Abstract
Age is the greatest risk factor for cardiovascular disease. Telomere length is shorter in the hearts of aged mice compared to young mice, and short telomere length has been associated with an increased risk of cardiovascular disease. One year of voluntary wheel running exercise attenuates the age-associated loss of telomere length and results in altered gene expression of telomere length maintaining and genome stabilizing proteins in heart tissue of mice. Understanding the early adaptive response of the heart to an endurance exercise bout is paramount to understanding the impact of endurance exercise on heart tissue and cells. To this end we studied mice before (BL), immediately post (TP1) and one-hour following (TP2) a treadmill running bout. We measured the changes in expression of telomere related genes (shelterin components), DNA damage sensing (p53, Chk2) and DNA repair genes (Ku70, Ku80), and MAPK signaling. TP1 animals had increased TRF1 and TRF2 protein and mRNA levels, greater expression of DNA repair and response genes (Chk2 and Ku80), and greater protein content of phosphorylated p38 MAPK compared to both BL and TP2 animals. These data provide insights into how physiological stressors remodel the heart tissue and how an early adaptive response mediated by exercise may be maintaining telomere length/stabilizing the heart genome through the up-regulation of telomere protective genes.
Introduction
Age is the number one risk factor for both males and females in the development of cardiovascular diseases (Dominguez et al., 2006). Prevention of cardiovascular diseases (CVD) is associated with lifestyle choices such as cessation of smoking, reduction in body mass, and maintaining physical activity (Dominguez et al., 2006). The long-recognized positive benefits or ‘cardioprotective’ effects of exercise on reduction of CVD risk include reduced resting blood pressure, lowered basal heart rate, reduced cholesterol levels and improved vascular health (e.g., reduced atherosclerosis) (Neufer et al., 2015). However, these improvements following regular endurance exercise training do not fully explain the cardioprotective effects of exercise (Neufer et al., 2015) and questions about the biological underpinnings of the effects of exercise remain unanswered.
Certain cell types in the adult heart can regenerate and are proliferative (van Berlo & Molkentin, 2014; Bergmann et al., 2015). Neonatal mouse hearts can fully regenerate after partial surgical resection or induced myocardial infarction (Porrello et al., 2011). However, the ability of the heart to regenerate in this fashion is rapidly lost in the first week of post-natal life and is concurrent with massive changes in gene expression of the heart tissue (Porrello et al., 2011). Further, the regenerative capacity of the heart is reduced with age (Bergmann et al., 2015) and the mutational load of the heart cells increases with age as well, leading to genome instability and tissue dysfunction (Dolle et al., 2000). Thus, understanding how age and aging per say influence the heart and finding means to slow, prevent, or reverse age-related changes in the heart tissue is paramount.
A hallmark of molecular aging is the progressive shortening of telomeres with advancing age (Lopez-Otin et al., 2013; Bar et al., 2014). Telomeres are repetitive DNA elements (5’-TTAGGGn) at the ends of linear chromosomes that when sufficiently long prevent DNA ends from being recognized as DNA double strand breaks (Shay & Wright, 2010). Telomeres shorten with each cell division until the protective effect of telomere DNA is diminished, thus telomere length limits the regenerative capacity of a cell/tissue (Bodnar et al., 1998). Short telomere length is associated with many age-related diseases and is an independent risk factor for mortality (Ludlow & Roth, 2011; Blackburn et al., 2015). Short telomeres in human and mouse tissues and cells are also associated with increased genome instability and tissue dysfunction (O'Sullivan & Karlseder, 2010). Importantly, several lines of evidence indicate that human telomeres shorten at nearly equal rates across tissues with aging, even in low turnover tissues such as the heart (Daniali et al., 2013). A recently identified additional function of telomere length in cells is that of telomere length dependent chromosome looping that regulates gene expression or telomere position effect over long distances (Robin et al., 2014). Telomeres can also shorten due to unrepaired DNA damage at the telomeres caused by oxidative stress or other genotoxic insults (Ludlow et al., 2014). Thus, understanding how exercise can maintain telomere length in cardiac tissue is important from several vantage points including genome stability and gene expression regulation.
Telomere length is maintained in some cells by the reverse transcriptase enzyme telomerase that consists of two main components, a protein component, telomerase reverse transcriptase (TERT), and an RNA template, telomerase RNA component (TERC; (Shay & Wright, 2007)). Telomere length and genome stability are also regulated by a DNA binding complex found at the ends of chromosomes called shelterin (de Lange, 2010), which consists of six proteins that bind to telomere DNA and regulate telomerase at the telomeres (de Lange, 2010). Telomere repeat binding factors 1 and 2 (TRF1 and TRF2) bind to double stranded telomere DNA and regulate telomere length, while protection of telomeres 1 (POT1) regulates telomerase action at the telomere. Additionally, DNA damage response proteins that transiently associate with telomeres (KU70/80, CHK2 and p53) can aid in genome stability and modify the telomere end structure.
Previously, we showed that long-term (44 weeks) voluntary wheel running in rodents maintained telomeres at a similar length compared to young animals and were significantly longer compared to age-match sedentary animals in heart tissue (Ludlow et al., 2012b). In a follow up study we showed that treadmill running induces an early adaptive response of the telomere length maintenance system in the highly activated skeletal muscles (plantaris) but not the lesser activated muscles (tibialis anterior) driven in part by activation of p38 MAPK (Ludlow et al., 2012a). Whether a similar phenomenon of upregulation of genes related to maintenance of telomeres occurs in the cardiac tissue following an acute bout of endurance type exercise is currently unknown.
To begin to understand the early adaptive response of endurance type exercise on the telomere maintenance system in rodent cardiac tissue we determined the effects of an acute bout of treadmill running on gene expression and protein content of telomere-related proteins in mouse cardiac muscle. Further, we investigated MAPK (p38, ERK, and JNK) activation as potential pathways through which exercise may result in telomere length maintenance in cardiac tissue. We hypothesized that acute exercise would increase the expression of telomere length-maintaining proteins in parallel with increased activation of MAPKs in mouse myocardium.
Methods
Ethical Approval
The procedures used in the present study were approved by the University of Maryland Institutional Animal Care and Use Committee and conformed to the National Institutes of Health’s Guide for the Use and Care of Laboratory Animals (NIH Pub. No. 85-23, revised, 1996). The authors of this study understand the ethical principles of the journal and we made every attempt to ensure that this work complies with the animal ethics checklist. C57Bl/6J mice were assigned to a baseline (BL) group that was not exposed to exercise or to one of two groups exposed to an acute treadmill running bout and sacrificed immediately (TP1) or one hour (TP2) following the exercise. Animals were kept on an ad libitum diet and on a standard 12 hours light : 12 hours dark schedule. Animals were first anesthetized by isoflurane and euthanized by cardiac excision. Cardiac gene expression of telomere-related proteins (Trf1, Trf2, Pot1a, Pot1b, Tert, Ku70, Ku80, p53 and Chk2) was analyzed via RT-PCR and TRF1, TRF2 and p38 MAPK protein content was analyzed via immunoblotting.
Animals
Twenty-two female C57Bl/6J mice were purchased from Jackson Laboratory (Bar Harbor, Maine) and housed at 25°C on a 12 hour light-dark cycle with access to standard laboratory mouse chow (Prolab RMH 3000, 5P00, LabDiet by Purina, Nestlé S.A., Vevey, Switzerland) and water ad libitum. We choose to use only female C57Bl/6J mice because they have a greater propensity to run compared to male mice (Lightfoot et al., 2004; Turner et al., 2005). Mice from the two exercise groups were acclimated to the treadmill for two weeks before the tests were performed, beginning at 6 weeks of age. The acclimation period was comprised of 7 progressive exercise sessions, the first involving a 5 min running bout at 2 m/min, no shock and the last one involving a 10 min running bout at 15–20 m/min, moderate shock (details shown in Table 2). All mice were sacrificed at 8 weeks of age to limit the amount of influence age could have on telomere-related variables. Data on the skeletal muscles from these animals has been published (Ludlow et al., 2012a).
Table 2.
Primer sequences for gene expression analysis.
| Gene name | Primer sequence |
|---|---|
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) F |
5'- GTG TCC GTC GTG GAT CTG 3’ |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) R |
5'- CCT GCT TCA CCA CCT TCT TG 3’ |
| 18S F | 5' GAT CCA TTG GAG GGC AAG TCT 3' |
| 18S R | 5' CCA AGA TCC AAC TAC GAG CTT TTT 3' |
| Telomere repeat binding factor 1 (TRF1) F | 5'-CAT GGA CTA CAC AGA CTT AC-3' |
| Telomere repeat binding factor 1 (TRF1) R | 5'-ATC TGG CCT ATC CTT AGA CG-3' |
| Telomere repeat binding factor 2 (TRF2) F | 5'-TGT CTG TCG CGC ATT GAA GA-3' |
| Telomere repeat binding factor 2 (TRF2) R | 5'-GCT GGA AGA CCT CAA TAG GAA-3' |
| Protection of telomeres 1a (POT1a) F | 5'-CCC TGA ATC TAC TCA AGG AAG-3' |
| Protection of telomeres 1a (POT1a) R | 5'-GAA GCG AAC AAT GTC TCC AA-3' |
| Protection of telomeres 1b (POT1b) F | 5'- CTT TAA GCC TCC GGC CTT AAG CAA AG-3' |
| Protection of telomeres 1b (POT1b) R | 5'- CTT GGA CAT GAT TAT CAG CAA CGA CA-3' |
| mouse telomerase reverse transcriptase (mTert) F |
5'- GCC TTG AGC ACA ATG ACC -3' |
| mouse telomerase reverse transcriptase (mTert) R |
5'- ATA TCC GTG GTG GCA CAA AT -3' |
Incremental treadmill exercise test
Forty-eight hours after the last acclimation session animals from the exercise groups were subjected to an incremental exercise test for assessment of their peak treadmill running speed. First, the mouse was placed for 2 min on the stationary treadmill belt. Thereafter, the treadmill speed was set at 6m/min and increased 3m/min every 2 minutes until refusal to run (animal seated on the shock pad for more than 30 seconds). The speed of the last stage completed was recorded as the peak treadmill running speed.
Acute treadmill exercise bout
Forty-eight hours after the incremental exercise test, the mice were exposed to 30 minutes of treadmill running at approximately 70% of their peak speed and were sacrificed immediately (TP1; n = 8) or 1h following (TP2; n = 8) the running bout. The duration and intensity of the treadmill exercise for the mice were chosen to mimic the current exercise recommendations of the American College of Sports medicine for maintenance of human health (Balady GJ, 2000). The treadmill was kept at 7° incline throughout the experiment. To encourage the animals to continue to run a shock grid at the back of the treadmill was used (detail shown in Table 1). All animals completed the 30-minute treadmill bout without stopping.
Table 1.
Treadmill acclimation procedures.
| Session | Time (min) | Belt speed (m*min−1) |
Shock pad | Days |
|---|---|---|---|---|
| 1 | 0 – 5 | 0 | No shock | 1–2 |
| 5 –15 | 2 | No shock | ||
| 2 | 0 – 2 | 0 | No shock | 4–5 |
| 2 – 5 | 2 | No shock | ||
| 5 – 10 | 5 | Light | ||
| 10 – 15 | 10 | Light | ||
| 3 | 0 – 2 | 0 | No shock | 7 |
| 2 – 5 | 5 | Light | ||
| 5 – 10 | 15 | Light | ||
| 10 – 15 | 20 | Moderate | ||
| 4 | 0 – 2 | 0 | No shock | 8; 10 |
| 2 – 5 | 5 | Light | ||
| 5 – 10 | 15 | Moderate | ||
| 10 – 15 | 20 – 24 | High |
Light shock (1.02 mA; 2.7 pulses*sec−1); Moderate shock (2.04 mA; 2.7 pulses*sec−1); High shock (3.06 mA; 2.7 pulses*sec−1). Intensity in milliamperes (0.34 to 3.4mA) and repetition rate at 200msec pulses at a rate of 0.3 to 3 pulses per second. m*min−1 = meters per minute.
Cardiac muscle collection and preparation
At the specified time points, the animals were anesthetized (3% isoflurane) and euthanized by cardiac excision. The hearts were flash frozen and powdered with a mortar and pestle in liquid nitrogen prior to separation into four vials for RNA, DNA, protein, and telomerase enzyme activity. The samples were stored at −80°C until further analysis.
RNA extraction and reverse transcription–polymerase chain reaction
Total RNA was extracted from the frozen samples using TRIzol reagent (Invitrogen Technologies, San Diego, CA) on the basis of previously described techniques (Chomczynski & Sacchi, 1987). One µg of total RNA was reverse transcribed using the High Capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA). Gene expression analyses were performed for all target genes using 1µL of each reverse transcription reaction product and normalized to 18S and Glycerol-aldehyde 3-dehydrogenase (Gapdh); however, expression of the target genes did not differ based on normalization to Gapdh or to 18S (data not shown). Specific primer sequences are shown in Table 2. The PCR products were separated on a 2.0% agarose gel and visualized using ethidium bromide. Band intensities were analyzed by densitometry using Image J software (Rasband, 1997–2011).
Protein Expression
Protein was extracted using a lysis buffer (50 mM Hepes (pH 7.4), 0.1% Triton X-100, 4 mM EGTA, 10 mM EDTA, 15 mM Na4P2O7•H2O, 100 mM – glycerophosphate, and a protease inhibitor cocktail - complete mini EDTA-free tablet (Roche, Indianapolis, IN)) and protein content was determined using bicinchoninic acid protein assay (Pierce, Rockford, IL). Fifty micrograms of total protein was prepared and resolved on 10% (TRF1 and 2) or 7.5% (MAPKs) SDS-PAGE gels, transferred to PVDF membranes, and blocked in 5% non-fat dry milk for 30 min. Immunoblotting was performed for TRF1 (1:200, C-19, SC 1977, Santa Cruz Biotechnologies, Santa Cruz, CA), TRF2 (1:200, H-300, SC 9143, Santa Cruz Biotechnologies, Santa Cruz, CA), phosphorylated p38 MAPK (1:500, Cell Signaling 9211, Thr180/Tyr182, Danvers, MA), total p38 MAPK (1:500, Cell Signaling 9212, Danvers, MA), phosphorylated ERK1/2 (P44 ERK1/ p42 ERK2, Thr202/Tyr204, Cell Signaling 9101, 1:1000), total ERK1/2 (Cell Signaling 9102, 1:1000), phosphorylated SAPK/JNK1/2 (p46 JNK1, p54 JNK2, Thr183/Tyr185, Cell Signaling 9251, 1:1000), total SAPK/JNK1/2 (Cell Signaling 9251, 1:1000) and GAPDH (1:1000, Cell signaling, 14C10 Rabbit mAb # 2118, Danvers, MA). Products were visualized using species appropriate horseradish-peroxidase linked secondary antibodies and visualized on an enhanced chemiluminescence (ECL) imager (Syngene Bio Imaging, Fredrick, MD). Band intensities were analyzed by densitometry using Image J software (Rasband, 1997–2011). For protein content, GAPDH was used as a loading reference.
Telomerase
Telomerase enzyme activity was measured using a commercially available kit utilizing the telomere repeat amplification protocol (TRAP; Quantitative Telomerase Detection Kit; US Biomax, Rockville, MD). Protein concentration was determined (as above) and 1 µg of protein was added to the reaction according to the recommendations of the manufacturer and as previously performed in our lab (Ludlow et al., 2008). In addition to the standards provided in the kit we assayed heat-treated samples as a negative control. Heat-treated samples were concluded to be telomerase negative if the mean of the critical threshold (Ct) for the heat-treated sample duplicates was three standard deviations above that of the telomerase positive sample (this criteria was also used to determine a telomerase positive sample). We also assayed a human cancer cell line known to be telomerase positive (HeLa, ATCC, CCL-2, Manassas, VA) to ensure sensitivity of the assay. Due to sample processing and limitations in the assay (heat treated negative control was not three standard deviations different from lysate), we had to exclude two animals from the post exercise groups. This exclusion reduced the power of our analysis and may have hindered the detection of biologically relevant and statistically significant differences.
Statistical analysis
All values are presented as means ± standard deviation of the mean. One-way analysis of variance (ANOVA) with Tukey’s honest significant difference was performed for all analyses. Statistical significance was accepted at p ≤ 0.05.
Results
Body mass, peak and submaximal treadmill running speeds attained for each group are presented in Table 3.
Table 3.
Body weight and running speeds for the experimental groups.
| BL (n = 4) | TP1 (n = 8) | TP2 (n = 8) | |
|---|---|---|---|
| Body mass (g) | 19.7 ± 1.8 | 18.3 ± 2.8 | 17.4 ± 2.3 |
| Peak treadmill running speed (m*min−1) |
- | 36.8 ± 14.4 | 32.6 ± 6.5 |
| Average running speed during acute treadmill bout (m*min−1) |
- | 24.8 ± 11.0 | 21.0 ± 4.0 |
Data are presented as means ± standard deviation. BL: Baseline; TP1: immediately after acute treadmill running bout; TP2: 1-hour after acute treadmill running bout. g = grams. m*min−1 = meters per minute.
Cardiac shelterin gene expression and protein content increase in response to acute exercise
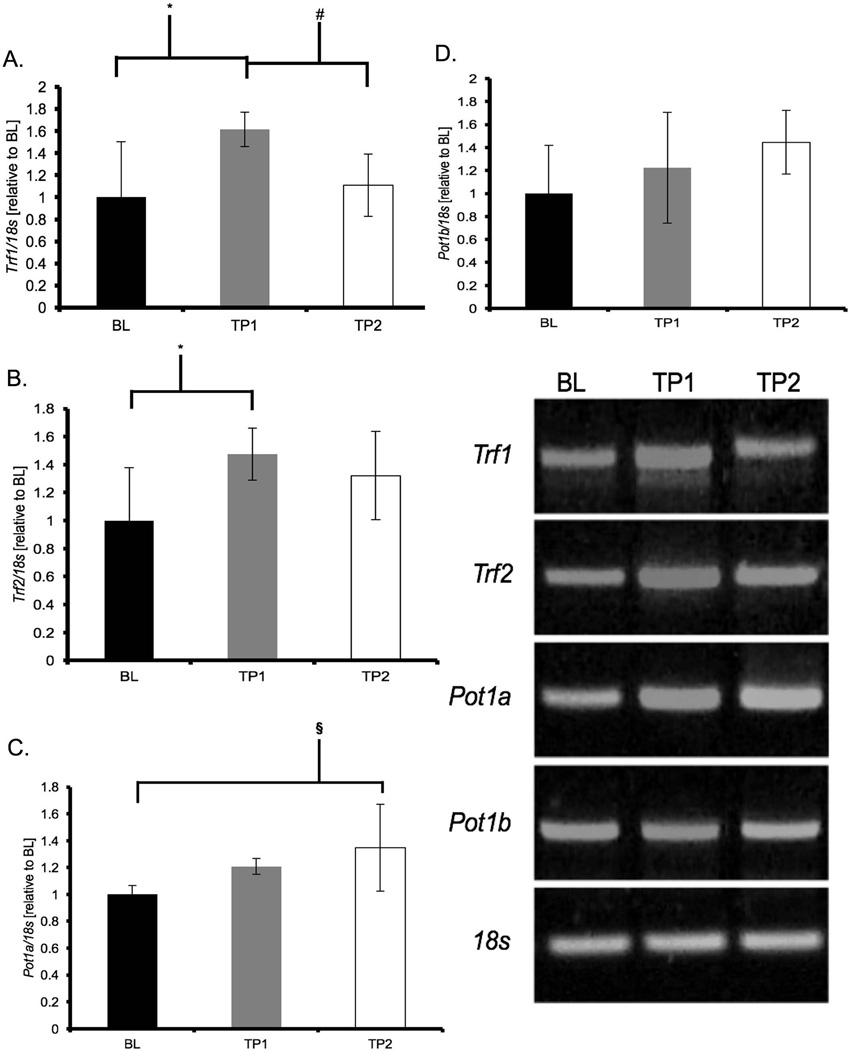
Acute treadmill running resulted in a significant up-regulation of cardiac gene expression of shelterin components (Figure 1). TP1 animals showed increases in Trf1 (+61%, p = 0.01) and Trf2 (+47%, p = 0.04) mRNA while TP2 animals showed increases in Pot1a mRNA expression (+35%, p = 0.04) when compared to BL. There were no differences at any time point for Pot1b gene expression (Figure 1).
Figure 1. Acute treadmill running increased shelterin gene expression in cardiac muscle.
mRNA abundance was assessed by RT-PCR and target genes were normalized to 18S and expressed relative to BL. Results of densitometric analysis are shown. Data are presented as means ± S.D. BL: baseline (n = 6); TP1: immediately after the exercise bout (n = 8); TP2: one-hour after the exercise bout (n = 8). * TP1 significantly different than BL (p < 0.05). # TP1 significantly different than TP2 (p < 0.05). § TP2 significantly different than BL (p < 0.05).
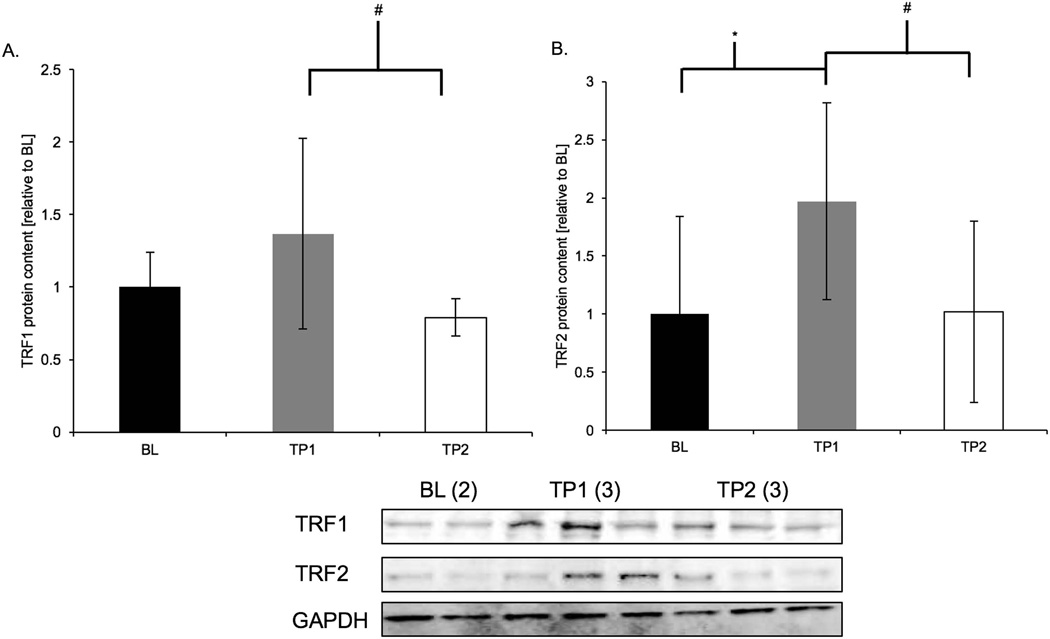
Acute treadmill running induced an increase in protein content of shelterin components at TP1. TRF1 protein content was greater in TP1 animals compared to TP2 animals (+58%, p = 0.002). TRF2 protein content was greater in TP1 animals compared to both BL (+97%, p = 0.05) and TP2 animals (+95%, p = 0.03; Figure 2a and b).
Figure 2. TRF1 and TRF2 protein content were increased following acute treadmill running.
Results of densitometric analysis and representative immunoblots of TRF1, TRF2, and GAPDH are shown; GADPH was a loading reference. Data are presented as means ± S.D. BL: baseline (n = 6); TP1: immediately after the exercise bout (n = 8); TP2: one-hour after the exercise bout (n = 8). GAPDH: glyceraldehyde 3-phosphate dehydrogenase. * TP1 significantly different than BL (p < 0.05). # TP1 significantly different than TP2.
Telomerase enzyme activity and mTert gene expression following acute exercise
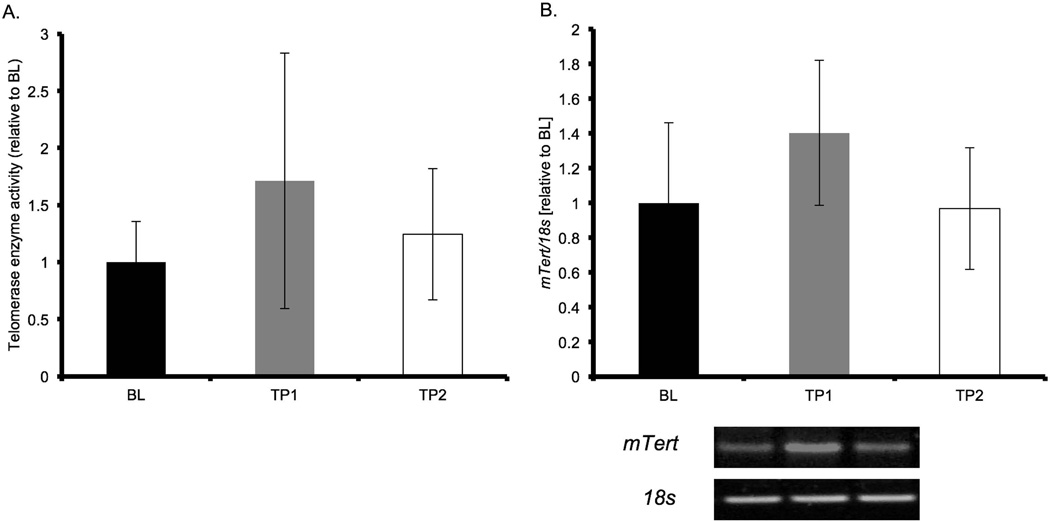
mTert gene expression regulates telomerase activity (Armstrong et al., 2000) and we hypothesized that an increase in mTert gene expression would correlate with an increase in telomerase enzyme activity. ANOVA analysis of mTert mRNA expression did not detect an effect (p = 0.1, Figure 3b.). Telomerase enzyme activity was not different in the ANOVA analysis (p = 0.2, Figure 3a.).
Figure 3. Activity and expression of telomerase increased after acute treadmill running.
Telomerase enzyme activity (left) and mTert gene expression (right) are shown. mRNA abundance for mTert was assessed with RT-PCR, normalized to 18S and expressed relative to BL. Results of densitometric analysis are shown. Data are presented as means ± S.D. BL: baseline (n = 4); TP1: immediately after the exercise bout (n = 6); TP2: one-hour after the exercise bout (n = 6). mTert = mouse telomerase reverse transcriptase.
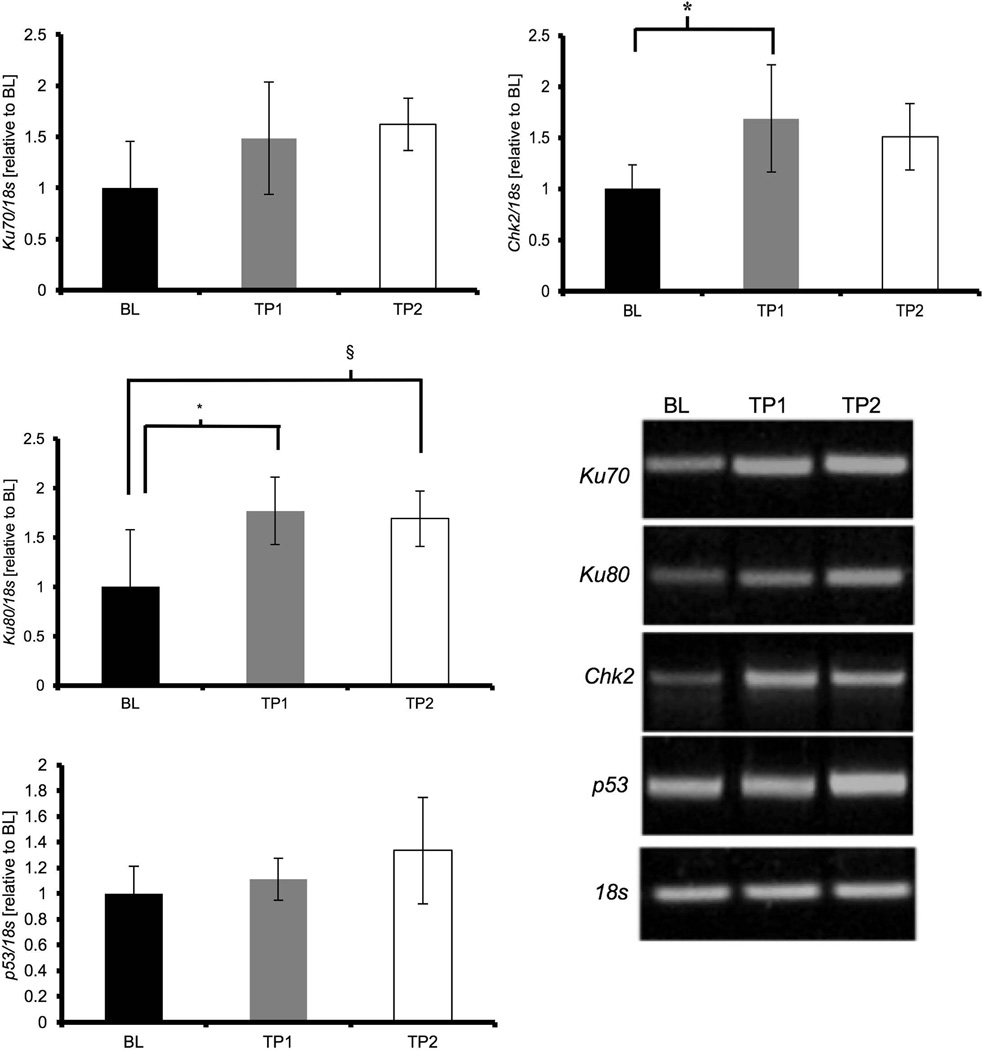
DNA damage repair and response gene expression in cardiac muscle increases following acute exercise
Previous literature has implicated the accumulation of DNA damage and p53 signaling in the aging heart, therefore we tested the influence of acute exercise on DNA damage and repair and DNA damage signaling genes. The expression of DNA damage repair protein Ku70 was not different (p = 0.08, Figure 4a), while Ku80 gene expression was significantly increased in TP1 and TP2 animals compared to BL (p = 0.01; and p = 0.02, Figure 4b, respectively). Expression of the mRNA of DNA damage response protein p53 was not different between groups (p = 0.17, Figure 4c), but Chk2 mRNA expression was significantly increased at TP1 compared to BL animals (p = 0.03, Figure 4d).
Figure 4. Acute exercise increases gene expression of DNA damage repair and response factors.
mRNA abundance was assessed by RT-PCR and target genes were normalized to 18S and expressed relative to BL. Results of densitometric analysis are shown. Data are presented as means ± S.D. BL: baseline (n = 6); TP1: immediately after the exercise bout (n = 8); TP2: one-hour after the exercise bout (n = 8). * TP1 significantly different than BL (p < 0.05). § TP2 significantly different than BL (p < 0.05).
p38 MAPK phosphorylation is altered in parallel with changes in gene expression
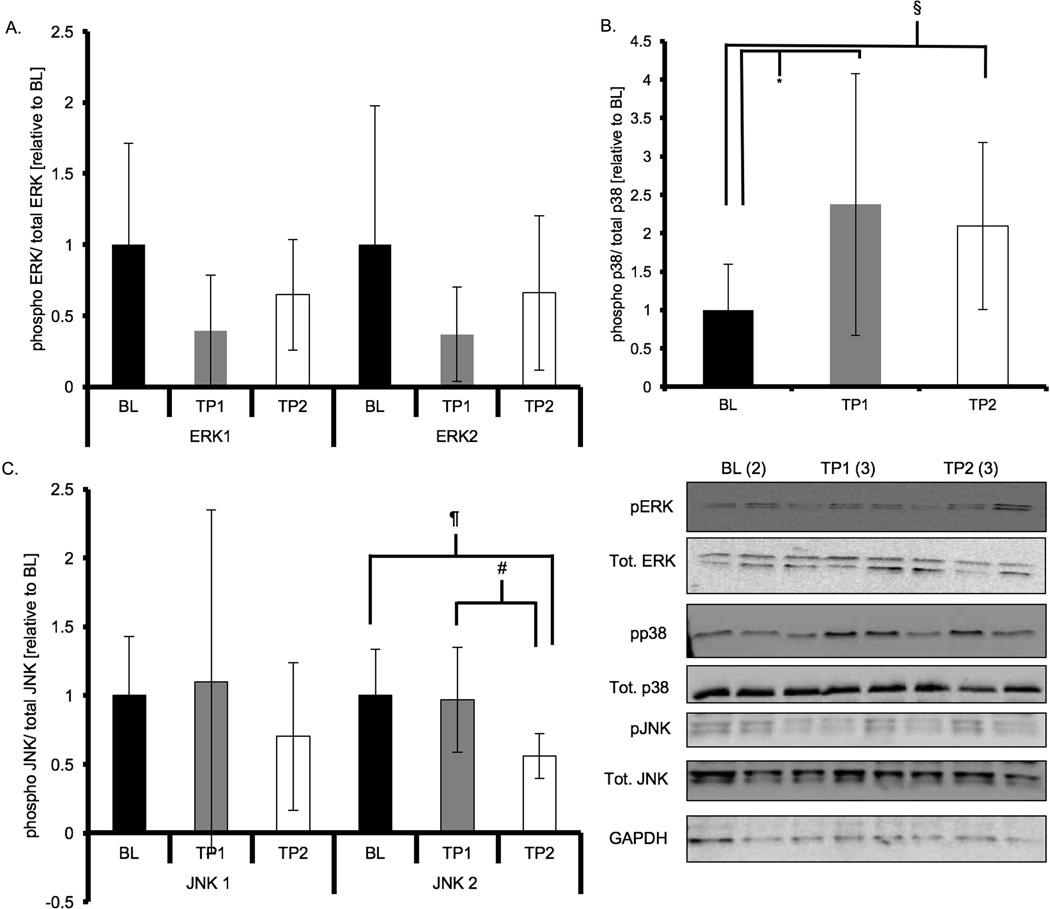
To test if mitogen-activated protein kinases were activated during acute exercise in parallel with alterations in shelterin components we measured the phosphorylation status of three MAPKs: p38 MAPK, ERK1/2 and JNK1/2. ERK1 (p44) phosphorylation levels were significantly reduced immediately following exercise (p = 0.02, Figure 5) and were similar to BL at TP2, while ERK2 (p42) phosphorylation levels were not significantly different at any time point (p = 0.16). p38 MAPK phosphorylation levels were greater in TP1 animals compared to BL animals (p = 0.04), and tended to be greater in TP2 compared to BL animals (p = 0.10), but were not different between TP1 and TP2 (p = 0.63, Figure 5). Phosphorylated levels of JNK1 (p46) were not different at any time point (p = 0.59), but levels of phosphorylated JNK2 (p54) were significantly reduced at TP2 following exercise compared to both BL and TP1 (p = 0.001).
Figure 5. p38 MAPK phosphorylation in cardiac muscle is increased following acute treadmill running.
Results of densitometric analysis and representative immunoblots of MAPKs (total and phosphorylated) and GAPDH are shown; GADPH was a loading reference. Phosphorylated to total protein ratios were derived and expressed relative to BL. Data are presented as means ± S.D. BL: baseline; TP1: immediately after the exercise bout; TP2: one-hour after the exercise bout. * TP1 significantly different than BL (p < 0.05). # TP1 significantly different than TP2 (p < 0.05). § TP2 tended to be different than BL (p < 0.1).
Discussion
Our laboratory has previously determined that chronic voluntary wheel running exercise in mice resulted in telomere length maintenance and increased expression of shelterin and DNA damage response and repair genes in cardiac tissue (Ludlow et al., 2012a; Ludlow et al., 2012b). The purpose of the current study was to begin to elucidate the acute effects of exercise on cardiac tissue gene expression of shelterin proteins and DNA damage response and repair genes. We describe for the first time in C57Bl/6J mice that an acute bout of treadmill exercise results in upregulation of cardiac telomere length maintaining proteins that occurs in parallel with activation of the p38 MAPK signaling pathway. These data provide important insights into a potential pathway mediating telomere length maintenance and genome stability in response to chronic exercise in cardiac tissue. We also show that key DNA damage response and repair proteins are increased following acute exercise. Together, these results support a ‘telomere length-protective’ effect of exercise in cardiac tissue and indicate that the initial adaptation may be associated with altered p38 MAPK signaling (Spallarossa et al., 2009). Maintenance of longer telomeres in cardiac tissues/cell types may result in a ‘youthful’ gene expression program and longer healthy heart function with chronological aging (Robin et al., 2014; Robin et al., 2015). We hypothesize that the chronic effects of exercise on telomere length may be the cumulative effect of each bout and that understanding the pathways associated with the adaptive response to exercise may lead to improved therapeutics/exercise prescription for the prevention of cardiovascular disease.
The regenerative capacity of the heart cell types, particularly the cardiomyocyte, is limited (van Berlo & Molkentin, 2014); however, recent reports indicate that rare neonatal-like adult cardiomyocytes can divide (Porrello et al., 2011; Canseco et al., 2015; Kimura et al., 2015). DNA damage signaling (p53, p21 and p16) and mitochondrial dysfunction occurs with aging in the heart and leads to pathological conditions such as heart failure and myocardial infarcts, indicating that the turnover of heart cells is not sufficient to replace cells under physiological wear and tear (Keller & Howlett, 2016; Narasimhan & Rajasekaran, 2016). Further, it has been shown that telomere length in the adult human heart and in mouse heart tissue does shorten with aging, suggesting that some level of cellular turnover and/or telomeric DNA damage is occurring with age (Werner et al., 2008; Werner et al., 2009; Wong et al., 2010; Ludlow et al., 2012b). Exercise training is associated with substantial cardiovascular health benefits and we recently showed that long-term wheel running in mice maintained telomere length and increased expression of telomere length maintaining proteins in heart tissue of active animals compared to controls (Ludlow et al., 2012b).
Previous research has indicated that exercise training is associated with increased gene expression of shelterin in cardiac tissue (Werner et al., 2008; Werner et al., 2009; Ludlow et al., 2012b). In our previous chronic exercise study, we observed statistically significant decreases in Trf1, Trf2, Pot1a and Pot1b mRNA levels in hearts of aged mice that were attenuated by exercise training (Ludlow et al., 2012b). TRF1 and TRF2 are double-stranded telomere binding proteins that regulate telomere length (de Lange, 2010). POT1a and POT1b interact with the single-stranded G-rich overhang of telomeres and interact with another shelterin protein TPP1, that together recruit telomerase to telomeres and control telomere repeat processivity (the ability of telomerase to successively add telomere repeats to chromosome ends) (Nandakumar et al., 2012; Nandakumar & Cech, 2013; Schmidt & Cech, 2015). Maintenance of shelterin component expression levels is critical to maintaining telomere length homeostasis and to prevent DNA damage signaling at the telomere. Here, consistent with our previous data that shelterin adapts to exercise training, we show that a single bout of exercise is able to increase the expression of TRF1, TRF2 (both protein and mRNA), and Pot1a (mRNA), but not Pot1b. These data indicate that exercise stress related signaling results in an up regulation of shelterin components in cardiac muscle and that with training (cumulative effect of each individual exercise bout) mRNA levels may be maintained over time. The immediate response of TRF1 and TRF2 protein level increase is likely due to an increase in protein stability or a reduction in protein degradation, while the increase in mRNA levels are an adaptive response caused by early adaptive signaling events. Briefly, studies of master’s athletes have shown increased TRF2 levels (protein and mRNA) compared to age-matched healthy, sedentary individuals (Werner et al., 2009). A study of ultra-endurance athletes’ white blood cells (WBCs) 24 hours following seven marathons in seven days showed an increase in TRF1 and TRF2 mRNA levels compared to baseline (Laye et al., 2011). To the contrary, an acute endurance exercise bout (30 min cycling, 80% max HR) was associated with reduced TRF2 mRNA levels in human WBCs 60 min after exercise (Chilton et al., 2014). The authors also measured miRNAs and found several upregulated miRNAs that could target TRF2, indicating the exercise induced regulation of TRF2 could be very tightly controlled and depend on the timing of measurement and intensity and duration of the exercise stimulus. Pot1a mRNA levels increased one-hour following exercise. POT1a in rodents suppresses the ATR-dependent DNA damage response at telomeres (Palm et al., 2009). These findings also extend our previous results that chronic voluntary wheel running resulted in increased Pot1a gene expression in hearts of 1-year old animals compared to sedentary age-matched animals (Ludlow et al., 2012b). Laye et al. (Laye et al., 2011) also showed an increase in POT1 levels compared to baseline levels in WBCs following the ultra-marathon event described above. Thus, the upregulation of shelterin with acute exercise may accumulate over time (i.e., a training effect or repeated bout effect) resulting in increased shelterin and aiding in the telomere length maintenance phenotype observed in chronically trained animals.
The major telomere length maintenance enzyme complex, telomerase, has also been shown to be upregulated following short term (three weeks) and long-term (six months) exercise training in rodent heart tissue (Werner et al., 2008; Werner et al., 2009). We observed a slight but not statistically significant increase in telomerase activity and in mTert (mouse telomerase reverse transcriptase) gene expression following exercise. Our data indicate that a single acute bout of exercise does not significantly increase telomerase activity levels and that the effect of exercise on telomerase may be a repeat bout effect, with telomerase adapting after several bouts of exercise. Several human studies to date have observed mixed results concerning the acute effects of exercise on hTERT gene expression. In a study of human WBCs, hTERT mRNA levels increased 60 minutes following a bout of cycling compared to resting levels (Chilton et al., 2014). While in the ultra-marathon study mentioned above, no change in hTERT mRNA levels was observed in WBCs (Laye et al., 2011). Most recently, a study of WBCs in young healthy individuals following a 30 min treadmill run showed an increase in telomerase activity compared to the rested state (Zietzer et al., 2016). These mixed results point to the need for more research on exercise and telomerase. Several considerations about how exercise could be influencing telomerase and hTERT should be made as follows. In order for telomerase to maintain telomeres two different steps must occur, first there must be active enzyme (i.e., TERT must be expressed) and second the active enzyme must be recruited to the telomeres. Thus, an increase in either process could initiate telomere length maintenance. These data could suggest that the initial adaptation to exercise may be to increase telomerase recruitment to telomeres and the training adaptation is to increase telomerase enzyme activity as observed in previous studies (Werner et al., 2008; Werner et al., 2009). However, further research into the activation, assembly, and recruitment of telomerase (i.e., TPP1 levels) and mTert/hTERT gene expression regulation as well as the cell cycle/division kinetics and types of cells expressing telomerase following exercise on telomere length maintenance in cardiac tissue of rodents and humans is warranted.
Since longer telomeres are associated with increased genome stability and previously we have shown an upregulation of DNA damage response and repair genes in cardiac tissue of trained animals, we investigated the effects of acute exercise on DNA damage response and repair genes. We observed that Ku80 and Chk2 gene expression increased immediately following exercise and returned to baseline levels by one-hour post exercise. Ku80 is a heterodimer protein that has been shown to transiently associate with telomeres and is involved in the DNA damage response (Lopez et al., 2011; Pfingsten et al., 2012). Further, (Laye et al., 2011) observed increased gene expression of Ku70/Ku80 in both immune cells and skeletal muscle in humans after running 7 marathons in 7 days. Moreover, we have previously observed greater Ku70/Ku80 gene expression in cardiac tissue of one-year old mice that voluntarily ran for 44 weeks compared to sedentary aged-matched animals (Ludlow et al., 2012b). Combined, these data indicate that exercise training and acute exercise in both trained and untrained individuals increases gene expression related to enhanced DNA damage response and repair. We hypothesize that improved DNA damage response and repair, in concert with increased shelterin expression and telomerase activity, would result in telomere length maintenance and an anti-apoptotic and anti-senescent cellular environment in exercise-trained compared to sedentary individuals.
To address possible signaling mechanisms, we measured a common stress response pathway in relation to the observed changes in gene expression. We measured activation of p38MAPK, ERK1/2 and JNK1/2, which are known to be a part of cardiac growth pathways (Rose et al., 2010). We observed a significant upregulation in p38MAPK phosphorylation following exercise in conjunction with gene expression changes, while we observed decreased activation (phosphorylation) of ERK1/2 and JNK1/2. We recently reported that activation of p38 MAPK in skeletal muscle was directly related to altered levels of shelterin components (Ludlow et al., 2012a). Similar to our skeletal muscle data and other reports in cardiac muscle we observed significant p38 MAPK phosphorylation following an acute bout of exercise. Activation of these three MAPKs has been associated with cardiac disease and pathophysiological hypertrophy (Asrih et al., 2013). Further, MAPKs have been associated with exercise and aging responses, as well as expression of telomere binding proteins in cardiac tissue (Iwasa et al., 2003; Iemitsu et al., 2006; Collado et al., 2007; Spallarossa et al., 2009). In a model of artificially selected rats bred for low or high ability to run, an acute treadmill running bout activated all three MAPKs (Hunter et al., 2008). These discrepant results may be due to the exercise stress or the rodent model used; however, both interventions resulted in significant activation of p38MAPK. Activation of p38 MAPK results in changes in activation and localization (i.e., cytoplasmic versus nuclear) of a variety transcription factors (Rose et al., 2010) that are likely related to the altered gene expression of shelterin components. While only descriptive in nature in the current experiment, we and others have previously shown links between p38MAPK and shelterin gene expression (Spallarossa et al., 2009; Ludlow et al., 2012a). The response of p38 MAPK is likely due to transient increases in calcium levels, oxidative stress and local concentration of trophic factors (growth hormone and IGF-1) in the cardiac tissue and cells (Rose et al., 2010). While activation of the MAPKs has been implicated as being related to pathophysiological cardiac remodeling of the heart, we propose that activation of p38 MAPK alone may trigger an early adaptive response to increase genome stabilizing gene expression. Future experiments investigating the exercise associated activation of p38 MAPK, which isoform is specifically activated, and the regulatory roles that the p38MAPK stress response pathway plays in cardiac telomere length maintenance related gene expression are necessary.
This study is not without limitations that must be considered for appropriate interpretation of the presented data. We did not separate out the different p38 MAPK isoforms to determine which specific isoform is responsible to the observed changes in shelterin expression following acute exercise. To help prevent acute bout effects from carrying over from the acclimation or testing period, animals were placed in their normal cages for 48hrs prior to the acute exercise bout and sacrifice, which should minimize the effects of the acclimation and testing, but these effects cannot be ruled out. Additionally, we were limited by sample availability and assay sensitivity (qPCR based TRAP assay), thus while we were close to significance in several instances, we failed to detect differences due to these limitations. Future studies with greater sample sizes and more robust measures of telomerase activity may detect significant differences. Further, translation of these data from the rodent model to humans should be done with caution, as laboratory mice have significantly longer telomeres compared to human telomeres. That being said the regulation of shelterin in vivo in humans and mice appears to be similar, thus providing evidence for the translational value of shelterin and telomere data obtained in mice (Werner et al., 2008; Werner et al., 2009; Laye et al., 2011). Again, we emphasize that this is the first report describing a novel gene expression phenotype and potential association between shelterin and p38 MAPK in cardiac tissue. We did not measure telomere length, as we would not anticipate such a short-term exercise stimulus to alter telomere length in a meaningful or detectable fashion.
Understanding how telomere length is regulated in cardiac tissue with exercise training and how each bout of exercise results in a cardioprotective phenotype is important research for improving the healthspan of humans. Telomere length is one aspect of aging tissues that regulates genomic stability and, as elucidated recently, chromatin structure and gene expression. How exercise results in longer telomeres and perhaps influences gene expression with aging in cardiac tissue and how the altered gene expression effects specific cell types and the increase in fibrotic cells in the heart with aging makes these findings and future research particularly important to human cardiovascular health. Ultimately, understanding the positive benefits of exercise training on these phenotypes may lead to novel therapies in preventative and personalized medicine.
New Findings.
A positive association between telomere length and exercise training has been shown in cardiac tissue of mice. It is currently unknown how each bout of exercise influences telomere length regulating proteins. We sought to determine how a bout of exercise altered the expression of telomere length regulating genes and a related signaling pathway in cardiac tissue of mice.
Acute exercise altered the expression of telomere length regulating genes in cardiac tissue and may be related to altered MAPK signaling. These findings are important in understanding how exercise provides a cardio-protective phenotype with aging.
Acknowledgments
This work was supported by the Department of Kinesiology Graduate Research Initiative Fund and NIH predoctoral training grant AG000268 (A.T. Ludlow, UMD), NIH/NCI T32 CA124334-07 (A.T. Ludlow UTSWMC), NIH/NCI K99/R00 Pathway to Independence CA197672-01 (A.T. Ludlow UTSWMC), and by Brazilian CAPES Foundation PDEE scholarship BEX1714090 (L.C.J. Lima). The animals used in this study were also used in a concurrent study of skeletal muscle.
Footnotes
Author Contributions:
Andrew T. Ludlow: Designed and executed the study. Performed laboratory measures, collected and analyzed the data. Drafted and edited the manuscript.
Laila C. J. Lima: Designed and executed the study. Performed laboratory measures, collected and analyzed the data. Drafted and edited the manuscript.
Lindsay W. Ludlow: Drafted and edited the manuscript.
Espen E. Spangenburg: Designed the study. Edited the manuscript.
Stephen M. Roth: Designed the study. Drafted and edited the manuscript.
Disclosures
None.
References
- Armstrong L, Lako M, Lincoln J, Cairns PM, Hole N. mTert expression correlates with telomerase activity during the differentiation of murine embryonic stem cells. Mechanisms of Development. 2000;97:109–116. doi: 10.1016/s0925-4773(00)00423-8. [DOI] [PubMed] [Google Scholar]
- Asrih M, Mach F, Nencioni A, Dallegri F, Quercioli A, Montecucco F. Role of mitogen-activated protein kinase pathways in multifactorial adverse cardiac remodeling associated with metabolic syndrome. Mediators of Inflammation. 2013;2013:367245. doi: 10.1155/2013/367245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balady GJBK, Golding LA, Gordon NF, Mahler DA, Myers JN, Sheldahl, Lois M. American College of Sports Medicine: ACSM's Guidelines for Exercise Testing and Prescription. Philadephia: Lippincott, Williams and Wilkins; 2000. [Google Scholar]
- Bar C, Bernardes de Jesus B, Serrano R, Tejera A, Ayuso E, Jimenez V, Formentini I, Bobadilla M, Mizrahi J, de Martino A, Gomez G, Pisano D, Mulero F, Wollert KC, Bosch F, Blasco MA. Telomerase expression confers cardioprotection in the adult mouse heart after acute myocardial infarction. Nat Commun. 2014;5:5863. doi: 10.1038/ncomms6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andra M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisen J. Dynamics of Cell Generation and Turnover in the Human Heart. Cell. 2015;161:1566–1575. doi: 10.1016/j.cell.2015.05.026. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Epel ES, Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350:1193–1198. doi: 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- Canseco DC, Kimura W, Garg S, Mukherjee S, Bhattacharya S, Abdisalaam S, Das S, Asaithamby A, Mammen PP, Sadek HA. Human ventricular unloading induces cardiomyocyte proliferation. Journal of the American College of Cardiology. 2015;65:892–900. doi: 10.1016/j.jacc.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton WL, Marques FZ, West J, Kannourakis G, Berzins SP, O'Brien BJ, Charchar FJ. Acute exercise leads to regulation of telomere-associated genes and microRNA expression in immune cells. PloS One. 2014;9:e92088. doi: 10.1371/journal.pone.0092088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical biochemistry. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Daniali L, Benetos A, Susser E, Kark JD, Labat C, Kimura M, Desai K, Granick M, Aviv A. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. doi: 10.1038/ncomms2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T. How shelterin solves the telomere end-protection problem. Cold Spring Harbor Symposia on Quantitative Biology. 2010;75:167–177. doi: 10.1101/sqb.2010.75.017. [DOI] [PubMed] [Google Scholar]
- Dolle ME, Snyder WK, Gossen JA, Lohman PH, Vijg J. Distinct spectra of somatic mutations accumulated with age in mouse heart and small intestine. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8403–8408. doi: 10.1073/pnas.97.15.8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez LJ, Galioto A, Ferlisi A, Pineo A, Putignano E, Belvedere M, Costanza G, Barbagallo M. Ageing, lifestyle modifications, and cardiovascular disease in developing countries. The Journal of Nutrition, Health & Aging. 2006;10:143–149. [PubMed] [Google Scholar]
- Hunter CJ, Koch LG, Britton SL, Boluyt MO. Initial signaling response to acute exercise bout is similar in hearts of rats bred for divergent exercise capacities. Frontiers in Bioscience. 2008;13:347–355. doi: 10.2741/2684. [DOI] [PubMed] [Google Scholar]
- Iemitsu M, Maeda S, Jesmin S, Otsuki T, Kasuya Y, Miyauchi T. Activation pattern of MAPK signaling in the hearts of trained and untrained rats following a single bout of exercise. Journal of Applied Physiology. 2006;101:151–163. doi: 10.1152/japplphysiol.00392.2005. [DOI] [PubMed] [Google Scholar]
- Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes to cells : devoted to molecular & cellular mechanisms. 2003;8:131–144. doi: 10.1046/j.1365-2443.2003.00620.x. [DOI] [PubMed] [Google Scholar]
- Keller KM, Howlett SE. Sex Differences in the Biology and Pathology of the Aging Heart. Canadian Journal of Cardiology. 2016 doi: 10.1016/j.cjca.2016.03.017. [DOI] [PubMed] [Google Scholar]
- Kimura W, Xiao F, Canseco DC, Muralidhar S, Thet S, Zhang HM, Abderrahman Y, Chen R, Garcia JA, Shelton JM, Richardson JA, Ashour AM, Asaithamby A, Liang H, Xing C, Lu Z, Zhang CC, Sadek HA. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature. 2015;523:226–230. doi: 10.1038/nature14582. [DOI] [PubMed] [Google Scholar]
- Laye MJ, Solomon TP, Karstoft K, Pedersen KK, Nielsen SD, Klarlund Pedersen B. Increased Shelterin mRNA Expression in Peripheral Blood Mononuclear Cells and Skeletal Muscle Following an Ultra Long-Distance Running Event. Journal of Applied Physiology. 2011 doi: 10.1152/japplphysiol.00997.2011. [DOI] [PubMed] [Google Scholar]
- Lightfoot JT, Turner MJ, Daves M, Vordermark A, Kleeberger SR. Genetic influence on daily wheel running activity level. Physiological Genomics. 2004;19:270–276. doi: 10.1152/physiolgenomics.00125.2004. [DOI] [PubMed] [Google Scholar]
- Lopez CR, Ribes-Zamora A, Indiviglio SM, Williams CL, Haricharan S, Bertuch AA. Ku must load directly onto the chromosome end in order to mediate its telomeric functions. PLoS Genet. 2011;7:e1002233. doi: 10.1371/journal.pgen.1002233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow AT, Lima LC, Wang J, Hanson ED, Guth LM, Spangenburg EE, Roth SM. Exercise alters mRNA expression of telomere-repeat binding factor 1 in skeletal muscle via p38 MAPK. Journal of Applied Physiology. 2012a;113:1737–1746. doi: 10.1152/japplphysiol.00200.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow AT, Roth SM. Physical activity and telomere biology: exploring the link with aging-related disease prevention. Journal of aging research. 2011;2011:790378. doi: 10.4061/2011/790378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow AT, Spangenburg EE, Chin ER, Cheng WH, Roth SM. Telomeres shorten in response to oxidative stress in mouse skeletal muscle fibers. Journals of Gerontology. Series A: Biological Sciences and Medical Sciences. 2014;69:821–830. doi: 10.1093/gerona/glt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow AT, Witkowski S, Marshall MR, Wang J, Lima LC, Guth LM, Spangenburg EE, Roth SM. Chronic exercise modifies age-related telomere dynamics in a tissue-specific fashion. The journals of gerontology. Series A, Biological sciences and medical sciences. 2012b;67:911–926. doi: 10.1093/gerona/gls002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow AT, Zimmerman JB, Witkowski S, Hearn JW, Hatfield BD, Roth SM. Relationship between physical activity level, telomere length, and telomerase activity. Med Sci Sports Exerc. 2008;40:1764–1771. doi: 10.1249/MSS.0b013e31817c92aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandakumar J, Bell CF, Weidenfeld I, Zaug AJ, Leinwand LA, Cech TR. The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature. 2012;492:285–289. doi: 10.1038/nature11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandakumar J, Cech TR. Finding the end: recruitment of telomerase to telomeres. Nature reviews. Molecular cell biology. 2013;14:69–82. doi: 10.1038/nrm3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan M, Rajasekaran NS. Exercise, Nrf2 and Antioxidant Signaling in Cardiac Aging. Frontiers in Physiology. 2016;7:241. doi: 10.3389/fphys.2016.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufer PD, Bamman MM, Muoio DM, Bouchard C, Cooper DM, Goodpaster BH, Booth FW, Kohrt WM, Gerszten RE, Mattson MP, Hepple RT, Kraus WE, Reid MB, Bodine SC, Jakicic JM, Fleg JL, Williams JP, Joseph L, Evans M, Maruvada P, Rodgers M, Roary M, Boyce AT, Drugan JK, Koenig JI, Ingraham RH, Krotoski D, Garcia-Cazarin M, McGowan JA, Laughlin MR. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metabolism. 2015;22:4–11. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- O'Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nature Reviews: Molecular Cell Biology. 2010;11:171–181. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm W, Hockemeyer D, Kibe T, de Lange T. Functional dissection of human and mouse POT1 proteins. Molecular and Cellular Biology. 2009;29:471–482. doi: 10.1128/MCB.01352-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingsten JS, Goodrich KJ, Taabazuing C, Ouenzar F, Chartrand P, Cech TR. Mutually exclusive binding of telomerase RNA and DNA by Ku alters telomerase recruitment model. Cell. 2012;148:922–932. doi: 10.1016/j.cell.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. U. S. National Institutes of Health; 1997–2011. [Google Scholar]
- Robin JD, Ludlow AT, Batten K, Gaillard MC, Stadler G, Magdinier F, Wright WE, Shay JW. SORBS2 transcription is activated by telomere position effect-over long distance upon telomere shortening in muscle cells from patients with facioscapulohumeral dystrophy. Genome Research. 2015;25:1781–1790. doi: 10.1101/gr.190660.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin JD, Ludlow AT, Batten K, Magdinier F, Stadler G, Wagner KR, Shay JW, Wright WE. Telomere position effect: regulation of gene expression with progressive telomere shortening over long distances. Genes and Development. 2014;28:2464–2476. doi: 10.1101/gad.251041.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiological Reviews. 2010;90:1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JC, Cech TR. Human telomerase: biogenesis, trafficking, recruitment, and activation. Genes and Development. 2015;29:1095–1105. doi: 10.1101/gad.263863.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay JW, Wright WE. Hallmarks of telomeres in ageing research. Journal of Pathology. 2007;211:114–123. doi: 10.1002/path.2090. [DOI] [PubMed] [Google Scholar]
- Shay JW, Wright WE. Telomeres and telomerase in normal and cancer stem cells. FEBS Letters. 2010;584:3819–3825. doi: 10.1016/j.febslet.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spallarossa P, Altieri P, Aloi C, Garibaldi S, Barisione C, Ghigliotti G, Fugazza G, Barsotti A, Brunelli C. Doxorubicin induces senescence or apoptosis in rat neonatal cardiomyocytes by regulating the expression levels of the telomere binding factors 1 and 2. American Journal of Physiology: Heart and Circulatory Physiology. 2009;297:H2169–H2181. doi: 10.1152/ajpheart.00068.2009. [DOI] [PubMed] [Google Scholar]
- Turner MJ, Kleeberger SR, Lightfoot JT. Influence of genetic background on daily running-wheel activity differs with aging. Physiological Genomics. 2005;22:76–85. doi: 10.1152/physiolgenomics.00243.2004. [DOI] [PubMed] [Google Scholar]
- van Berlo JH, Molkentin JD. An emerging consensus on cardiac regeneration. Nature Medicine. 2014;20:1386–1393. doi: 10.1038/nm.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner C, Furster T, Widmann T, Poss J, Roggia C, Hanhoun M, Scharhag J, Buchner N, Meyer T, Kindermann W, Haendeler J, Bohm M, Laufs U. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 2009;120:2438–2447. doi: 10.1161/CIRCULATIONAHA.109.861005. [DOI] [PubMed] [Google Scholar]
- Werner C, Hanhoun M, Widmann T, Kazakov A, Semenov A, Poss J, Bauersachs J, Thum T, Pfreundschuh M, Muller P, Haendeler J, Bohm M, Laufs U. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. Journal of the American College of Cardiology. 2008;52:470–482. doi: 10.1016/j.jacc.2008.04.034. [DOI] [PubMed] [Google Scholar]
- Wong LS, van der Harst P, de Boer RA, Huzen J, van Gilst WH, van Veldhuisen DJ. Aging, telomeres and heart failure. Heart Failure Reviews. 2010;15:479–486. doi: 10.1007/s10741-010-9173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zietzer A, Buschmann EE, Janke D, Li L, Brix M, Meyborg H, Stawowy P, Jungk C, Buschmann I, Hillmeister P. Acute physical exercise and long-term individual shear rate therapy increase telomerase activity in human peripheral blood mononuclear cells. Acta Physiologica (Oxford, England) 2016 doi: 10.1111/apha.12820. [DOI] [PubMed] [Google Scholar]