Abstract
Background
Inbred mouse strains are differentially sensitive to the acute effects of ethanol and are useful tools for examining how unique genomes differentially affect alcohol-related behaviors and physiology. DBA/2J mice have been shown to be sensitive to the acute anxiolytic effects of alcohol as well as the anxiogenic effects of withdrawal from chronic alcohol exposure, while B6 mice are resistant to both. Considering that the basolateral amygdala is an important brain region for the acute and chronic effects of ethanol on fear and anxiety related behaviors, we hypothesized that there would be strain-dependent differences in the acute effects of ethanol in BLA slices.
Methods
We utilized patch clamp electrophysiology in BLA coronal slices from four inbred mouse strains (A/J, BALBcJ, C57BL/6J and DBA2/J) to examine how genetic background influences acute ethanol effects on synaptic vesicle recycling and post-tetanic potentiation in response to low (2 Hz) and high (40 Hz) frequency stimulation.
Results
We found that ethanol inhibited synaptic vesicle recycling in a strain- and stimulation frequency-dependent manner. Vesicle recycling in DBA/2J and BALBcJ cells was inhibited by acute ethanol during both low and high frequency stimulation while recycling measured from AJ cells was sensitive only during high frequency stimulation. Recycling at C57BL/6J synapses was insensitive to ethanol regardless of stimulation frequency. We additionally found that cells from DBA/2J and BALBcJ mice were sensitive to ethanol-mediated inhibition of post-tetanic potentiation.
Conclusions
Acute ethanol application inhibited vesicle recycling and post-tetanic potentiation at glutamatergic synapses in both a strain- and frequency-dependent fashion. Several presynaptic proteins that contribute to synaptic vesicle priming in addition to post-tetanic potentiation have been implicated in alcohol-related behaviors, including Munc13, Munc18, and RIM proteins, making them potential candidates for the molecular mechanism controlling these effects.
Key Words/Phrases: Vesicle priming, vesicle recycling, presynaptic, acute ethanol, post-tetanic potentiation
Introduction
Alcohol use disorders (AUDs) are extremely prevalent and often lead to the development of severe mental and physical health issues. Although a few therapeutic options are available for the treatment of AUDs, the majority of addicted individuals are unable to remain abstinent from alcohol for prolonged periods of time. Some of the major barriers preventing successful treatment outcomes are an incomplete understanding of the diverse physiological effects of ethanol and the role that genetic diversity plays in the heterogeneity of AUDs. For example, of the few available pharmacological treatments, efficacy has often been linked with specific genetic mutations including Naltrexone (OPRM1), Acamprosate (GRIN2B), and Topiramate (GRIK1) (Oslin et la., 2003, Karpyak et al., 2014, Kranzler et al., 2014). Furthermore, the efficacy of behavioral interventions like the 12-step facilitation program has been linked to mutations in the GABRA2 gene (Bauer et al., 2007). These highlight the importance of understanding how genetic background influences the effects of alcohol.
Inbred mouse strains are valuable tools for determining the role of specific genotypes in producing different behavioral traits associated with AUDs as well as understanding the physiological underpinnings that contribute to these different genetic vulnerabilities. The DBA/2J (D2), C57BL/6J (B6), A/J (AJ), and BALBcJ (BALBc) strains have been commonly used in the alcohol field and differ in several alcohol-related behaviors including consummatory behavior, acute ethanol intoxication, and withdrawal-related phenotypes. For example, B6 mice have been widely used for their high intake and preference for alcohol containing solutions, while D2, BALBc, and AJ mice are known to have significantly lower consumption and preference for alcohol (Lê et al., 1994, Gill and Boyle, 2005). Conversely, D2, BALBc and AJ mice appear to be more sensitive to the acute effects of alcohol relative to the B6 strain as they show enhanced locomotor stimulation in response to low doses of ethanol (Kiianmaa et al., 1983, Cunningham et al., 1992, Crawley et al., 1997, Gill et al., 2000). D2 mice also exhibit increased sedation in response to high doses of ethanol relative to B6 mice (Elston et al., 1982). Given these abundant strain-dependent differences in alcohol-related behaviors, it is likely these strains display several unique neurophysiological responses to alcohol.
Alcohol acutely modulates emotional states as well as long-term emotional processing. In part, these effects occur through direct actions of alcohol on the basolateral (BLA) and extended amygdala. For example, presynaptic function of GABAergic synapses within the BLA is robustly potentiated by ethanol (Silberman et al., 2008, 2012). Acute ethanol also robustly inhibits post-synaptic kainate receptor-mediated responses in this brain region (Läck et al., 2008). These effects likely contribute to ethanol’s acute anxiolysis. However, relatively less is understood about the potential effects of acute ethanol on presynaptic glutamatergic function within the amygdala. Our lab has previously demonstrated that chronic exposure to alcohol enhances BLA presynaptic glutamatergic function in an input-specific manner (Läck et al., 2007, Christian et al., 2012, Christian et al., 2013). Furthermore, relative to B6 mice, D2 mice are more sensitive to both the anxiolytic effects of ethanol intoxication and the anxiogenic effects of withdrawal from chronic ethanol exposure (McCool and Chappell, 2015). Together, these studies suggest that acute ethanol might act on presynaptic glutamatergic BLA synapses in a strain-dependent fashion.
While acute ethanol effects on postsynaptic glutamate receptors are well characterized, much less is known about ethanol modulation of presynaptic glutamate release. But our findings that chronic ethanol modulates presynaptic function (Läck et al., 2007, Christian et al., 2013) suggest these compartments may also be targeted by acute ethanol. Consistent with this, recent studies in non-mammalian model systems have shown that presynaptic proteins involved in vesicle trafficking, priming, and release are directly modulated by acute ethanol (Kapfhamer et al., 2008, Das et al., 2013, Johnson et al., 2013). In order to evaluate the role of ethanol exposure on synaptic vesicle trafficking and function in mice, we have utilized two different stimulation protocols. First, we used a low frequency (2 Hz) protocol to measure vesicle recycling over a prolonged period of stimulation (400 pulses) as well as characteristics of short-term plasticity. Then, we used a high frequency (40 Hz) protocol to directly and separately examine the effects of acute ethanol on the readily releasable pool of vesicles (RRP) and synaptic vesicle recycling. Using this methodology, we show a novel, strain-dependent effect of ethanol that specifically inhibits synaptic vesicle recycling and, subsequently, short-term plasticity. Together, these strain-dependent presynaptic effects of ethanol may contribute to some of the observed strain-dependent differences in sensitivity to the acute effects of alcohol.
Materials and Methods
Animals
5 week old male C57BL/6J (B6), DBA/2J (D2), BLABcJ (BALB), and A/J (AJ) mice were obtained from The Jackson Laboratories (Bar Harbor, ME) and given access to food and water ad libitum. Mice were group housed for 1–2 weeks in a facility maintained by institutional animal resource personnel with housing conditions consistent with the NIH Guidelines for the Care and Use of Laboratory Animals (68–74°F, 30–70% relative humidity) prior to the experiments. All experimental procedures were approved by the WFUSM Animal Care and Use Committee.
Slice Preparation
Animals were anesthetized with isoflurane and decapitated. Brains were quickly removed and incubated for 5 minutes in an ice-cold sucrose modified artificial cerebral spinal fluid (aCSF) containing (in mM): 180 sucrose, 30 NaCl, 4.5 KCl, 1 MgCl2·6H2O, 26 NaHCO3, 1.2 NaH2PO4, 0.10 ketamine, and 10 glucose, equilibrated with 95% O2 and 5% CO2. Coronal slices containing the BLA were obtained (300 μm) using a VT1200 S vibrating blade microtome (Leica, Buffalo Grove, IL) and were incubated for at least 1 h in room temperature (~25°C) in oxygenated standard aCSF containing (in mM): 126 NaCl, 3 KCl, 1.25 NaH2PO4, 2 MgSO4, 2 CaCl2, 26 NaHCO3, and 10 glucose, before initiation of recordings.
Whole cell patch clamp recordings
BLA slices were transferred to a submersion-type recording chamber and perfused with room temperature (~25°C) aCSF (2.0 ml/min) for whole-cell voltage clamp recordings similar to previously published reports (Christian et al., 2013). Data were acquired via Axopatch 700B (Molecular Devices, Foster City, CA) and analyzed offline via pClamp software (Molecular Devices, version 10.5). Inclusion criteria for presumptive principal neurons included high membrane capacitance (>100 pF) and low access resistance in the whole-cell configuration (<20 MΩ, Washburn et al., 1992). Recordings in which access resistance or capacitance changed ≥20% during the record or with changes in resting membrane currents ≥100pA were excluded from analysis. Glutamatergic responses were pharmacologically isolated using 100μM picrotoxin (a GABAA receptor antagonist) in the bath aCSF and were recorded with electrodes filled with an internal solution containing (in mM): 145 Cs-gluconate, 10 EGTA, 5 NaCl, 1 MgCl2, 10 HEPES, 0.4 QX314, 1 CaCl2, 4 Mg-ATP, and 0.4 Na3-GTP. Osmolarity of internal solution was corrected to ~285mOsm with sucrose and pH was adjusted to~7.25 with D-gluconic acid. Synaptic responses were electrically evoked using concentric bipolar stimulating electrodes (FHC Inc, Bowdoin, ME) placed medial to the BLA within the stria terminalis since these synapses are facilitated by chronic ethanol (Christian et al., 2012, 2013).
Stimulation Protocols
2 Hz Stimulation Protocol
Stria terminalis inputs to the BLA were stimulated in the following sequence: 10 pulses 0.1 Hz (pre-train stimulation), 400 pulses 2 Hz, followed by three single stimulations at 1, 5 and 25 seconds after the 2 Hz train. These final stimulations were used to assess any post-tetanic potentiation (PTP). Ethanol was then washed on for 10 minutes in the absence of stimulation followed by a repetition of the protocol: 10 pulses 0.1 Hz (pre-train stimulation), 400 pulses 2 Hz, 3 single pulses at 1, 5 and 15 seconds after the 2 Hz train (PTP).
40 Hz Stimulation Protocol
Medial inputs were stimulated for 100 pulses at 40 Hz, followed by a 10 minute ethanol application period in the absence of stimulation. An additional 100 pulses at 40 Hz were delivered to assess the effects of ethanol application on the size of the RRP and vesicle recycling rate (see below). In these studies we did not attempt to wash out the ethanol from the recording chamber to see if the effects on vesicle recycling were reversible. This was because; in a previous study we found that acute ethanol exposure leads to the delayed release of endocannabinoids in the BLA which result in presynaptic inhibition. Although these effects were not present after a 10 minute exposure, as used in this study, they were present around 25 minutes and could therefore complicate interpretations of ethanol washout.
Data Analysis
The 2.5 second 40 Hz trains of stimuli were used to determine the size of the RRP as well as the synaptic vesicle refilling/recycling rate as described previously (Schneggenburger et al., 2002, Gioia et al., 2016). Briefly, response amplitudes were summed across the stimulation trained and used to construct cumulative amplitude plots for each cell during baseline and ethanol exposure. Linear regressions passing through the final 10 data points were used to define a y-intercept which serves as an estimate of the size of the RRP. We then subtracted the apparent size of the RRP from the total cumulative amplitude to estimate the contribution of recycling synaptic vesicles that refill the releasable pool during much of the stimulation train. Consistent with previous literature (Hagler & Goda, 2001), we found that release became more asynchronous as the 40 Hz stimulation progressed. This would sometimes interfere with the ability to measure baselines before each stimulus. In order to avoid this issue, baseline synaptic transmission measurements were taken immediately before the initiation of each train.
Statistics
All graphs are plotted as mean ± SEM of each group. Primary statistical analyses were conducted using 2-Way ANOVA, One-Way ANOVA, or t-tests (GraphPad, GraphPad Software Inc, La Jolla, CA) according to the experimental design as described, with posttests as appropriate.
Drug Preparation
Picrotoxin (Tocris) was prepared fresh daily and dissolved in DMSO then added to aCSF with final concentrations of DMSO in the perfusate <0.05%. Alcohol was mixed with aCSF and delivered to the recording chamber through a calibrated syringe pump to obtain the desired concentration for recording.
Results
Experiments using prolonged stimulation have been valuable in determining synaptic strength in the context of high neuronal demand as well as for assessing different characteristics of short-term plasticity. During prolonged stimulation synaptic vesicles gradually become depleted from the presynaptic terminal when the stimulation frequency becomes higher than the recycling/refilling rate. We have previously shown that medial inputs to the BLA fatigue at stimulation frequencies >2 Hz (Gioia et al., 2016), and therefore, wanted to examine the effects of ethanol during non-depleting (2 Hz) and depleting (40 Hz) frequencies. Notably, neurons dynamically alter their firing rates in response to different stimuli so it was also important to examine ethanol effects across a range of stimulation frequencies.
Low Frequency Stimulation of Medial BLA Inputs Reveals Strain-Dependent Inhibition by Ethanol
In order to determine if ethanol modulates the ability of synapses to communicate during non-depleting sustained activity, we utilized a prolonged (400 pulses) 2 Hz stimulation protocol. We delivered two 400-pulse trains of electrical stimuli to the medial inputs of the BLA with a 10 minute period in between during which we bath applied 80mM ethanol to the slices. Through analysis of the total cumulative amplitude attained during these trains, we found that cumulative synaptic amplitudes were inhibited by acute ethanol when recording from D2 cells (Fig. 1D; t-test, p<0.01, n=7). Recordings from BALBc cells showed a trend towards inhibition (Fig. 1C; t-test p=0.0896, n=9), while cells from AJ (Fig. 1A) and B6 mice (Fig. 1B) were insensitive (t-tests respectively, p=0.58, p=0.71, n=7). Ethanol inhibition appeared to be independent from the amount of response ‘run down’ during the stimulation train as these values were not significantly correlated (p>0.05 in all strains, not shown).
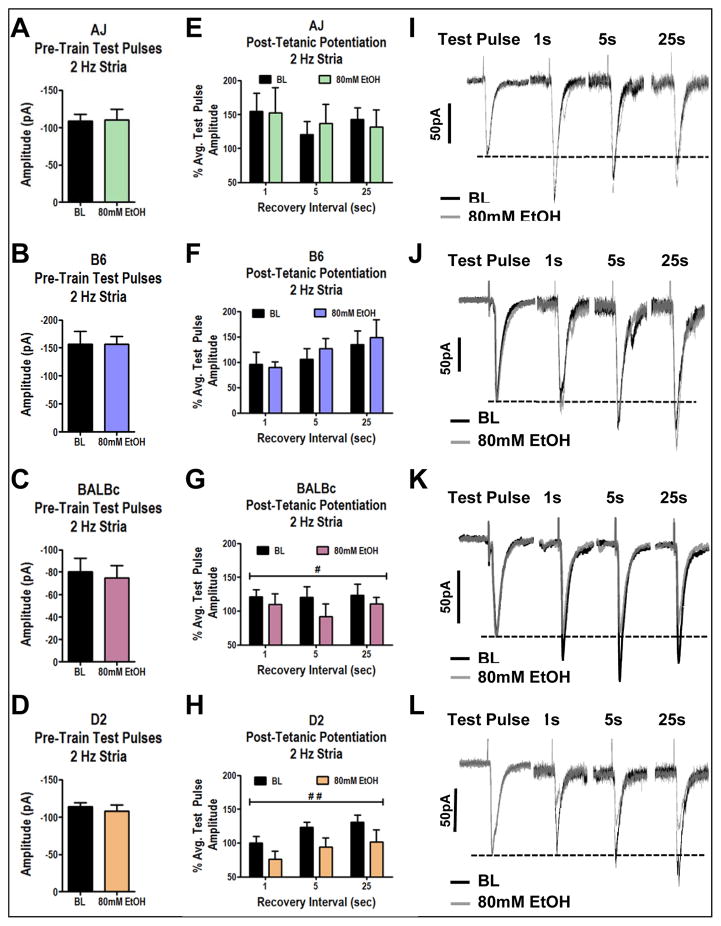
Figure 1. 2 Hz Stimulation of Medial BLA Inputs Reveals Strain-Dependent Inhibition by Ethanol.
A–D) Cumulative amplitude plots of 2 Hz stimulation of stria terminalis inputs to the BLA at baseline and during exposure to 80mM ethanol in (A) AJ mice, (B) B6 mice, (C) BALBc mice, and (D) D2 mice. Cumulative amplitudes are significantly inhibited by 80mM ethanol in D2 strain (t-test, p<0.01). E–H) Comparison of ethanol effects on the initial 10 pulses of the 2 Hz train compared with the last 100 pulses. (E) AJ mice are insensitive to ethanol effects during both early and late phases of the 2 Hz train (2-Way ANOVA, with ethanol, p=0.49, and early/late, p=0.86, as main factors). (F) B6 mice are insensitive to ethanol effects at both time points (2-Way ANOVA, Ethanol, p=0.83, and early/late, p=0.12). (G) BALBc mice are sensitive to ethanol inhibition during the later portions of the 2 Hz train (repeated measures 2-way ANOVA, Ethanol, p<0.05, and early/late, p=0.43, with Bonferroni post-test significant at the later stimulations, p<0.05). (H) D2 mice were sensitive to inhibition by ethanol specifically in the later time point (2-Way ANOVA, Ethanol (p<0.01) early vs late (p=0.15) Bonferroni posttest was significant at the later stimulations (p<0.05). I–L) Exemplar traces showing the first and last 10 pulse bins of baseline and alcohol exposure: (I) AJ cells, (J) B6 cells, (K) BALBc cells (L) D2 cells. * indicates significant difference with t-test (p<0.05) ** (p<0.01), # indicates significant difference with bonferroni posttest (p<0.05).
We then compared ethanol effects during the beginning of the train and at the end of the train. Importantly, we found that none of the strains were sensitive to ethanol during the first 10 pulses. This is consistent with many previous studies showing no acute effect of ethanol on ‘basal’ glutamate release. However, when we examined the amplitude of the final 100 EPSCs of each train, when the cells are presumably releasing newly recruited vesicles, we found that 80mM ethanol inhibited responses recorded during these later portions of the train from BALBc (Fig. 1G, 2-Way ANOVA, ethanol (p<0.05) early/late (p=0.43), Bonferroni post-test p<0.05 at last 100 pulses, n=9) and D2 cells (Fig. 1H, 2-Way ANOVA, ethanol (p<0.01), early/late (p=0.15), Bonferroni p<0.05 at the last 100 pulses, n=7), while AJ cells (Fig 1E, 2-Way ANOVA, Ethanol (p=0.49), early/late (p=0.86), n=7) and B6 cells were insensitive (Fig 1F, 2-Way ANOVA, Ethanol (p=0.83), early/late (p=0.12), n=7). These results strongly suggest that ethanol inhibition occurs through interference with synaptic vesicle refilling or recycling during later portions of the train. However, higher frequency stimulation (40 Hz) is required to rapidly deplete the RRP and adequately examine the recycling rates in isolation when in the presence of ethanol.
Ethanol Inhibits Post-Tetanic Potentiation in a Strain Dependent Manner
Repetitive stimulation will often produce short-term plasticity that can last from several seconds to tens of minutes after stimulation. Therefore, we also examined the effects of ethanol on synaptic plasticity during these 2 Hz experiments. In order to ensure that long-term plasticity induced by the initial train did not confound our measures of ethanol modulation, we examined synaptic responses at 0.1 Hz immediately before each 2 Hz train, with the second train separated by a 10 min period with no stimulation. Here we found that none of the strains expressed long-term plasticity following the initial 2 Hz stimulation as the amplitudes of pre-train stimuli were not different between the baseline and ethanol conditions (Fig. 2A–D; t-tests within strain, p>0.05 in all strains). This further supports the hypothesis that ethanol inhibition occurs through disruption of cellular processes occurring during the latter part of the stimulus train which is also consistent with others finding no effect of ethanol on glutamatergic release probability in the BLA following a 10 minute exposure to alcohol (Robinson et al., 2016).
Figure 2. Ethanol Inhibits the Expression of Post-Tetanic Potentiation.
A–D) Test pulses were obtained at 0.1 Hz before each 2 Hz train to determine if ethanol mediated effects were a result of plasticity induced by the initial 2 Hz train. Values represent the average of 10 responses during 0.1 Hz stimulation. Ethanol had no significant effects on the amplitude of EPSCs during pre-train test pulses in (A) AJ mice (t-test, p=0.82, n=7), (B) B6 mice (t-test, p=0.99, n=7), (C) BALBc mice (t-test, p=0.30, n=9), (D) D2 mice (t-test, p=0.37, n=7). E–H) Single events were evoked 1 second, 5 seconds, and 25 seconds following 2 Hz stimulation to examine effects of ethanol on short-term plasticity. E) AJ mice were insensitive to ethanol effects on short-term plasticity (2-Way ANOVA, time after stimulus (p=0.74) ethanol (p=0.95), n=7). F) B6 mice were insensitive to ethanol effects on short-term plasticity (2-Way ANOVA, time after stimulus (p=0.10) ethanol (p=0.66), n=7). G) 80mM ethanol significantly interferes with short-term plasticity in BALBc mice (2-Way ANOVA, time after stimulus (p=0.83) ethanol (p<0.05), n=9). H) 80mM ethanol significantly interferes with short-term plasticity in D2 mice (2-Way ANOVA, time after stimulus (p=0.15) ethanol (p<0.01). I–L) Exemplar traces comparing average pre-train test pulses with events evoked 1, 5, and 25 seconds after the 2 Hz train. I) AJ cells, J) B6 cells, K) BALBc cells, L) D2 cells. # indicates significant main effect of ethanol (p<0.05) ## (p<0.01).
PTP is a form of short-term plasticity expressed exclusively in the presynaptic compartment that generally lasts from 10s of seconds to a couple of minutes following repetitive stimulation (Zucker & Regehr, 2002). Single synaptic events were evoked 1 second, 5 seconds, and 25 seconds following the 2 Hz stimulation and compared to the amplitude of the pre-train stimuli in order to examine PTP. Like the effect on cumulative amplitude, we found that ethanol had no significant effects on PTP in AJ and B6 cells (Fig 2E, AJ, 2-Way ANOVA, time after stimulus (p=0.74) ethanol (p=0.95), n=7; Fig 2F, B6, 2-Way ANOVA, time after stimulus (p=0.10) ethanol (p=0.66), n=7). Conversely, ethanol significantly inhibited PTP in BALBc cells, a 2-Way ANOVA revealed a significant main effect of ethanol (Fig. 2G, p<0.05, n=9) but no effect on time after stimulus (p=0.83). Additionally, ethanol significantly inhibited PTP in D2 cells, a 2-Way ANOVA revealed a significant main effect of ethanol (Fig. 2H, p<0.01, n=7) but no effect on time after stimulus (p=0.15). These results raise the interesting possibility that the ethanol-sensitive mechanisms potentially involved in synaptic vesicle recycling may also be involved in PTP.
Ethanol Inhibits Synaptic Vesicle Recycling During High Frequency Stimulation in a Strain-Dependent Manner
We used high frequency (40 Hz) stimulation in order to distinguish between effects of ethanol on the RRP and the vesicle recycling rate. 40 Hz trains of stimuli delivered to medial BLA inputs rapidly deplete the RRP within the first 10 stimuli (Gioia et al., 2016) after which continued stimulation leads to a mixture of EPSCs and failures dictated by the rate of activity-dependent refilling of the RRP. In order to quantify the size of the RRP and synaptic vesicle recycling rates, we used previously reported procedures that are detailed in the methods section above (Schneggenburger et al., 2002, Gioia et al., 2016).
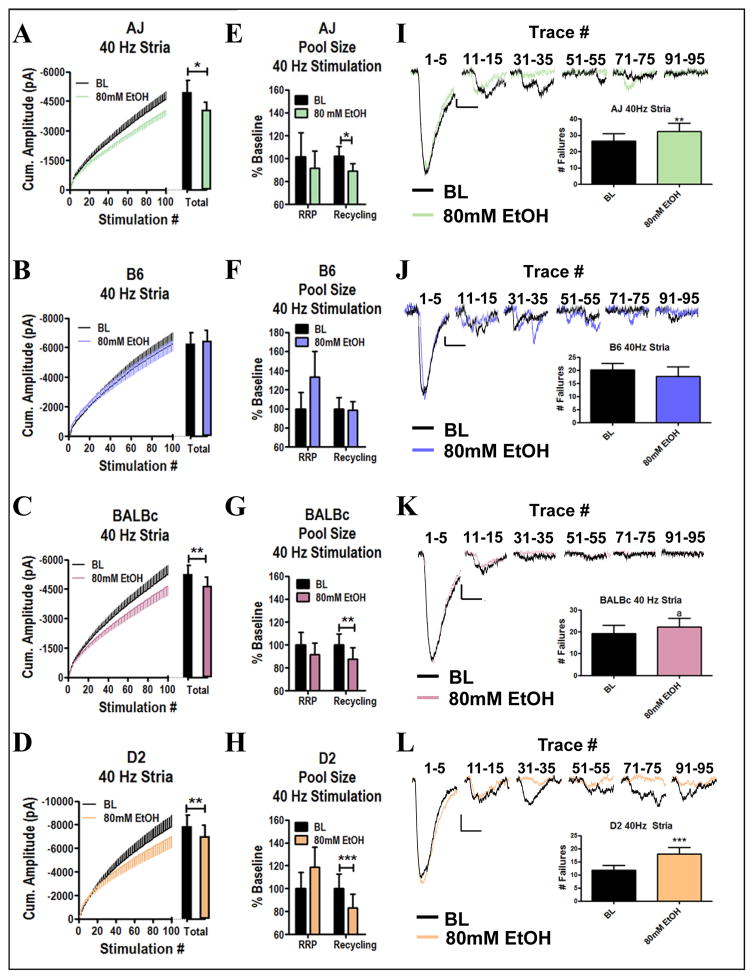
In these experiments, we examined effects of ethanol during high frequency stimulation by delivering two 100-pulse trains at 40 Hz to the medial inputs of the BLA with a 10 minute period in between during which we bath applied 80mM ethanol to the slices. Again, through analysis of the total cumulative amplitude measured during these trains, we found that 80mM ethanol inhibited cumulative amplitudes of AJ, BALBc and D2 cells (Fig. 3A, C, & D, within-strain t-tests, p<0.05, p<0.01, p<0.01 respectively, n=14, 15, 14), while B6 cells remained insensitive (Fig. 3B, t-test, p= 0.72, n=13). These results are particularly interesting in that they suggest a frequency-dependent effect of ethanol inhibition in the AJ strain. Thus, behaviors or neurophysiological processes involving high-frequency firing in-vivo may be disrupted in the AJ strain while those requiring lower frequency firing may be insensitive to ethanol. We also used the cumulative amplitude plots to segregate contributions from the readily releasable and recycling pools (see Methods) and found that ethanol specifically disrupted synaptic vesicle recycling. Cells from AJ, BALBc, and D2 mice all showed ethanol-mediated inhibition of recycling (Fig. 3E, G, & H; t-tests respectively, p<0.05, p<0.01, p<0.001) with no effects on the size of the RRP (t-tests respectively, p=0.32, p=0.20, p=0.06). Conversely, cells from B6 mice were insensitive to ethanol mediated effects on vesicle recycling (Fig. 3F, t-test, p=0.85). These results are consistent with the 2 Hz studies where ethanol inhibition occurred at the end of the train and directly suggest that ethanol inhibits synaptic vesicle recycling. Furthermore, ethanol inhibition again appeared to be independent from the amount of response amplitude ‘run down’ during the stimulation train as these values were not significantly correlated (p>0.05 in all strains). The traces illustrated in figure 3 show that vesicular release transitions to a more asynchronous form of release and frequently induces a tonic glutamate current due to the buildup of synaptic glutamate. Importantly, the effect of ethanol on synaptic vesicle recycling is highly correlated with the reduction of the tonic current (p<0.0001, r=0.51) consistent with the interpretation that inhibition of vesicle recycling decreases synaptic glutamate concentration.
Figure 3. Ethanol Inhibits Synaptic Vesicle Recycling During 40 Hz Stimulation of Medial BLA Inputs.
A–D) Cumulative amplitude plots of 40 Hz stimulation of stria terminalis inputs to the BLA at baseline and during exposure to 80mM ethanol. (A) Cells from AJ mice were sensitive to inhibition by ethanol during 40 Hz stimulation (t-test, p<0.05), (B) Cells from B6 mice were insensitive to ethanol during 40 Hz stimulation (t-test, p=0.72), (C) Cells from BALBc mice were sensitive to inhibition by 80mM ethanol during 40 Hz stimulation (t-test, p<0.01), (D) Cells from D2 mice were sensitive to inhibition by 80mM ethanol during 40 Hz stimulation (t-test, p<0.01). E–H) Quantification of ethanol effects on the size of the RRP and recycling pool (E) The recycling pool was significantly inhibited by ethanol in AJ mice (t-test, p<0.05) while the RRP was not (t-test, p=0.32), (F) The recycling pool was not altered by ethanol in B6 mice (t-test, p=0.85) and neither was the RRP (t-test, p=0.07), (G) The recycling pool was significantly inhibited in BALBc mice (t-test, p=0.006) while the RRP was not (t-test, p=0.20) (H) The recycling pool was significantly inhibited by ethanol in D2 mice (t-test, p=0.0002) while the RRP was not (t-test, p=0.06). I–L) Exemplar traces of 40 Hz stimulation of medial inputs (I) AJ, (J) B6, (K) BALBc, (L) D2.
Ethanol Inhibition of Synaptic Vesicle Recycling in D2 Cells is Dose-Dependent
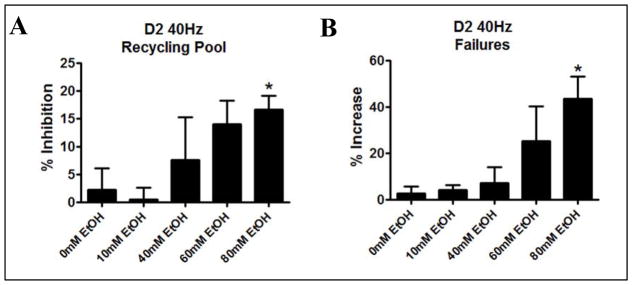
In order to determine if ethanol mediated effects on synaptic vesicle recycling occurred at behaviorally relevant concentrations of ethanol, we repeated the 40 Hz experiments at 0, 10, 40, and 60 mM ethanol in D2 and B6 mice. Here we found that ethanol effects on the recycling pool were significant in D2 mice (Fig. 4A, 1-Way ANOVA p=0.0128), but not in B6 mice (Fig 4B, 1-Way ANOVA, p=0.62). In D2 mice, ethanol effects on synaptic vesicle recycling were significant at 60mM ethanol (t-test, p= 0.017) and had a trend towards significance at 40mM (p=0.19), while there were no effects at 10mM (p=0.78) and 0mM (p=0.51). Cells from B6 mice were insensitive to ethanol at every concentration tested (t-tests, p>0.05). Ethanol had no effect on RRP size at any concentration tested in D2 mice (t-tests, p>0.05) and in B6 mice (t-tests, p>0.05). These results suggest that relatively high concentrations of ethanol are required to produce consistent effects on synaptic vesicle recycling in D2 mice while B6 mice are insensitive
Figure 4. Ethanol Inhibits Synaptic Vesicle Recycling in a Dose-Dependent Manner in D2 but not B6 Mice.
A) Dose effect curve for ethanol mediated inhibition of the entire 40 Hz train in D2 mice (LogIC50=−1.376, IC50=42mM ethanol, SEM ±1.5mM). (B) Dose effect graph for ethanol effects on the recycling pool in B6 cells. These values could not be fit to a non-linear regression but are shown for comparison between strains.
Discussion
In these studies we identified synaptic vesicle recycling as a novel target for acute inhibition by ethanol. This strain-dependent effect is amplified in responses to higher frequency stimulation, suggesting that either accumulation of second messengers within the presynaptic terminal, or simply higher demand placed upon presynaptic recycling machinery, may facilitate inhibition by ethanol. Importantly, we have previously shown these BLA glutamatergic inputs respond to chronic ethanol exposure by greatly increasing their function (Christian et al., 2013). The current work suggests that adaptations within alcohol-sensitive processes within presynaptic terminals themselves could explain this presynaptic ‘plasticity’ following chronic ethanol. This would be consistent with many studies showing that acute ethanol inhibition frequently leads to rebound up-regulation of various systems during withdrawal (Lowery-Gionta et al., 2015, Pleil et al., 2015). Therefore, it is possible that repeated chronic exposure to alcohol and its inhibition of synaptic vesicle recycling leads to the development of presynaptic facilitation, offsetting acute inhibition. We also found that ethanol acutely inhibited synaptic vesicle recycling in D2 cells during high frequency (40 Hz) stimulation with an IC50 of approximately 42mM (Fig. 4). This concentration is clearly outside the range of blood levels caused by casual ethanol consumption but is not outside the range attained by heavy drinkers or alcohol-dependent individuals. Given that synaptic vesicle recycling is an integral process at all synapses, these results may likely have broader implications for a wide variety of brain regions and could explain why ethanol produces robust allostatic adaptations to glutamatergic signaling in so many brain regions. Finally, we found that there were strain-dependent differences in the inhibition of PTP by ethanol. PTP is a form of short-term plasticity that occurs over tens to hundreds of seconds following repetitive stimulation and is regulated by several presynaptic proteins and second messengers which are also involved in vesicle recycling. Therefore, it is possible that the effects on PTP and vesicle recycling are controlled by similar mechanisms.
In these studies we examined ethanol effects during both high and low frequency stimulation; in part we utilized these different frequencies as a means to identify the mechanisms involved in presynaptic inhibition, while also understanding that neurons dynamically alter their firing rates to accommodate for different tasks. Medial inputs to the BLA contain fibers from the mPFC, hippocampus and thalamus; cells in all of these regions are all known to operate in both low and high frequency ranges (Milad & Quirk, 2002, Wang et al., 2011, Galvan et al., 2012). For example, neurons in the mPFC fire at low frequencies during fear conditioning and higher frequencies during extinction (Milad & Quirk, 2002) while low frequency oscillations between the mPFC and BLA are required for the initiation of freezing behavior during fear conditioning (Karalis et al., 2016). Ethanol sensitive recycling at glutamate synapses from D2 but not B6 mice (Fig. 1, 3) may provide a mechanism for the observation that chronic ethanol withdrawal alters amygdala dependent delay fear conditioning in D2 mice but not in B6 mice (Tipps et al., 2015). Additionally, BLA neurons dynamically alter their firing rate in-vivo from 0–30 Hz in response to anxiogenic stimuli, with frequency being positively correlated with anxiety-like behavior (Wang et al., 2011). Further, D2 mice are sensitive to the acute anxiolytic effects of ethanol intoxication while B6 mice are not (McCool and Chappell, 2015); ethanol inhibition of responding during high frequency trains (Figs 3–4) may contribute to this as well. Finally, alcohol disrupts gamma band (30–80 Hz) oscillations in the hippocampus of rats (Wang et al., 2016) and the visual cortex of humans (Campbell et al., 2014); and ethanol mediated disruption of synaptic vesicle recycling offers a potential cellular substrate for this phenomenon as well. These studies together highlight the importance of examining ethanol across a range of presynaptic stimuli.
We demonstrate in these studies that ethanol inhibits synaptic responses during low frequency trains in cells from D2 and BALBc mice while cells from AJ and B6 mice were insensitive (Fig. 1). Interestingly, cells from AJ mice became sensitive to inhibition by ethanol when the stimulation frequency was increased (Fig. 3). This raises that possibility that AJ mice may be sensitive to ethanol effects on behaviors that require higher frequency neuronal activity in vivo such as conditioned fear and protected from those mediated by low frequency activity such as unconditioned anxiety. Furthermore, we found that cells from B6 mice were insensitive across both frequencies; and this is consistent with their low sensitivity to many of the acute intoxicating effects of ethanol. Together our data offer specific insight into strain-dependent neurophysiological effects of ethanol.
Ethanol Inhibition of Synaptic Vesicle Recycling
During the low frequency experiments we found that ethanol inhibited EPSCs specifically at the end of the trains but not the beginning (Fig. 1) which suggested to us that ethanol may be interfering with activity dependent refilling/recycling into the RRP. We were able to confirm this hypothesis using high frequency stimulation which allowed us to quantify the size of the RRP and the recycling/refilling rate. Here we found that ethanol selectively inhibited the recycling component of the train without interfering with the RRP (Fig 3). The relative insensitivity of the RRP to acute ethanol compliments a previous study from our lab using a paired-pulse protocol that found no acute effect of ethanol on initial glutamate release probability when bath applied for 10 minutes (Robinson et al., 2016). Our results demonstrate that multiple stimuli are required to observe presynaptic inhibition at this synapse.
The presynaptic terminal contains several different pools of synaptic vesicles that can be distinguished based on their molecular interactions and proximity to the plasma membrane. The RRP contains mature vesicles that are docked and primed for release at the active zone. These readily releasable vesicles undergo SNARE complex mediated fusion with the plasma membrane in response to terminal calcium influx. After the vesicle has released its contents it becomes part of the plasma membrane until endocytic processes internalize the membrane for reuse. This endocytic process also removes a number of proteins from the membrane and clears up space in the active zone for new vesicles to dock (Wu et al., 2014). After the vesicles dock to the plasma membrane they need to be primed before they can be released (Varoqueaux et al., 2002). Therefore, endocytosis, refilling of the RRP and priming of synaptic vesicles (together referred to as vesicle recycling) are rate limiting processes during high frequency activity.
A wide variety of proteins and second messengers within the presynaptic terminal interact during vesicle recycling and could potentially influence ethanol inhibition. Fortunately, a number of molecular and genetic studies have identified some presynaptic proteins that are associated with alcohol-related behaviors and are also involved in vesicle release and recycling. Firstly, acute ethanol exposure has been shown to inhibit N/P/Q type voltage gated calcium channels (VGCCs) (Maldve et al., 2004) which are important for synaptic vesicle release as well as short-term depression (Xu et al., 2005). However, this effect is unlikely to explain the current results as initial pulses in the trains were not influenced by ethanol as would be expected through a direct effect on VGCCs. Secondly, Munc13 proteins are required for vesicle priming in glutamatergic neurons (Varoqueaux et al., 2002) and have been shown to bind alcohol in their C1 domain (Das et al., 2013). Interestingly, there is a non-synonymous single nucleotide polymorphism (SNP) in the C1 domain of Munc13-1 that is shared between D2 and BALBc mice but not B6 or AJ mice (GeneNetwork SNP Browser, www.genenetwork.org). This SNP potentially alters ethanol binding affinity and/or the ability of ethanol to displace diacylglycerol which also binds to Munc13 C1 domains. Thirdly, Munc18-1 proteins are involved in vesicle priming and have been implicated in acute ethanol sensitivity (Johnson et al., 2013). In one study, using a panel of BXD mice, a Munc18-1 polymorphism was found to be associated with ethanol drinking/preference (Fehr et al., 2005); however, this mutation is unlikely to directly regulate the effect of ethanol on vesicle recycling as the mutation did not segregate across strains consistently with the ethanol sensitivity in the strains we used in the current study. That is, this mutation is shared between BALBc (here sensitive) and B6 (insensitive) mice while D2 (sensitive) and AJ (frequency-dependent sensitivity) mice express the other allelic variant. Importantly, Munc13 proteins interact with Munc18-1 during vesicle priming so mutations in either protein could contribute to strain-dependent differences. Lastly, the Rab3A protein has been associated with the acute effects of ethanol in a non-mammalian system (Kapfhamer et al., 2008) and is known to interact with priming related proteins including Munc13-1, RIM-1 and Munc18-1. Together, these studies suggest that inhibition of vesicle recycling may occur through disruption of the synaptic vesicle priming complex consisting of Munc13-1, RIM-1, Munc18-1 and Rab3A.
Ethanol Inhibition of Post-Tetanic Potentiation
Chronic exposure to ethanol has been shown to interfere with various forms of long- and short-term synaptic plasticity throughout the CNS. For example, chronic exposure to ethanol has been shown to disrupt LTP in the hippocampus (reviewed in Zorumski et al., 2014) and lateral amygdala (Stephens et al., 2005) and has been shown to produce LTP-like effects through presynaptic and postsynaptic mechanisms in the BLA (Läck et al., 2007, Christian et al., 2012, Christian et al., 2013). Furthermore, chronic exposure has also been shown to modulate short-term plasticity including paired-pulse facilitation at both glutamatergic and GABAergic synapses (reviewed in McCool, 2011) as well as the magnitude and duration of PTP (Gage & Hubbard, 1966, Traynor et al., 1976).
In our experiments, we observed an ethanol mediated inhibition of PTP in D2 and BALBc cells (Fig. 2). At the beginning of our low frequency experiments, we used a 10 pulse/0.1 Hz pre-train stimulus to determine if any long term (>10min) plasticity occurred in response to the first train. We did not find differences in the size of these pre-train stimuli indicating that LTP or LTD were not expressed under these stimulation conditions (Fig. 2). However, we did observe ethanol mediated-inhibition of PTP, a presynaptic form of short-term plasticity that occurs immediately following a tetanic stimulation that lasts for 10s to 100s of seconds (Zucker & Regehr, 2002). We examined EPSC amplitudes evoked at 1, 5 and 25 second intervals following the 2 Hz tetanic stimulation. Here, we found that BALBc and D2 mice had reduced EPSC amplitudes in the presence of 80mM ethanol while AJ and B6 mice were unaffected (Fig. 2). Given that the same strains were sensitive to PTP inhibition and vesicle recycling inhibition, it is likely that the same or overlapping mechanisms are involved in both processes.
PTP is regulated by a number of different presynaptic proteins providing a variety of potential ethanol targets. Again, Munc13 proteins appear to be attractive candidates as they are highly implicated in PTP. Following repetitive stimulation, calmodulin accumulates in the presynaptic terminal and binds to Munc13 proteins to enhance vesicle priming and produce PTP (Junge et al., 2004). Additionally, diacylglycerol also accumulates during repetitive stimulation and initiates PTP through both Munc13- and PKC-dependent processes, both of which involve downstream effects on Munc18-1 proteins (Wierda et al., 2007, Brager et al., 2003). RIM proteins, which are important regulators of vesicle docking and VGCC positioning within the active zone (Han et a., 2011), have also been shown to be important for regulating the magnitude of PTP (Schoch et al., 2002) through direct interactions with Munc13 proteins (Deng et al., 2011). Finally, mGluR-mediated, PLD-dependent signaling is required for PTP in BLA medial inputs (Krishnan et al., 2016). In this case, PLD activation can modulate Munc13 membrane association through the generation of phosphatidic acid and its conversion to DAG (Song et al., 1999). Together, these results are consistent with a role of ethanol acting through synaptic vesicle priming machinery, including Munc13-1, Munc18-1 and RIM1 proteins, to modulate PTP.
Conclusion
In conclusion, we have demonstrated a novel strain-dependent effect of ethanol acting on presynaptic glutamatergic terminals that involves inhibition of synaptic vesicle recycling and post-tetanic potentiation. Furthermore, we suggest that these effects are related to ethanol interactions with the molecular mechanisms involved in synaptic vesicle priming, including Munc13, Munc18-1, and RIM proteins. These effects may contribute to strain-dependent differences in alcohol-related behaviors including the acute anxiolytic and intoxicating effects of alcohol, disruption of amygdala dependent fear conditioning, and sensitivity to withdrawal related seizures and anxiogenesis (Elston et al., 1982, Kiianmaa et al., 1983, Crawley et al., 1997, Gill et al., 2000, McCool & Chappell, 2015). Future studies should examine vesicle recycling at other synapses since downstream vesicle priming is integral to release at most synapses.
Acknowledgments
NIH Grants R01 AA023999, R01 AA014445, U01 AA020942, T32 AA007565
Contributor Information
Dominic A Gioia, Wake Forest School of Medicine, 27157.
Brian A McCool, Wake Forest School of Medicine, 27157.
References
- Bauer LO, Covault J, Harel O, Das S, Gelernter J, Anton R, Kranzler HR. Variation in GABRA2 predicts drinking behavior in project MATCH subjects. Alcohol Clin Exp Res. 2007 Nov;31(11):1780–7. doi: 10.1111/j.1530-0277.2007.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager DH, Cai X, Thompson SM. Activity-dependent activation of presynaptic protein kinase C mediates post-tetanic potentiation. Nat Neurosci. 2003 Jun;6(6):551–2. doi: 10.1038/nn1067. [DOI] [PubMed] [Google Scholar]
- Campbell AE, Sumner P, Singh KD, Muthukumaraswamy SD. Acute effects of alcohol on stimulus-induced gamma oscillations in human primary visual and motor cortices. Neuropsychopharmacology. 2014 Aug;39(9):2104–13. doi: 10.1038/npp.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, Robinson S, McCool BA. Chronic intermittent ethanol and withdrawal differentially modulate basolateral amygdala AMPA-type glutamate receptor function and trafficking. Neuropharmacology. 2012 Jun;62(7):2430–9. doi: 10.1016/j.neuropharm.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, McCool BA. Thalamic glutamatergic afferents into the rat basolateral amygdala exhibit increased presynaptic glutamate function following withdrawal from chronic intermittent ethanol. Neuropharmacology. 2013 Feb;65:134–42. doi: 10.1016/j.neuropharm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997 Jul;132(2):107–24. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl) 1992;107(2–3):385–93. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- Das J, Xu S, Pany S, Guillory A, Shah V, Roman GW. The pre-synaptic Munc13-1 binds alcohol and modulates alcohol self-administration in Drosophila. J Neurochem. 2013 Sep;126(6):715–26. doi: 10.1111/jnc.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Kaeser PS, Xu W, Südhof TC. RIM proteins activate vesicle priming by reversing autoinhibitory homodimerization of Munc13. Neuron. 2011 Jan 27;69(2):317–31. doi: 10.1016/j.neuron.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston SF, Blum K, DeLallo L, Briggs AH. Ethanol intoxication as a function of genotype dependent responses in three inbred mice strains. Pharmacol Biochem Behav. 1982 Jan;16(1):13–5. doi: 10.1016/0091-3057(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Fehr C, Shirley RL, Crabbe JC, Belknap JK, Buck KJ, Phillips TJ. The syntaxin binding protein 1 gene (Stxbp1) is a candidate for an ethanol preference drinking locus on mouse chromosome 2. Alcohol Clin Exp Res. 2005 May;29(5):708–20. doi: 10.1097/01.alc.0000164366.18376.ef. [DOI] [PubMed] [Google Scholar]
- Gage PW, Hubbard JI. An investigation of the post-tetanic potentiation of end-plate potentials at a mammalian neuromuscular junction. J Physiol. 1966 May;184(2):353–75. doi: 10.1113/jphysiol.1966.sp007919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hu X, Smith Y, Wichmann T. In vivo optogenetic control of striatal and thalamic neurons in non-human primates. PLoS One. 2012;7(11):e50808. doi: 10.1371/journal.pone.0050808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GeneNetwork. University of Tennessee; 2003. [Accessed September 15, 2016]. Database online. updated March 19, 2008. Available at: www.genenetwork.org. [Google Scholar]
- Gill K, Boyle AE. Genetic analysis of alcohol intake in recombinant inbred and congenic strains derived from A/J and C57BL/6J progenitors. Mamm Genome. 2005 May;16(5):319–31. doi: 10.1007/s00335-004-2239-x. [DOI] [PubMed] [Google Scholar]
- Gill K, Boyle A, Lake K, Desaulniers N. Alcohol-induced locomotor activation in C57BL/6J, A/J, and AXB/BXA recombinant inbred mice: strain distribution patterns and quantitative trait loci analysis. Psychopharmacology (Berl) 2000 Jul;150(4):412–21. doi: 10.1007/s002130000458. [DOI] [PubMed] [Google Scholar]
- Gioia D, Alexander N, McCool B. Differential expression of Munc13-2 produces unique synaptic phenotypes in the basolateral amygdala of C57BL/6J and DBA/2J mice. [Accepted August 2016];J Neurosci. doi: 10.1523/JNEUROSCI.1785-16.2016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Kaeser PS, Südhof TC, Schneggenburger R. RIM determines Ca2+ channel density and vesicle docking at the presynaptic active zone. Neuron. 2011 Jan 27;69(2):304–16. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Goda Y. Properties of synchronous and asynchronous release during pulse train depression in cultured hippocampal neurons. J Neurophysiol. 2001 Jun;85(6):2324–34. doi: 10.1152/jn.2001.85.6.2324. [DOI] [PubMed] [Google Scholar]
- Johnson JR, Kashyap S, Rankin K, Barclay JW. Rab-3 and unc-18 interactions in alcohol sensitivity are distinct from synaptic transmission. PLoS One. 2013 Nov 14;8(11):e81117. doi: 10.1371/journal.pone.0081117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge HJ, Rhee JS, Jahn O, Varoqueaux F, Spiess J, Waxham MN, Rosenmund C, Brose N. Calmodulin and Munc13 form a Ca2+ sensor/effector complex that controls short-term synaptic plasticity. Cell. 2004 Aug 6;118(3):389–401. doi: 10.1016/j.cell.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Kapfhamer D, Bettinger JC, Davies AG, Eastman CL, Smail EA, Heberlein U, McIntire SL. Loss of RAB-3/A in Caenorhabditis elegans and the mouse affects behavioral response to ethanol. Genes Brain Behav. 2008 Aug;7(6):669–76. doi: 10.1111/j.1601-183X.2008.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalis N, Dejean C, Chaudun F, Khoder S, Rozeske RR, Wurtz H, Bagur S, Benchenane K, Sirota A, Courtin J, Herry C. 4-Hz oscillations synchronize prefrontal-amygdala circuits during fear behavior. Nat Neurosci. 2016 Apr;19(4):605–12. doi: 10.1038/nn.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpyak VM, Biernacka JM, Geske JR, Jenkins GD, Cunningham JM, Rüegg J, Kononenko O, Leontovich AA, Abulseoud OA, Hall-Flavin DK, Loukianova LL, Schneekloth TD, Skime MK, Frank J, Nöthen MM, Rietschel M, Kiefer F, Mann KF, Weinshilboum RM, Frye MA, Choi DS. Genetic markers associated with abstinence length in alcohol-dependent subjects treated with acamprosate. Transl Psychiatry. 2014 Oct 7;4:e462. doi: 10.1038/tp.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiianmaa K, Hoffman PL, Tabakoff B. Antagonism of the behavioral effects of ethanol by naltrexone in BALB/c, C57BL/6, and DBA/2 mice. Psychopharmacology (Berl) 1983;79(4):291–4. doi: 10.1007/BF00433403. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Covault J, Feinn R, Armeli S, Tennen H, Arias AJ, Gelernter J, Pond T, Oncken C, Kampman KM. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014 Apr;171(4):445–52. doi: 10.1176/appi.ajp.2013.13081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan B, Scott MT, Pollandt S, Schroeder B, Kurosky A, Shinnick-Gallagher P. Fear potentiated startle increases phospholipase D (PLD) expression/activity and PLD-linked metabotropic glutamate receptor mediated post-tetanic potentiation in rat amygdala. Neurobiol Learn Mem. 2016 Feb;128:65–79. doi: 10.1016/j.nlm.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läck AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007 Dec;98(6):3185–96. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läck AK, Ariwodola OJ, Chappell AM, Weiner JL, McCool BA. Ethanol inhibition of kainate receptor-mediated excitatory neurotransmission in the rat basolateral nucleus of the amygdala. Neuropharmacol. 2008 Oct;55(5):661–8. doi: 10.1016/j.neuropharm.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Ko J, Chow S, Quan B. Alcohol consumption by C57BL/6, BALB/c, and DBA/2 mice in a limited access paradigm. Pharmacol Biochem Behav. 1994 Feb;47(2):375–8. doi: 10.1016/0091-3057(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Marcinkiewcz CA, Kash TL. Functional alterations in the dorsal raphe nucleus following acute and chronic ethanol exposure. Neuropsychopharmacology. 2015 Feb;40(3):590–600. doi: 10.1038/npp.2014.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldve RE, Chen X, Zhang TA, Morrisett RA. Ethanol selectively inhibits enhanced vesicular release at excitatory synapses: real-time visualization in intact hippocampal slices. Alcohol Clin Exp Res. 2004 Jan;28(1):143–52. doi: 10.1097/01.ALC.0000106304.39174.AD. [DOI] [PubMed] [Google Scholar]
- McCool BA. Ethanol modulation of synaptic plasticity. Neuropharmacology. 2011 Dec;61(7):1097–108. doi: 10.1016/j.neuropharm.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Chronic intermittent ethanol inhalation increases ethanol self-administration in both C57BL/6J and DBA/2J mice. Alcohol. 2015 Mar;49(2):111–20. doi: 10.1016/j.alcohol.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002 Nov 7;420(6911):70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O’Brien CP. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003 Aug;28(8):1546–52. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Pleil KE, Lowery-Gionta EG, Crowley NA, Li C, Marcinkiewcz CA, Rose JH, McCall NM, Maldonado-Devincci AM, Morrow AL, Jones SR, Kash TL. Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology. 2015 Dec;99:735–49. doi: 10.1016/j.neuropharm.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SL, Alexander NJ, Bluett RJ, Patel S, McCool BA. Acute and chronic ethanol exposure differentially regulate CB1 receptor function at glutamatergic synapses in the rat basolateral amygdala. Neuropharmacology. 2016 Sep;108:474–84. doi: 10.1016/j.neuropharm.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Sakaba T, Neher E. Vesicle pools and short-term synaptic depression: lessons from a large synapse. Trends Neurosci. 2002 Apr;25(4):206–12. doi: 10.1016/s0166-2236(02)02139-2. [DOI] [PubMed] [Google Scholar]
- Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Südhof TC. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002 Jan 17;415(6869):321–6. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Shi L, Brunso-Bechtold JK, Weiner JL. Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther. 2008 Jan;324(1):251–60. doi: 10.1124/jpet.107.128728. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Weiner JL. β1-adrenoceptor activation is required for ethanol enhancement of lateral paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther. 2012 Nov;343(2):451–9. doi: 10.1124/jpet.112.196022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Ailenberg M, Silverman M. Human munc13 is a diacylglycerol receptor that induces apoptosis and may contribute to renal cell injury in hyperglycemia. Mol Biol Cell. 1999 May;10(5):1609–19. doi: 10.1091/mbc.10.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Ripley TL, Borlikova G, Schubert M, Albrecht D, Hogarth L, Duka T. Repeated ethanol exposure and withdrawal impairs human fear conditioning and depresses long-term potentiation in rat amygdala and hippocampus. Biol Psychiatry. 2005 Sep 1;58(5):392–400. doi: 10.1016/j.biopsych.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Tipps ME, Raybuck JD, Buck KJ, Lattal KM. Acute ethanol withdrawal impairs contextual learning and enhances cued learning. Alcohol Clin Exp Res. 2015 Feb;39(2):282–90. doi: 10.1111/acer.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor ME, Woodson PB, Schlapfer WT, Barondes SH. Sustained tolerance to a specific effect of ethanol on posttetanic potentiation in Aplysia. Science. 1976 Aug 6;193(4252):510–11. doi: 10.1126/science.181841. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002 Jun 25;99(13):9037–42. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DV, Wang F, Liu J, Zhang L, Wang Z, Lin L. Neurons in the amygdala with response-selectivity for anxiety in two ethologically based tests. PLoS One. 2011 Apr 11;6(4):e18739. doi: 10.1371/journal.pone.0018739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhao J, Liu Z, Guo F, Wang Y, Wang X, Zhang R, Vreugdenhil M, Lu C. Acute Ethanol Inhibition of γ Oscillations Is Mediated by Akt and GSK3β. Front Cell Neurosci. 2016 Aug 17;10:189. doi: 10.3389/fncel.2016.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci. 1992 Oct;12(10):4066–79. doi: 10.1523/JNEUROSCI.12-10-04066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierda KD, Toonen RF, de Wit H, Brussaard AB, Verhage M. Interdependence of PKC-dependent and PKC-independent pathways for presynaptic plasticity. Neuron. 2007 Apr 19;54(2):275–90. doi: 10.1016/j.neuron.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Wu LG, Hamid E, Shin W, Chiang HC. Exocytosis and endocytosis: modes, functions, and coupling mechanisms. Annu Rev Physiol. 2014;76:301–31. doi: 10.1146/annurev-physiol-021113-170305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wu LG. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron. 2005 May 19;46(4):633–45. doi: 10.1016/j.neuron.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Mennerick S, Izumi Y. Acute and chronic effects of ethanol on learning-related synaptic plasticity. Alcohol. 2014 Feb;48(1):1–17. doi: 10.1016/j.alcohol.2013.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]