Abstract
Background
Maternal thyroid function is a critical mediator of fetal brain development. Pregnancy-related physiologic changes and handling conditions of blood samples may influence thyroid hormone biomarkers. We investigated the reliability of thyroid hormone biomarkers in plasma of pregnant women under various handling conditions.
Methods
We enrolled 17 pregnant women; collected serum and plasma were immediately frozen. Additional plasma aliquots were subjected to different handling conditions before the analysis of thyroid biomarkers: storage at room temperature for 24 or 48 hours before freezing and an extra freeze–thaw cycle. We estimated free thyroid hormone indices in plasma based on T3 uptake.
Results
High correlations between plasma and serum (>0.94) and intra-class correlation coefficients for plasma handling conditions (0.96 to 1.00) indicated excellent reliability for all thyroid hormone biomarkers.
Conclusion
Delayed freezing and freeze–thaw cycles did not affect reliability of biomarkers of thyroid function in plasma during pregnancy.
INTRODUCTION
Prenatal maternal thyroid function is a critical mediator of fetal brain development1,2. The relationship between maternal thyroid hormone concentrations measured in stored blood samples and child neurodevelopmental outcomes is of particular interest in birth cohorts that have adequate statistical power to investigate rarer outcomes such as cerebral palsy, autism, or attention-deficit hyperactivity disorder (e.g. Norwegian Mother and Child Cohort Study and Danish National Birth Cohort)2–7. Among maternal thyroid hormone biomarkers, thyroid stimulating hormone (TSH) is of paramount importance8, but free thyroid hormone levels (free thyroxine, free T4, and free triiodothyronine, free T3) have relevance to the study of offspring neurodevelopmental impairments and disorders9–13. In pregnant women, because of changes in plasma binding proteins and circulating thyroid hormone levels, immunoassays for free thyroid hormone levels perform poorly14,15. However, the gold standard assay for free T4, incorporating equilibrium dialysis, can only be performed on serum8. In the two birth cohorts mentioned above, however, maternal plasma but not serum was stored4,16. And as with many large-scale birth cohort studies, including the Norwegian Mother and Child Cohort Study and the Danish National Birth Cohort, the protocol was streamlined and cost-efficient, with unrefrigerated shipment of samples before long-term freezing at a central biorepository, and samples subjected to various processing schedules, transport durations and temperatures (eTable 1). Furthermore, analyses of stored biospecimens will necessarily be performed on thawed samples, with specimens having potentially experienced several freeze–thaw cycles due to aliquoting for various analytical purposes. Although effects of some handling conditions and storage on thyroid hormone measurements in blood have been studied17–21, few data on pregnant women are available.
Thus, the goals of the present study were to evaluate in blood specimens from pregnant women the reliability of thyroid hormone biomarkers in plasma versus serum, including an alternate estimate of free thyroid hormone levels in plasma, and the effects of storage at ambient temperature and freeze–thaw cycles on plasma biomarkers.
METHODS
We enrolled 17 pregnant women who were having blood drawn as part of their routine prenatal care at the North Carolina Women’s Hospital (Chapel Hill, North Carolina, USA), and obtained informed consent. Eligible women were in their third trimester of pregnancy, age 18 or older, spoke English, and had no known thyroid-related condition. All samples were given anonymously. This study was approved by the Institutional Review Board of UNC Chapel Hill.
From each woman, we collected 25 mL of blood and processed it within one hour of collection. Bloods were spun and separated into one 1.5 mL serum and five 1.5 mL plasma aliquots. Serum and one plasma aliquot were stored immediately at −80°C, representing the optimal treatment condition. One aliquot of plasma was left unrefrigerated at room temperature for 24 hours, and one for 48 hours, before being stored at −80°C. After overnight storage at −80°C, one plasma aliquot from each time point was allowed to thaw completely (two hours at 4°C) and re-frozen at −80°C (Figure 1).
Figure 1.
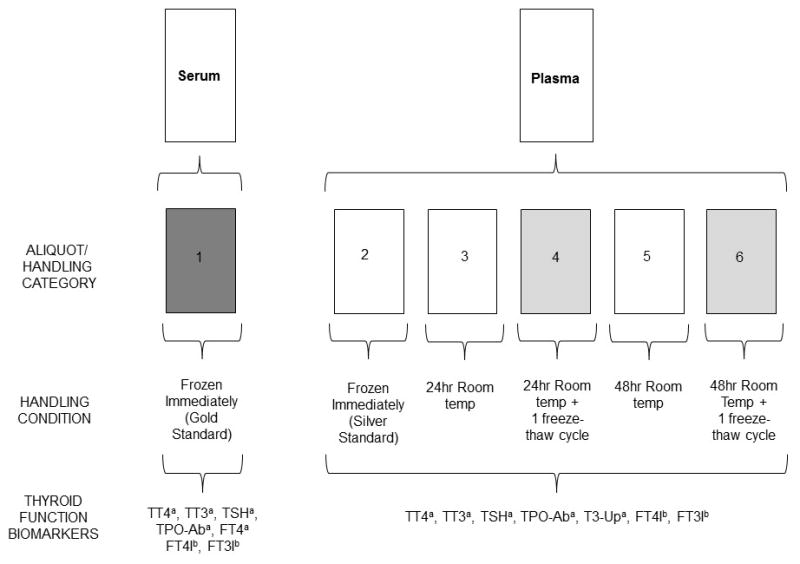
Schematic overview of handling conditions of serum and plasma from 17 pregnant women and the thyroid function biomarkers that were analyzed.
TT4=Total thyroxine, TT3=Total triiodothyronine, FT4=free thyroxine, FT4I= free thyroxine index, FT3I=free triiodothyronine index, TSH=Thyroid stimulating hormone (thyrotropin), TPO-Ab = Thyroxine peroxidase antibody, T3-Up=T3 uptake
aTT4, TT3, and T3-Up were measured by electrochemiluminescent immunoassay (ECLIA). TSH and TPO-Ab were measured by chemiluminescent immunoassay (CLIA). FT4 in serum was measured by equilibrium dialysis with high-performance liquid chromatography-tandem mass spectrometry (ED-HPLC-MS/MS).
bFT4I and FT3I in plasma were calculated from T3 uptake.
Biomarker measurements in serum and plasma were conducted at ARUP Laboratories (Salt Lake City, Utah, USA) using immunoassays (see eAppendix 1): total T3, total T4, TSH, T3 uptake, and thyroid peroxidase antibody. Additionally, free T4 was measured by equilibrium dialysis followed by high performance liquid chromatography-tandem mass spectrometry in serum only. T3 uptake, which is an indirect measure of thyroxine binding globulin binding capacity22, was used to calculate the indices of free T4 and free T3 (eAppendix 2)29.
We used Spearman’s rank correlation to examine the strength of association between serum and plasma biomarker levels, and Wilcoxon signed-rank test to investigate potential differences between serum and optimally treated plasma. For parametric analysis, variables were natural log-transformed if necessary to achieve normal distributions. The 95% confidence intervals (CI) were computed from the bootstrap distribution.
As a measure of reliability across the five plasma handling conditions (Figure 1), we calculated intra-class correlation coefficients (two-way random effects model) for each thyroid hormone variable (except free T4). The intra-class correlation coefficient reflects the proportion of the intra-individual variance attributed to sample handling. A value close to one indicates a high reliability across sample handling categories.
To investigate the effect of time spent at ambient temperature and an additional freeze–thaw cycle on plasma thyroid hormones, we fitted a linear mixed-effect model (random slope model) to the data for each measured variable using R and lme423,24. Consistent with other studies17,25 we assumed a linear relationship between time and freeze–thaw with hormone levels. We performed analyses on a long file containing thyroid hormone levels as the dependent variable (Ncases=17×5). The fixed effects were time (delay before freezing: 0, 24 and 48 hours), freeze (1 vs 2 freeze–thaw cycles), and the interaction between time and freeze.
RESULTS
Spearman’s rho was > 0.94 for all measured biomarkers comparing plasma to serum (Table 1). The average value of T3 uptake was somewhat higher in serum as compared to optimally treated plasma (Table 1; eFigure 1). Across the five plasma handling conditions (see Figure 1) intra-class correlation coefficients were high; ranging from 0.98 to 1.00 for total T4, total T3, T3 uptake, thyroid peroxidase antibody, and TSH (Table 1). We found no fixed effects of time or an additional freeze–thaw cycle on T3 uptake, total T4, TSH or thyroid peroxidase antibody in plasma (Table 2). There was, however, a positive effect of time on total T3 levels, increasing levels by about 1.35 ng/dL for each 24 hours (95% CI 0.01–2.66). No interactions between time and freeze–thaw cycles were found.
Table 1.
Intra-class correlation coefficient of thyroid hormones variables across all five plasma handling categories (see Figure 1), and comparing serum and optimal treated plasma from 17 pregnant women (only 14 women had thyroxine peroxidase antibody above detection) using Spearman’s rank correlation and Wilcoxon signed-rank test.
| Thyroid variable | Serum vs. plasma | Plasma (handling category 2–5) |
|---|---|---|
|
| ||
| Spearman’s rho (rs) (95% CI lower, upper) | Intra-class correlation coefficient (95% CI lower, upper) | |
| Total thyroxine, total T4 | 0.97 (0.84, 1.00) | 0.98 (0.95, 0.99) |
| Total triiodothyronine, total T3 | 0.99 (0.93, 1.00) | 0.98 (0.95, 0.99) |
| Thyroid stimulating hormone, TSH | 1.00 (0.96, 1.00) | 1.00 (0.999, 1.000) |
| Thyroxine peroxidase antibody, TPO-Ab | 0.94 (0.78, 0.99) | 0.98 (0.96, 0.99)a |
| T3 uptake | 0.98 (0.95, 1.00)b | 0.98 (0.95, 0.99) |
| Free T4 index | 0.97 (0.84, 1.00) | 0.97 (0.95, 0.99)a |
| Free T3 index | 0.99 (0.95, 1.00)c | 0.96 (0.93, 0.99)a |
variable was ln-transformed to approximate normal distribution before analyses
Difference plasma vs. serum (Wilcoxon signed-rank test; 95% CI −1.0000, −0.9999)
Difference plasma vs. serum (Wilcoxon signed-rank test; 95% CI −7.0, −1.5)
Table 2.
Fixed effects of time left unrefrigerated and freeze–thaw cycle(s) on measured thyroid hormone biomarkers in plasma of pregnant women
| Time Left Unrefrigerateda | Freeze–Thawa | Interaction between time and freeze–thawb | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Fixed effect | 95% Confidence Interval | Fixed effect | 95% Confidence Interval | Fixed effect | 95% Confidence Interval | |
| TT3 | 1.35 | 0.01, 2.66 | 0.41 | −2.07, 2.72 | −0.88 | −4.08, 2.17 |
| T3 uptake | 0.12 | −0.36, 0.26 | −0.06 | −0.06, 0.28 | 0.29 | −0.13, 0.77 |
| TT4 | 0.03 | −0.04, 0.10 | −0.05 | −0.16, 0.05 | 0.05 | −0.10, 0.21 |
| TSH | 0.01 | −0.01, 0.02 | −0.01 | −0.02, 0.00 | −0.01 | −0.03, 0.02 |
| TPO-Abc | 0.001 | −0.01, 0.02 | −0.02 | −0.02, −0.01 | 0.04 | −0.01, 0.10 |
Model includes only fixed effect of time left unrefrigerated (ordinal categorical) or freeze–thaw (dichotomous) respectively.
Model includes fixed effects of time left unrefrigerated, freeze-thaw, and time*freeze thaw interaction term.
The variable was natural log-transformed to approximate normal distribution
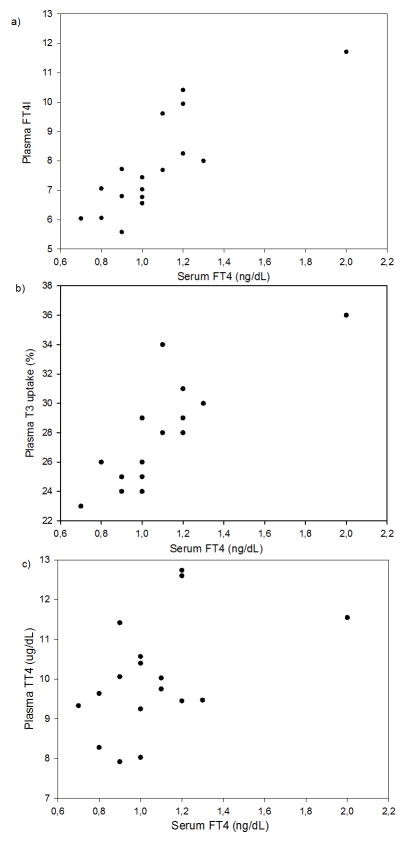
Free T4 levels in serum measured using the equilibrium dialysis approach showed strong, positive associations with T3 uptake and the calculated free T4 index in optimally treated plasma (Figures 2a–b). There was a weaker association between serum free T4 with total T4 in optimally treated plasma (Figure 2c). Free T4 showed a similar pattern of association with serum levels of free T3 index, T3 uptake and total T4 (eFigure 2). Furthermore, intra-class correlation coefficients for free T4 and free T3 indices across plasma handling categories were high (> 0.96; Table 1). Free T3 index was higher in serum versus optimally treated plasma, following the pattern of T3 uptake (Table 1).
Figure 2.
Scatter-plots of free thyroxine (free T4, FT4) in serum of 17 pregnant women measured by “gold standard” method equilibrium dialysis with high-performance liquid chromatography–tandem mass spectrometry in relation to a) Free T4 index (FT4I) in optimally treated plasma (Spearman’s rho, rs =0.81, CI 0.52–0.94), b) T3 uptake (T3-Up) in optimally treated plasma (rs=0.79; CI 0.49–0.92)1 and c) Total thyroxine (total T4, TT4) in optimally treated plasma (rs=0.45, CI −0.02–0.73).
1 Only 15 points are showing in plot b) due to overlapping points for two samples on two occasions.
DISCUSSION
The high correlation of all measured biomarkers between serum and optimally treated plasma, and high intra-class correlation coefficients across the plasma handling conditions, indicates that plasma collected from large-scale birth cohort studies subjected to variable handling conditions are likely to provide reliable measures of total T4, total T3, TSH, T3 uptake, and thyroid peroxidase antibody. Although a small, positive fixed effect of time at ambient temperature was found for total T3 levels, even with this increase for 48 hours the change was < 2% of the median.
To our knowledge, no other studies have investigated the effect of time spent at ambient temperature and freeze–thaw cycles on total T3 levels in plasma or serum, although other thyroid function biomarkers have been examined. Various handling conditions in repeated samples from the same healthy, non-pregnant individuals (n=10) showed no effect on free T3, free T4, or TSH in plasma kept 0, 2, 4, 6, 24 or up to 72-hours at room temperature17. No differences were found in measured levels of TSH, free T4, thyroid peroxidase antibody, or thyroglobulin antibody in sera samples from non-pregnant women (n=8) stored up to 6 days at 4°C25. Additionally, comparisons of frozen (n=50) versus fresh sera (n=50) from pregnant subjects showed that a freeze–thaw cycle resulted in only a minor increase in free T325. In a study of pooled serum samples from various patients, TSH appeared stable in serum stored up to 4 days at room temperature20.
It may be important to distinguish between the effects of time delays both pre- and post-blood separation. Our study specifically addressed post-separation time delays, as was typical for plasma in the Norwegian Mother and Child Cohort Study16,26 (eTable 1). Although pre-separation delay may be more relevant for the Danish National Birth Cohort 4,27, a previous study has indicated that thyroid hormone biomarkers in plasma and sera are similarly stable in room temperature with up to 72 hours delay in blood separation17.
Accurate assessment of free T4 and free T3 during pregnancy is of great importance to the study of offspring neurodevelopmental outcomes9–13. However, pregnancy-induced elevations in thyroxine binding globulin and total thyroid hormones and decreases in blood albumin interfere with immunoassays of free thyroid hormone levels and the equilibrium dialysis approach in serum is recommended8,22. Maternal plasma, and not serum, is stored in birth cohorts such as the Norwegian Mother and Child Cohort Study and the Danish National Birth Cohort. We investigated a surrogate biomarker, T3 uptake, that measures the relative amount of unbound thyroxine binding globulin20 and can be used to calculate free T4 and free T3 indices 28. These indices may provide a more accurate estimate of free thyroid hormones than immunoassays since they mathematically account for the increased thyroxin binding globulin levels during pregnancy14,15,22. We found that the calculated free T4 index in serum and plasma, and plasma free T3 index, were within normal values for non-pregnant29 and pregnant women14,30, and the free T4 index showed a high, positive correlation with serum free T4. Additionally, the high intra-class correlation coefficients across plasma handling conditions suggest these calculated indices provide a reliable estimate of free T4 and free T3 during pregnancy.
In conclusion, plasma levels of total T4, total T3, TSH, T3 uptake, and thyroid peroxidase antibody are reliable biomarkers even when subjected to extended time at ambient temperature and an additional freeze–thaw cycle.
Supplementary Material
eTable 1. Table presenting plasma handling methods typical of large birth cohorts.
eTable 2. Table presenting results of thyroid biomarker analysis in serum and plasma of 17 pregnant women.
eFigure 1. Figures with box plots presenting thyroid function biomarkers in 1 serum and 5 plasma aliquots sampled from pregnant women.
eFigure 2. Figures with scatterplots of free T4 measured in serum in relation to serum levels of free T4 index, T3 uptake and total T4.
eAppendix 1. Detailed method description of thyroid biomarker analysis.
eAppendix 2. Formula for calculation of free T4 and T3 indices.
Acknowledgments
Source of Funding:
This study was supported in part by R01ES021777, the Intramural Research Program, The National Institutes of Environmental Health Sciences (NIEHS), the National Institutes of Health (NIH), and The Norwegian Institute of Public Health.
Footnotes
Conflicts of Interest:
There is no conflict of interest concerning this study.
References
- 1.Patel J, Landers K, Li H, Mortimer RH, Richard K. Thyroid hormones and fetal neurological development. Journal of Endocrinology. 2011;209:1–8. doi: 10.1530/JOE-10-0444. [DOI] [PubMed] [Google Scholar]
- 2.Rovet J, Willoughby K. Maternal Thyroid Function During Pregnancy: Effects on the Developing Fetal Brain. In: Zimmerman AW, Connors SL, editors. Maternal Influences on Fetal Neurodevelopment. Springer; New York: 2010. pp. 55–77. [Google Scholar]
- 3.Williams GR. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol. 2008;20:784–94. doi: 10.1111/j.1365-2826.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- 4.Olsen J, Melbye M, Olsen SF, Sorensen TI, Aaby P, Andersen AM, Taxbol D, Hansen KD, Juhl M, Schow TB, Sorensen HT, Andresen J, Mortensen EL, Olesen AW, Sondergaard C. The Danish National Birth Cohort--its background, structure and aim. Scand J Public Health. 2001;29:300–7. doi: 10.1177/14034948010290040201. [DOI] [PubMed] [Google Scholar]
- 5.Magnus P, Stoltenberg C. The Norwegian mother and child cohort study (MoBa) - past, present and future. Norsk Epidemiologi. 2014;24:3–6. doi: 10.5324/nje.v24i1-2.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa) International Journal of Epidemiology. 2006;35:1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 7.Liew Z, Ritz B, von Ehrenstein OS, Bech BH, Nohr EA, Fei CY, Bossi R, Henriksen TB, Bonefeld-Jorgensen EC, Olsen J. Attention Deficit/Hyperactivity Disorder and Childhood Autism in Association with Prenatal Exposure to Perfluoroalkyl Substances: A Nested Case-Control Study in the Danish National Birth Cohort. Environmental Health Perspectives. 2015;123:367–373. doi: 10.1289/ehp.1408412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP, Sullivan S, Wiersinga W American Thyroid Association Taskforce on Thyroid Disease During P, Postpartum. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kooistra L, Crawford S, Van Baar AL, Brouwers EP, Pop VJ. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics. 2006;117:161–167. doi: 10.1542/peds.2005-0227. [DOI] [PubMed] [Google Scholar]
- 10.Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, Vulsma T, Wiersinga WM, Drexhage HA, Vader HL. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50:149–55. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 11.Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59:282–8. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 12.Andersen SL, Laurberg P, Wu CS, Olsen J. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG: An International Journal of Obstetrics & Gynaecology. 2014;121:1365–74. doi: 10.1111/1471-0528.12681. [DOI] [PubMed] [Google Scholar]
- 13.Pakkila F, Mannisto T, Pouta A, Hartikainen AL, Ruokonen A, Surcel HM, Bloigu A, Vaarasmaki M, Jarvelin MR, Moilanen I, Suvanto E. The impact of gestational thyroid hormone concentrations on ADHD symptoms of the child. Journal of Clinical Endocrinology and Metabolism. 2014;99:E1–E8. doi: 10.1210/jc.2013-2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee RH, Spencer CA, Mestman JH, Miller EA, Petrovic I, Braverman LE, Goodwin TM. Free T4 immunoassays are flawed during pregnancy. American Journal of Obstetrics and Gynecology. 2009;200:260.e1–260.e6. doi: 10.1016/j.ajog.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 15.Thienpont LM, Van Uytfanghe K, Poppe K, Velkeniers B. Determination of free thyroid hormones. Best Practice & Research Clinical Endocrinology & Metabolism. 2013;27:689–700. doi: 10.1016/j.beem.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Ronningen KS, Paltiel L, Meltzer HM, Nordhagen R, Lie KK, Hovengen R, Haugen M, Nystad W, Magnus P, Hoppin JA. The biobank of the Norwegian mother and child cohort Study: A resource for the next 100 years. European Journal of Epidemiology. 2006;21(8):619–625. doi: 10.1007/s10654-006-9041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oddoze C, Lombard E, Portugal H. Stability study of 81 analytes in human whole blood, in serum and in plasma. Clinical Biochemistry. 2012;45:464–469. doi: 10.1016/j.clinbiochem.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Clark S, Youngman LD, Palmer A, Parish S, Peto R, Collins R. Stability of plasma analytes after delayed separation of whole blood: implications for epidemiological studies. International Journal of Epidemiology. 2003;32:125–130. doi: 10.1093/ije/dyg023. [DOI] [PubMed] [Google Scholar]
- 19.Gislefoss RE, Grimsrud TK, Mørkrid L. Stability of selected serum hormones and lipids after long-term storage in the Janus Serum Bank. Clinical Biochemistry. 2015;48(6):364–369. doi: 10.1016/j.clinbiochem.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Koliakos G, Gaitatzi M, Grammaticos P. Stability of serum TSH concentration after non refrigerated storage. Panminerva Med. 1999;41:99–101. [PubMed] [Google Scholar]
- 21.Oddie TH, Klein AH, Foley TP, Fisher DA. Variation in values for iodothyronine hormones, thyrotropin, and thyroxine-binding globulin in normal umbilical-cord serum with season and duration of storage. Clin Chem. 1979;25:1251–3. [PubMed] [Google Scholar]
- 22.Stockigt JR. Free thyroid hormone measurement - A critical appraisal. Endocrinology and Metabolism Clinics of North America. 2001;30:265–289. doi: 10.1016/s0889-8529(05)70187-0. [DOI] [PubMed] [Google Scholar]
- 23.Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 24.Bates DMM, Bolker B, Walker S. R package version 1.1–7. 2014. lme4: Linear mixed-effects models using Eigen and S4. [Google Scholar]
- 25.Mannisto T, Surcel HM, Bloigu A, Ruokonen A, Hartikainen AL, Jarvelin MR, Pouta A, Vaarasmaki M, Suvanto-Luukkonen E. The effect of freezing, thawing, and short- and long-term storage on serum thyrotropin, thyroid hormones, and thyroid autoantibodies: implications for analyzing samples stored in serum banks. Clin Chem. 2007;53:1986–7. doi: 10.1373/clinchem.2007.091371. [DOI] [PubMed] [Google Scholar]
- 26.Paltiel L, Haugan A, Skjerden T, Harbak K, Baekken S, Stensrud NK, Knudsen GP, Magnus P. The biobank of the Norwegian mother and child cohort study - Present status. Norsk Epidemiologi. 2014;24:29–35. [Google Scholar]
- 27.Bach CC, Liew Z, Bech BH, Nohr EA, Fei C, Bonefeld-Jorgensen EC, Henriksen TB, Olsen J. Perfluoroalkyl acids and time to pregnancy revisited: An update from the Danish National Birth Cohort. Environ Health. 2015;14:59. doi: 10.1186/s12940-015-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunlap DB. Thyroid Function Tests. In: Walker HKHW, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3. Chapter 140. Boston: Butterworths; 1990. [PubMed] [Google Scholar]
- 29.Sawin CT, Chopra D, Albano J, Azizi F. The free triiodothyronine (T3) index. Ann Intern Med. 1978;88:474–7. doi: 10.7326/0003-4819-88-4-474. [DOI] [PubMed] [Google Scholar]
- 30.Roti E, Gardini E, Minelli R, Bianconi L, Flisi M. Thyroid-Function Evaluation by Different Commercially Available Free Thyroid-Hormone Measurement Kits in Term Pregnant-Women and Their Newborns. Journal of Endocrinological Investigation. 1991;14:1–9. doi: 10.1007/BF03350244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Table presenting plasma handling methods typical of large birth cohorts.
eTable 2. Table presenting results of thyroid biomarker analysis in serum and plasma of 17 pregnant women.
eFigure 1. Figures with box plots presenting thyroid function biomarkers in 1 serum and 5 plasma aliquots sampled from pregnant women.
eFigure 2. Figures with scatterplots of free T4 measured in serum in relation to serum levels of free T4 index, T3 uptake and total T4.
eAppendix 1. Detailed method description of thyroid biomarker analysis.
eAppendix 2. Formula for calculation of free T4 and T3 indices.