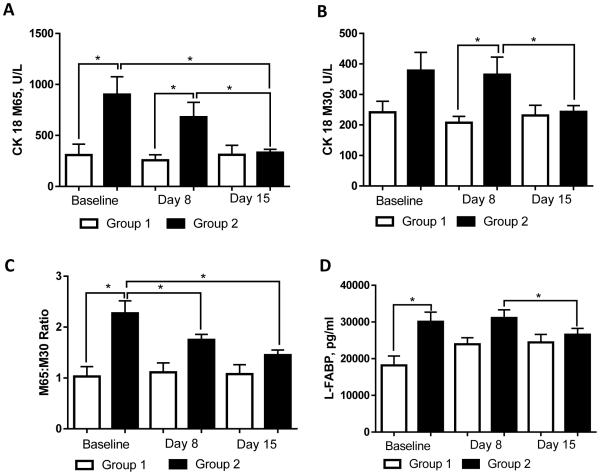
At baseline (T1), patients in Group 2 had significantly higher levels of CK18 M65 (890.3 ± 164.6 vs 308.1 ± 105.9 U/L, p < 0.05; Fig. 2A) compared to Group 1, and a moderate increase in CK18 M30 (378.2 ± 59.6 vs 241.6 ± 36.1 U/L; p = ns; Fig. 2B). Positive correlations were observed in Group 2 at T1 between both CK18 M65 and ALT (r = 0.473, p = 0.005) and CK18 M30 and ALT (r = 0.357, p = 0.041). CK18 M30 was positively correlated with average drinks per drinking day at T1 in males (r = 0.503, p = 0.007). The CK18 M65:M30 ratio provides an estimate of the type of cell death (necrotic vs apoptotic) (Kramer et al., 2004). The CK18 M65:M30 ratio was slightly greater than 2 at T1 in patients with liver injury (Group 2; Fig. 2C), and was significantly higher than in subjects without liver damage (Group 1), suggesting a greater contribution of necrotic rather than apoptotic hepatocyte cell death in Group 2 patients. While both CK18 M65 and CK18 M30 remained elevated in Group 2 compared to Group 1 at T2, these levels were reduced at T3 and were similar to the levels of Group 1. When comparing male and female subgroups, both CK18 M65 and CK18 M30 were higher in females compared with males (Table 2).
Fig. 2.
Plasma levels of liver injury markers in alcohol dependent subjects during alcohol detoxification program. (A) CK18 M65, (B) CK18 M30, (C) CK18 M65:M30 ratio, and (D) L-FABP. Analysis of variables was performed at the onset (baseline), day 8, and day 15 of the program. The data are expressed as means ± SEM, * p < 0.05. CK18, Cytokeratin 18; L-FABP, liver fatty acid-binding protein.
Table 2.
Characteristics of Liver Injury and Plasma Cytokine Levels in Male and Female Alcohol-dependent Individuals
| Study Data/Time Point | Group 1 (ALT < 40) | Group 2 (ALT ≥ 40) | |||
|---|---|---|---|---|---|
| Males | Females | Males | Females | ||
| CK-18 M65, U/L |
Baseline | 327.2±199.7 | 291.4±109.8 | 832.7±177.8 | 1149±444.3 |
| Day 8 | 275.3±70.4 | 239.8±86.4 | 552.3±79.9 | 1188±668.4 | |
| Day 15 | 383.4±165.0 | 247.7±100.0 | 312.2±21.6 * | 426.0±137.4 | |
| CK18 M30, U/L |
Baseline | 236.4±59.3 | 246.7±46.1 | 337.9±63.01 | 559.6±155.4 |
| Day 8 | 206.2±20.9 | 208.6±37.3 | 301.8±32.7 | 638.4±260.5 | |
| Day 15 | 256.9±58.4 | 205.2±34.3 | 213.3±12.5 | 366.7±79.2 | |
| L-FABP, pg/ml |
Baseline | 16397±4198 | 19718±3202 | 29668±3086& | 31917±3945 |
| Day 8 | 22726±2778 | 25005±2411 | 31387±2550 | 29850±4490 | |
| Day 15 | 24826±4374 | 24093±1884 | 26392±1806 | 27233±4922 | |
| TNF-alpha, pg/ml |
Baseline | 1.56±0.26 | 1.34±0.27 | 1.94±0.13 | 2.74±0.62 |
| Day 8 | 2.26±0.35 | 1.69±0.24 | 2.51±0.18 | 2.81±0.54 | |
| Day 15 | 1.77±0.28 | 1.75±0.26 | 2.44±0.14 | 2.87±0.46 | |
| PAI-1, ng/ml | Baseline | 38.7±5.4 | 40.3±4.5 | 46.3±5.7 | 40.9±9.0 |
| Day 8 | 35.0±7.7 | 28.3±4.4 | 27.7±2.2 | 29.2±5.0 | |
| Day 15 | 34.2±6.8 | 34.4±4.4 | 32.5±2.7 | 28.8±4.9 | |
Analysis of variables was performed at the onset (baseline), day 8, and day 15 of the detoxification program. Abbreviations: ALT, alanine aminotransferase; CK, cytokeratin; L-FABP, liver fatty acid binding protein, PAI-1, plasminogen activator inhibitor type 1; TNF-α, tumor necrosis factor alpha.
p < 0.05 in comparison to baseline;
p < 0.05, Gr 1 vs Gr 2 at given time point.
Another marker of liver injury, plasma L-FABP was modestly, but significantly elevated at admission (T1) in Group 2 patients with mild liver injury compared to Group 1 alcohol-dependent subjects without liver damage (30077 ± 2607 vs 18168 ± 2541 pg/ml, p < 0.05; Fig. 2D). L-FABP levels were similarly higher in Group 2 males and females compared to Group 1 males and females (Table 2). In Group 2, L-FABP positively correlated at T1 with other markers of liver damage, including ALT (r = 0.474, p = 0.005), CK18 M65 (r = 0.737, p < 0.0001), and CK 18 M30 (r = 0.493, p = 0.003). While L-FABP levels in Group 2 remained unchanged at day 8 (T2) and slightly reduced at day 15 (T3), L-FABP levels in Group 1 were increased (but not significantly), resulting in similar L-FABP levels in both groups by the end of the observed period.
Induction of Plasma Cytokines in Alcohol-dependent Patients with and without Mild Alcoholic Liver Disease
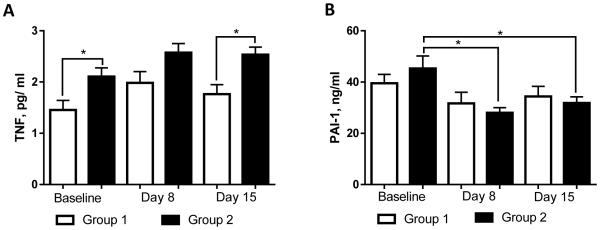
Alcohol-induced liver injury is known to be associated with systemic inflammation, and TNF-α is a pro-inflammatory cytokine playing a critical role in ALD development and progression (McClain et al., 1998). In our study, the levels of TNF-α were significantly increased at T1 in patients with liver injury (Group 2) compared to those without liver damage (Group 1) (2.10 ± 0.1 vs 1.45 ± 0.1 pg/ml, p < 0.05) and remained similarly increased throughout the 2 weeks of abstinence (Fig. 3A). In Group 2, levels of TNF-α positively correlated at T1 with the markers of liver injury, including ALT (r = 0.427, p = 0.019), CK18 M65 (r = 0.525, p = 0.003), and CK18 M30 (r = 0.609, p < 0.001). When comparing male and female subgroups, levels of TNF-α in Group 2 females were higher compared to Group 2 males, although not significantly (Table 2). PAI-1, a cytokine that plays an important role in hepatic inflammation caused by ethanol (Beier and Arteel, 2012) was similar at T1 in both Group 1 and Group 2 with a minor nonsignificant elevation in Group 2 (39.55 ± 3.4 vs 45.27 ± 4.9 ng/ml, p = ns; Fig. 3B). A decrease in PAI-1 levels occurred in both groups during treatment, reaching a significant reduction in subjects with elevated ALT levels at T2, and remained at those levels at T3. PAI-1 levels did not differ significantly between males and females (Table 2). There were no noticeable differences between Group 1 and Group 2, or between males and females in the levels of other pro-inflammatory cytokines, including IL-1β, IL-6, IL-8, and MCP-1 (data not shown).
Fig. 3.
Plasma cytokine levels in alcohol dependent patients during alcohol detoxification program. (A) TNF-α, and (B) PAI-1 levels. Analysis of variables was performed at the onset (baseline), day 8 , and day 15 of the program. The data are expressed as means ± SEM, * p < 0.05. PAI-1, Plasminogen activator inhibitor type 1; TNF-α, tumor necrosis factor alpha
Liver Injury was Associated with Elevated Endotoxemia in Alcohol-dependent Patients with Mild Alcoholic Liver Disease
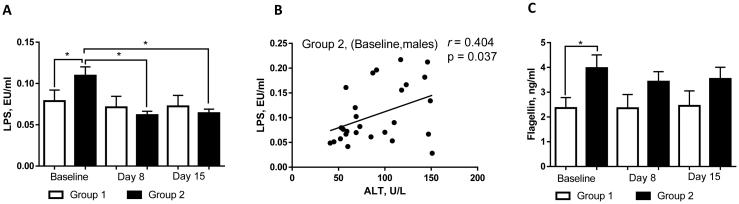
Numerous clinical and experimental studies have shown that alcohol consumption (chronic and acute) impairs intestinal barrier integrity resulting in increased endotoxemia, a critical factor contributing to alcohol-mediated liver disease (Szabo, 2015). Because only a subset of alcohol-dependent subjects admitted to the treatment program presented with indications of liver injury, we examined whether endotoxemia was associated with liver injury in these individuals. At admission (T1), the levels of plasma LPS (a well-known gram-negative bacteria-derived product contributing to ALD) were higher in those patients with elevated ALT compared to patients with normal ALT levels (Fig. 4A). Elevated LPS levels significantly decreased by day 8 (T2), and persisted at those levels at day 15 (T3). Interestingly, while LPS levels were reduced in both sexes by the day 8 (T2) compared to initial values (T1), a statistically significant drop was noted only in males (Table 3). A positive correlation between LPS and ALT levels was also observed at T1 in males (r = 0.404, p = 0.037; Fig. 4B) but not in females (possibly due to low sample size in the female group). We did not find any correlations between LPS and TNF-α or LPS and PAI-1 in either males or females. There were no significant differences observed at the onset of therapy between Group 1 and Group 2 or between males and females for other markers of endotoxemia, including sCD14 and LBP (data not shown). It is worth noting that, in parallel with elevated LPS, we observed increased levels of flagellin, a gram-negative bacteria-derived product similar to LPS, in patients with liver injury compared to those without liver damage (Fig. 4C). Flagellin levels were slightly higher in males compared to females (Table 3).
Fig. 4.
Plasma markers of endotoxemia in alcohol dependent subjects during alcohol detoxification program. (A) LPS levels were increased in patients with liver injury (Group 2) compared to patients without liver damage (Group 1). (B) LPS levels were positively correlated with ALT levels at T1 in Group 2 males. (C) Flagellin levels were significantly increased at T1 in patients with liver injury compared to individuals without liver damage. Analysis of variables was performed at the onset (baseline), day 8, and day 15 of the program. The data are expressed as means ± SEM, * p < 0.05. Correlation analysis was performed at the onset of detoxification therapy. ALT, alanine aminotransferase; LPS, lipopolysaccharides.
Table 3.
Characteristics of Endotoxemia in Male and Female Alcohol-dependent Individuals
| Study Data/Time Point | Group 1 (ALT < 40) | Group 2 (ALT ≥ 40) | |||
|---|---|---|---|---|---|
| Males | Females | Males | Females | ||
| LPS, EU/ml | Baseline | 0.07±0.02 | 0.07±0.01 | 0.10±0.01 | 0.11±0.02 |
| Day 8 | 0.06±0.01 | 0.07±0.02 | 0.05±0.004 * | 0.07±0.01 | |
| Day 15 | 0.06±0.01 | 0.07±0.01 | 0.05±0.004 * | 0.08±0.01 | |
| Flagellin, ng/ml |
Baseline | 2.8±0.6 | 2.02±0.5 | 3.9±0.5 | 2.2±0.5 |
| Day 8 | 2.6±1.1 | 2.09±0.5 | 3.5±0.4 | 2.8±0.8 | |
| Day 15 | 3.2±1.2 | 1.8±0.4 | 3.7±0.5 | 2.6±0.8 | |
Analysis of variables was performed at the onset (baseline), day 8, and day 15 of the detoxification program. Abbreviations: ALT, alanine aminotransferase; LPS – lipopolysaccharides.
p < 0.05 in comparison to baseline
DISCUSSION
Our study evaluated alcohol-dependent subjects admitted to an alcohol withdrawal program. A subset of these patients (69% of the cohort, 81% of whom were males) had mild (sub-clinical) ALD. The fact that AST/ALT ratio, which increases with the severity of AH, was not significantly elevated in these patients was consistent with our hypothesis that this patient cohort had early ALD. Liver injury in these patients was characterized not only by elevated ALT and AST levels (classic markers of liver damage), but also by increased CK18, a sensitive marker of hepatocyte cell death in different types of liver disease, including ALD (Cave et al., 2011; Feldstein et al., 2009; Gonzalez-Quintela et al., 2006; Sowa et al., 2014). In previous studies, elevated serum levels of CK18 fragment (CK M30) were found in alcoholic hepatitis patients (Li et al., 2010), which were positively correlated with the hepatic apoptotic score (Gonzalez-Quintela et al., 2009). Increased levels of CK18 correlated with hepatocyte ballooning, the presence of Mallory-Denk bodies (a hallmark of ALD), and hepatic TNF-α and TGF-β expression in patients with alcoholic liver fibrosis (Lavallard et al., 2011). We observed an increase in both soluble full-length CK18 M65 (which is released from cells undergoing necrosis (Kramer et al., 2004)) and the caspase cleaved fragment, CK18 M30 (a surrogate biomarker of cell apoptosis). The high CK18 M65:M30 ratio suggested a predominantly necrotic mode of hepatocyte cell death contributing to the liver injury in our patient cohort. CK18 levels decreased with abstinence, indicating a reduction in hepatocyte death. We also examined plasma L-FABP, which was recently proposed as a biomarker of liver injury (Pelsers et al., 2005). In our study, elevated L-FABP levels were positively correlated with increased plasma ALT, as well as elevated CK18 M65 and CK18 M30 levels in patients with mild ALD. L-FABP is predominantly expressed in the liver, as well as in the intestine and kidney in minor amounts, with its primary function to facilitate transport of intracellular fatty acids. Given that no differences were observed in the plasma levels of I-FABP (as a marker of intestinal injury, data not shown), the elevation of L-FABP most likely reflects its liver origin. However, the utility of plasma L-FABP as a biomarker of alcohol-induced liver injury remains to be determined.
In our study, all patients (with and without liver disease) were long-term alcohol consumers with similar levels of alcohol consumption (drinks per day). However, the liver injury subset group had greater endotoxemia, which is considered to be a critical factor in ALD pathogenesis (Szabo, 2015). We also found a significant positive correlation between LPS and ALT levels at admission in patients with mild ALD, more so in males than in females (possibly due to low sample size in the female group). In patients with mild ALD, the sensitive biomarkers of liver cell death, CK18 M65 and M30, were improved following the decrease in endotoxemia, supporting a role for LPS in the liver injury observed in these individuals. Further, our data showed that liver injury was associated with systemic inflammation, specifically, elevated plasma TNF-α levels. Although, we did not find any correlations between LPS and TNF-α in our patient cohort with mild ALD, the strong correlation between endotoxemia and TNF-α has been previously reported by others in patients with more advanced liver disease (e.g., alcoholic cirrhosis) (Hanck et al., 1998). Alcohol-induced endotoxemia has been well documented in patients with different stages of ALD, especially in those with advanced alcoholic hepatitis and cirrhosis (Bode et al., 1987; Fukui, 2005; Parlesak et al., 2000; Schafer et al., 1997). There are also studies reporting equally increased endotoxin levels in patients with mild and severe ALD (Schafer et al., 2002; Urbaschek et al., 2001), possibly due to the limitations in LPS measurement protocol (Schafer et al., 2002), or the fact that the cirrhotic patients were less intoxicated, as significantly higher endotoxin levels were found in those patients who were highly intoxicated than in those who were clinically less intoxicated at admission, even with the same degree of ALD (Urbaschek et al., 2001). The observation from this work and studies from other investigators (Bala et al., 2014; Fukui et al., 1991) provide evidence that endotoxemia in humans might be particularly high after acute ethanol exposure. Indeed, recent work from Szabo’s laboratory demonstrated a rapid increase in serum endotoxin levels in healthy individuals acutely administered alcohol to simulate binge drinking, with slightly higher endotoxin levels occurring in females (Bala et al., 2014). There was also an increase in other enteric bacterial elements such as 16S rDNA in the serum following acute alcohol ingestion (Bala et al., 2014). In our study we also found elevated levels of other gram-negative bacteria-derived products similar to LPS, namely flagellin (a ligand for Toll-Like Receptor 5 (TLR-5)), in patients with liver injury compared to those without liver damage. Very few reports exist on the role of flagellin in liver diseases. For example, over-activation of TLR-5 signaling by high-dose flagellin caused acute inflammatory responses, neutrophil accumulation and oxidative stress in murine livers (Xiao et al., 2015). Our group recently demonstrated that flagellin could induce TNF-α production in human peripheral blood monocyte-derived macrophages (Wang et al., 2013). Further studies on the role of flagellin in ALD are warranted.
Alcohol-induced increased intestinal permeability has been postulated as the primary cause of elevated endotoxemia in ALD. An early study from Germany by Parlesak, et al., (Parlesak et al., 2000) reported that gut permeability, as assessed by recovery of high molecular weight polyethylene glycols, was increased in patients with ALD, and this was correlated with elevated LPS levels. A short period of abstinence led to a marked reduction in gut permeability, especially in those ALD patients with initially high values (Parlesak et al., 2000). Other studies by Keshavarzian and coworkers evaluated gut permeability in healthy volunteers, alcoholics without liver disease, alcoholics with liver disease (mainly cirrhosis), and patients with liver disease of non-alcoholic origin (Keshavarzian et al., 1999). In that particular point-in-time study, gut permeability was monitored using urinary sugar probes. They showed that patients with ALD had significantly increased gut permeability compared to healthy controls and alcoholics without ALD. The authors concluded that “a ‘leaky gut’ may be a necessary cofactor for the development of chronic liver injury in heavy drinkers”. Dr. Keshavarzian’s group performed a subsequent cross-sectional study in healthy volunteers and in alcoholics with and without mild liver disease (Mutlu et al., 2012). In that elegant study, serum endotoxin levels were elevated to a similar degree in alcoholics with and without liver disease. Given that intestinal microbiota are the sources of circulating endotoxins, the microbiome was evaluated in this study. Endotoxemia was associated with alterations in the gut microbiome, specifically, lower abundance of Bacteriodetes and higher levels of Proteobacteria. Our group demonstrated that restoration of alcohol-mediated alterations in the gut microbiome by oral supplementation with probiotics was associated with improvement in liver injury in patients with mild alcohol-induced liver injury admitted to an alcohol detoxification unit (Kirpich et al., 2008). Although LPS levels were not evaluated in those patients, they most likely had increased gut permeability and endotoxemia, given that gut dysbiosis is a well-documented factor contributing to both alcohol-induced “leaky gut” and endotoxemia. Indeed, recent work from Delzenne’s group in Belgium demonstrated increased intestinal permeability (as assessed by chromium EDTA) associated with the gut microbiota alterations in a subset of alcohol-dependent individuals who were admitted for alcohol detoxification and rehabilitation program (Leclercq et al., 2014). These same investigators evaluated alcohol-dependent individuals over a three-week period and found initial elevated plasma LPS levels in parallel to increased gut permeability that normalized by the end of the treatment period (Leclercq et al., 2012). The increased gut permeability and endotoxemia significantly correlated with depression, anxiety and craving. The group having higher gut permeability and endotoxemia also showed more severe psychological disturbances, which only partially improved with abstinence and attenuated gut permeability and endotoxemia (Leclercq et al., 2012; Leclercq et al., 2014). Patients with liver fibrosis, as assessed by FibroScan, were excluded from these studies, but more detailed analysis of subtle liver disease was not described.
Consistent with the notion that females are more susceptible to alcohol-induced liver injury than males (Becker et al., 1996), females in our study demonstrated higher levels of biomarkers of liver injury (e.g., ALT and AST levels, CK18 M65 and CK18 M30) compared to males, although they had lower daily alcohol intake and shorter lifetime duration of heavy drinking. The higher susceptibility to toxic effects of alcohol in females compared to males may be explained, in part, by the observation that females drinking equal amounts of alcohol exhibit higher blood alcohol levels than males due to lower levels of gastric alcohol dehydrogenase (Frezza et al., 1990), and, possibly, due to an effect of estrogens (Eagon, 2010). In the present study, we observed no significant differences in LPS levels between males and females with early ALD, which is in agreement with the previously published studies (Schafer et al., 2002; Urbaschek et al., 2001). However, we did find reduced levels of flagellin in females compared to males. The reason for this is not clear; the females had significantly elevated levels of AST compared to males and therefore might be expected to have increased flagellin rather than decreased. Our finding of essentially similar levels of inflammatory cytokines and relatively reduced liver inflammation in males compared to females suggests that the females may adapt differently to changes in the gut induced by chronic alcohol consumption. Further research on the immunological differences between males and females may help explain the sex differences that appear to drive a more rapidly progressive course of alcohol related liver damage in females.
The present study had several limitations. One of the potential limitations was that we were not able to analyze the ALT and AST dynamics during the detoxification period, as limited data points were available in the clinical laboratory database. Another limiting aspect was the lack of a control group comprised of healthy individuals without alcohol dependence. However, the primary goal of the study was to compare two groups of alcohol-dependent individuals with the presence or absence of alcohol-mediated liver injury based on elevated serum markers of liver damage. Therefore, we believe that the absence of control group of healthy alcohol-independent individuals was an appropriate approach to achieve our goal. We also did not compare our patients (receiving medical management) with a comparable (non-treatment seeking) cohort to determine whether or not medical management itself contributed to lowering endotoxemia. Because of the exploratory nature of our study, additional research is warranted.
In conclusion, our study and studies from other investigators demonstrating that mild ALD in humans is associated with endotoxemia, provide a rationale for the use of LPS-targeted therapies to modify or neutralize the systemic endotoxin pool, as well as for interventions to modulate gut microbiota and intestinal permeability (e.g., probiotics or dietary factors) as a potential clinical approach in the management of early ALD.
ACKNOWLEDGEMENTS
The authors thank LCTS clinical staff at NIAAA NIH for the medical management of the patients. The authors thank Ms. Marion McClain for proofreading the manuscript.
Funding: The study was supported by NIH grants R21 AA020849 (IAK), R01 AA024102 (IAK), Z99-AA999999 (VV), U01AA022489 (CJM), 1U01AA021901-01 (CJM), 1U01AA021893-01 (CJM), R01AA023681 (CJM), the Department of Veterans Affairs BX000350 (CJM), the Department of Defense W81XWH-11-1-0595 (CJM). Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM113226 (CJM), and the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number P50AA024337 (CJM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: The authors have no conflict of interest to declare
Author Contribution: CJM and JCU are project PIs and contributed equally to the study. MS and VV assembled clinical data. IAK, KCF, MP, LZ, CH performed sample analysis. CJM, JCU, IAK, and VV interpreted data. IAK and CJM wrote the manuscript, VV described the patient population. CJM, JCU, IAK, VV, and DTG contributed scientifically.
REFERENCES
- Anstee QM, Daly AK, Day CP. Genetics of Alcoholic Liver Disease. Seminars in liver disease. 2015;35:361–74. doi: 10.1055/s-0035-1567832. [DOI] [PubMed] [Google Scholar]
- Askgaard G, Gronbaek M, Kjaer MS, Tjonneland A, Tolstrup JS. Alcohol drinking pattern and risk of alcoholic liver cirrhosis: a prospective cohort study. J Hepatol. 2015;62:1061–7. doi: 10.1016/j.jhep.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Bala S, Marcos M, Gattu A, Catalano D, Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One. 2014;9:e96864. doi: 10.1371/journal.pone.0096864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker U, Deis A, Sorensen TI, Gronbaek M, Borch-Johnsen K, Muller CF, Schnohr P, Jensen G. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23:1025–9. doi: 10.1002/hep.510230513. [DOI] [PubMed] [Google Scholar]
- Beier JI, Arteel GE. Alcoholic liver disease and the potential role of plasminogen activator inhibitor-1 and fibrin metabolism. Exp Biol Med (Maywood) 2012;237:1–9. doi: 10.1258/ebm.2011.011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M, Saveria Croce L, Sasso F, Pozzato G, Cristianini G, Brandi G. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut. 1997;41:845–50. doi: 10.1136/gut.41.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987;4:8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- Broitman SA, Gottlieb LS, Zamcheck N. Influence of Neomycin and Ingested Endotoxin in the Pathogenesis of Choline Deficiency Cirrhosis in the Adult Rat. J Exp Med. 1964;119:633–42. doi: 10.1084/jem.119.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave M, Falkner KC, Henry L, Costello B, Gregory B, McClain CJ. Serum cytokeratin 18 and cytokine elevations suggest a high prevalence of occupational liver disease in highly exposed elastomer/polymer workers. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2011;53:1128–33. doi: 10.1097/JOM.0b013e31822cfd68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave M, Falkner KC, Ray M, Joshi-Barve S, Brock G, Khan R, Bon Homme M, McClain CJ. Toxicant-associated steatohepatitis in vinyl chloride workers. Hepatology. 2010;51:474–81. doi: 10.1002/hep.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusi K, Chang Z, Harrison S, Lomonaco R, Bril F, Orsak B, Ortiz-Lopez C, Hecht J, Feldstein AE, Webb A, Louden C, Goros M, Tio F. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;60:167–74. doi: 10.1016/j.jhep.2013.07.042. [DOI] [PubMed] [Google Scholar]
- D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–31. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Eagon PK. Alcoholic liver injury: influence of gender and hormones. World journal of gastroenterology. 2010;16:1377–84. doi: 10.3748/wjg.v16.i11.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072–8. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. The New England journal of medicine. 1990;322:95–9. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Uemura M, Nakatani Y, Tsujita S, Hoppo K, Tamagawa T, Kitano H, Kikukawa M, Ann T, Ishii Y, Kojima H, Sakurai S, Tanaka R, Namisaki T, Noguchi R, Higashino T, Kikuchi E, Nishimura K, Takaya A, Fukui H. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcoholism, clinical and experimental research. 2000;24:48S–54S. [PubMed] [Google Scholar]
- Fukui H. Relation of endotoxin, endotoxin binding proteins and macrophages to severe alcoholic liver injury and multiple organ failure. Alcoholism, clinical and experimental research. 2005;29:172S–79S. doi: 10.1097/01.alc.0000189278.30237.e9. [DOI] [PubMed] [Google Scholar]
- Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12:162–9. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Quintela A, Abdulkader I, Campos J, Fernandez-Hernandez L, Lojo S. Serum levels of keratin-18 fragments [tissue polypeptide-specific antigen (TPS)] are correlated with hepatocyte apoptosis in alcoholic hepatitis. Digestive diseases and sciences. 2009;54:648–53. doi: 10.1007/s10620-008-0371-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Quintela A, Garcia J, Campos J, Perez LF, Alende MR, Otero E, Abdulkader I, Tome S. Serum cytokeratins in alcoholic liver disease: contrasting levels of cytokeratin-18 and cytokeratin-19. Alcohol. 2006;38:45–9. doi: 10.1016/j.alcohol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–66. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanck C, Rossol S, Bocker U, Tokus M, Singer MV. Presence of plasma endotoxin is correlated with tumour necrosis factor receptor levels and disease activity in alcoholic cirrhosis. Alcohol and alcoholism. 1998;33:606–8. doi: 10.1093/alcalc/33.6.606. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. The American journal of gastroenterology. 1999;94:200–7. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- Kirpich IA, Feng W, Wang Y, Liu Y, Barker DF, Barve SS, McClain CJ. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res. 2012;36:835–46. doi: 10.1111/j.1530-0277.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, Soloviev AG, Barve SS, McClain CJ, Cave M. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675–82. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer G, Erdal H, Mertens HJ, Nap M, Mauermann J, Steiner G, Marberger M, Biven K, Shoshan MC, Linder S. Differentiation between cell death modes using measurements of different soluble forms of extracellular cytokeratin 18. Cancer research. 2004;64:1751–6. doi: 10.1158/0008-5472.can-03-2455. [DOI] [PubMed] [Google Scholar]
- Lavallard VJ, Bonnafous S, Patouraux S, Saint-Paul MC, Rousseau D, Anty R, Le Marchand-Brustel Y, Tran A, Gual P. Serum markers of hepatocyte death and apoptosis are non invasive biomarkers of severe fibrosis in patients with alcoholic liver disease. PLoS One. 2011;6:e17599. doi: 10.1371/journal.pone.0017599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq S, Cani PD, Neyrinck AM, Starkel P, Jamar F, Mikolajczak M, Delzenne NM, de Timary P. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun. 2012;26:911–8. doi: 10.1016/j.bbi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Starkel P, Windey K, Tremaroli V, Backhed F, Verbeke K, de Timary P, Delzenne NM. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111:E4485–93. doi: 10.1073/pnas.1415174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang Y, Wu K, Fan D. Serum cytokeratin-18 fragment level: a noninvasive biomarker for not only nonalcoholic steatohepatitis, but also alcoholic steatohepatitis. Hepatology. 2010;51:1865–6. doi: 10.1002/hep.23433. [DOI] [PubMed] [Google Scholar]
- Lippai D, Bala S, Catalano D, Kodys K, Szabo G. Micro-RNA-155 deficiency prevents alcohol-induced serum endotoxin increase and small bowel inflammation in mice. Alcoholism, clinical and experimental research. 2014;38:2217–24. doi: 10.1111/acer.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain CJ, Barve S, Barve S, Deaciuc I, Hill DB. Tumor necrosis factor and alcoholic liver disease. Alcoholism, clinical and experimental research. 1998;22:248S–52S. doi: 10.1097/00000374-199805001-00006. [DOI] [PubMed] [Google Scholar]
- Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. American journal of physiology Gastrointestinal and liver physiology. 2012;302:G966–78. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan JP. The role of intestinal endotoxin in liver injury: a long and evolving history. Hepatology. 2010;52:1829–35. doi: 10.1002/hep.23917. [DOI] [PubMed] [Google Scholar]
- Page A, Paoli PP, Hill SJ, Howarth R, Wu R, Kweon SM, French J, White S, Tsukamoto H, Mann DA, Mann J. Alcohol directly stimulates epigenetic modifications in hepatic stellate cells. J Hepatol. 2015;62:388–97. doi: 10.1016/j.jhep.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–7. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- Pelsers MM, Hermens WT, Glatz JF. Fatty acid-binding proteins as plasma markers of tissue injury. Clin Chim Acta. 2005;352:15–35. doi: 10.1016/j.cccn.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Pelsers MM, Morovat A, Alexander GJ, Hermens WT, Trull AK, Glatz JF. Liver fatty acid-binding protein as a sensitive serum marker of acute hepatocellular damage in liver transplant recipients. Clin Chem. 2002;48:2055–7. [PubMed] [Google Scholar]
- Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160–8. doi: 10.1016/j.jhep.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Schafer C, Greiner B, Landig J, Feil E, Schutz ET, Bode JC, Bode C. Decreased endotoxin-binding capacity of whole blood in patients with alcoholic liver disease. J Hepatol. 1997;26:567–73. doi: 10.1016/s0168-8278(97)80422-9. [DOI] [PubMed] [Google Scholar]
- Schafer C, Parlesak A, Schutt C, Bode JC, Bode C. Concentrations of lipopolysaccharide-binding protein, bactericidal/permeability-increasing protein, soluble CD14 and plasma lipids in relation to endotoxaemia in patients with alcoholic liver disease. Alcohol and alcoholism. 2002;37:81–6. doi: 10.1093/alcalc/37.1.81. [DOI] [PubMed] [Google Scholar]
- Schwartz JM, Reinus JF. Prevalence and natural history of alcoholic liver disease. Clinics in liver disease. 2012;16:659–66. doi: 10.1016/j.cld.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Connors GJ, Agrawal S. Assessing drinking outcomes in alcohol treatment efficacy studies: selecting a yardstick of success. Alcoholism, clinical and experimental research. 2003;27:1661–6. doi: 10.1097/01.ALC.0000091227.26627.75. [DOI] [PubMed] [Google Scholar]
- Sowa JP, Atmaca O, Kahraman A, Schlattjan M, Lindner M, Sydor S, Scherbaum N, Lackner K, Gerken G, Heider D, Arteel GE, Erim Y, Canbay A. Non-invasive separation of alcoholic and non-alcoholic liver disease with predictive modeling. PLoS One. 2014;9:e101444. doi: 10.1371/journal.pone.0101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30–6. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, Doty L, Adams LJ, Vengeliene V, Spanagel R, Zhang Y, Shen J, George DT, Hommer D, Heilig M. Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch Gen Psychiatry. 2010;67:1069–77. doi: 10.1001/archgenpsychiatry.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbaschek R, McCuskey RS, Rudi V, Becker KP, Stickel F, Urbaschek B, Seitz HK. Endotoxin, endotoxin-neutralizing-capacity, sCD14, sICAM-1, and cytokines in patients with various degrees of alcoholic liver disease. Alcoholism, clinical and experimental research. 2001;25:261–8. [PubMed] [Google Scholar]
- Wakim-Fleming J, Mullen KD. Long-term management of alcoholic liver disease. Clinics in liver disease. 2005;9:135–49. doi: 10.1016/j.cld.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu Y, Kirpich I, Ma Z, Wang C, Zhang M, Suttles J, McClain C, Feng W. Lactobacillus rhamnosus GG reduces hepatic TNFalpha production and inflammation in chronic alcohol-induced liver injury. The Journal of nutritional biochemistry. 2013;24:1609–15. doi: 10.1016/j.jnutbio.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Liu F, Yang J, Zhong M, Zhang E, Li Y, Zhou D, Cao Y, Li W, Yu J, Yang Y, Yan H. Over-activation of TLR5 signaling by high-dose flagellin induces liver injury in mice. Cellular & molecular immunology. 2015;12:729–42. doi: 10.1038/cmi.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]