Abstract
Paragangliomas are rare extra-adrenal neuroendocrine tumors that are derived from embryonic neural crest cells. They are classified as functioning or nonfunctioning based on their ability to produce catecholamines. If nonfunctional, they are mostly asymptomatic and typically discovered as incidental lesions. If functional, they may secrete catecholamines, leading to clinical presentations similar to adrenal pheochromocytomas, including episodic headaches, hypertension, sweating and tachycardia. Most paragangliomas are benign, however, 15 to 35 percent may eventually become metastatic.1 Standard treatment for paraspinal paragangliomas below the neck is surgical resection with adjuvant radiation therapy on an individualized basis. In spite of complete excision, 20 percent of patients with primary extra-adrenal paragangliomas below the neck may develop recurrence.2 Leptomeningeal dissemination is a rare phenomenon, seen only in a small number of cases worldwide.3
Keywords: Paraganglioma, Spinal Neoplasm, Neuroendocrine Tumor, Metastasis
Case Presentation
A 61-year-old woman with no history of malignancy and a past medical history of hypertension and type 2 diabetes mellitus presented to her primary care physician with low back pain in late 2011. She had no focal neurologic signs or symptoms on initial presentation. MRI revealed an intrathecal tumor of the lumbar spine at the L3 level (Figure 1), without evidence of abnormalities throughout the rest of the spine or brain. The 4.6 cm tumor was resected en bloc and histopathologically determined to be a WHO grade 1 paraganglioma. Germline genetic testing revealed no clinically significant alteration in the SDHB, SDHC or SDHD genes; however, a variant of unknown clinical significance, p.H50R or c.149A>G, in exon 2 of the SDHD gene was identified. This variant is felt to be a rare polymorphism rather than a pathogenic mutation.4 It is not currently confirmed to be deleterious or causative of classic hereditary paraganglioma syndrome, and it is known to exist in the general population of healthy individuals with a frequency of approximately 3%.5 Site-specific genetic testing for this alteration was offered to the patient’s parents through research to clarify from which side of the family it was inherited and possibly determine if it may be associated with her paraganglioma, however, this offer was declined. Plasma metanephrines were measured to be within normal limits (<0.10 nmol/L), as were urine and serum normetanephrine (0.81 nmol/L in both samples).
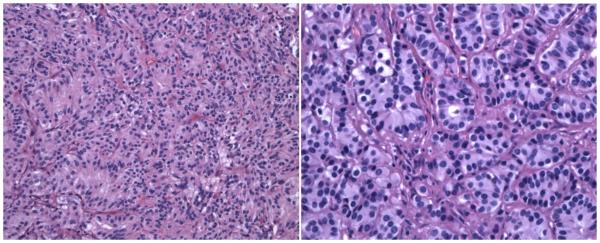
Figure 1.
Hematoxylin and eosin (H&E) staining of the tumor reveals nests of cells (the classic Zellballen pattern) consisting of small ovoid nuclei and eosinophilic cytoplasm separated by thin, fibrovascular septae (H&E × 200 - Figure 1a (left)). Higher power reveals the bland, stippled chromatin pattern and scattered nucleoli (H&E × 400 - Figure 1b (right)).
Operation
An L2-L4 laminectomy was performed in February 2012. Thinly noted dura and arachnoid over the underlying tumor were opened to immediately reveal an encapsulated tumor originating from the filum. Arachnoid adhesions were carefully cauterized after which a gross total en-bloc resection of the extramedullary mass was performed without violating its capsule. Following the surgery, she experienced complete resolution of her low back pain, but she did note residual rectal numbness and weakness without loss of bowel or bladder function.
Pathology
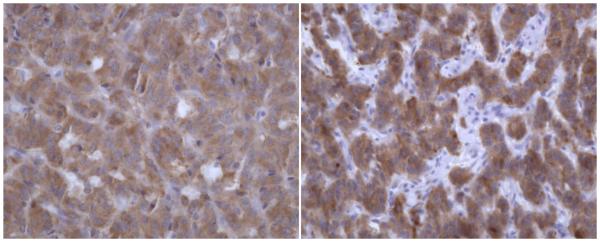
Neuropathology examination demonstrated a thinly encapsulated, pink-tan to maroon tumor that was 4.6 × 2.0 × 1.8 cm in size. The tumor came within 0.6 cm of the nearest margin without evidence of dural sac invasion. It was estimated that complete macroscopic surgical removal was attained. With hematoxylin and eosin staining, the tumor contained relatively uniform, round-to-oval shaped neoplastic cells arranged in nests (the classically described “Zellballen” pattern). Focal pseudorosetting patterns were seen. The nuclei were centrally located and displayed “salt and pepper” type chromatin. Moderate amounts of granular cytoplasm were present (Figure 1). The tumor nests were surrounded by delicate monolayers of thin, elongated sustentacular cells. Fragments of bone were included in the tumor matrix. Immunohistochemical stains performed on the tumor revealed it to be positive for non-specific enolase and synaptophysin (Figure 2), both stains of neuroendocrine origin, and negative for glial fibrillary acidic protein and epithelial membrane antigen. The histopathology was diagnostic of a paraganglioma.
Figure 2.
The tumor is positive by immunohistochemical staining for non-specific enolase (NSE × 400 – Figure 2a (left)) and synaptophysin (synaptophysin × 400 – Figure 2b (right)).
Postoperative course
A follow-up MRI at 6 months revealed no residual tumor. In June 2013, the patients developed acute-onset, severe low-back pain radiating down her right thigh and new-onset numbness of her right buttock. An MRI revealed multiple, extensive leptomeningeal foci along the conus medullaris and nerve roots, spreading upward to level of T1 (See Figure 3), with no evidence of metastatic disease in the cervical spine or brain. Three separate lumbar punctures were performed with cytologies negative for malignancy. Given the location and size of the recurrent disease, biopsy was not feasible to confirm diagnosis.
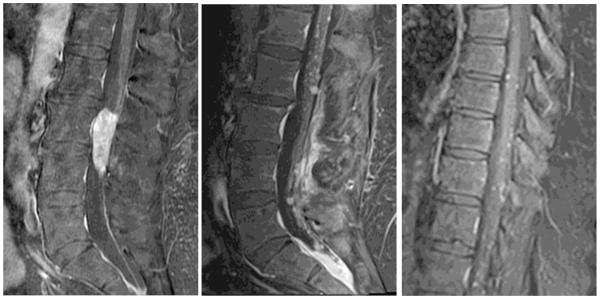
Figure 3.
Sagittal T1 post-contrast images depict an enhancing mass at presentation (left) and enhancing recurrence at 17 months in the lumbar spine (middle), including dependently in the inferior thecal sac, in addition to post-operative changes from prior mass resection. (Right) Sagittal T1 post-contrast image depicting thoracic metastases at 17 months.
18F-FDOPA is known to accumulate within neuroendocrine tumors. By synthetically attaching a radiotracer fluorine-18 isotope to the F-DOPA molecule and allowing cellular uptake via the neutral amino acid transporter (LAT1/4F2hc), a PET/CT can then be utilized for precise localization of tumors.6 68Ga-DOTA-analogs PET/CT may also be used for the detection of recurrent or metastatic paragangliomas.7,16 Our patient was referred to the National Institute of Health, where an 18F-FDOPA PET/CT was positive for recurrent paraganglioma in the same distribution as the MRI findings (Figure 4). Also tested at NIH were plasma chromogranin A, catecholamine, and metanephrine levels that were found to be within normal limits.
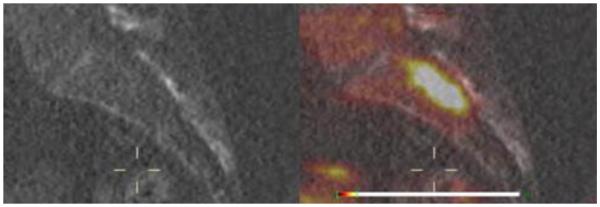
Figure 4.
Sagittal CT Scan (left) compared with 18F-FDOPA PET/CT uptake scan (right) demonstrating recurrence of paraganglioma at 6 months.
Subsequent MRI studies revealed continued, insidious progression and dissemination of leptomeningeal disease. The patient was observed until March, 2014 when she developed new-onset numbness of her lower extremities and accompanying worsening MRI findings. An Ommaya reservoir was placed for intrathecal delivery of ThioTEPA, which was given twice weekly for 6 weeks, followed by weekly treatments for 8 weeks. In August, 2014, she developed progressive neurologic symptoms in the S1 distribution, similar to those previously experienced. Progression of her disease in the sacral spine was confirmed by MRI, with multiple foci involving nerve roots of the cauda equina. The patient then received radiation therapy to her T12 through sacral spinal canal (45 Gy) with L2-sacral canal boost (50Gy) from September to October 2014, which improved her neurologic symptoms.
Because her thoracic spine metastases had been stable on the intrathecal ThioTEPA, the sacral progression was thought to be due to poor penetration of the drug into the bulky sacral recurrence. Therefore, after the lumbosacral radiation, intrathecal ThioTEPA was resumed and serial MRIs revealed stable disease. Intrathecal ThioTEPA was continued monthly until August 2015, when an MRI revealed continued dissemination superiorly throughout her cervical leptomeninges and into the cerebellum, where a 3 mm enhancing lesion was seen on the left side. Treatment was then changed to capecitabine and temozolomide (CAPTEM) and repeat MRIs through May, 2016revealed stable disease.
Discussion
Paragangliomas are mostly benign but do have significant recurrence rates that differ with site of disease, and can recur decades after surgical resection.8 Some studies indicate metastasis rates of 28% to 42% for retroperitoneal paragangliomas.9,10 Carotid body tumors have been found to have the lowest metastatic rates (2%-9%).11,12 Local recurrence after surgical excision is more common in patients with genetic predilection compared with sporadic pathology, and if tumor is of extra-adrenal origin compared with adrenal tumors.2 Due to the small number of instances where dissemination of recurrent, macroscopically resected tumor has occurred, treatment must be determined on an individual basis.
Several targeted therapies are being developed for the treatment of metastatic paragangliomas. Peptide receptor radionuclide therapy (PRRT) has been shown to be effective in treating other neuroendocrine tumors and possibly effective in the treatment of metastatic paragangliomas.17 However, somatostatin analogs employed in PRRT do not cross the blood-brain barrier and are thus ineffective for the treatment of CNS disease. Paraganglioma cell-surface ATP-synthase has been demonstrated as another potential therapeutic target.18 Resveratrol, as well as other ATP synthase targets, has been shown to potentially cross the blood-brain barrier.25 Further development and study of these and other novel systemic and localized treatment modalities is necessary for the advancement of treating metastatic PGLs.
For paragangliomas located below the neck, radiation therapy is typically employed only for painful bony lesions or tumors with rapid growth.13 Although paragangliomas were once thought to be resistant to radiation,26 our case and others’ demonstrate that radiation can effectively palliate symptoms and stop growth of paraganglioma.23, 27
A review of the literature was performed to identify all reported cases of leptomeningeal dissemination of paraganglioma. A comparison of our case with that of two similar cases from Switzerland3 and Sweden14 reveals many similarities (See Table 1). In all three circumstances, the paragangliomas were initially small and encapsulated and were located in the lumbosacral area, making total surgical resection plausible. Given the anatomic locations of these lesions, local recurrence and dissemination likely occurred via the spinal subarachnoid space rather than the cerebrospinal fluid. In all three instances, metastatic disease was found within the cerebellum. These cases highlight the imperative role of complete surgical resection, where possible, in the treatment of all paragangliomas. In two cases, radiation was successful at controlling progressive metastases. Our case also highlights that alkylating agents that penetrate the CNS can produce disease stability in leptomeningeal paraganglioma.
Table 1.
A comparison of three cases of metastatic paragangliomas.
| Case | Location of Primary |
Time to Dissemination |
En bloc Resection (Y/N?) |
Sites of Dissemination |
Treatment of recurrence |
Survival |
|---|---|---|---|---|---|---|
| Thomson, et al. |
Intrathecal at L3 |
6 months | Yes | Conus medullaris, cauda equina nerve root, thoracic spine, cerebellum |
XRT, Intrathecal ThioTEPA, and CAPTEM |
Alive as of May 2016 (5.5 years after Dx) |
| Strommer, et al. |
Intradural at L3 |
22 years | Yes | Multiple posterior fossa metastases: cerebellar declive, culmen and right flocculus, left inferior colliculus, as well as recurrence at original site, and in the cauda equina at L4-S1 |
Median suboccipital craniotomy at which time cystic midline cerebellar lesion and tumor were removed but left behind the other lesions. Later (1993), hemilaminectomy of L4-5 and S-1 with gross total resection performed. |
Alive as of 1994 when manuscript was submitted. |
| Roche, et al. |
Intraspinal from L4 to S1 |
3 years | No | Conus medullaris, right cerebello- pontine angle (CPA), cerebellar culmen, thoracic spinal cord, conus. |
Gross total removal of CPA tumor, however, small sub-arachnoidal lesions were identified but not resected. XRT to the cranio-spinal drop neoplasms. |
4.5 years after initial surgery. (6 mos. after CPA recurrence, suffered from fluid and electrolyte imbalance following surgical intervention of small bowel obstruction and died.) |
In all three cases, histological or genetic characteristics were initially indistinguishable from other benign paragangliomas, giving no hint of their later leptomeningeal spread.15 Moreover, en bloc resection did not prevent leptomeningeal dissemination in two of these cases.
Identification of new biomarkers for the aggressiveness of these tumors is warranted. A high Ki-67 index has been proposed as a potential prognostic factor. A recent study evaluated 18F-FLT PET/CT, a PET proliferation tracer, as a potential radioimaging agent in a series of paraganglioma patients with varying genetic backgrounds in order to compare 18F-FLT uptake with 18F-FDG PET/CT and evaluate classic factors of aggressiveness. There was no superiority of 18F-FLT uptake in progressive lesions suggesting the possibility that proliferation may not be a major indicator of aggressiveness.19 Genetic biomarkers are also being developed as prognostic indicators. A recent study demonstrated that 53% of patients with at least one extra-adrenal paraganglioma had an identified germline mutation.20 The most commonly mutated susceptibility gene associated with pheochromocytomas and paragangliomas is succinate dehydrogenase subunit B (SDHB), which also carries the highest risk of malignancy.20,22 In patients with SDHB mutations, primary tumor size has been found to be an age-independent predictor of patient survival and metastasis.22 Age at diagnosis has also been found in these patients to be a size-independent predictor of patient survival.22 Thus, in order to achieve the best possible clinical outcome, patients discovered to have any SDHB mutation are recommended to undergo genetic counseling24 and early and regular evaluations for the development of PCC/PGL.
To date there are no large studies describing specific outcomes of metastatic paragangliomas initially located below the neck. This is, in part, due to the indolent, insidious nature of the disease, even in the setting of dissemination. In addition, the optimal therapeutic strategy for disseminated low-grade paragangliomas has yet to be defined. From our experience, we believe alkylating chemotherapeutic agents and radiation therapy are both active modalities. It is possible that gene-targeted radiotherapeutics and other novel targeting moieties will be employed in the future.21 Further studies are warranted to assess treatment outcomes in patients with this uncharacteristic modality of disease metastasis, with comparison of current treatment protocols and newly-developed models as they become available. We also wish to emphasize the important utility of 18F-FDOPA scans in the diagnosis of unresectable, small-sized disease where biopsy is not feasible. Documentation of atypical cases such as this one is central to clinician awareness in addition to the promotion of developing novel biomarkers for malignant potential and original treatment protocols.
Footnotes
Consent
Written, informed consent was obtained from this patient for the publication of this case report with its accompanying images and data.
Competing Interests / Disclosures
The authors report no conflict of interest concerning the materials or findings presented in this paper.
References
- 1.Lee JA, Duh QY. Sporadic paraganglioma. World J Surg. 2008;32(5):683–7. doi: 10.1007/s00268-007-9360-4. [DOI] [PubMed] [Google Scholar]
- 2.Amar L, Servais A, et al. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab. 2005;90(4):2110. doi: 10.1210/jc.2004-1398. [DOI] [PubMed] [Google Scholar]
- 3.Strommer KN, Brandner S, et al. Symptomatic cerebellar metastasis and late local recurrence of a cauda equina paraganglioma. J Neurosurg. 1995;83:166–69. doi: 10.3171/jns.1995.83.1.0166. [DOI] [PubMed] [Google Scholar]
- 4.Cascón A, Ruiz-Llorente S, et al. G12 and H50R Variations in SDHD. Genes, Chromosomes & Cancer. 2003;37:220–1. doi: 10.1002/gcc.10212. [DOI] [PubMed] [Google Scholar]
- 5.Cascón A, Ruiz-Llorente S, et al. Identification of novel SDHD mutations in pateitns with phaeochromocytoma and/or paraganglioma. Eur J Hum Genet. 2002;10:457–461. doi: 10.1038/sj.ejhg.5200829. [DOI] [PubMed] [Google Scholar]
- 6.Santhanam P, Taieb D. Role of 18F-FDOPA PET/CT imaging in endocrinology. Clinical Endocrinology. 2014;81:789–98. doi: 10.1111/cen.12566. [DOI] [PubMed] [Google Scholar]
- 7.Janssen I, Chen CC, Millo CM, Ling A, Taieb D, Lin FI, et al. PET/CT comparing 68Ga-DOTATATE and other radiopharmaceuticals and in comparison with CT/MRI for the localization of sporadic metastatic pheochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2016 Mar 21; doi: 10.1007/s00259-016-3357-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Slycke S, Caiazzo R, et al. Local-regional recurrence of sporadic or syndromic abdominal extra-adrenal paraganglioma: incidence, characteristics, and outcome. Surgery. 2009 Dec;146(6):986–92. doi: 10.1016/j.surg.2009.10.055. [DOI] [PubMed] [Google Scholar]
- 9.Lack EE, Cubilla AL, Woodruff JM, et al. Extra-adrenal paragangliomas of the retroperitoneum. A clinicopathologic study of 12 tumors. Am J Surg Pathol. 1980;4:109–120. doi: 10.1097/00000478-198004000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Olson JR, Abell MR. Nonfunctional, non-chromaffin paragangliomas of the retroperitoneum. Cancer. 1969;23:1358–67. doi: 10.1002/1097-0142(196906)23:6<1358::aid-cncr2820230618>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Nora JD, Hallett JW, Jr, O’Brien PC, et al. Surgical resection of carotid body tumors : long term survival, recurrence and metastasis. Mayo Clin Proc. 1988;63:348–52. doi: 10.1016/s0025-6196(12)64856-3. [DOI] [PubMed] [Google Scholar]
- 12.Shamblin WR, ReMine WH, Sheps SG, et al. Carotid body tumor (chemodectoma). Clinicopathologic analysis of ninety cases. Am J Surg. 1971;12:732–39. doi: 10.1016/0002-9610(71)90436-3. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Sippel RS, O’Dorisio MS, et al. The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors : pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas. 2010;39(6):775–83. doi: 10.1097/MPA.0b013e3181ebb4f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roche PH, Figarella-Branger D, Regis J, Peragut JC. Cauda Equina Paraganglioma with Subsequent Intracranial and Intraspinal Metastases. Acta Neurochir (Wien) 1996;138:475–479. doi: 10.1007/BF01420312. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhofer G, Tischler AS, de Krijger RR. Diagnostic tests and biomarkers for pheochromocytoma and extra-adrenal paraganglioma: from routine laboratory methods to disease stratification. Endocr Pathol. 2012 Mar;23(1):4–14. doi: 10.1007/s12022-011-9188-1. [DOI] [PubMed] [Google Scholar]
- 16.Jain TK, Basher RK, et al. Ga-68 DOTA-NOC PET/CT for the Detection of Residual/Recurrence in a Rare Case of Sacral Spinal Canal Paraganglioma. World J Nucl Med. 2016 Jan-Apr;15(1):71–72. doi: 10.4103/1450-1147.167575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinato DJ, Black JRM, Ramaswami R, Tan TM, Adjogatse D, Sharma R. Peptide receptor radionuclide therapy for metastatic paragangliomas. Medical Oncology. 2016 May;33(5):47. doi: 10.1007/s12032-016-0737-9. [DOI] [PubMed] [Google Scholar]
- 18.Fliedner SMJ, Yang C, Thompson E, Abu-Asab M, Hsu CM, Lampert G, et al. Potential therapeutic target for malignant paragangliomas: ATP synthase on the surface of paraganglioma cells. Am J Cancer Res. 2015;5(4):1558–70. [PMC free article] [PubMed] [Google Scholar]
- 19.Blanchet EM, Taieb D, Millo C, Martucci V, Chen CC, Merino M, et al. 18F-FLT PET/CT in the Evaluation of Pheochromocytomas and Paragangliomas: A Pilot Study. J Nucl Med. 2015 Dec;56(12):1849–54. doi: 10.2967/jnumed.115.159061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fishbein L, Merrill S, Fraker DL, Cohen DL, Nathanson KL. Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann Surg Oncol. 2013 May;20(5):1444–50. doi: 10.1245/s10434-013-2942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castinetti F, Kroiss A, Kumar R, Pacak K, Taieb D. 15 YEARS OF PARAGANGLIOMA: Imaging and imaging-based treatment of pheochromocytoma and paraganglioma. Endocr Relat Cancer. 2015 Aug;22(4):T135–45. doi: 10.1530/ERC-15-0175. [DOI] [PubMed] [Google Scholar]
- 22.Schovanek J, Martucci V, Wesley R, Fojo T, Del Rivero J, Huynh T, et al. The size of the primary tumor and age at initial diagnosis are independent predictors of the metastatic behavior and survival of patients with SDHB-related pheochromocytoma and paraganglioma: a retrospective cohort study. BMC Cancer. 2014 Jul 21;14:523. doi: 10.1186/1471-2407-14-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogel J, Atanacio AS, Prodanov T, Turkbey BI, Adams K, Martucci V, et al. External beam radiation therapy in treatment of malignant pheochromocytoma and paraganglioma. Front Oncol. 2014 Jun 27;4:166. doi: 10.3389/fonc.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raygada M, King KS, Adams KT, Stratakis CA, Pacak K. Counseling patients with succinate dehydrogenase subunit defects: genetics, preventive guidelines, and dealing with uncertainty. J Pediatr Endocrinol Metab. 2014 Sep;27(9-10):837–44. doi: 10.1515/jpem-2013-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, et al. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002 Dec 27;958(2):439–47. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- 26.Drasin H. Treatment of Malignant Pheochromocytoma. West J Med. 1978 Feb;128(2):106–11. [PMC free article] [PubMed] [Google Scholar]
- 27.Fishbein L, Bonner L, Torigian DA, Nathanson KL, Cohen DL, Pryma D, et al. External beam radiation therapy (EBRT) for patients with malignant pheochromochytoma and non-head and neck paraganglioma: combination with 131I-MIBG. Horm Metab Res. 2012 May;44(5):405–10. doi: 10.1055/s-0032-1308992. [DOI] [PMC free article] [PubMed] [Google Scholar]