Abstract
Background
B cells play many roles in health and disease. However, little is known about the mechanisms that drive B cell responses in the airways, especially in humans. Chronic rhinosinusitis (CRS) is an inflammatory disease of the upper airways that affects 10% of Europeans and Americans. A subset of CRS patients develop nasal polyps (NP), which are characterized by type 2 inflammation, eosinophils and group 2 innate lymphoid cells (ILC2). We have reported that NP contain elevated levels of B cells and antibodies, making NP an ideal system for studying B cells in the airways.
Objective
We sought to determine the mechanisms that drive B cell activation and antibody production during chronic airway inflammation.
Methods
We analyzed B cells from NP or tonsil, or after ILC2 co-culture, by flow cytometry. Antibody production from tissue was measured using Luminex assays, and the frequency of antibody-secreting cells by ELISpot. Formation of B cell clusters was assessed using immunohistochemistry. Expression of genes associated with B cell activation and class switch recombination was measured by qRT-PCR.
Results
NP contained significantly elevated frequencies of plasmablasts, especially those that expressed the extra follicular marker Epstein-Barr virus-induced protein 2 (EBI2), but significantly fewer germinal center (GC) B cells compared to tonsil. Antibody production and the frequency of antibody-secreting cells were significantly elevated in NP, and there was evidence for local class switch recombination in NP. Finally, ILC2s directly induced EBI2 expression on B cells in vitro.
Conclusions and Clinical Relevance
Our data suggest there is a unique B cell activation environment within NP that is distinct from classic GC-mediated mechanisms. We show for the first time that ILC2s directly induce EBI2 expression on B cells, indicating that ILC2s may play an important role in B cell responses. B cell-targeted therapies may provide new treatment options for CRSwNP.
Introduction
B cells play a critical role in adaptive immune responses. Not only can these cells become activated to produce high affinity antibodies that are critical for immunity to infectious organisms, but B cells themselves are known to play important roles in immune regulation, including regulatory cell function, and co-stimulation and activation of antigen-specific T cell responses [1, 2]. It is also clear that disturbances in B cell responses can play a role in disease, especially in autoimmune disorders that are driven by the production of pathogenic autoantibodies. The classical view is that B cells are activated during a germinal center reaction in secondary lymphoid organs, resulting in the formation of long-lived memory B cells and plasma cells that produce high affinity antibodies. More recent studies have indicated that similar germinal center reactions can be observed at peripheral sites during acute inflammatory responses, such as in the lung during infection with influenza [3, 4]. Studies also suggest that peripheral activation of B cells can be important in the pathogenesis of autoimmune disease [5]. Unfortunately, there is little known about the mechanisms that drive B cell responses at peripheral sites during chronic inflammatory responses, especially in human systems. Accordingly, the goal of this study is to understand the mechanisms of peripheral activation of B cells, regardless of whether their generated antibodies are autoreactive or protective. To do this, we employ tissue samples from patients with chronic rhinosinusitis (CRS), which we, and others, have shown previously to have robust B cell responses ([6, 7] and see below).
Chronic rhinosinusitis is a chronic inflammatory disease of the upper airways that affects 10% of the population in Europe and the United States [8]. This disease results in significant patient morbidity and is associated with over $8 billion spent annually on medical and surgical management in the United States [9]. CRS is typically divided into two distinct patient subsets, those that develop nasal polyps (CRSwNP) and those that do not (CRSsNP). Because patients with CRS often have surgery to remove their inflamed sinus tissues as a routine part of treatment, the fresh tissue removed provides a unique opportunity to study the mechanisms involved in an ongoing inflammatory response at a mucosal site in humans. Nasal polyps (NP) generally have elevated type 2 responses, including significant increases in eosinophils, mast cells, basophils and type 2 innate lymphoid cells (ILC2s) [9, 10]. Previous work from our group has demonstrated that B cells, plasma cells, and antibodies are also highly elevated in NP compared to control uncinate tissue (UT) from non-CRS subjects [6]. We have also reported elevated levels of autoantibodies in NP, but not systemically, in CRSwNP patients [11, 12]. However, the activation state of the B cells, and the identity of the antibody-secreting cells, in NP have not been elucidated. In addition, we found that expression of Epstein-Barr virus-induced protein 2 (EBI2), a marker of extrafollicular plasmablasts, which are B cells that are activated outside of the germinal center, was highly elevated in NP tissue extracts, but this work did not examine expression of EBI2 on B cells in NP specifically [6]. Overexpression of EBI2 in murine models is associated with increased antibody production and formation of plasmablasts in secondary lymphoid organs, while knockout of this molecule diminishes early antibody responses, suggesting that it plays an important role in B cell responses [13]. Presently, little is known about the function or regulation of EBI2 on B cells in humans or during mucosal immune responses, or about the expression patterns of EBI2 on human B cell subsets.
In order to determine the mechanisms that may drive the activation of B cells at a peripheral site during chronic inflammation, such as in NP, we compared B cell responses in NP to those within tonsils. While our previous work did find elevated levels of EBI2 in NP, it did not examine the cell-specific expression of EBI2 in NP. Due to the importance of EBI2 in B cell responses, we also sought to determine how EBI2 expression is regulated on NP B cells. Tonsil serves as a valuable comparator tissue because it represents a classical secondary lymphoid organ in which B cells are activated and expanded in a germinal center (GC) reaction. Herein, we report that the B cell response in NP is characterized by a low frequency of GC B cells, and an increase in EBI2+ plasmablasts, presumably generated independent of germinal centers and the negative selection that they provide. In addition, and for the first time, we demonstrate that group 2 innate lymphoid cells (ILC2s), which are known to be elevated in NP, directly induced EBI2 expression on B cells, suggesting that they may play a role in the induction of these extrafollicular B cell responses occurring in NP during chronic inflammation in the airways.
Methods
Patient Tissue Samples
This study was approved by the Northwestern University Feinberg School of Medicine Institutional Review Board, and all patients provided informed consent. Polyp tissue was collected during routine endoscopic sinus surgery from patients with CRSwNP. Control uncinate tissue (UT) was collected from non-CRS patients undergoing skull-base surgical procedures for tumor removal or septoplasy. Adult tonsils were removed during routine tonsillectomy. Patients with aspirin-exacerbated respiratory disease, established immunodeficiency, pregnancy, coagulation disorder, classic allergic fungal sinusitis, or cystic fibrosis were excluded.
Cell Isolation
For B cell phenotyping, 100–250mg of tissue was cut using a scalpel and placed in a Gentle MACS tube (Miltenyi Biotech) and incubated in 5ml of RPMI+1mg/ml Collagenase Type I and 30ug/ml DNase I (Worthington Biochemicals) at 4°C overnight on a shaker. Tissues were dissociated using a Gentle MACS machine. Cells were isolated through a 70um nylon mesh (BD Biosciences), and red blood cells were lysed using 1× red blood cell lysis buffer (eBiosciences). For ILC2 and B cell co-cultures, PBMCs were first isolated from peripheral blood leukopaks (Stem Cell Technologies) using Ficoll density centrifugation, and remaining red blood cells were lysed as above. 10% of the PMBCs were reserved for B cell sorting, and the remaining cells were labeled for magnetic purification of lineage negative cells. Cells were stained with a cocktail of FITC-conjugated lineage markers, including CD3, CD19, CD20, CD56, CD16, CD14 and CD11c according to the manufacturer’s instructions. After washing, cells were incubated with anti-FITC beads and lineage negative cells were collected after magnetic separation according to the manufacturer’s instructions (Stem Cell Technologies).
Flow Cytometry
All antibody staining was performed for 15 minutes at room temperature, compensation beads (BD Biosciences) were used for single stained controls, and appropriate fluorescence minus one (FMO) controls were used to determine gates. All antibodies, clones, and final dilutions are listed in Table 1. The anti-human EBI2-Alexa647 antibody was a kind gift from Dr. Andreas Sailer. For co-culture experiments, ILC2s were defined as lineagenegCD127+CRTH2+ cells, and B cells were defined as CD19+CD3neg cells. Cells were sorted using a BD FACS Aria in the Flow Cytometry Core Facility at Northwestern University.
Table 1.
Flow cytometry antibody information
| Target | Fluorochrome | Clone | Dilution | Company |
|---|---|---|---|---|
| CD3 | Alexa700 | UCHT1 | 1:40 | eBioscience |
| CD11c | FITC | Bu15 | 1:20 | BioLegend |
| CD19 | APC-Cy7 | SJ25C1 | 1:20 | BD Biosciences |
| CD27 | PE-Cy7 | M-T271 | 1:33 | BD Biosciences |
| CD38 | PerCP-Cy5.5 | HIT2 | 1:40 | BD Biosciences |
| CD127 | Alexa647 | A019D5 | 1:20 | BioLegend |
| CRTH2 | PE | BM16 | 1:20 | BioLegend |
| EBI2 | Alexa647 | 607b | 1:100 | Gift |
| IgD | FITC | IA6-2 | 1:10 | BD Biosciences |
| Lineage | FITC | 348801* | 1:5 | BioLegend |
| Live/Dead | Aqua | n/a | 1:1000 | Life Technologies |
Catalog number; item contains multiple clones
Ex Vivo Tissue Culture and Antibody Measurements
100–250mg pieces of tissue were cultured in 5ml of RPMI+penicillin/streptomycin+1mM sodium pyruvate+10%FCS for 4 days as previously described.6 Cell-free supernatants were collected and stored at −20°C for antibody measurements. Antibodies were measured from supernatants using the Human Isotyping and Human IgE kits from Millipore, according to the manufacturer’s instructions. Antibody levels in each sample were normalized to total protein, as measured by the BCA assay (Pierce), as previously described.6
CD20 Immnunohistochemistry
Immunohistochemistry was performed as previously described.13 Briefly, 3um sections were cut from paraffin embedded formalin fixed tissues. Sections were stained with anti-human CD20 (Thermo Fisher Scientific, clone L26; 1:250) overnight at 4°C. Slides were incubated with biotinylated horse anti-mouse secondary antibody (Vector Laboratories; 1:500) at room temperature for 1 hour, followed by incubation with avidin-peroxidase complex (Vectastain Elite ABC Kit) at room temperature for 1 hour. Slides were then incubated with DAB-plus reagent (ThermoFisher) at room temperature for 10 minutes. Finally, slides were counter stained with Gill modified hematoxylin (EMD Millipore) for 5 to 10 seconds. CD20 staining was assessed using a Zeiss Axiostar plus Transmitted-Light Microscope. Follicles were defined as a dense group of >300 CD20+ cells in a 200µm×200µm area; clusters were defined as a group of 100–299 CD20+ cells in a 200µm×200µm section. In order to thoroughly assess follicle formation, we assessed CD20 staining throughout the entire tissue sample by counting CD20+ cells in serial sections spanning the entire width of the tissue spaced 100µm apart from 13 UT and 17 polyp samples, In addition, to account for the large difference in tissue size between NP and UT samples, we normalized the total number of follicles or clusters from each sample to the total tissue area for that sample, and thus we were able to obtain a measurement of the frequency of follicles and clusters in NP and control UT tissue.
ELISpot Assays
Total IgG, IgA and IgE ELISpot assays were performed using kits from MabTech, according to the manufacturer’s instructions. All samples were assayed in triplicate on .45um hydrophobic high protein binding immobilon-P 96 well plates (Millipore). ELISpot plates were analyzed using a CTL-Immunospot S6 Analyzer.
qRT-PCR
RNA and cDNA were isolated from tissue samples as previously described.14 RNA quality was assessed using the Agilent Bioanalyzer 2100 system, and only samples with a RIN >7 were used for cDNA synthesis and qRT-PCR. The following primer pairs were used15, 16, 17: aicdaF: 5’-agaggcgtgacagtgctaca-3’, aicdaR: 5’-tgtagcggaggaagagcaat-3’, g1F: 5’-ccagggcagggtcagca-3’, g1R: 5’-ggtgctcttggaggaggg-3’, g4F: 5’-ccagggcagggtcagca-3’, g4R: 5’-ggcagcccagggcg-3’, a1F: 5’-ctcagcactgcgggccctcca-3’, a1R: 5’-gttcccatctggctgggtgctgca-3’, a2F: 5’-ctcagcactgcgggccctcca-3’, a2R: 5’-gttcccatcttggggggtgctgtc-3’, eF: 5’-ggccacacatccacaggc-3’, eR: 5’-ggggtgaagtccctggagc-3’, GAPDHF: 5’-gaaggtgaaggtcggagtc-3’, GAPDHR: 5’-gaafatggtgatgggatttc-3’. qRT-PCR was done using SYBR Green master mix (Applied Biosystems), 1µM primer pair mix, and 7.5µl of cDNA in 20µl final volume. All samples were run in duplicate on an Applied Biosystems Step One Plus PCR machine for 40 cycles, and a melt curve analysis. aicda, α1, α2, ε and GAPDH were run with a 60°C extension phase, while γ1 and γ4 were run with a 62°C extension phase. GLT expression was normalized to GAPDH and expressed as 2−dCt.
ILC2-B Cell Co-cultures
After isolation, cells were cultured at a 1:1 ratio in triplicate in 96-well round bottom plates in 200µl of RPMI+penicillin/streptomycin+1mM sodium pyruvate+10%FCS and 10U/ml IL-2 for 5 days. Triplicates were pooled, and cell free supernatants were collected and stored at −20°C. Samples were stained and analyzed to assess B cell phenotypes as above.
Statistical Analysis
Mann-Whitney U test was used for comparison between two groups, and the Kruskal-Wallis test with Dunn’s correction was used for comparison of >2 groups. All analysis was done using Graph Pad Prism v5.0b software and p<0.05 was considered statistically significant.
Results
Nasal Polyps Contain Elevated Frequencies of Activated B Cell Subsets
While our previous work had demonstrated elevated levels of total B cells and plasma cells, it did not provide information on the activation state of those cells. Thus, in order to understand how B cell responses in NP were generated, we used flow cytometry to assess the B cell subsets in NP and adult tonsil tissue to determine their frequency and activation state. Figure 1A illustrates our gating strategy for each B cell subset, within the CD19+ gate. As expected, we found that total CD19+ B cells were elevated in tonsils compared to NP (Figure 1B; p<0.0001). In addition, while we found no differences in the frequencies of memory B cells (CD19+IgDnegCD27+CD38neg), the frequency of naïve B cells (CD19+IgD+CD27neg) was significantly higher in tonsils (p<0.0001; Figure 1B). We next characterized the frequencies of highly activated B cell subsets, including GC B cells (CD19+IgDnegCD38+) [14] and plasmablasts (CD19+IgDnegCD27+CD38hi). Interestingly, while the frequency of GC B cells was significantly higher in tonsils (p<0.0001), the frequency of plasmablasts was significantly higher in NP (p<0.0001; Figure 1C).
Figure 1.
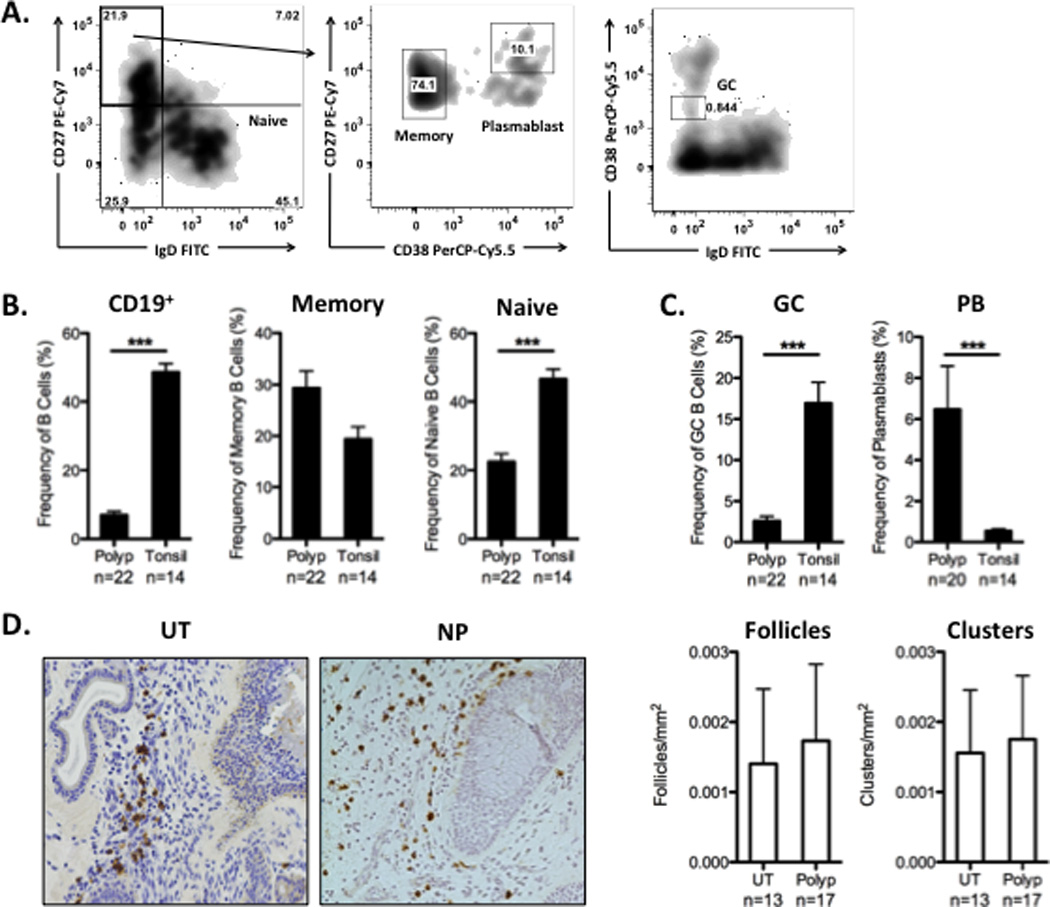
The frequency of B cell subsets in NP and tonsil. A. Identification of B cell subsets by flow cytometry. All cells were identified after gating on single alive cells. Total CD19+ frequency was calculated from the CD3neg gate. The frequency of naïve, memory, GC, and plasmablasts (PB) are expressed as a % of total CD19+ cells. B. Tonsils contained elevated levels of total B cells and naïve B cells. C. The frequency of activated B cell subsets was distinct between NP and tonsil. NP contained a significantly higher frequency of plasmablasts, while tonsil contained a higher frequency of GC B cells. D. Representative 20× images of CD20 staining in a control UT and NP sample. Immunohistochemical staining of CD20+ cells revealed no increase in the frequency of B cell follicles (group of >300 CD20+ cells in a 200µm×200µm area) or clusters (group of 100–299 CD20+ cells in a 200µm×200µm area) in NP compared to normal sinus tissue from non-CRS patients. Data represent mean ± SEM; *p<0.05, **p<0.01; ***p<0.001 by Mann-Whiney U test.
In order to further confirm our results regarding a low frequency of GC B cells in NP tissue, we used immunohistochemistry to assess the formation of tertiary lymphoid tissues and B cell clusters in NP and control UT. UT serves as an appropriate control for these studies because it represents normal sinus tissue, and it provides insights into the levels of such structures in healthy tissues. While we did find the formation of clusters and follicles in NP, we did not find evidence that the frequency of the formation of these structures was any higher than what was found in control UT (Figure 1D). Together, these data suggest that the mechanisms that drive B cell activation in NP are distinct from classic GC-mediated mechanisms.
B Cells From Nasal Polyps Secrete High Levels of Antibodies in vitro
To further assess the level of activation of B cells in NP, we measured antibody production by B cells in vitro. Previously, we found that NP contained highly elevated levels of antibodies of all isotypes, but it was not clear whether all of these antibodies were being produced locally [6]. In order to address this, we first cultured NP or tonsil tissue explants for 4 days, as previously described [6], and we measured total antibody levels in culture supernatants. We found that B cells in NP produced significantly higher levels of IgG1, IgG2, IgG4, IgM, IgA, and IgE compared to tonsil (Figure 2A; p<0.05). We next quantitated the frequency of antibody secreting cells in NP and tonsil using ELISpot assays. In line with the results above, we found that the frequency of IgG-, IgA-, and IgE-secreting cells in NP was significantly elevated compared to tonsils (p<0.05; Figure 2B). Finally, we assessed whether B cells were being activated locally to undergo class switch recombination by measuring the expression of the enzyme activation induced cytadine deaminase (AID) and germline transcripts for IgG, IgA, and IgE. As expected, expression of AID was significantly elevated in tonsil tissues, but it was also detectible in NP (Figure 2C). We also found detectible levels of expression of germline transcripts for IgE, confirming a previous report [7], as well as for IgG1, IgG4, IgA1, and IgA2 in NP tissues (Figure 2C). Together, these data support the hypothesis that B cells in NP are highly activated and secrete large amounts of antibodies.
Figure 2.
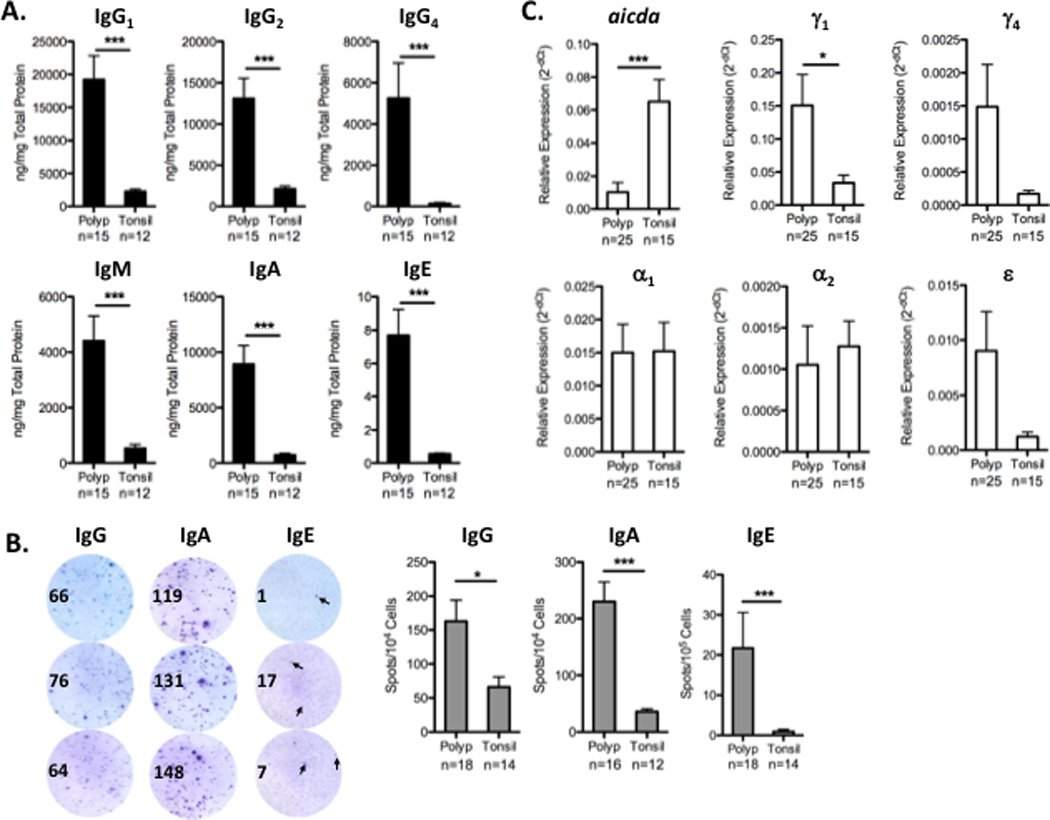
Antibody production in NP and tonsil. A. Tissue explants from NP or tonsil were cultured in vitro for 4 days, and total antibody production was measured from the culture supernatants. NP produced significantly higher levels of all antibody isotypes compared to tonsil. B. The frequency of antibody-secreting cells was determined by ELISpot assays. Representative triplicate ELISpot wells from NP are shown; arrows in the IgE wells indicate representative positive spots. NP contained significantly elevated frequencies of IgG-, IgA-, and IgE-secreting cells compared to tonsil. C. Expression of AID and germline transcripts was measured using qRT-PCR. AID expression was highest in tonsil, but detectible in NP. Expression of germline transcripts for IgE, IgG1, IgG4, IgA1 and IgA2 was detectible in NP. Data represent mean ± SEM; *p<0.05, **p<0.01; ***p<0.001 by Mann-Whiney U test.
EBI2+ Plasmablasts are Elevated in Nasal Polyps
Previously, we demonstrated that expression of EBI2 was elevated in NP tissue extracts compared to UT from control subjects. Because EBI2 expression is associated with the formation of plasmablasts and increased antibody production in mice, but little is known about its role in humans, we next examined EBI2 expression on B cell subsets from NP and tonsil. As expected, we found that the frequency of EBI2+ cells was highest in the plasmablast subset, while naïve and memory B cells had very low EBI2 expression (Figure 3A). Interestingly, when we assessed the proportion of plasmablasts that were EBI2+ in the two tissues, we found that the frequency of EBI2+ plasmablasts in NP was significantly higher than in tonsil (p<0.0001; Figure 3B). These results suggest that B cell activation in polyps may be more akin to an extrafollicular response, and not driven by local GC formation.
Figure 3.
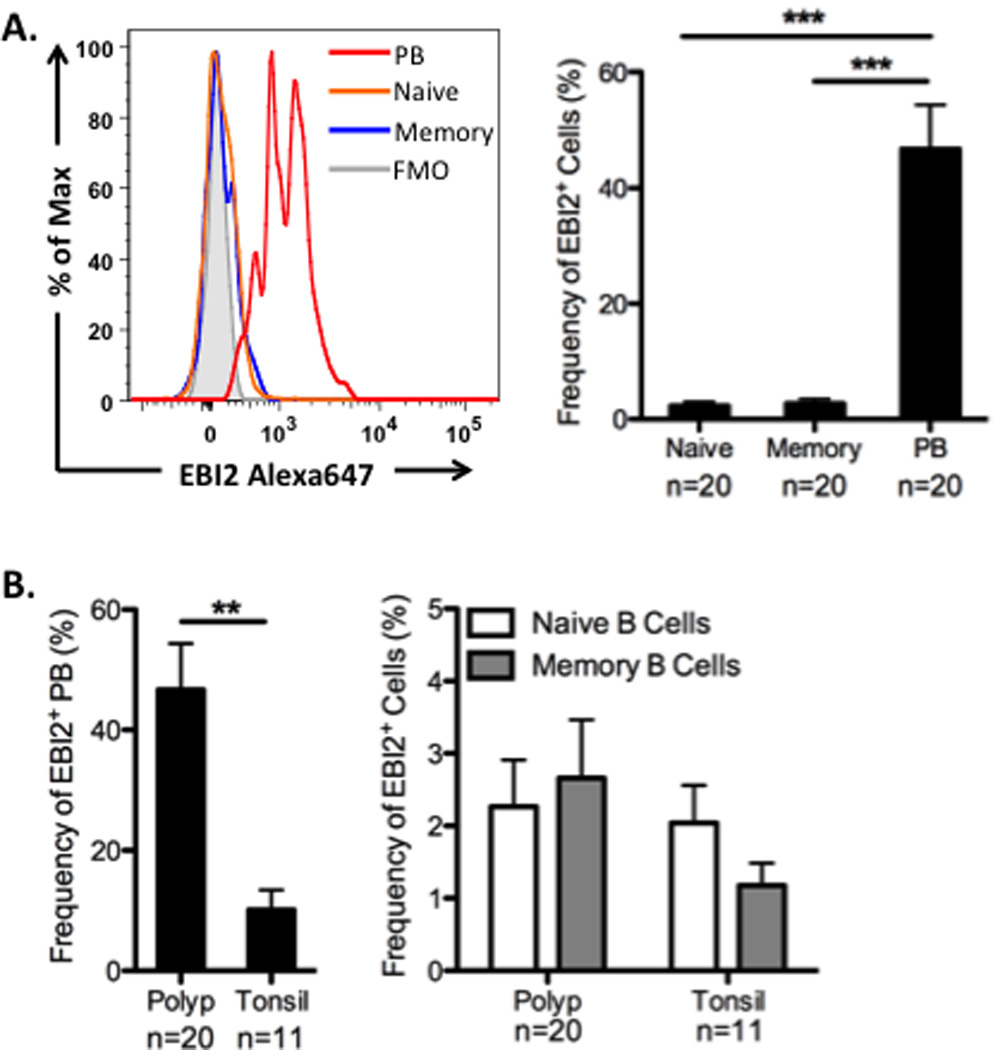
EBI2 expression on B cell subsets. A. Representative histograms from one NP sample showing EBI2 expression on plasmablast (PB), naïve and memory B cells. The frequency of EBI2 expressing cells was highest in the plasmablasts in NP. B. The frequency of EBI2+ plasmablasts, as a percentatge of total plasmablasts, was significantly higher in NP compared to tonsil, but the frequency of EBI2+ cells was not different in naïve or memory B cells. Data represent mean ± SEM; *p<0.05, **p<0.01; ***p<0.001 by Kruskal-Wallis test with Dunn’s correction (A) or Mann-Whiney U test (B).
ILC2s Directly Induce EBI2 Expression on B Cells in vitro
Altogether, our data suggest that there is a unique B cell activation environment within NP that results in highly increased levels of extrafollicular B cells and active secretion of antibodies. The exact mechanism that drives this response in NP is unclear. It is well established that EBI2 plays a critical role in the development of extrafollicular plasmablast responses at early time points after infection. However, the mechanisms by which EBI2 expression is induced on B cells have not been reported. Recently, ILC2s have been reported to be elevated in NP [10]. In order to determine whether ILC2s could play a role in B cell activation, we cultured peripheral blood B cells with or without autologous ILC2s for 5 days. We found that a significantly higher percentage of B cells expressed EBI2 when cultured in the presence of ILC2s (p<0.01; Figure 4). These data provide the first evidence that human ILC2s can directly influence B cells, and that they induce an extrafollicular B cell phenotype.
Figure 4.
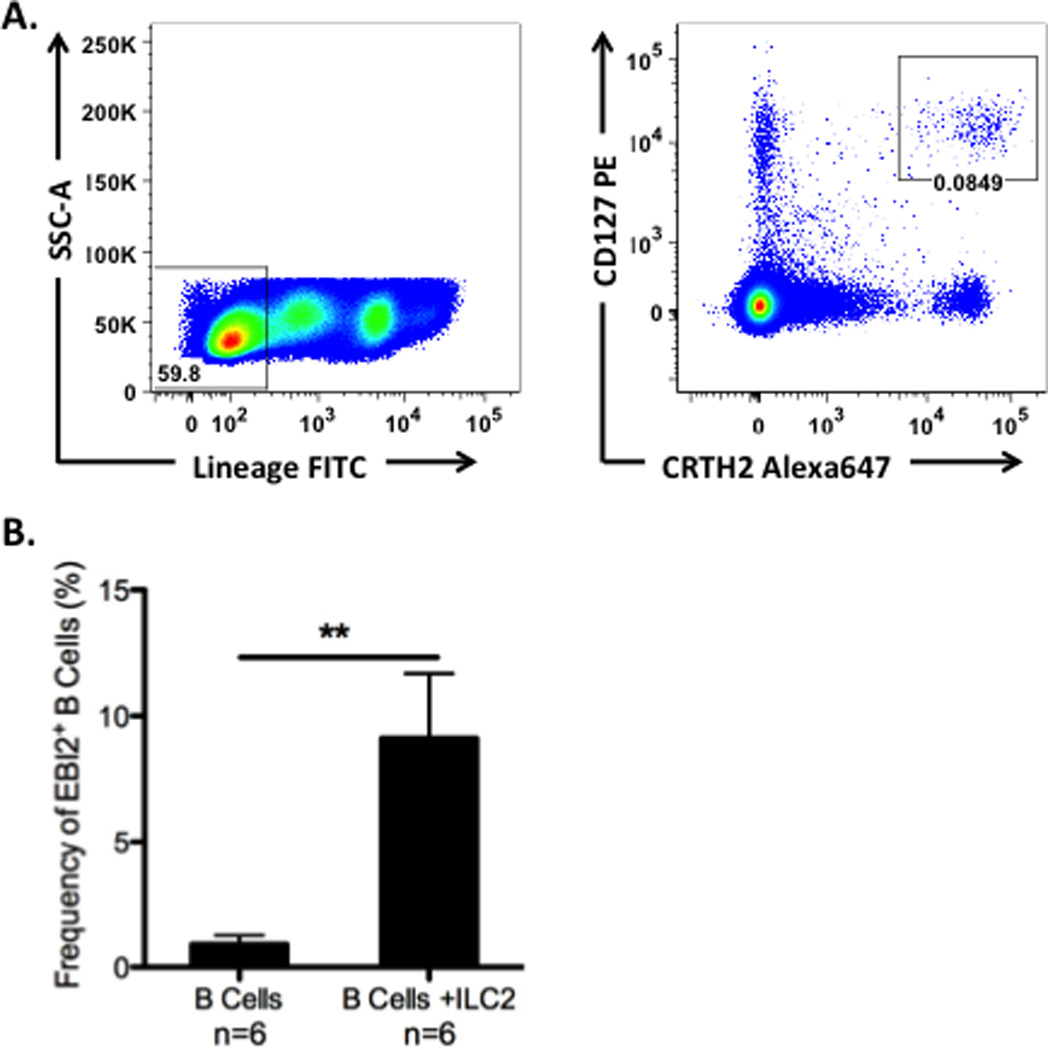
ILC2s induce EBI2 expression on B cells. A. Gating strategy for the identification of ILC2s in peripheral blood by flow cytometry. Data are representative of n=6 samples. B. The frequency of EBI2+ cells was significantly enhanced in B cells co-cultured with autologous ILC2s. Data represent mean ± SEM; *p<0.05, **p<0.01; ***p<0.001 by Mann-Whiney U test.
Discussion
B cell responses are often overlooked in the study of adaptive immunity, especially outside of their role as antibody producing cells. It is clear that antibody production plays a critical role in both health and disease, but B cells can also perform other important immune functions [1, 2]. B cells express MHC class II, along with co-stimulatory molecules, and thus play a key role in the activation of T cell responses [1]. Some subsets of B cells are also known to produce IL-10 and function as important regulators of inflammation [15]. Despite the clear importance of B cells in inflammatory responses, there is a lack of data regarding the mechanisms that drive B cell activation and antibody production at peripheral sites during inflammation, especially in the airways.
The data presented herein demonstrate that B cell activation during chronic inflammatory responses within NP is highly distinct from the classical B cell activation that occurs in GC reactions in secondary lymphoid organs, such as tonsils. This is a novel extension of our previous work [6], which provides important details on the activation state of B cells in NP and insights into the mechanisms that drive this activation and antibody production during disease. We demonstrate that B cell responses in NP are characterized by elevations of EBI2+ plasmablasts and a lack of GC B cells (Figures 1 and 3). In addition, NP contained highly elevated frequencies of antibody-secreting cells, and antibody production was elevated (Figure 2). This suggests that the activation and regulation of B cell responses within a chronic inflammatory environment is distinct from that which occurs in secondary lymphoid organs. Further, our data demonstrate that ILC2s were capable of directly inducing an extrafollicular B cell phenotype in vitro (Figure 4), and suggest that ILC2s may actively participate in the activation of B cells and antibody production during type 2 immune responses, has not been described in a human system.
There are two important novel features of our findings. First, we show that plasmablasts are activated to make substantial quantities of antibodies in NP tissue, presumably via extrafollicular activation. Extrafollicular B cell responses have been well characterized in secondary lymphoid organs [16], but little is known about these responses at peripheral sites. Because extrafollicular activation of B cells does not include the same negative selective pressures present in a GC reaction, it is possible that this mechanism may account for the increased production of autoantibodies in NP, which could be pathogenic [11, 17]. In addition, while our data suggest an important role for ILC2s in the induction of extrafollicular B cell responses in NP, they do not exclude a potential role for T cells in this response. T cells are also highly elevated in NP[18], and they likely play an important role in the development of extrafollicular B cell responses in secondary lymphoid organs. Thus, it will be important to investigate the potential role of T cells in this response in NP in future studies. Our study also confirms the local class switching to IgE in NP that was previously reported in NP [7, 19], and we extend these findings to show that switching to IgG and IgA also occurs in NP. This is important, since we find elevations of all antibody isotypes in NP, not just IgE, and these data indicate that local production of antibodies is not limited to IgE. However, our study does not find convincing evidence for the increased formation of tertiary lymphoid structures, or even any increase in the clustering of B cells, within NP, suggesting that B cell activation during chronic inflammation may be independent of these other, more well-described, mechanisms. This is in contrast to previous studies, which have reported the formation of tertiary lymphoid structures in NP, and suggested that B cell activation in NP occurs in these structures [7, 19]. Although we agree that these structures are found in NP, importantly, our study included an analysis of B cell clusters in control sinus tissues, an element lacking in the previous studies. While we did find evidence of B cell follicle and cluster formation in NP, in agreement with these previous studies, we did not find that these structures appear more frequently in NP compared to normal tissue. We interpret this to mean that the formation of lymphoid follicles, or large aggregates, in NP doesn’t account for the large increases in B cell levels, B cell activation, or antibody production found in NP. Coupled with our data regarding the elevated expression of EBI2 on plasmablasts in NP, these data strongly suggest that B cells in NP are activated independently of classic germinal center reactions.
The second important novel feature our findings is that they highlight a potentially exciting role for ILC2s in the induction of early B cell activation and antibody production. It is interesting to speculate that exposure to proteases or other PAMPS that can activate ILC2s might directly activate B cell responses. Since ILC2s and B cells are both highly elevated in NP, this interaction is likely to occur in CRSwNP. Currently, ILC2s are known to play an important role in the initiation of allergic inflammatory responses, both in the clearance of helminths, and in allergic airway inflammation [20], but it has not been previously demonstrated that they can directly influence B cell responses. Several other innate immune effector cells have been shown to directly influence B cell activation and antibody production in the bone marrow, lymph nodes and spleen, including eosinophils, macrophages and mast cells [21, 22]. Activation of B cells by such innate effector cells results in the formation an extrafollicular plasmablast response, and can also lead to the induction of AID expression and class switch recombination [22]. Thus, it is not unreasonable to speculate that ILC2s might play a similar role, especially in the context of type 2 immune responses within the airways. We are currently investigating the mechanisms that are responsible for the interactions between ILC2s and B cells. Interactions between ICOS and ICOSL on T cells and B cells, respectively, are known to play an important role in B cell activation and antibody production. ILC2s are also known to express ICOS, and a recent report demonstrated ICOS+ ILC2s in NP [23], suggesting that this molecule may be important for the interaction between ILC2s and B cells as well. The effect of ICOS, as well as other potential mediators, such as IL-13, on B cell activation, class switching, and antibody production are all areas of active investigation.
Together, this study demonstrates that B cell activation in the airways during inflammatory responses is quite distinct from the classical B cell activation observed in secondary lymphoid organs. Our data support the hypothesis that B cells are activated locally in the tissue, and that ILC2s may play a critical role in this process. Targeting these B cell responses may prove to be beneficial for the treatment of CRSwNP, as well as for other diseases involving local B cell responses.
Acknowledgments
Funding: NIH Grants: K12 HD055884, R37 HL068546, U19 AI106683, RO1 HL078860, R01 AI072570, R01 AI104733, and the Ernest S. Bazley Trust
Footnotes
Conflict of Interest
There are no conflicts of interest from any of the authors to disclose.
References
- 1.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4(+) T cell immunity. Nat Rev Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiLillo DJ, Horikawa M, Tedder TF. B-lymphocyte effector functions in health and disease. Immunol Res. 2011;49:281–292. doi: 10.1007/s12026-010-8189-3. [DOI] [PubMed] [Google Scholar]
- 3.Randall TD. Bronchus-associated lymphoid tissue (BALT) structure and function. Advances in immunology. 2010;107:187–241. doi: 10.1016/B978-0-12-381300-8.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richert LE, Harmsen AL, Rynda-Apple A, Wiley JA, Servid AE, Douglas T, Harmsen AG. Inducible bronchus-associated lymphoid tissue (iBALT) synergizes with local lymph nodes during antiviral CD4+ T cell responses. Lymphat Res Biol. 2013;11:196–202. doi: 10.1089/lrb.2013.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiorina P, Sayegh MH. B cell-targeted therapies in autoimmunity: rationale and progress. F1000 Biol Rep. 2009;1:39. doi: 10.3410/B1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hulse KE, Norton JE, Suh L, Zhong Q, Mahdavinia M, Simon P, Kern RC, Conley DB, Chandra RK, Tan BK, Peters AT, Grammer LC, 3rd, Harris KE, Carter RG, Kato A, Schleimer RP. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gevaert P, Nouri-Aria KT, Wu H, Harper CE, Takhar P, Fear DJ, Acke F, De Ruyck N, Banfield G, Kariyawasam HH, Bachert C, Durham SR, Gould HJ. Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy. 2013;68:55–63. doi: 10.1111/all.12054. [DOI] [PubMed] [Google Scholar]
- 8.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, Georgalas C, Goossens H, Harvey R, Hellings P, Hopkins C, Jones N, Joos G, Kalogjera L, Kern B, Kowalski M, Price D, Riechelmann H, Schlosser R, Senior B, Thomas M, Toskala E, Voegels R, Wang de Y, Wormald PJ. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology Supplement. 2012:3. preceding table of contents, 1–298. [PubMed] [Google Scholar]
- 9.Hulse KE, Stevens WW, Tan BK, Schleimer RP. Pathogenesis of nasal polyposis. Clin Exp Allergy. 2015;45:328–346. doi: 10.1111/cea.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 11.Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, Zhou J, Norton J, Carter R, Hinchcliff M, Harris K, Peters A, Grammer LC, Kern RC, Mohan C, Schleimer RP. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:1198 e1–1206 e1. doi: 10.1016/j.jaci.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeffe JS, Seshadri S, Hamill KJ, Huang JH, Carter R, Suh L, Hulse KE, Norton J, Conley D, Chandra R, Kern R, Jones J, Schleimer RP, Tan BK. A Role for Anti-BP180 Autoantibodies in Chronic Rhinosinusitis. Laryngoscope. 2013;123:2104–2111. doi: 10.1002/lary.24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatto D, Paus D, Basten A, Mackay CR, Brink R. Guidance of B cells by the orphan G protein-coupled receptor EBI2 shapes humoral immune responses. Immunity. 2009;31:259–269. doi: 10.1016/j.immuni.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 14.Jackson SM, Harp N, Patel D, Wulf J, Spaeth ED, Dike UK, James JA, Capra JD. Key developmental transitions in human germinal center B cells are revealed by differential CD45RB expression. Blood. 2009;113:3999–4007. doi: 10.1182/blood-2008-03-145979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 16.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 17.DeFranco AL. Germinal centers and autoimmune disease in humans and mice. Immunol Cell Biol. 2016 doi: 10.1038/icb.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derycke L, Eyerich S, Van Crombruggen K, Perez-Novo C, Holtappels G, Deruyck N, Gevaert P, Bachert C. Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PLoS One. 2014;9:e97581. doi: 10.1371/journal.pone.0097581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang YN, Song J, Wang H, Wang H, Zeng M, Zhai GT, Ma J, Li ZY, Liao B, Wang BF, Zhen Z, Wang N, Cao PP, Lin P, Ning Q, Liu Z. Nasal IL-4(+)CXCR5(+)CD4(+) T follicular helper cell counts correlate with local IgE production in eosinophilic nasal polyps. J Allergy Clin Immunol. 2016;137:462–473. doi: 10.1016/j.jaci.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Kim BS, Artis D. Group 2 Innate Lymphoid Cells in Health and Disease. Cold Spring Harbor perspectives in biology. 2015 doi: 10.1101/cshperspect.a016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerutti A, Puga I, Cols M. New helping friends for B cells. Eur J Immunol. 2012;42:1956–1968. doi: 10.1002/eji.201242594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinuesa CG, Chang PP. Innate B cell helpers reveal novel types of antibody responses. Nat Immunol. 2013;14:119–126. doi: 10.1038/ni.2511. [DOI] [PubMed] [Google Scholar]
- 23.Bal SM, Bernink JH, Nagasawa M, Groot J, Shikhagaie MM, Golebski K, van Drunen CM, Lutter R, Jonkers RE, Hombrink P, Bruchard M, Villaudy J, Munneke JM, Fokkens W, Erjefalt JS, Spits H, Ros XR. IL-1beta, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol. 2016;17:636–645. doi: 10.1038/ni.3444. [DOI] [PubMed] [Google Scholar]


