Summary
Background
Increased von Willebrand factor (VWF) and reduced ADAMTS13 activity are associated with arterial thrombosis. This may also be the culprit mechanism implicated in delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage (SAH).
Objective
To determine plasma VWF and ADAMTS13 in patients with SAH and healthy subjects; and to explore the levels of those markers and outcome after SAH.
Methods
40 consecutive patients were enrolled between September 2007 and April 2014 in a pilot study. Plasma samples were collected from SAH patients on post-bleed day (PBD) 0, 1, 3, 5, 7 and 10 and healthy controls. VWF antigen (VWFAg) and VWF activity (VWFAc) were determined by enzyme-linked immunoassay and collagen binding assay, respectively. ADAMTS13 activity was determined by the cleavage of a fluorescent substrate. Univariate descriptive statistics and cluster analyses were performed based on outcomes in the group with SAH only.
Results
Mean age of SAH patients was 52.4 years (26–84 years) and 30 (75%) were women. 12/40 (30%) had a high Hunt and Hess grade (IV–V) and 25 (62.5%) were treated with coil embolization. Plasma VWFAg and VWFAc were significantly higher in SAH patients than those in healthy subjects on each PBD (p<0.0001). Concurrently, plasma ADAMTS13 activity in SAH patients was significantly lower than that in healthy subjects (p<0.0001). Among those with SAH, cluster analysis demonstrated that patients with higher VWFAg and VWFAc and/or lower ADAMTS13 activity might be at risk of increased mortality.
Conclusions
The relative deficiency of plasma ADAMTS13 activity in SAH patients may associate with worse outcome.
Keywords: Subarachnoid hemorrhage, delayed cerebral ischemia, von Willebrand factor, ADAMTS13, platelet hypercoagulability, microvascular thrombosis, and brain aneurysm
Introduction
von Willebrand factor (VWF) is a multimeric protein that plays a critical role in hemostasis. In plasma, VWF binds and transports clotting factor VIII, helps platelets tether to injured endothelium, and promotes platelet adhesion and aggregation under flow conditions. Plasma VWF is primarily synthesized and secreted from vascular endothelial cells and its expression and release are increased when endothelial cells are activated or injured (1). Therefore, increased plasma levels of VWF may signify endothelial dysfunction and/or predispose to thrombosis. Recent studies have demonstrated that an accumulation of circulating ultra large VWF may be associated with arterial thrombotic disorders including myocardial infarction (2, 3) and ischemic cerebral stroke (2, 4–6).
ADAMTS13, a plasma metalloprotease, regulates the function of ultra large VWF by proteolytic cleavage of VWF at its central A2 domain (7). This cleavage results in reduction in circulating VWF multimer size, thereby reducing its thrombotic potential while retaining its hemostatic activity. Newly synthesized ultra large VWF is stored in the Weibel-Palade bodies of endothelial cells and released upon stimulation by inflammatory cytokines (8, 9). These ultra large VWF multimers are highly thrombogenic, which can form string-like structures and recruit platelets to the site of injury (10). Plasma ADAMTS13 is highly efficient in removing ultra large VWF anchored on endothelial cells and/or during the thrombus growth (11). The importance of ADAMTS13 and VWF interaction is clearly demonstrated by the overwhelming burden of spontaneous microvascular thrombi in small arterioles and capillaries in patients with thrombotic thrombocytopenic purpura (TTP) (12, 13). This is a potentially fatal syndrome caused by severe deficiency (usually less than 10% of normal) of plasma ADAMTS13 activity, resulting from acquired autoantibodies against ADAMTS13.
Subarachnoid hemorrhage (SAH) is a devastating neurological condition that affects nearly 30,000 people annually in the United States. Although this subtype comprises only 3–5% of all strokes, it accounts for up to 25% of stroke-related deaths (14). A major morbidity of SAH is delayed cerebral ischemia (DCI), which is thought to be due in part to microvascular and macrovascular thrombosis (15, 16). DCI may be reversible, or it may progress to cerebral infarction, which is associated with increased mortality and poor functional outcome (17).
There is increasing evidence suggesting that the pathophysiology of DCI may be primarily platelet-mediated. Autopsy studies demonstrate accumulations of platelet thrombi as opposed to erythrocyte-rich thrombi in parenchymal small vessels after SAH (18). Platelet-mediated microvascular thrombosis after SAH has been demonstrated in animal models (18, 19). Increased levels of β-thromboglobulin, thromboxane B2, P-selectin, and platelet activating factor suggest increased platelet activation, possibly initiated by subarachnoid blood-induced endothelial injury (20). However, the role of the relative deficiency of plasma ADAMTS13 activity and increased levels of plasma VWF in pathogenesis and outcomes after SAH has not been investigated. Therefore, the goals of this pilot study are: 1) to determine the plasma levels of VWF and ADAMTS13 in comparison with those in healthy subjects and to explore the difference in the levels of those biomarkers and mortality rates only in patients with SAH; 2) to provide information regarding effect size on outcomes to aid with sample size calculations in designing future large or multicenter studies.
Methods
Patients
Forty consecutive patients with moderate-to-severe SAH (defined as Fisher group III) were prospectively enrolled in a study of inflammatory biomarkers at the University of Pennsylvania, Philadelphia, PA, a tertiary care academic hospital. Approval from the Institutional Review Board (IRB) was obtained. Criteria for inclusion were diffuse thick subarachnoid clot on admission head computed tomography (CT) (e.g. Fisher group III), hospital admission within 24 hours of ictus, and projected intensive care unit (ICU) care of greater than 24 hours. Patients who received treatment using investigational drugs were excluded from analysis. Patients with SAH from antecedent head trauma, ischemic or hemorrhagic stroke, vascular malformation, or other secondary causes were also excluded.
Demographic data, including age and sex as well as clinical and laboratory data were recorded. Hunt and Hess classifications were determined by the admitting physicians and based on the clinical examination obtained in the Emergency Department. Fisher group was recorded on the basis of the initial head CT. Aneurysm size and location were recorded based on information from catheter angiography and operative reports.
All patients were treated according to a local standard protocol that included aggressive pre-hospital and preoperative resuscitation, early aneurysm occlusion, and aggressive prevention, and treatment of intracranial hypertension and delayed cerebral ischemia according to published recommendations (21). No patient received anti-fibrinolytic treatment. Transcranial Doppler (TCD) ultrasound studies were performed daily from admission to hospital day 14 for all patients with adequate temporal bone windows. Mean velocities of >200 cm/s in the middle cerebral arteries and Lindegaard ratios of >3 were recorded as evidence of TCD vasospasm. Continuous electroencephalography (cEEG) for alpha variability was performed for all patients between post bleed days (PBD) 2 and 10. Patients experiencing DCI received hypertensive euvolemic therapy. Intra-arterial vasodilator therapy, balloon angioplasty, and/or intraventricular administration of nicardipine were considered if TCD elevations persisted despite induced hypertension, if cEEG demonstrated worsening alpha variability, or if neurological state declined. Intracranial hypertension was treated according to a step-wise local protocol. Patients were treated for hypovolemia to a goal of normal volume status. Sodium levels were monitored serially and maintained >135 g/dL. In addition, for comparison, plasma from 40 healthy adult individuals was collected and stored until analysis.
Plasma VWFAg by ELISA
Plasma VWF antigen was determined by a laboratory-developed enzyme-linked immunosorbent assay (ELISA) as described previously (22). Briefly, maxisorp plates (Nalge Nunc International, Rochester, NY) were coated with 1:2,000 dilution of polyclonal rabbit anti-human VWF antibody (Dako, Denmark) at 4 °C for overnight. Plasma samples at various dilutions and standards were incubated at room temperature for 2 hours after blocking with 1% casein in PBS. After washing with phosphate buffered saline (PBS) containing 0.05% Tween-20 for 3 times, a horseradish peroxidase (HRP) conjugated polyclonal rabbit anti-human VWF (Dako, Denmark) antibody at 1:3,000 was applied as the detection antibody. Following the final wash steps, one single tetramethylbenzidine (TMB) solution (Invitrogen, Camarillo, CA) was used for color development. The absorbance at 450 nm was determined with a ThermoMax190 micro-titer plate reader (Molecular Device, Sunnyvale, CA). Pooled normal human plasma (NHP, George King Bio-Medical, Overland Park, KS) was used as the standard, defined as having 100% of VWFAg.
Plasma VWF activity by CBA
Plasma VWFAc was determined by the ELISA-based collagen-binding assay (CBA) (23), similarly to VWF antigen measurement with the only difference in the first coating step. The coating reagent (100 μl) for the CBA was 6 μg/ml of type III collagen (Southern Research Institute, Birmingham, AL) in 20 mM of acetic acid instead of primary antibody (rabbit) against human VWF. The rest of steps remained the same as measurement of VWFAg as described as above. Same NHP was used for calibration.
Plasma ADAMTS13 activity by FRETS
Recombinant human VWF73 peptide derived from the central A2 domain of VWF (i.e. rF-VWF73) was expressed and fluorescein-labeled, and purified to homogeneity according to the protocol described previously (24). The final concentrations of rF-vWF73 peptides were determined by using a NanoDrop spectrophotometer (Thermo Scientific) at the absorbance of 280 nm with a correction at 495 nm. Purified rF-VWF73 (1 μmol/L) was incubated with either 2.5 μl of NHP or patient’s plasma in 5 mmol/L Bis-Tris, pH 6.0, 25 mmol/L CaCl2, and 0.005% Tween-20 at 25 °C. The rate of fluorescence generation was monitored every 2 min for 60 min on a GeminiXPS microtiter plate reader (Molecular Devices, Sunnyvale, CA, USA). Relative activity (%) was determined based on the standard curve generated with pooled NHP. ADAMTS13 activity in pooled NHP was defined as having 100% of proteolytic activity.
Statistical analysis
Because of the difference in the nature of two main goals in this pilot study, different statistical methods were employed. The analysis of variance (ANOVA) was used to determine the differences among group means of plasma levels of VWFAg, VWFAc, and ADAMTS13 activity between SAH patients and healthy subjects. For exploratory analysis between each of the biomarkers and mortality outcome in patients with SAH, the statistical analyses concentrated on hypothesis generation (such as effect size for sample size calculation) for a subsequent larger study. Primarily, using only the data from patients with SAH, univariate analysis was performed with descriptive statistics to summarize both quantitative and categorical variables. The means and standard deviations were reported for quantitative variables. Likewise, frequencies and percentages were reported for categorical variables. The non-parametric Wilcoxon group comparison p-values were reported with adjusted multiple testing using false discovery rates (FDR) (25). Per Sullivan and Feinn’s recommendation, we report the effect size (Cohen’s D equivalent) which “is the main finding of a quantitative study” and represents the magnitude of difference between 2 variables (26). We also follow the guidelines to quantify the effect size as low (0.2–0.5), moderate (0.5–0.8), and high (>0.8) (27). Furthermore, we calculated the simultaneous confidence intervals of the reported effects sizes using the FDR method (28) at 10% level with Fisher’s Z transformation for categorical variables (29). Overall for the univariate analysis, rather than just reporting p-values, we also follow recommended practice and report FDR and effect sizes along with simultaneous confidence intervals (30). Therefore, the p-values are used only for reporting purposes in exploratory analyses.
Following univariate analysis, we explored the data for repeated measures of VWFAg, VWFAc, and ADAMTS13 in SAH patients on mortality, the primary outcome, using cluster analysis. Briefly, cluster analysis is an exploratory data analysis tool to sort data into groups in a way that the degree of association between data is maximal if they belong to the same group and minimal otherwise. As a first step in cluster analysis, we used the ‘elbow method,’ which is one of the most common techniques used to estimate the number of clusters. This involved using a plot of within-cluster error measure wherein the location of the ‘elbow’ provides the optimal number of clusters (31). Subsequently, a k-means clustering algorithm was fit to the data. All statistical analyses were performed using R statistical software (Vienna, Austria). Cohen’s D was calculated using the lsr package. The confidence interval for effect size d was calculated using MBESS package. Everitt & Hothorn (2009) R function was used to plot the cluster elbows.
Results
Patient characteristics
In this cohort of 40 consecutive patients with SAH, the mean age was 52.4 years (range 26–84 years). Thirty of 40 (75%) patients were women. Twelve (30%) patients had a high Hunt and Hess grade (IV–V) on admission and more than half (25/40) were treated with coil embolization. 60% of patients had a clinical history of hypertension, while 68% (26/40) endorsed current or prior tobacco use. The observed incidence of mortality in this cohort was 18% (7/40) (Table 1).
Table 1.
Univariate analysis between mortality outcome and clinical parameters
| Categorical variables | Levels | N=40 | % | Mortality | p value | FDRa | db (90% CI) | |
|---|---|---|---|---|---|---|---|---|
| No (n=33) | Yes (n=7) | |||||||
| Sex | Men | 10 | 0.8 | 24 | 6 | 0.65 | 0.77 | 0.07 |
| Women | 30 | 0.3 | 9 | 1 | (−.21,0.34) | |||
| Hunt-Hess grade | Low | 28 | 0.7 | 26 | 2 | 0.02 | 0.19 | 0.36 |
| High | 12 | 0.3 | 7 | 5 | (−.03,0.66) | |||
| Fisher | Mild | 2 | 0.1 | 2 | 0 | 0.43 | 0.67 | 0.12 |
| Moderate | 28 | 0.7 | 24 | 4 | (−.17,0.41) | |||
| Severe | 10 | 0.3 | 7 | 3 | ||||
False discovery rate;
Effect size cohen’s d (standardized mean or mean rank difference) with 90% simultaneous CI.
Plasma levels of VWFAg, VWFAc, and ADAMTS13 activity in patients with SAH vs. healthy subjects
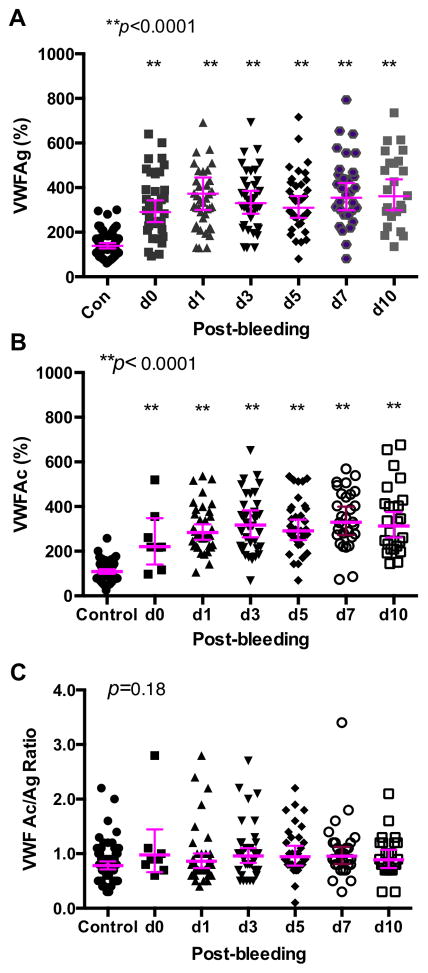
Plasma levels of VWFAg were significantly elevated in patients with SAH compared to healthy subjects on every PBD (p<0.0001) (Fig. 1A). There was a progressive increase in plasma VWFAg levels over the study period; the averaged levels increased by 9 μg/mL per time point [coefficient 0.089; 95% CI (1.1–1.66), p=0.025] when clustered by patient using regression analysis. Plasma levels of VWFAc were also significantly higher in the SAH group than in the healthy subject on every PBD (Fig. 1B). There was no significant difference in the ratio of VWFAc to VWFAg between patients with SAH and healthy subjects (p=0.31) and the ratios remained stable throughout the 10-day hospitalization (Fig. 1C). These results suggest that both VWF antigen and VWF activity are proportionally increased after subarachnoid hemorrhage.
Fig. 1. Plasma VWFAg, VWFAc, and the ratio of VWFAc to VWFAg in patients with SAH and healthy controls.
Plasma levels of VWFAg (A), VWFAc (B), and the ratio of VWFAc/VWFAg (C) were determined by a modified ELISA as described in the Method. The horizontal lines indicate the median ± 95% confidential interval. ANOVA with Kruskal-Wallis test was used to determine the statistical significance between the each PBD and the control. P values <0.05 and <0.01 are considered to be statistically significant and highly significant, respectively.
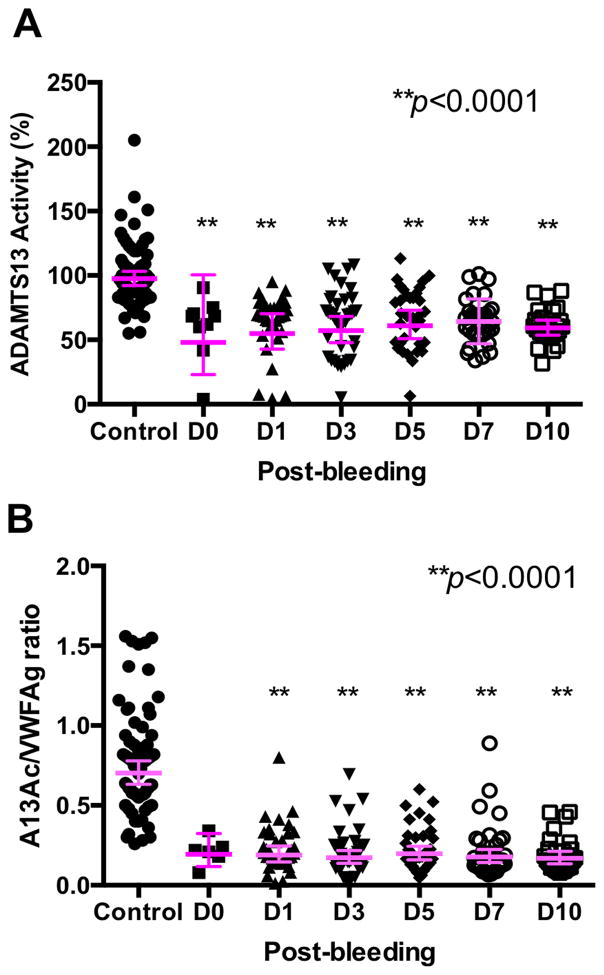
Reduced plasma ADAMTS13 activity and the ratio of ADAMTS13 to vWF in patients with SAH compared with healthy subjects
Plasma ADAMTS13 activity in SAH patients and healthy subjects were determined by FRETS described in the Methods. Plasma ADAMTS13 activity in SAH patients was significantly lower than that in healthy subjects (p<0.0001) and remained persistently low throughout 10 PBDs (Fig. 2A). The ratio of plasma ADAMTS13 activity to VWF antigen was strikingly lower in SAH patients than in healthy subjects (p<0.0001) (Fig. 2B). These results suggest the persistent relative deficiency of plasma ADAMTS13 activity in patients suffered from SAH.
Fig. 2. Plasma ADAMTS13 activity and the ratio of ADAMTS13 activity to VWFAg in patients with SAH and healthy controls.
Plasma ADAMTS13 activity (A) was determined by the cleavage of rF-VWF73 as described in the Method. The ratios of plasma ADAMTS13 activity to VWFAg in patients and controls are shown in B. ANOVA with Kruskal-Wallis test was used to determine the statistical significance between the each PBD and the control. All p values were <0.0001, indicating that the difference was statistically highly significant between patients at each PBD and control.
Exploratory analysis between plasma VWFAg, VWFAc, and ADAMTS13 activity in patients with SAH and mortality outcome
Using only the data from patients with SAH, univariate analysis of both quantitative and categorical variables is categorized by mortality (Tables 1 & 2). Only were the laboratory parameters obtained on PBD1 and PBD3 used in the statistical analysis because of high frequency of missing data on other days of hospitalization. The entire patient cohort including 10 men and 30 women were available for analysis: 1 man and 6 women died. Five of 7 (72%) non-survivors had a high Hunt and Hess grade (p<0.02) (Table 1), consistent with the association of severe injury with mortality.
Table 2.
Univariate analysis between the mortality and plasma VWFAg, VWFAc, and ADAMTS13 activity
| Category | N | Missing data (%) | Mean | SD | Mortality (No)
|
Mortality (Yes)
|
p | FDRa | db (90% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||||||||
| D1-VWFAg | 36 | 0.12 | 322.9 | 142.3 | 30 | 307.9 | 149.5 | 6 | 397.7 | 62.4 | 0.06 | 0.228 | 0.87 |
|
| |||||||||||||
| (−0.15,1.88) | |||||||||||||
|
| |||||||||||||
| D3-VWFAg | 39 | 0.05 | 372.5 | 225.1 | 32 | 330.8 | 134.5 | 7 | 563.2 | 419.2 | 0.04* | 0.19 | 0.88 |
|
| |||||||||||||
| (−0.13,1.89 | |||||||||||||
|
| |||||||||||||
| D1-VWFAc | 36 | 0.12 | 301.4 | 105.2 | 30 | 291.1 | 97.6 | 6 | 353.2 | 135.6 | 0.43 | 0.67 | 0.35 |
|
| |||||||||||||
| (−0.48,1.19) | |||||||||||||
|
| |||||||||||||
| D3-VWFAc | 38 | 0.07 | 394.1 | 444.4 | 32 | 320.6 | 128.9 | 6 | 786.2 | 1068.0 | 0.24 | 0.57 | 0.53 |
|
| |||||||||||||
| (−0.38,1.44) | |||||||||||||
|
| |||||||||||||
| D1-A13 | 36 | 0.12 | 63.69 | 21.8 | 30 | 62.61 | 23.3 | 6 | 69.07 | 11.0 | 0.66 | 0.78 | 0.19 |
|
| |||||||||||||
| (−0.57,0.97) | |||||||||||||
|
| |||||||||||||
| D3-A13 | 39 | 0.05 | 63.61 | 24.4 | 32 | 65.87 | 25.1 | 7 | 53.27 | 18.8 | 0.14 | 0.38 | 0.62 |
|
| |||||||||||||
| (−0.25,1.50) | |||||||||||||
Indicates significance at 0.05;
FDR-False Discovery Rate;
Effect size Cohen’s d (standardized mean or mean rank difference) with 90% simultaneous CI; N, total number of patients; SD, standard deviation; moderate and large effect sizes (>0.5) are shown in bold; A13, ADAMTS13; VWFAg and VWFAc are VWF antigen and collagen-binding activity, respectively.
Because of low number of patient samples on PBD0 and other subsequent days available for analysis, we chose the results of PBD1 and PBD3 for further analysis. As shown in Table 2, the mean plasma levels of VWFAg on PBD1 and PBD3 were 322.9% and 372.5%, respectively. The mean values of VWFAc on PBD1 and PBD3 were 301.4% and 394.1%, respectively. The mean plasma ADAMTS13 activity on PBD1 and PBD3 were the same (~63%). The patient group with mortality had a higher mean value of plasma VWFAg on both PBD1 (397.7% vs. 307.9%, p=0.06) and PBD3 (563.2% vs. 330.8%, p<0.05) as compared with those survived (Table 2). However, after accounting for multiple testing, the false discovery rate (FDR) did not show any statistical significance. Nonetheless, the effect sizes (d) for plasma VWFAg on PBD1 and PBD3 were 0.87 (90% CI: −0.15, 1.88) and 0.88 (90%CI: −0.13, 1.89), respectively (Table 2). These d values are large enough to indicate that these differences have practical significance and may reach statistical significance if sample size is increased.
Similarly, the plasma VWFAc on PBD1 (353.2% vs. 291.1%, p=0.43) and PBD3 (786.2% vs. 320.6%, p=0.24) was much higher in the group with mortality than in those without (Table 2). While there was no statistically significant difference between the two groups, the effect size (Cohen’s d) revealed low to moderate values for both PBD1 (d=0.35, 95% CI: −0.48, 1.19) and PBD3 (d=0.53, 90% CI: −0.38, 1.44) (Table 2). The mean plasma levels of ADAMTS13 activity on PBD3 in the group with mortality (53.3%) was lower compared to those without (65.9%) with a moderate effect size (Cohen’s d=0.62, 90% CI: −0.25, 1.50) (Table 2). These results suggest the association of low plasma ADAMTS13 activity with mortality.
Clustering analysis
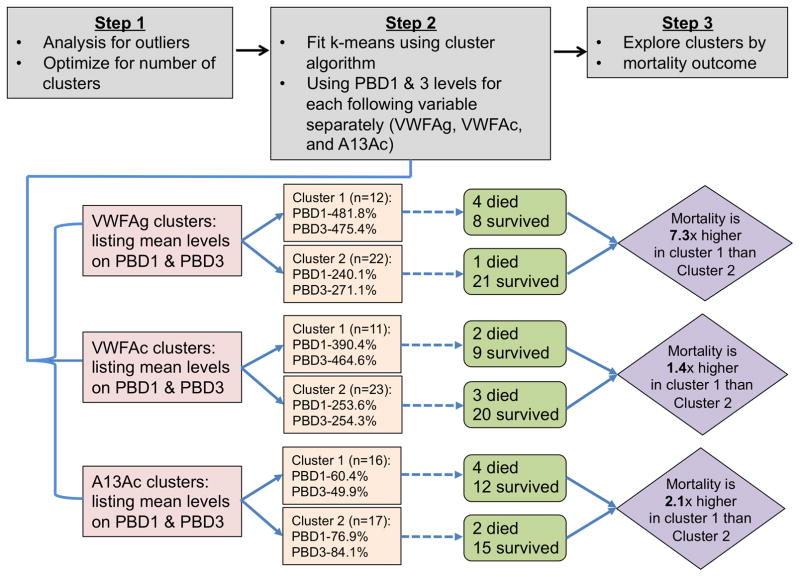
Prior to performing cluster analysis, simple outlier analysis using ranges of each quantitative variable reveled a few outliers in ADAMTS13 activity (both on PBD1 and PBD3) and one outlier in VWFAc (on PBD1 and PBD3). After removing outlier (values outside the 2 standard deviation) and performing a list-wise deletion by individual combination of variables (e.g. VWFAc PBD1 and PBD3, VWFAg PBD1 and PBD3, and ADAMTS13 PBD1 and PBD3), there were a total of 34 usable observations in VWFAc, VWFAg, and 33 usable observations in ADAMTS13.
The scree plots (not shown) for within group sums of squares versus the number of clusters for VWFAc, VWFAg, and ADAMTS13 for PBD1 and PBD3 indicated an optimal solution containing 2 clusters. Consequently, a k-means clustering algorithm with 2 clusters was fit individually for observations of PBD1 and PBD3 on the same variables (Fig. 3). For plasma VWFAg, the first cluster average for PBD1 (n=12) was 481.8% and 240.1% for cluster 2 (n=22). Similarly, for PBD3 the first cluster average was 475.4% and second cluster average was 254.3%. This indicates that first cluster had higher average VWFAg compared to second cluster for both PBD1 and PBD3 and there were 4/8 (80%) mortalities in cluster 1 compared to 1/21 (20%) in cluster 2. In other words, the mortality risk was 7.33 times higher if the patients had high mean VWFAg levels. For plasma VWFAc, cluster 1 (n=11) had higher values (~1.5 – 1.8 times higher) compared to cluster 2 (n=23) for both PBD1 and PBD3. Again, the mortality risk is higher in cluster 1 compared to cluster 2 (1.39 times higher). Finally, for ADAMTS13, for PBD1, cluster 1 had an average of 60.4% and 76.9% for cluster 2. The size of the cluster was almost equally split (Cluster 1=16 and Cluster 2=17). For PBD3, cluster 1 had an average of 49.9% and cluster 2 had an average of 84.1%. This clearly indicates that cluster 1 had lower ADAMTS13 level compared to cluster 2 for both PBD1 and PBD3. Thus, it is not surprising that the mortality rates were higher in cluster 1 (66%) compared to cluster 2 (33%). Specifically, the risk of mortality increased by 2.1 times in patients with lower ADAMTS13 levels on PBD1 and PBD3 (Fig. 3). The mean plasma VWFAg was not significantly higher in those with angiographic vasospasm [OR=1.08 95% CI (0.67 – 1.81); p=0.78] or sonographic vasospasm [OR= 0.96 95% CI (0.58 – 1.58); p=0.87] compared with those without (data not shown). In summary, exploratory analysis using cluster analysis suggested the association between mortality and increased levels of plasma VWFAg and VWFAc or reduced plasma ADAMTS13 activity.
Fig. 3. Cluster analysis.
This analysis demonstrated that mortality risk was higher in patients with high plasma levels of VWFAg and VWFAc levels (~7.3 and 1.4 times, respectively) and low plasma levels of ADAMTS13 activity (2.1 times).
Discussion
In the first part of this pilot study, plasma VWFAg and VWFAc, as well as ADAMTS13 activity were determined in a cohort of patients with SAH and compared with healthy subjects. In the second part, various exploratory statistical analyses have been performed to determine the correlations or effect sizes between each biomarker and mortality outcome in patients with SAH. We demonstrate the statistically significant elevation of plasma VWFAg and VWFAc levels and reduced plasma ADAMTS13 activity when compared with the healthy subjects (p<0.0001). The elevated plasma VWFAg levels on PBD1 and PBD3 or the mean levels of plasma VWFAg of all 10-PBDs (not shown) were significantly associated with in-hospital mortality. Additionally, we found a significant reduction of plasma ADAMTS13 activity in SAH patients compared with healthy subjects, which is potentially correlated with increased mortality and disease severity.
Persistently high plasma VWFAg levels over the 10-day study period are very surprising to us and are of potentially clinical significance, as this period after SAH is often marked by microvascular and macrovascular thrombosis, similar to what has been previously observed with the elevations of VWFAg levels (4, 6, 32). The increased plasma VWFAg and VWFAc levels were associated with a concomitant decrease in plasma ADAMTS13 activity levels, resulting in relatively moderate to severe deficiency of plasma ADAMTS13 activity in patients with SAH. These findings are consistent with clinical and experimental studies demonstrating a relationship between elevated VWFAg levels and decreased ADAMTS13 activity in ischemic stroke.
The pathophysiological role of VWF in ischemic stroke has recently gained increased attention. In fact, deficiency of VWF results in reduced ischemic volume compared to healthy controls in a VWF knock out murine stroke model (33). Animal studies using the transient middle cerebral artery occlusion model have demonstrated involvement of the collagen-VWF-glycoprotein (GP) Ib axis in progression of ischemia (34). A recent meta-analysis of prospective cohort studies assessing the relationship between plasma VWFAg and ischemic stroke (n=1,500 patients) demonstrated an odds ratio of 1.17 [95% CI (1.08 – 1.26)]. If all case-controlled studies (n=2,532) were included in the analysis, the odds ratio increased to 1.55 [95% CI (1.31–1.83)] (2).
The relationship between VWF and thrombosis in part depends on VWF multimer size. VWF, synthesized in endothelial cells and megakaryocytes, undergoes considerable processing, including multimerization and lateral association under flow to form UL-VWF forms that are hyperactive. These ULVWF multimers can recruit circulating platelets to the site of injury (35). Smaller multimers of VWF, which represent newly synthesized proteins and processed by plasma ADAMTS13, are typically found in circulation and are hemostatic, but not prothrombotic. While the mechanisms underlying altered ratio of ADAMTS13 to VWF after SAH are not fully understood, inflammatory cytokinesis and damage-associated molecular pattern molecules (DAMPs) are known to stimulate a massive release of VWF from endothelial cells and suppress the biosynthesis of ADAMTS13 in the liver (12, 36).
The inability to cleave ULVWF resulting from severe deficiency of ADAMTS13 activity leads to a potential fatal blood disorder TTP. Patients with TTP are found to develop disseminated microvascular thrombosis in the brain, heart, pancreas, kidneys, and intestine, resulting in multi-organ failure (12, 36). The absence of VWF cleavage allows for the accumulation of ULVWF and platelets (as opposed to thrombi composed of erythrocytes) that eventually occlude arterioles and capillaries. Although TTP results from severe reductions (<10% baseline) in ADAMTS13 activity, a tendency toward thrombosis may be observed with milder reductions in activity. Reduced plasma ADAMTS13 activity may be a risk factor for myocardial infarction (2, 5), ischemic stroke (4, 5). In a longitudinal cohort study of nearly 6,000 Swiss patients, with a median follow up of 10.7 years, patients with reduced baseline ADAMTS13 levels were at an increased risk of ischemic stroke (37). Those with ADAMTS13 levels in the lowest quartile had an absolute increase in stroke risk of 11%. This corroborates associations observed in smaller cohort studies (5, 38).
One of the putative mechanisms of thrombosis after SAH is platelet-hyperactivity. Platelet aggregation and isolated thromboemboli have been observed after SAH in the cortical arteries of experimental animals (39, 40) and in patients at autopsy (41) Subarachnoid hemorrhage results in considerable injury to the vascular endothelium (40, 42), resulting in elevation of markers of endothelial activation in blood, cerebral dialysate, and cerebrospinal fluid. A surrogate for platelet activation, platelet activating factor (PAF), has also been shown to be elevated post SAH. Elevations in PAF on PBD4~14 as well as transient increases in systemic inflammatory markers, such as IL-6 and IL-1 beta from internal jugular vein samples of patients have been observed after SAH (43).
Increased ADAMTS13 expression has also been shown to reduce ischemic burden in experimental stroke (44). In contrast, reduced ADAMTS13 levels may increase both the incidence and size of ischemic strokes (38). In our study, both reduced ADAMTS13 activity and increased VWFAg associated with mortality by cluster analysis. Thus, this corroborates a hypercoagulable state after SAH. Although only a few studies have been performed evaluating ADAMTS13 levels in patients with ischemic stroke, a meta-analysis of 616 patients demonstrated a clear association between reduced ADAMTS13 levels and increased risk of stroke [OR 2.72; 95% CI (1.52 – 4.86)] (2). Experimental murine models of SAH demonstrate that Adamts13−/− mice sustain larger areas of microvascular thrombosis than do wild-type mice, but that with recombinant ADAMTS13 treatment, the knock out mice can experience smaller thrombosis areas, similar to wild type mice (45). Therefore, recombinant ADAMTS13 may be a putative treatment option in SAH patients at risk for DCI.
There are some limitations to this study. Firstly, our analysis was performed at a single center with a limited sample size and therefore, the conclusions should be regarded as hypothesis generating and not causal. However, despite a small cohort of patients in this pilot study, cluster analysis demonstrated that high plasma VWFAg levels and low plasma ADAMTS13 activity levels may associate with increased mortality after SAH. Secondly, while our data demonstrate a potential relationship between VWFAg elevation and mortality after SAH, this relationship may not be unique to SAH. Other forms of brain injury (e.g. traumatic brain injury) may incite a similar response, and not consequently DCI, alone. Further studies in other brain injury models would help clarify this issue. Third, the hypercoagulability noted in this study could have been in part due to surgical clipping of the aneurysm. However, the surgical intervention did not alter the relationship between elevated VWF and mortality. Similarly, aneurysms were not identified in 5 patients from this prospective study. However, removal of these patients from analysis did not change the association between VWF and in-hospital mortality. Therefore, these patients were included in the study. Lastly, given the lack of a clear temporal relationship between the studies variables, these data do not imply causality, only association.
Conclusions
Our study suggests that plasma VWF antigen levels and VWF activity are significantly increased and plasma ADAMTS13 activity is concomitantly reduced in patients with SAH when comparing with healthy subjects. Furthermore, an increase in plasma VWFAg and VWFAc and a concomitant reduction of ADAMTS13 activity after SAH are associated with mortality in patients with SAH. This effect may be mediated through micro- and macrovascular thrombosis as the VWF elevation was also associated with evidence of radiographic infarction. The relatively severe deficiency of plasma ADAMTS13 activity may be associated with cerebral ischemic and thrombosis after SAH. Further larger study is warranted to determine whether ADAMTS13 supplementation could mitigate microvascular thrombosis and ischemia in patients with SAH. The effect sizes between different laboratory parameters and mortality outcome provided in this pilot study should facilitate sample size calculations for such future multicenter studies.
What is known on this topic?
Increased von Willebrand factor (VWF) and reduced ADAMTS13 activity are risk factors for arterial thrombosis.
Whether those are also the culprit mechanisms underlying delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage (SAH) is not known.
What this paper adds?
This study demonstrates that plasma levels of VWF antigen and activity are significantly increased with concomitant reduction in plasma ADAMTS13 activity in patients with SAH comparing to the healthy subjects.
In the SAH group alone, such changes in plasma VWF levels are associated with mortality.
The effect sizes (Cohen’s d) between various laboratory parameters and mortality in SAH patients provide valuable information for the sample size determination in future multicenter studies.
Acknowledgments
The study was in part supported by grants from NIH R01 HL126724 and R01 HL115187 (to X.L.Z.).
Footnotes
Authorship Statement
MK, WC, and XLZ designed and performed experiments, interpreted results, and wrote manuscript. JM, HPP, and DR performed data analysis and revised manuscript. KN, KM EMW, SF, MJS, DK, EEZ, JMS, SEK, and JML recruited patients and revised manuscript.
Conflict of interest statement
XLZ receives a grant support from Alexion and serves in the speaker’s bureau of Alexion and performs consultation for Ablynx.
References
- 1.Ruggeri ZM, Ware J. The structure and function of von Willebrand factor. Thromb Haemost. 1992;67:594–9. [PubMed] [Google Scholar]
- 2.Sonneveld MA, de Maat MP, Leebeek FW. Von Willebrand factor and ADAMTS13 in arterial thrombosis: a systematic review and meta-analysis. Blood Rev. 2014;28:167–78. doi: 10.1016/j.blre.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Horii M, Uemura S, Uemura M, et al. Acute myocardial infarction as a systemic prothrombotic condition evidenced by increased von Willebrand factor protein over ADAMTS13 activity in coronary and systemic circulation. Heart Vessels. 2008;23:301–7. doi: 10.1007/s00380-008-1053-x. [DOI] [PubMed] [Google Scholar]
- 4.Wieberdink RG, van Schie MC, Koudstaal PJ, et al. High von Willebrand factor levels increase the risk of stroke: the Rotterdam study. Stroke. 2010;41:2151–6. doi: 10.1161/STROKEAHA.110.586289. [DOI] [PubMed] [Google Scholar]
- 5.Andersson HM, Siegerink B, Luken BM, et al. High VWF, low ADAMTS13, and oral contraceptives increase the risk of ischemic stroke and myocardial infarction in young women. Blood. 2012;119:1555–60. doi: 10.1182/blood-2011-09-380618. [DOI] [PubMed] [Google Scholar]
- 6.Bongers TN, de Maat MP, van Goor ML, et al. High von Willebrand factor levels increase the risk of first ischemic stroke: influence of ADAMTS13, inflammation, and genetic variability. Stroke. 2006;37:2672–7. doi: 10.1161/01.STR.0000244767.39962.f7. [DOI] [PubMed] [Google Scholar]
- 7.Zheng XL. ADAMTS13 and von Willebrand Factor in Thrombotic Thrombocytopenic Purpura. Annu Rev Med. 2015;66:211–25. doi: 10.1146/annurev-med-061813-013241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernardo A, Ball C, Nolasco L, et al. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood. 2004;104:100–6. doi: 10.1182/blood-2004-01-0107. [DOI] [PubMed] [Google Scholar]
- 9.Sporn LA, Marder VJ, Wagner DD. von Willebrand factor released from Weibel-Palade bodies binds more avidly to extracellular matrix than that secreted constitutively. Blood. 1987;69:1531–4. [PubMed] [Google Scholar]
- 10.Arya M, Anvari B, Romo GM, et al. Ultralarge multimers of von Willebrand factor form spontaneous high-strength bonds with the platelet glycoprotein Ib-IX complex: studies using optical tweezers. Blood. 2002;99:3971–7. doi: 10.1182/blood-2001-11-0060. [DOI] [PubMed] [Google Scholar]
- 11.Dong JF, Moake JL, Nolasco L, et al. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–9. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 12.Moake JL. Thrombotic thrombocytopenic purpura: the systemic clumping “plague”. Annu Rev Med. 2002;53:75–88. doi: 10.1146/annurev.med.53.082901.103948. [DOI] [PubMed] [Google Scholar]
- 13.Zheng X, Majerus EM, Sadler JE. ADAMTS13 and TTP. Curr Opin Hematol. 2002;9:389–94. doi: 10.1097/00062752-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart Disease and Stroke Statistics--2011 Update: A Report From the American Heart Association. Circulation. 123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peltonen S, Juvela S, Kaste M, et al. Hemostasis and fibrinolysis activation after subarachnoid hemorrhage. J Neurosurg. 1997;87:207–14. doi: 10.3171/jns.1997.87.2.0207. [DOI] [PubMed] [Google Scholar]
- 16.Nina P, Schisano G, Chiappetta F, et al. A study of blood coagulation and fibrinolytic system in spontaneous subarachnoid hemorrhage. Correlation with hunt-hess grade and outcome. Surg Neurol. 2001;55:197–203. doi: 10.1016/s0090-3019(01)00402-5. [DOI] [PubMed] [Google Scholar]
- 17.Rabinstein AA, Friedman JA, Weigand SD, et al. Predictors of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke. 2004;35:1862–6. doi: 10.1161/01.STR.0000133132.76983.8e. [DOI] [PubMed] [Google Scholar]
- 18.Vergouwen MD, Vermeulen M, Coert BA, et al. Microthrombosis after aneurysmal subarachnoid hemorrhage: an additional explanation for delayed cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:1761–70. doi: 10.1038/jcbfm.2008.74. [DOI] [PubMed] [Google Scholar]
- 19.Bell JD, Thomas TC, Lass E, et al. Platelet-mediated changes to neuronal glutamate receptor expression at sites of microthrombosis following experimental subarachnoid hemorrhage. J Neurosurg. 2014;121:1424–31. doi: 10.3171/2014.3.JNS132130. [DOI] [PubMed] [Google Scholar]
- 20.Juvela S, Ohman J, Servo A, et al. Angiographic vasospasm and release of platelet thromboxane after subarachnoid hemorrhage. Stroke. 1991;22:451–5. doi: 10.1161/01.str.22.4.451. [DOI] [PubMed] [Google Scholar]
- 21.Raya AK, Diringer MN. Treatment of subarachnoid hemorrhage. Crit Care Clin. 2014;30:719–33. doi: 10.1016/j.ccc.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Jin SY, Skipwith CG, Shang D, et al. von Willebrand factor cleaved from endothelial cells by ADAMTS13 remains ultralarge in size. J Thromb Haemost. 2009;7:1749–52. doi: 10.1111/j.1538-7836.2009.03570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerritsen HE, Turecek PL, Schwarz HP, et al. Assay of von Willebrand factor (vWF)-cleaving protease based on decreased collagen binding affinity of degraded vWF: a tool for the diagnosis of thrombotic thrombocytopenic purpura (TTP) Thromb Haemost. 1999;82:1386–9. [PubMed] [Google Scholar]
- 24.Zhang L, Lawson HL, Harish VC, et al. Creation of a recombinant peptide substrate for fluorescence resonance energy transfer-based protease assays. Anal Biochem. 2006;358:298–300. doi: 10.1016/j.ab.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 25.Yoav B, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach To Multiple Testing. J Roy Stat Soc. 2005;57:289–300. [Google Scholar]
- 26.Sullivan GM, Feinn R. Using Effect Size—or Why the P Value Is Not Enough. J Grad Med Educ. 2012;4:279–82. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- 28.Benjamini Y, Yekutieli D. Quantitative trait Loci analysis using the false discovery rate. Genetics. 2005;171:783–90. doi: 10.1534/genetics.104.036699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armitage P, Colton T. Encyclopedia of Biostatistics. 2. Hoboken, NJ: Wiley Interscience; 2005. [Google Scholar]
- 30.Kelley K. Confidence Intervals for standardized effect sizes: THeory, application, and implementation. J Stat Soft. 2007;20:1–24. [Google Scholar]
- 31.Gordon Ad. Null models in cluster validation. Springer; 1996. [Google Scholar]
- 32.De Meyer SF, Schwarz T, Deckmyn H, et al. Binding of von Willebrand factor to collagen and glycoprotein Ibalpha, but not to glycoprotein IIb/IIIa, contributes to ischemic stroke in mice--brief report. Arterioscler Thromb Vasc Biol. 2010;30:1949–51. doi: 10.1161/ATVBAHA.110.208918. [DOI] [PubMed] [Google Scholar]
- 33.Kleinschnitz C, De Meyer SF, Schwarz T, et al. Deficiency of von Willebrand factor protects mice from ischemic stroke. Blood. 2009;113:3600–3. doi: 10.1182/blood-2008-09-180695. [DOI] [PubMed] [Google Scholar]
- 34.De Meyer SF, Stoll G, Wagner DD, et al. von Willebrand factor: an emerging target in stroke therapy. Stroke. 2012;43:599–606. doi: 10.1161/STROKEAHA.111.628867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dopheide SM, Maxwell MJ, Jackson SP. Shear-dependent tether formation during platelet translocation on von Willebrand factor. Blood. 2002;99:159–67. doi: 10.1182/blood.v99.1.159. [DOI] [PubMed] [Google Scholar]
- 36.Zheng XL. ADAMTS13 and von Willebrand factor in thrombotic thrombocytopenic purpura. Annu Rev Med. 2015;66:211–25. doi: 10.1146/annurev-med-061813-013241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonneveld MA, de Maat MP, Portegies ML, et al. Low ADAMTS13 activity is associated with an increased risk of ischemic stroke. Blood. 2015;126:2739–46. doi: 10.1182/blood-2015-05-643338. [DOI] [PubMed] [Google Scholar]
- 38.Fujioka M, Hayakawa K, Mishima K, et al. ADAMTS13 gene deletion aggravates ischemic brain damage: a possible neuroprotective role of ADAMTS13 by ameliorating postischemic hypoperfusion. Blood. 2010;115:1650–3. doi: 10.1182/blood-2009-06-230110. [DOI] [PubMed] [Google Scholar]
- 39.Parra A, McGirt MJ, Sheng H, et al. Mouse model of subarachnoid hemorrhage associated cerebral vasospasm: methodological analysis. Neurol Res. 2002;24:510–6. doi: 10.1179/016164102101200276. [DOI] [PubMed] [Google Scholar]
- 40.Findlay JM, Weir BK, Kanamaru K, et al. Arterial wall changes in cerebral vasospasm. Neurosurgery. 1989;25:736–45. doi: 10.1097/00006123-198911000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki S, Kimura M, Souma M, et al. Cerebral microthrombosis in symptomatic cerebral vasospasm--a quantitative histological study in autopsy cases. Neurol Med Chir (Tokyo) 1990;30:309–16. doi: 10.2176/nmc.30.309. [DOI] [PubMed] [Google Scholar]
- 42.Iuliano BA, Pluta RM, Jung C, et al. Endothelial dysfunction in a primate model of cerebral vasospasm. J Neurosurg. 2004;100:287–94. doi: 10.3171/jns.2004.100.2.0287. [DOI] [PubMed] [Google Scholar]
- 43.Hirashima Y, Nakamura S, Endo S, et al. Elevation of platelet activating factor, inflammatory cytokines, and coagulation factors in the internal jugular vein of patients with subarachnoid hemorrhage. Neurochem Res. 1997;22:1249–55. doi: 10.1023/a:1021985030331. [DOI] [PubMed] [Google Scholar]
- 44.Zhao BQ, Chauhan AK, Canault M, et al. von Willebrand factor-cleaving protease ADAMTS13 reduces ischemic brain injury in experimental stroke. Blood. 2009;114:3329–34. doi: 10.1182/blood-2009-03-213264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vergouwen MD, Knaup VL, Roelofs JJ, et al. Effect of recombinant ADAMTS-13 on microthrombosis and brain injury after experimental subarachnoid hemorrhage. J Thromb Haemost. 2014;12:943–7. doi: 10.1111/jth.12574. [DOI] [PubMed] [Google Scholar]