Abstract
The prevalence of asthma is higher in pre-pubescent and aging males, and in post-pubertal females, strongly indicating that sex steroids (especially estrogen) may be an important modulator in lung disease. We recently demonstrated that airway smooth muscle (ASM) expresses both alpha and beta forms of the estrogen receptor (ERα and ERβ) in males and females, and that these receptors regulate intracellular [Ca2+] and ASM contractility. While both ERα and ERβ have multiple splice variants, it is unclear if and how the expression of these variants is modulated under conditions such as chronic inflammation/ asthma. In order to test the hypothesis that the differential expression of ERα and ERβ variants contributes to the pathogenesis of asthma, we profiled the expression of various ERα and ERβ genes in asthmatic and inflamed (TNFα- or IL-13-treated) ASM. Gene expression was assessed at both the mRNA and protein levels in asthmatic ASM cells or non-asthmatic cells treated with TNFα (20ng/ml) or IL-13 (50ng/ml). We observed marked variation in the expression of ER isoforms in response to inflammatory stimuli, and in non-asthmatic vs. asthmatic ASM. Changes in protein levels of ERα and ERβ corresponded with the observed differential mRNA patterns. Pharmacological studies implicate cytosolic (p42/44 MAPK and PI3K) and nuclear (NFκB, STAT6 and AP-1) signaling pathways as putative mechanisms that mediate and/or regulate effects of inflammation on ER expression. We conclude that variations in ASM ER expression profiles occur with inflammation and that ER variants could contribute to estrogen signaling in airway diseases such as asthma.
Keywords: estrogen, sex steroid signaling, airway inflammation, asthma
Introduction
Asthma is an airway disease characterized by airway thickening and hyperresponsiveness. Signals and factors that trigger and/or that are activated due to airway inflammation are major contributors to asthma development and progression. Such disease modulators include sex steroids, their relevance in the airway being important considering the well-known clinical differences between male and female asthma patients (Becklake and Kauffmann, 1999; Bjornson and Mitchell, 2000; Caracta, 2003; Carey et al., 2007b; Jensen-Jarolim and Untersmayr, 2008; Melgert et al., 2007) where asthma is more common among pre-pubescent males but the prevalence is higher in females following puberty. In the aging population, males (possibly because of an increase in aromatase-mediated conversion of testosterone to estrogen) develop asthma more frequently than do females. Given that there is an increased incidence of asthma in young women (Bottema et al., 2005; Melgert et al., 2007; Postma, 2007), sex steroids such as estrogen and progesterone are of potential importance, although their relative contributions to different aspects of airway structure and function in the context of asthma remain underexplored. At least in the context of estrogens (which appear to be important regardless of the role of progesterone), in order to elucidate the link with asthma pathophysiology, it is essential to first understand the estrogen receptor (ER) expression and signaling in the airway from a more general viewpoint than a sex-specific aspect.
Clinical and experimental data appear conflicted with regard to whether estrogens are protective or detrimental in asthma with some studies suggesting that estrogens enhance inflammation (Riffo-Vasquez et al., 2007; Sakazaki et al., 2008), while others associate estrogens with an asthma-mitigating role (Carey et al., 2007a; Dimitropoulou et al., 2009; Haggerty et al., 2003; Lieberman et al., 1995; Matsubara et al., 2008; Myers and Sherman, 1994). For example, data from multiple groups, including our own, have shown that estrogen has a bronchodilatory function (Dimitropoulou et al., 2009; Sathish et al., 2015a; Sathish et al., 2015b; Townsend et al., 2011; Townsend et al., 2012a; Townsend et al., 2012b; Townsend et al., 2010). The effects of estrogens in the airway certainly appear to be complex, cell type- and context-dependent, interactive and importantly not completely understood (Matthews and Gustafsson, 2003; O’Lone et al., 2007; Sathish et al., 2015b; Townsend et al., 2012a). In this regard, estrogen signaling via ERs in the airway is still largely uncharacterized. In humans, 17β-estradiol acts through ERα and ERβ, two full-length ERs of the nuclear receptor family of transcription factors (Kim et al., 2008; Miller and Duckles, 2008). The genomic effects of nuclear ERs or those translocating from the cytosol to the nucleus are well known; however, there is emerging interest in membrane-bound ERs, their atypical, non-genomic effects and their activation of downstream transcription factors. Furthermore, ERα-36 and ERα-46, the shorter (truncated) isoforms of ERα, lack the activation function (AF-1) domain, and are believed to impart complex effects on the full length ERα (ERα-FL). And finally, the mechanisms by which ERβ splice variants modulate estrogen signaling (Heldring et al., 2007) remain unclear.
In this study, we sought to characterize the expression of ER isoforms and variants, and whether and how such expression is altered during chronic inflammation, as a first step towards understanding the potential role of ER isoforms and variations in asthma pathophysiology. We quantified the expression of ERα-36, ERα-46 and the full-length isoforms of ERα, and the five known variants (V1–5) of ERβ, in asthmatic vs. non-asthmatic airway smooth muscle (ASM) cells or in non-asthmatic cells exposed to prototypical inflammatory agents relevant to asthma, TNFα and IL-13. Our results show an increased abundance of all ER isoforms (except ERβ-V5) in asthmatic ASM cells. Our data also indicates that expression of ER isoforms is modulated by inflammation via signaling pathways such as cytosolic p42/44 MAPK and PI3K as well as nuclear AP-1, NFκB, STAT6.
Materials and Methods
Chemicals, drugs/inhibitors, antibodies
Antibody against ERα was obtained from Santa Cruz Biotechnology. ERβ–MC-10 antibody was developed and characterized by Dr. Hawse’s laboratory (Wu et al., 2012). Fluorescent secondary antibodies were obtained from Li-Cor Biosciences (Lincoln, NE). Pharmacological inhibitors for the signaling pathways NFκB (SN50), P42/44 (PD98059), PI3K (Wortmannin) and P38 (SB203580) were obtained from EMD/Calbiochem (Culver City, CA), STAT6 (AS1517499) from Axon MedChem (Reston, VA), and AP1 (SR11302) from Tocris (Minneapolis, MN). TNFα and IL-13 were obtained from R&D systems (Minneapolis, MN). All other chemicals or antibodies were obtained from Sigma-Aldrich.
ASM cells
ASM cells were isolated from post-surgical lung resections from both asthmatic and non-asthmatic individuals, as previously described (Prakash et al., 2006; Prakash et al., 2009a; Prakash et al., 2009b). Briefly, 3rd–6th level bronchi were isolated from surgical samples of lung resections incidental to patient thoracic surgery at Mayo Clinic, Rochester, MN (all protocols approved by an Institutional Review Board and considered minimal risk; tissues were collected from surgical pathology following diagnosis). ASM was dissected from normal-looking areas, immersed in ice-cold HBSS (Hanks’ Balanced Salt Solution; 2 mM Ca2+), denuded of epithelium by blunt dissection, and cells isolated as described previously (Prakash et al., 2006; Prakash et al., 2009a; Prakash et al., 2009b). Cells were limited to ≤ 3 passages of subculture and were serum-deprived at least 24 h prior to all experiments. ASM phenotype was frequently verified by expression of smooth muscle markers (actin and myosin, Ca2+ channel regulatory proteins such TRPC3, CD38 and Orai1), and by the lack of expression of epithelial and fibroblast markers as previously described (Prakash et al., 2006; Prakash et al., 2009a; Prakash et al., 2009b).
Treatment with inflammatory cytokines
Cells were treated with TNFα (20ng/ml) or IL-13 (50ng/ml) for either 24h or 48h (based on the experiment) before total RNA or protein was isolated. Expression was assessed at both mRNA and protein levels.
qPCR
Total RNA was isolated using the RNeasy Kit (Qiagen, Valencia, CA), reverse transcribed using the Transcriptor system (Roche, Indianapolis, IN), and the resultant cDNAs were subjected to Quantitative Real Time PCR optimized for Roche LC480 Light Cycler, using the ribosomal protein S16 as the internal control (reference gene), and the primers listed in Table 1. The comparative C(t) [ΔΔC(t)] method was used to determine changes in expression of mRNA of interest; ‘No treatment’ control was used as the calibrator for quantification.
Table 1. Oligonucleotide primers for qPCR on human ASM.
Forward and reverse primers specific for human amplicons targeted in the current study were obtained from Integrated DNA Technologies. Primers were used at a final concentration of 500nM in the quantitative real-time PCR reactions.
| Primer ID | Sequence |
|---|---|
| hERα36 | 5′GAGAATCCTGAACTTGCATCCT3′/ 5′TTAGACACGAGGAAACCACTTG3′ |
| hERα46 | 5′GCTACCATTATGGAGTCTGGTCCTGT 3′/ 5′CCCACCTTTCATCATTCCCACTTCGT 3′ |
| hERα-FL | 5′TGCAAGGCCTTCTTCAAGAGA3′/ 5′TCAATGGTGCACTGGTTGGT 3′ |
| hERβ-V1 | 5′CTCCCAGCAGCAATCCATGCGCC3′/ 5′AGCAGGTCATACACTGGGACCACA3′ |
| hERβ-V2 | 5′CTCCCAGCAGCAATCCATGCGCC3′/ 5′ TCACTGCTCCATCGTTGCTTCAGG3′ |
| hERβ-V3 | 5′CTCCCAGCAGCAATCCATGCGCC 3′/ 5′TCATTCCCGTGGCTGTTGACAGGC3′ |
| hERβ-V4 | 5′CTCCCAGCAGCAATCCATGCGCC 3′/ 5′CTAAGATAACTTCAAATGAATGAATTG3′ |
| hERβ-V5 | 5′GCTGAACGCCGTGACCGATGCTT 3′/ 5′TTAGGGCGCGTACCTCGCATGCC3′ |
| hS16 | 5′CAATGGTCTCATCAAGGTGAACGG3′/ 5′CTGACGGATAGCATAAATCTGGGC3′ |
Immunoblotting
Total protein was isolated using standard protocols. Approximately 25μg total protein was loaded per lane of the gel. Protein expression detection and densitometry were performed on a Li-Cor Odyssey IR scanning system (Lincoln, NE). Band intensities were normalized against β-Actin.
Statistical analyses
All experiments were performed in duplicate using ASM cells isolated from at least 4 different individuals (i.e., N=4). No treatments/vehicle represent cells not exposed to TNFα/ IL-13 or to inhibitor drugs. Comparisons were made using independent Student’s t-test or two-way ANOVA as appropriate, with Bonferroni correction for repeated measures. Statistical significance was tested at the P<0.05 level. Values are reported as means ± SE. “N” values representing numbers of patient samples are provided in the figure legends.
Results
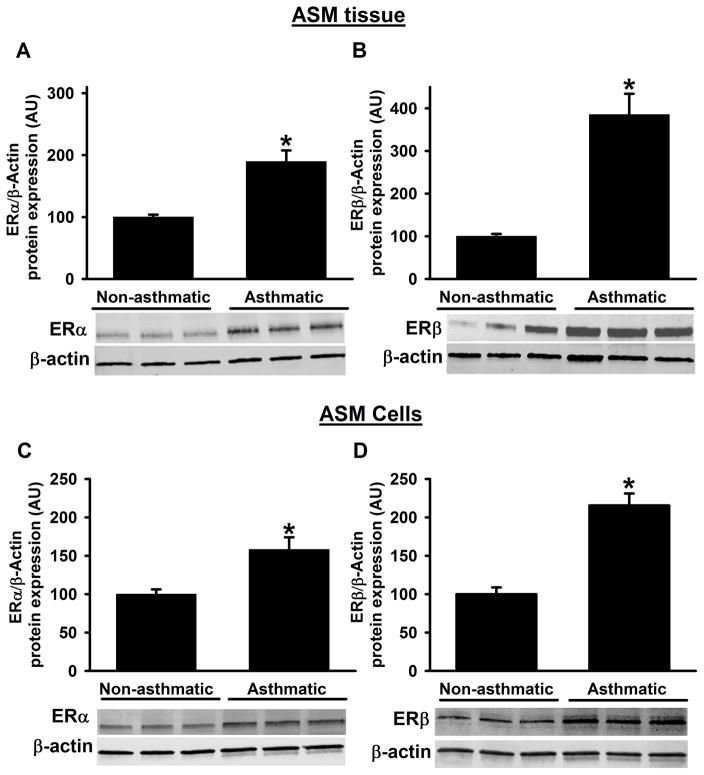
Epithelium-denuded ASM tissue samples from non-asthmatic and asthmatic patients were assessed for protein expression of ERα and ERβ by Western blot analysis and showed that baseline ERα and ERβ protein expression levels are significantly higher in asthmatic samples compared to non-asthmatics (Figure 1A&B). These data are further confirmed using primary ASM cells cultured from non-asthmatic and asthmatic samples, where we found similar increases with ER protein expression (Figure 1C&D). Notably, ERβ expression is multifold higher in asthmatic samples compared to ERα.
Figure 1. Protein expression of ERα and ERβ from asthmatic and non-asthmatic ASM.
Human ASM expresses both ERα and ERβ. At baseline, expression of ERα and ERβ in ASM tissue were significantly higher in asthmatic patients compared to non-asthmatic patients (A&B). In addition, human ASM cell lysates from asthmatics also showed the significant increase in the ERα and ERβ protein expression compared to non-asthmatics. N=4–6, each group; *indicates significant difference from ‘Non-asthmatic’ control; P<0.05.
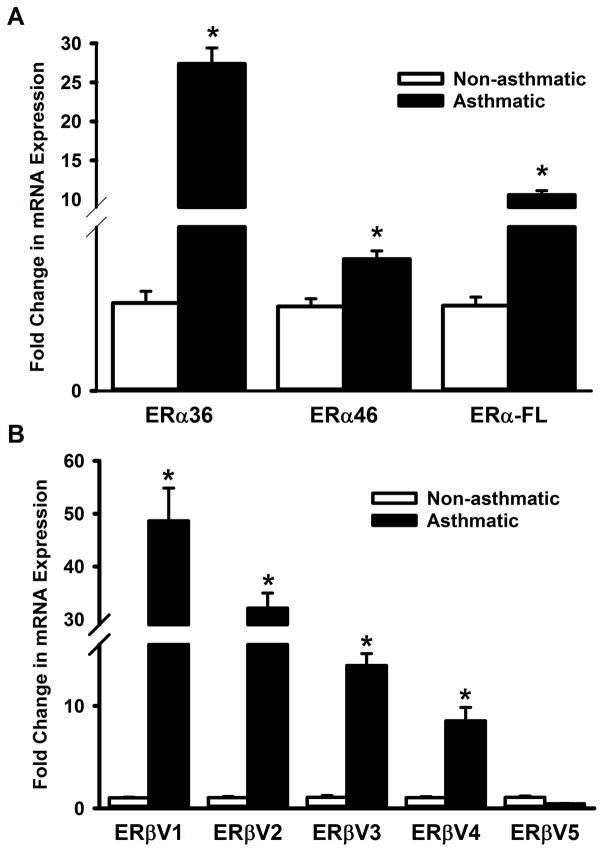
We next examined if the expression of ER isoforms and their variants are different between non-asthmatic and asthmatic individuals at the mRNA level. qPCR results show that expression of all three ERα variants (ERα-36, ERα-46 and ERα-FL) are elevated in ASM cells from asthmatic patients (Figure 2A). While a similarly high expression of ERβ variants V1, V2, V3 and V4 are observed in asthmatic ASM, the level of ERβ variant V5 mRNA seems comparable between non-asthmatic and asthmatic airways (Figure 2B).
Figure 2. Expression patterns of ERα and ERβ variants differ between asthmatic and non-asthmatic ASM cells.
Total RNA from ASM cells, isolated from non-asthmatic or asthmatic individuals, was reverse transcribed and the cDNA was used in qPCR to measure the expression of various ERα and ERβ isoforms. (A) Expression of all three ERα isoforms (ERα-36, ERα-46 and ERα-FL) were drastically elevated in asthmatic ASM. (B) A similar trend was seen in variants 1–4 of ERβ; however, variant 5 doesn’t seem to be increased in asthmatic ASM. N=4 each group; *indicates significant difference from ‘Non-asthmatic’ ASM; P<0.05.
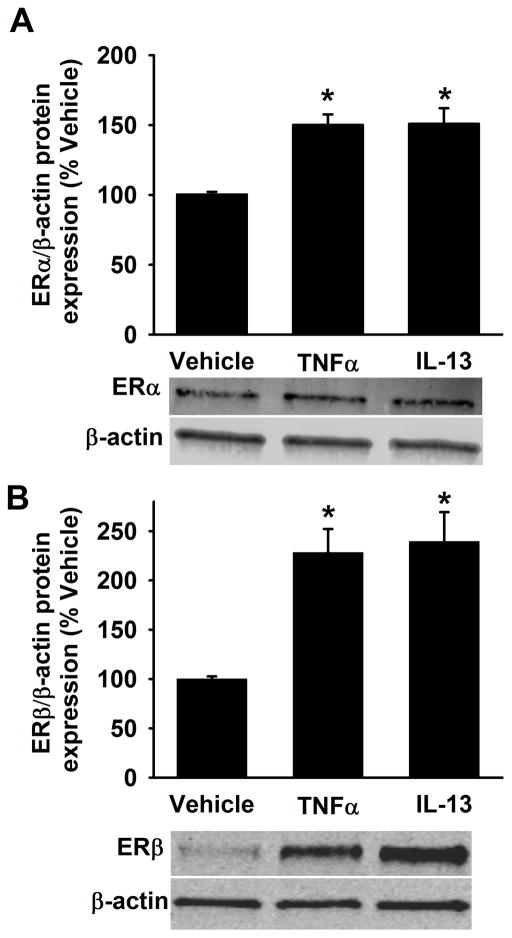
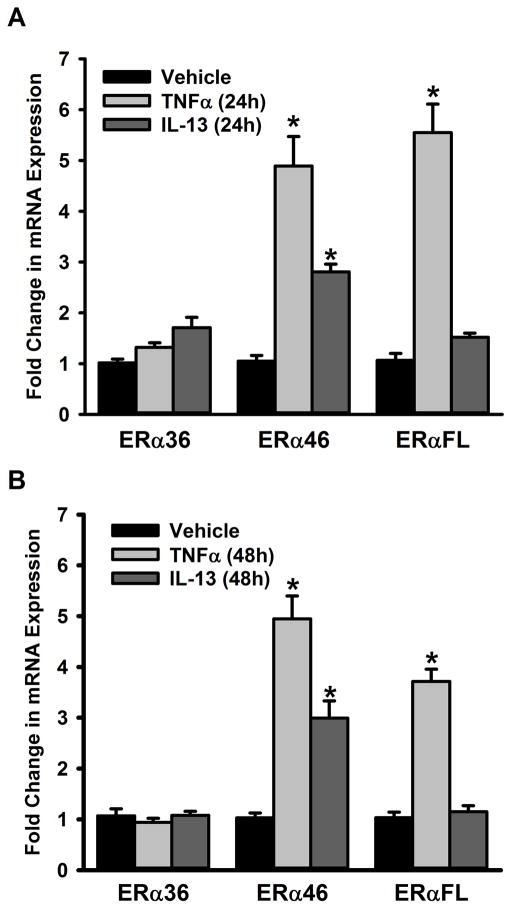
In separate analyses, non-asthmatic ASM cells were exposed to the pro-inflammatory cytokines TNFα or IL13 for 48h. The full length ERα and ERβ protein expression levels are significantly increased with TNFα or IL13 exposure compared to vehicle (Figure 3A&B). We also quantified mRNA expression of ER isoforms and as shown in Figure 4, neither a 24h (Figure 4A) nor a 48h (Figure 4B) exposure to TNFα or IL13 triggered a change in ERα-36 expression in non-asthmatic ASM cells. However, both 24h and 48h exposures to TNFα and IL13 caused the mRNA level of ERα-46 to increase. TNFα treatment resulted in upregulation of full length ER (ERα-FL) with little to no changes observed following exposure to IL-13 at either time point.
Figure 3. Inflammation increases ERα and ERβ protein expression in non-asthmatic ASM cells.
Human ASM cells from non-asthmatics were treated with pro-inflammatory cytokines either 20ng/ml TNFα or 50ng/ml IL-13 for 48h and harvested for protein expression studies using cell lysis buffer. Cytokine exposure significantly increased the expression of both ERα and ERβ in comparison to vehicle. N=4–6, each group; *indicates significant difference from vehicle; P<0.05.
Figure 4. Inflammation signals regulate the expression of ERα variants in the ASM.
Non-asthmatic ASM cells were treated with either 20ng/ml TNFα or 50ng/ml IL-13 for 24h or 48h before total RNA was isolated and reverse transcribed. The resultant cDNA was subjected to qPCR to measure the expression of various ERα isoforms. (A) While a 24h TNFα treatment increases the expression of ERα-46 and ERα-FL, there was no quantifiable change in ERα-36 expression; IL-13 treatment upregulates the expression of ERα-46 (but not that of the other isoforms) in that time period. (B) The same trend was observed 48h post treatment, as well. N=4, each group; *indicates significant difference from vehicle; P<0.05.
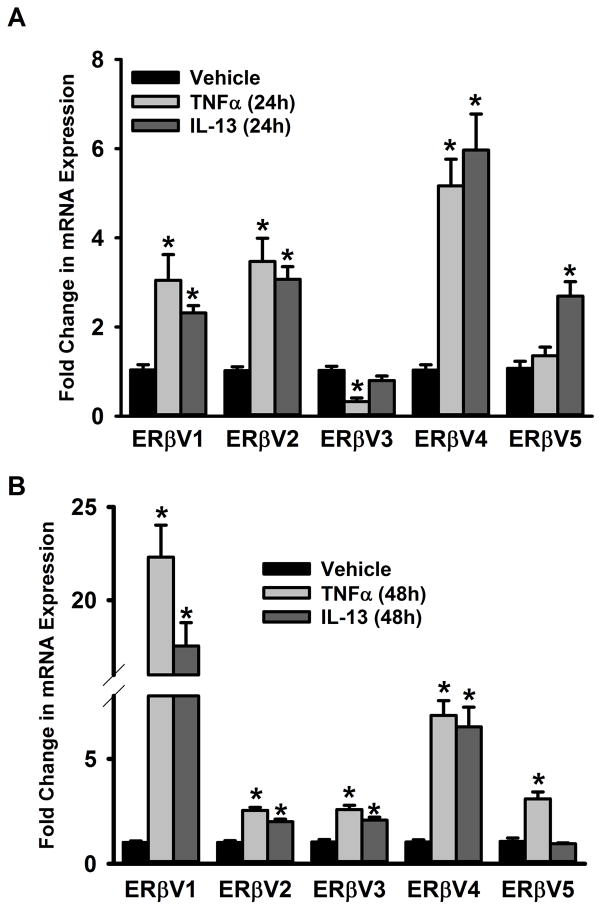
In the case of ERβ, expression of V1 and V2 increases after 24h or 48h exposure to either TNFα or IL-13 (Figure 5A&B). Interestingly, expression of ERβV3 increased only at 48h exposure to either TNFα or IL-13. Effects of TNFα or IL-13 on ERβV5 expression were variable.
Figure 5. Inflammation signals regulate the expression of ERβ variants in the ASM.
Non-asthmatic ASM cells were treated with either 20ng/ml TNFα or 50ng/ml IL-13 for 24h or 48h before total RNA was isolated and reverse transcribed. The resultant cDNA was subjected to qPCR to measure the expression of various ERβ isoforms. (A) While a 24h TNFα treatment increases the expression of ERβ variants 1, 2 and 4, there was no quantifiable change in ERβ variant 5 expression; interestingly, ERβ variant 3 expression was downregulated by this treatment. On the other hand, expression of variants 1, 2, 4 and 5 respond positively to a 24h IL-13 treatment while the variant 3 expressions remain unaffected. (B) The trend changes 48h post treatment: expression of all 5 isoforms increases after a 48h treatment with TNFα, while IL-13 treatment upregulates variants 1–4 but not variant 5. N=4, each group; *indicates significant difference from vehicle; P<0.05.
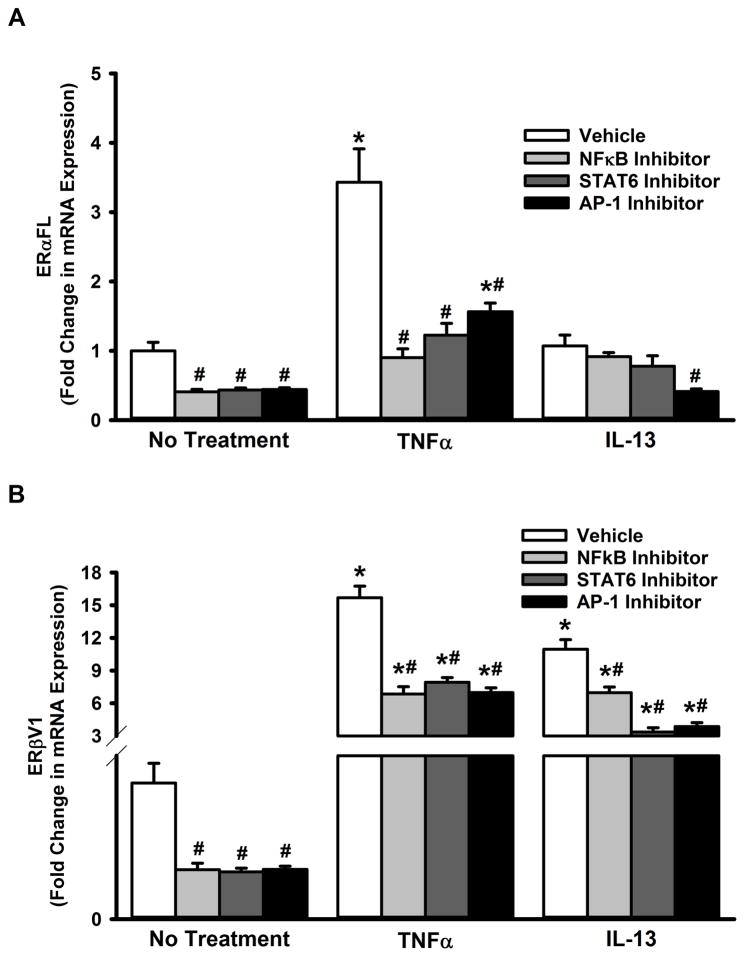
In order to understand the mechanisms that regulate ERα and ERβ expression with inflammation or disease, we tested the role of transcriptional regulation of their genes. Sequence analysis of the promoter regions suggested a putative role for transcription factors NFκB, AP1 and STAT6 (Table 2). Based on this, we pharmacologically inhibited NFκB, AP1 or STAT6 and assessed the resultant changes in expression of ERα and ERβ isoforms. We observed that TNFα-induced increase in both ERα-FL and ERβV1 expression is blunted when NFκB, AP1 or STAT6 is inhibited (Figure 6 A&B). Interestingly, only AP-1 inhibition blunted IL-13 effect on ERα-FL expression (Figure 6A). On the other hand, the transcription factors tested appear to be important for IL-13-induced changes in ERβV1 expression (Figure 6B).
Table 2. Putative transcription factor binding sites on ERα and ERβ genes.
Promoter analysis was done based on published consensus sequences for AP-1, NFkB and STAT6 binding sites in the regions up to 10KB upstream of initiation (ATG) codon on ERα and ERβ genes. Locations of transcription factor binding sites presented are distances upstream of the initiation codon.
| Gene | AP1 | NFkB | STAT6 |
|---|---|---|---|
| ESR1 (ERα) | −2202 to −2196 | −900 to −886 −2472 to −2421 −3110 to −3096 −3329 to −3319 |
−2827 to −2819 −2267 to −2260 −2121 to −2114 −397 to −389 |
| ESR2 (ERβ) | −1765 to −1759 | −1731 to −1715 −756 to −742 −602 to −587 |
−809 to −802 −408 to −398 |
Figure 6. Inflammation-associated nuclear signals regulate ERα and ERβ expression in the ASM.
Non-asthmatic ASM cells were treated with pharmacological inhibitors of transcription factors NFκB, AP1, and STAT6: NFκB was inhibited with 20μM SN-50, AP1 with 1μM SR11302, and STAT6 with 20nM AS1517499. After 2h, cells were exposed to either 20ng/ml TNFα or 50ng/ml IL-13 for 48h before total RNA was isolated and reverse transcribed. The resultant cDNA was subjected to qPCR to measure the expression of full-length ERα (ERα-FL) or ERβ variant 1. (A) Perturbing any of the transcription factor signals blocked TNFα-induced augmentation of ERα-FL expression. None of the inhibitors except the AP-1 blocker caused a significant change in ERα-FL expression, after IL-13 treatment. (B) In the case of ERβ, inhibiting any of the transcription factor signals decreased both TNFα- and IL-13-induced augmentation of ERβ-V1 expression. N=4, each group; *indicates significant difference from vehicle; ‘No Treatment’ control; #indicates significant difference between the vehicle and the TNFα/ IL-13-treated groups; P<0.05.
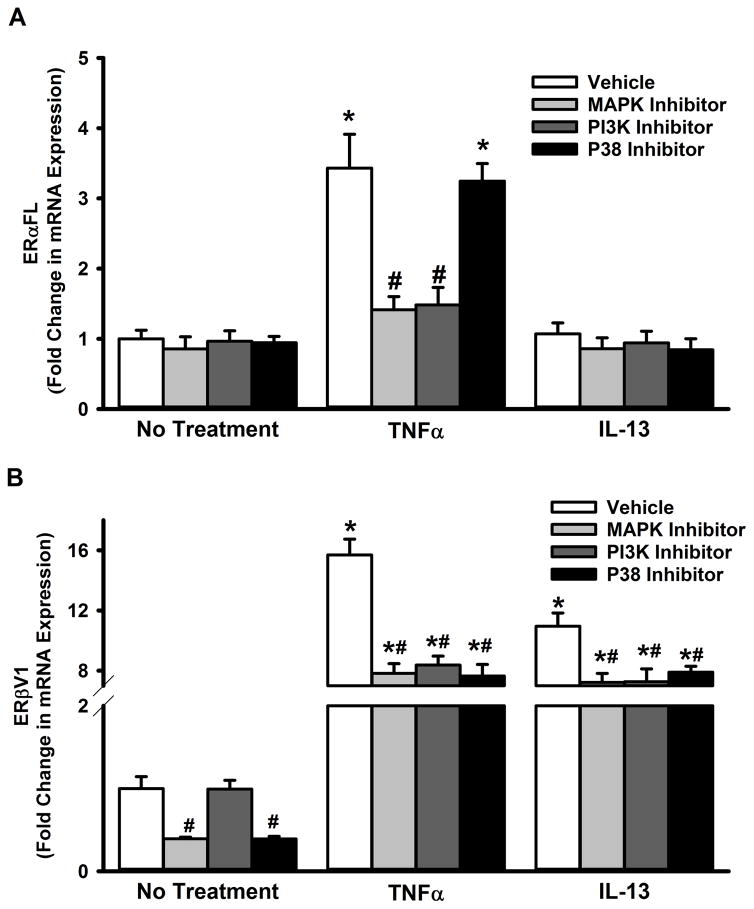
MAPKs and PI3K are among the major cytosolic signaling cascades known to contribute to inflammation effects in ASM (Lee and Yang, 2013; Prakash, 2013). To ascertain their role in controlling ER expression, we perturbed p42/44, PI3K and p38 pathways using pharmacological agents. As shown in Figure 7A, p42/44 MAPK and PI3K inhibition blunted TNFα-induced increases in ERα-FL expression, while p38 inhibition was without effect. All three pathways, however, seem to significantly contribute to both TNFα- and IL-13-induced increases in ERβV1 expression (Figure 7B).
Figure 7. Inflammation-associated cytosolic signaling cascades regulate ERα and ERβ expression in the ASM.
Non-asthmatic ASM cells were treated with pharmacological inhibitors of P38, MAPK42/44 or PI3K pathways: MAPK42/44 was inhibited with 2μM PD98059, PI3K with50nM Wortmannin, and P38 with 600nM SB203580. After 2h, cells were exposed to either 20ng/ml TNFα or 50ng/ml IL-13 for 48h before total RNA was isolated and reverse transcribed. The resultant cDNA was subjected to qPCR to measure the expression of full-length ERα (ERα-FL) or ERβ variant 1. (A) None of the inhibitors caused a change in the basal expression of ERα-FL. While inhibiting either MAPK(42/44) or PI3K pathway resulted in a significant reduction in TNFα-induced surge in ERα-FL expression, blocking the P38 pathway had no effect. IL-13 does not seem to affect ERα-FL expression, in the presence or absence of inhibitor drugs. (B) However, all three pathways tested (MAPK(42/44), PI3K and P38) seem to regulate TNFα or IL-13-mediated effects on ERβ expression: inhibiting any of these signals decreases both TNFα- and IL-13-induced augmentation of ERβ-V1 levels. N=4, each group; *indicates significant difference from vehicle; ‘No Treatment’ control; #indicates significant difference between the vehicle and the TNFα/ IL-13-treated groups; P<0.05.
Discussion
The current study focuses on understanding the potential role of sex steroid signaling in the context of asthma pathophysiology and sex differences. Here, understanding the specific contribution of estrogens may help explain clinical observations of greater asthma in women or in men under conditions of higher estrogen levels (e.g. in aging) vs. experimental observations of estrogen-induced bronchodilation for example. Given that there is limited information on the expression patterns of various ER isoforms in the airway, it is necessary to first address this issue. Our initial observation that almost all of the ERα and ERβ isoforms exhibit an aberrant expression in asthmatic ASM cells justify further exploration of how inflammation could potentially influence ER expression.
The lack of a consensus among published data has made it difficult to definitively describe a protective vs. detrimental role for estrogen signaling in the airway. For example, in premenstrual asthma, lower estrogen levels correlate with exacerbations of symptoms (Chhabra, 2005; Ensom et al., 2003; Haggerty et al., 2003), and asthma symptoms are abrogated during the third trimester (when circulating estrogen levels are the highest) in a third of pregnant women with preexisting asthma (Clark, 1993; Schatz, 1999). While the assumption has been that ER expression per se, particularly ERα should help explain estrogen effects, our current data show that different isoforms of ERα and ERβ are expressed in ASM during inflammation, and that ER variants should be considered.
Previous studies have implied that ERα and ERβ have different ligand affinities, are differentially expressed in a tissue-specific fashion and may act antagonistically, in contexts ranging from cancer progression in hormone-responsive tissues to neurodegenerative disease (Matthews and Gustafsson, 2003; O’Lone et al., 2007). Our data showing differential expression of ERα and ERβ in the presence of cytokines or with asthma suggest that complex effects of estrogen could involve the different pharmacology of ER isoforms. Interestingly, the expression of ERα-36, which has been shown to be potently activated by estrogen in neurons (Han et al., 2015), does not seem to be altered under TNFα/IL-13 treatment, in spite of showing an increased expression in asthmatic cells. Thus, this truncated isoform may serve to consistently transduce estrogen effects in the asthmatic airway Also noteworthy is the observation that IL-13 does not appear to have as robust an effect on ERα isoform expression as does TNFα. This implies that ERα may play a more active role in Th1 cytokine-induced inflammation than in Th2 cytokine function in the airway. The question then remains as to whether ERα functionality is maintained or not in airway inflammation or asthma.
Changes in ERβ expression are intriguing, and raise questions regarding what potential role this isoform could have in the airway. While variants V1–V4 are more abundant in asthmatic ASM, there is no significant difference in V5 expression between asthmatic and non-asthmatic cells. ERβ-V5 also appears to respond differently to the tested cytokines, while the other ERβ variants generally increase with longer cytokine treatments. What is not clear is the physiological significance of the different variants at baseline and with disease.
GPR30 is a transmembrane estrogen receptor which has been shown to mediate some of the effects of estrogen (Kim et al., 2008; Prossnitz et al., 2008). Although we found GPR30 mRNA in ASM cells, and both the asthmatic condition and cytokine treatment upregulate mRNA levels (data not shown), we have not detected GPR30 protein via immunoblotting. Accordingly, it remains unclear whether this estrogen receptor is of significance in ASM.
The mechanisms regulating expression of different ER isoforms with inflammation are relevant in two ways. First, pathways such as MAPK, PI3K/Akt and NFκB contribute to airway remodeling and asthma development, and our study suggests the same pathways may regulate ERα and ERβ expression during inflammation. Conversely, some of these pathways such as MAPK and PI3K are also activated by ERs, and thus could form a feed-forward loop for ER signaling. What is less clear is whether differential ER isoform functionality then controls how estrogen influences these pathways in the context of chronic inflammation.
In summary, the data presented here lay the foundation for future investigations on how estrogen signaling modulates ASM function with inflammation and asthma, particularly the impact of differential ERα and ERβ variant expression.
Acknowledgments
Supported by Young Clinical Scientist Award from the Flight Attendants Medical Research Institute (BA, SV), and R01 grants from the National Institutes of Health HL0123494 (SV), HL056470 (YSP), HL088029 (YSP).
References
- Becklake MR, Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax. 1999;54(12):1119–1138. doi: 10.1136/thx.54.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson CL, Mitchell I. Gender differences in asthma in childhood and adolescence. J Gend Specif Med. 2000;3(8):57–61. [PubMed] [Google Scholar]
- Bottema RW, Reijmerink NE, Koppelman GH, Kerkhof M, Postma DS. Phenotype definition, age, and gender in the genetics of asthma and atopy. Immunol Allergy Clin North Am. 2005;25(4):621–639. doi: 10.1016/j.iac.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Caracta CF. Gender differences in pulmonary disease. Mt Sinai J Med. 2003;70(4):215–224. [PubMed] [Google Scholar]
- Carey MA, Card JW, Bradbury JA, Moorman MP, Haykal-Coates N, Gavett SH, Graves JP, Walker VR, Flake GP, Voltz JW, Zhu D, Jacobs ER, Dakhama A, Larsen GL, Loader JE, Gelfand EW, Germolec DR, Korach KS, Zeldin DC. Spontaneous airway hyperresponsiveness in estrogen receptor-alpha-deficient mice. Am J Respir Crit Care Med. 2007a;175(2):126–135. doi: 10.1164/rccm.200509-1493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MA, Card JW, Voltz JW, Arbes SJ, Jr, Germolec DR, Korach KS, Zeldin DC. It’s all about sex: gender, lung development and lung disease. Trends Endocrinol Metab. 2007b;18(8):308–313. doi: 10.1016/j.tem.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra SK. Premenstrual asthma. Indian J Chest Dis Allied Sci. 2005;47(2):109–116. [PubMed] [Google Scholar]
- Clark SL. Asthma in pregnancy. National Asthma Education Program Working Group on Asthma and Pregnancy. National Institutes of Health, National Heart, Lung and Blood Institute. Obstet Gynecol. 1993;82(6):1036–1040. [PubMed] [Google Scholar]
- Dimitropoulou C, Drakopanagiotakis F, Chatterjee A, Snead C, Catravas JD. Estrogen replacement therapy prevents airway dysfunction in a murine model of allergen-induced asthma. Lung. 2009;187(2):116–127. doi: 10.1007/s00408-008-9129-z. [DOI] [PubMed] [Google Scholar]
- Ensom MH, Chong G, Beaudin B, Bai TR. Estradiol in severe asthma with premenstrual worsening. Ann Pharmacother. 2003;37(11):1610–1613. doi: 10.1345/aph.1D090. [DOI] [PubMed] [Google Scholar]
- Haggerty CL, Ness RB, Kelsey S, Waterer GW. The impact of estrogen and progesterone on asthma. Ann Allergy Asthma Immunol. 2003;90(3):284–291. doi: 10.1016/S1081-1206(10)61794-2. quiz 291–283, 347. [DOI] [PubMed] [Google Scholar]
- Han S, Zhao B, Pan X, Song Z, Liu J, Gong Y, Wang M. Estrogen receptor variant ER-alpha36 is involved in estrogen neuroprotection against oxidative toxicity. Neuroscience. 2015;310:224–241. doi: 10.1016/j.neuroscience.2015.09.024. [DOI] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87(3):905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Jensen-Jarolim E, Untersmayr E. Gender-medicine aspects in allergology. Allergy. 2008;63(5):610–615. doi: 10.1111/j.1398-9995.2008.01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Moriarty K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids. 2008;73(9–10):864–869. doi: 10.1016/j.steroids.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IT, Yang CM. Inflammatory signalings involved in airway and pulmonary diseases. Mediators of inflammation. 2013;2013:791231. doi: 10.1155/2013/791231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman D, Kopernic G, Porath A, Levitas E, Lazer S, Heimer D. Influence of estrogen replacement therapy on airway reactivity. Respiration. 1995;62(4):205–208. doi: 10.1159/000196448. [DOI] [PubMed] [Google Scholar]
- Matsubara S, Swasey CH, Loader JE, Dakhama A, Joetham A, Ohnishi H, Balhorn A, Miyahara N, Takeda K, Gelfand EW. Estrogen determines sex differences in airway responsiveness after allergen exposure. Am J Respir Cell Mol Biol. 2008;38(5):501–508. doi: 10.1165/rcmb.2007-0298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3(5):281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- Melgert BN, Ray A, Hylkema MN, Timens W, Postma DS. Are there reasons why adult asthma is more common in females? Curr Allergy Asthma Rep. 2007;7(2):143–150. doi: 10.1007/s11882-007-0012-4. [DOI] [PubMed] [Google Scholar]
- Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60(2):210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JR, Sherman CB. Should supplemental estrogens be used as steroid-sparing agents in asthmatic women? Chest. 1994;106(1):318–319. doi: 10.1378/chest.106.1.318. [DOI] [PubMed] [Google Scholar]
- O’Lone R, Knorr K, Jaffe IZ, Schaffer ME, Martini PG, Karas RH, Bienkowska J, Mendelsohn ME, Hansen U. Estrogen receptors alpha and beta mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol. 2007;21(6):1281–1296. doi: 10.1210/me.2006-0497. [DOI] [PubMed] [Google Scholar]
- Postma DS. Gender differences in asthma development and progression. Gend Med. 2007;4(Suppl B):S133–146. doi: 10.1016/s1550-8579(07)80054-4. [DOI] [PubMed] [Google Scholar]
- Prakash YS. Airway smooth muscle in airway reactivity and remodeling: what have we learned? American journal of physiology Lung cellular and molecular physiology. 2013;305(12):L912–933. doi: 10.1152/ajplung.00259.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Iyanoye A, Ay B, Mantilla CB, Pabelick CM. Neurotrophin effects on intracellular Ca2+ and force in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;291(3):L447–456. doi: 10.1152/ajplung.00501.2005. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Sathish V, Thompson MA, Pabelick CM, Sieck GC. Asthma and sarcoplasmic reticulum Ca2+ reuptake in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2009a;297(4):L794. doi: 10.1152/ajplung.00237.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Thompson MA, Pabelick CM. Brain-derived neurotrophic factor in TNF-alpha modulation of Ca2+ in human airway smooth muscle. Am J Respir Cell Mol Biol. 2009b;41(5):603–611. doi: 10.1165/rcmb.2008-0151OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- Riffo-Vasquez Y, Ligeiro de Oliveira AP, Page CP, Spina D, Tavares-de-Lima W. Role of sex hormones in allergic inflammation in mice. Clin Exp Allergy. 2007;37(3):459–470. doi: 10.1111/j.1365-2222.2007.02670.x. [DOI] [PubMed] [Google Scholar]
- Sakazaki F, Ueno H, Nakamuro K. 17beta-Estradiol enhances expression of inflammatory cytokines and inducible nitric oxide synthase in mouse contact hypersensitivity. Int Immunopharmacol. 2008;8(5):654–660. doi: 10.1016/j.intimp.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Sathish V, Freeman MR, Long E, Thompson MA, Pabelick CM, Prakash YS. Cigarette Smoke and Estrogen Signaling in Human Airway Smooth Muscle. Cell Physiol Biochem. 2015a;36(3):1101–1115. doi: 10.1159/000430282. [DOI] [PubMed] [Google Scholar]
- Sathish V, Martin YN, Prakash YS. Sex steroid signaling: implications for lung diseases. Pharmacol Ther. 2015b;150:94–108. doi: 10.1016/j.pharmthera.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz M. Interrelationships between asthma and pregnancy: a literature review. J Allergy Clin Immunol. 1999;103(2 Pt 2):S330–336. doi: 10.1016/s0091-6749(99)70258-7. [DOI] [PubMed] [Google Scholar]
- Townsend EA, Meuchel LW, Thompson MA, Pabelick CM, Prakash YS. Estrogen increases nitric-oxide production in human bronchial epithelium. J Pharmacol Exp Ther. 2011;339(3):815–824. doi: 10.1124/jpet.111.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev. 2012a;33(1):1–47. doi: 10.1210/er.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Sathish V, Thompson MA, Pabelick CM, Prakash YS. Estrogen effects on human airway smooth muscle involve cAMP and protein kinase A. Am J Physiol Lung Cell Mol Physiol. 2012b;303(10):L923–928. doi: 10.1152/ajplung.00023.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Thompson MA, Pabelick CM, Prakash YS. Rapid effects of estrogen on intracellular Ca2+ regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2010;298(4):L521–530. doi: 10.1152/ajplung.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Subramaniam M, Negron V, Cicek M, Reynolds C, Lingle WL, Goetz MP, Ingle JN, Spelsberg TC, Hawse JR. Development, characterization, and applications of a novel estrogen receptor beta monoclonal antibody. Journal of cellular biochemistry. 2012;113(2):711–723. doi: 10.1002/jcb.23443. [DOI] [PMC free article] [PubMed] [Google Scholar]