Abstract
Objective
In Alzheimer’s disease (AD), antipsychotic medications are often discontinued but which neuropsychiatric symptoms predict relapse is not established.
Methods
In the ADAD trial, 180 patients with AD and symptoms of agitation or psychosis received treatment with risperidone for 16 weeks, after which patients who responded (n=110) were randomized to: 1) continue risperidone for 32 weeks, 2) continue risperidone for 16 weeks followed by switch to placebo for 16 weeks or 3) placebo for 32 weeks. Discontinuation of risperidone was associated with a 2–4 fold increased risk of relapse over 16–32 weeks. In planned post hoc analyses, we examined associations between the 12 symptom domains in the Neuropsychiatric Inventory (NPI) and relapse in the first 16-week phase after randomization.
Results
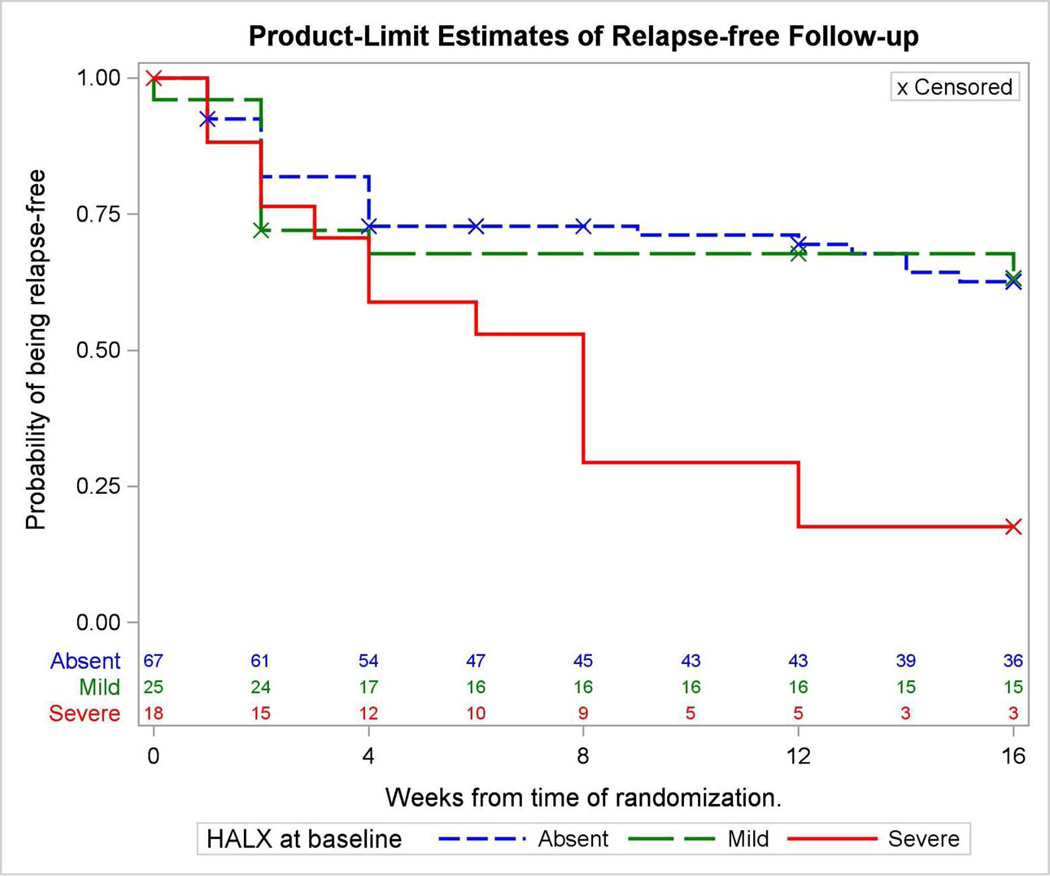
Compared to patients with mild hallucinations or no hallucinations, patients with severe hallucinations as a presenting symptom at baseline showed an increased likelihood of relapse (HR 2.96, 95% CI 1.52 to 5.76, p=0.001). This effect was present for the subsample with auditory, but not visual, hallucinations. Among patients with baseline hallucinations, 13 of 17 (76.5%) who discontinued risperidone relapsed, compared to 10 of 26 (38.5%) who continued risperidone (p<0.02). This group difference remained for severe (77.8%) compared to mild (36%) hallucinations (p=0.02). NPI domain scores after the initial open treatment phase (pre-randomization) were not associated with relapse.
Conclusion
Patients with severe baseline hallucinations were more likely to relapse after randomization, and the presence of baseline hallucinations was associated with increased risk of relapse after discontinuation of risperidone compared to continued risperidone treatment. For patients with hallucinations, particularly auditory hallucinations, antipsychotic discontinuation should be approached cautiously because of high relapse risk.
The study is registered as NCT00417482 at clinicaltrials.gov.
Introduction
Currently in the United States there are approximately 5 million individuals age 65 or older with Alzheimer’s disease (AD), and by the year 2050 that number will reach 13.8 million (1). During the course of illness, the majority of patients with AD suffer from behavioral disturbances (2, 3), with a lifetime risk of nearly 100% for the occurrence of these symptoms (4).
The FDA has not approved antipsychotics to treat agitation or psychosis in dementia or AD, but some countries, e.g., Germany, have approved risperidone for this type of indication. The lack of FDA approval leads to off-label use, usually because of clinical need. The concern about antipsychotic toxicity has led to Federal regulations that require discontinuation after a few months of antipsychotic treatment in nursing homes (5). There is a lack of knowledge about which types of neuropsychiatric symptoms increase the risk of relapse following discontinuation of antipsychotic treatment. In order for clinicians to make informed judgments about which patients can safely discontinue their antipsychotic medication and which patients need to continue treatment, it is important to identify which symptoms are associated with an increased likelihood of relapse after antipsychotic discontinuation. In our multicenter Antipsychotic Discontinuation in Alzheimer’s Disease (ADAD) trial, patients who responded to risperidone over 16 weeks of open-label treatment showed a 2–4 fold increased risk of relapse 16 to 32 weeks after discontinuation of risperidone compared to continuation on risperidone (6). In planned post hoc analyses from this trial, we examined the associations between neuropsychiatric symptoms, both at study baseline and immediately post-open risperidone treatment, and the likelihood of relapse after subsequent randomization to continuation risperidone treatment versus discontinuation to placebo.
Methods
ADAD Study Design
The rationale, design, and methods of the ADAD trial have been published previously (6). Briefly, to participate in this trial, outpatients or residents of assisted-living facilities or nursing homes needed to meet the following inclusion/exclusion criteria: DSM-IV criteria for dementia and probable AD based on the diagnostic criteria of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (7); Mini-Mental State Examination (MMSE) (8) score of 5 to 26 for outpatients or 2 to 26 if residing in a nursing home; Neuropsychiatric Inventory (NPI) (9) score of 4 or more on the delusions or hallucinations subscale (psychosis score) or the agitation–aggression subscale (agitation score). Scores on all NPI subscales range from 0 to 12 with higher scores indicating more pronounced symptoms. Patients were excluded if they had a history of stroke, transient ischemic attack, uncontrolled atrial fibrillation, or met DSM-IV criteria for any primary psychotic disorder predating the onset of AD. At baseline, subjects were assessed for the following cardiovascular risk factors as being present or absent: hypertension, hyperlipidemia, diabetes, and tobacco use. Subjects provided written informed consent after receiving a complete description of the study.
In Phase A (Week 0 to 16) of this trial, patients received open treatment with risperidone with dosage adjusted by the study physician as clinically indicated. At Week 16, patients were identified as responders to treatment if they had at least a 30% reduction in the core NPI score (sum of agitation-aggression, hallucinations, and delusions) and a score of 1 (very much improved) or 2 (much improved) on the Clinical Global Impression of Change (CGI-C) scale assessing behavioral symptoms. At Week 16, patients who responded to treatment in Phase A were then randomized into one of three groups in Phase B: Group 1 to continue risperidone for 32 weeks, Group 2 to continue risperidone for 16 weeks and then to receive placebo for 16 weeks, or Group 3 to receive placebo for 32 weeks. In Phase B, relapse was defined as an increase in the core NPI score of at least 30% or a 5-point increase in the core NPI score from the end of Phase A open treatment and a score of 6 (much worse) or 7 (very much worse) on the CGI-C at any study visit. Patients who met criteria for relapse immediately exited the protocol and received open treatment based on doctor’s choice.
Current sub-study
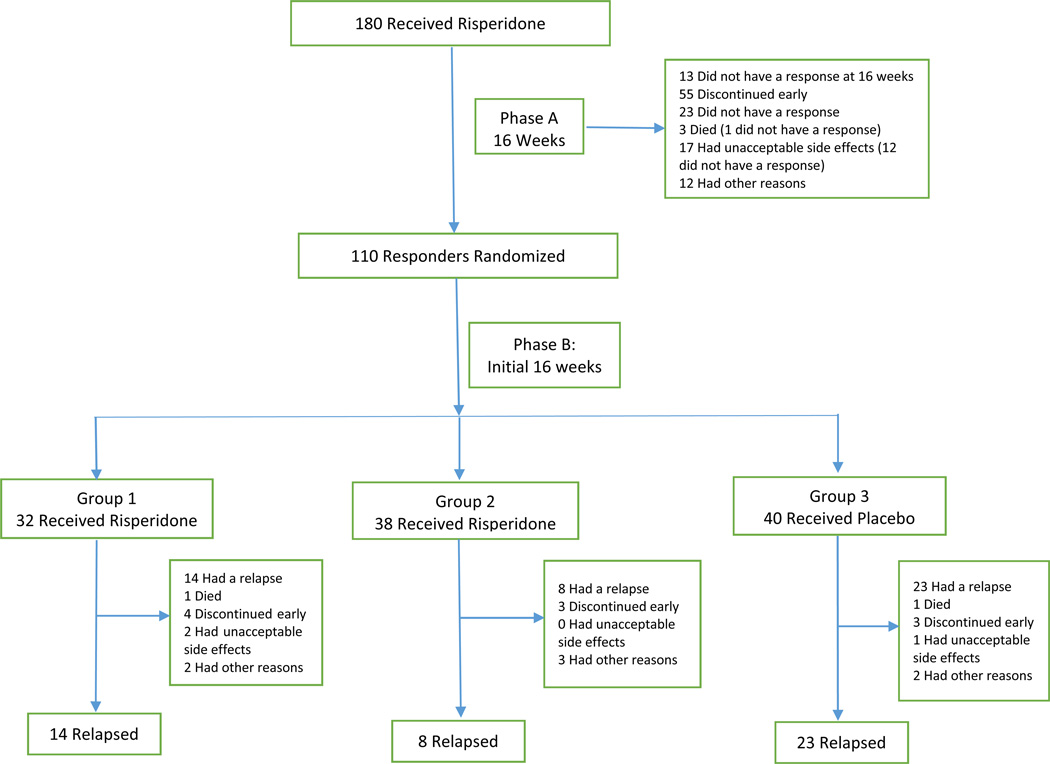
This sub-study included all patients who responded to risperidone in Phase A and were randomized at Week 16. Of the 112 patients who responded to treatment in Phase A, 110 were randomized at Week 16 to enter Phase B (Figure 1). Symptoms at initial presentation, prior to open label treatment, are referred to as baseline symptoms. Results from baseline until the first 16 weeks after randomization were examined. The final sixteen weeks (Week 32 to Week 48) were not analyzed for prediction of relapse due to relatively small sample size.
Figure 1. Patient flow through Phase A 16 weeks of open treatment and post-randomization initial 16 weeks of Phase B.
In Phase B (combining all groups), in addition to patient relapse, subjects exited the study because of death (n=2), early discontinuation (n=10), unacceptable side effects (n=3), or other reasons n=7). The final 16 weeks in the trial were not analyzed due to small sample size; full consort diagram has been previously published [6].
Statistical Analysis
Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC). Only randomized patients were included and analyses were conducted for all patients according to the intent-to-treat principle. NPI domains were evaluated at Week 0 (baseline) and at Week 16 (time-point of randomization) as potential predictors of relapse during the first 16 weeks after randomization (Weeks 16–32 in the trial).
Each individual NPI domain was dichotomized as present or absent at Week 0 and Week 16 and was compared for differences between relapsed and non-relapsed groups using Fisher’s exact test.
To examine clinically relevant relationships of the NPI domains in predicting relapse, the NPI domain scores were classified into three categories: 0 for not present, 1–6 for mild/moderate symptoms, and 7–12 for severe symptoms. This was done for all NPI domains at Week 0 and Week 16 respectively.
Individual NPI domain scores at Week 0 and Week 16 were analyzed using PROC PHREG (SAS v. 9.3) with the discrete method to address ties (10). Stratified Cox proportional hazard regression analyses were conducted to evaluate the associations between NPI domain scores at Week 0 and Week 16, respectively, and relapse between Weeks 16 and 32. All analyses were conducted with the two stratification variables employed in the randomization: presence or absence of psychosis, and residence in an assisted-living facility or nursing home versus residence at home (6). The Cox proportional hazard regression analysis was performed initially with dichotomized NPI domain scores, and then for NPI domain scores classified into three groups, based on severity, at Week 0 and Week 16 respectively. The twelve NPI domains were examined separately with treatment group and their interactions. Chi-square test was used to analyze the effect of treatment group on relapse. The proportional hazards assumption was evaluated by testing a non-zero slope in linear regression models of the Schoenfeld residuals as a function of event time. All models satisfied the assumption. Kaplan-Meier estimates of the risk of relapse were prepared for visual comparison.
Results
As previously reported, in Phase A open label treatment with risperidone for 16 weeks (n=180), NPI scores declined significantly for all 12 domains except for appetite. One hundred and twelve patients were responders, and 110 patients were randomized. Of these 110 patients, 32 patients received risperidone in Group 1, 38 patients received risperidone in Group 2 (combined Groups 1 and 2: n=70 on risperidone) and 40 patients received placebo in Group 3. In this subsample, the mean age was 79.97 (SD 7.83) years, 60% were female, the breakdown by race was 71.82% White, 19.09% African American, 8.18% Hispanic and 0.91% Other, and 50.9% were residing in a nursing home or assisted living facility. After randomization, patients who discontinued risperidone to receive placebo were more likely to relapse (n=24/40 or 60%) than patients who continued on risperidone (23/70 or 33%, p < .001)(6). Gender, race, and nursing home/outpatient status were not associated with relapse.
Baseline NPI domain scores and prediction of relapse
In the total randomized sample, in the stratified Cox models there was no significant association between any of the 12 baseline dichotomized NPI domain scores and time to relapse. Using the classification of absent, mild/moderate and severe symptoms, the only NPI domain that was significantly associated with relapse at Week 32 was hallucinations (p=0.005; Table 1). In Cox regression analysis, severe hallucinations were significantly associated with increased risk of relapse when compared to the group with no hallucinations (HR 2.70, 95% CI 1.19 to 6.12, p<0.02). Auditory hallucinations showed a significant effect in predicting relapse (HR 2.36, 95% CI 1.18 to 4.71, p < 0.02). Visual hallucinations were not predictive of relapse. Fourteen of 18 patients with severe hallucinations had severe delusions, and these two variables were associated with each other (Likelihood Ratio χ2=9.69, p< 0.05). Adding delusions into the model did not alter the significance of severe hallucinations in predicting relapse. The additional covariates of MMSE, number of cardiovascular risk factors, number of medications, and number of medications classified based on Beer’s Criteria that cover medications with potential toxicity in older adults (11) were included in the main analysis. None of these measures changed the significant effect of hallucinations in predicting relapse. Twenty-three patients were taking pain medications at baseline and they were significantly less likely to relapse (p< 0.02), but including the number of pain medications as a covariate did not alter the significant association between baseline hallucinations and the risk of relapse. Forty-eight patients were on other psychotropic medications at baseline, and class of psychotropic medication was not associated with the risk of relapse.
Table 1.
Neuropsychiatric Inventory (NPI) domain scores at baseline and at post-16 weeks of open-label risperidone treatment in the combined sample of 110 responders who were randomized to subsequent 16 weeks of continuation risperidone (n=70) or discontinuation to placebo (n=40).
| NPI Domains | Symptom Present Total ‘n’=110 |
Relapsed at week 32, % | Fisher’s exact test (p-value) |
||||
|---|---|---|---|---|---|---|---|
| N | % | NONE | Mild | Severe | |||
| Baseline (Week=0) |
Hallucinations** | 43 | 39.1 | 35.8 | 36 | 77.8 | 0.005 |
| Delusions | 95 | 86.4 | 33.3 | 35.1 | 50 | 0.28 | |
| Agitation/Aggression | 102 | 92.7 | 75 | 35.4 | 44.4 | 0.10 | |
| Depression/Dysphoria | 48 | 43.6 | 38.7 | 46.3 | 57.1 | 0.50 | |
| Anxiety | 56 | 50.9 | 50 | 32.5 | 43.8 | 0.23 | |
| Elation/Euphoria | 8 | 7.3 | 44.1 | 33.3 | 0 | 0.60 | |
| Apathy/Indifference | 44 | 40.0 | 43.9 | 34.6 | 50 | 0.59 | |
| Disinhibition | 53 | 48.2 | 42.1 | 44.7 | 40 | 0.96 | |
| Irritability/Lability | 82 | 74.5 | 35.7 | 42.6 | 48.6 | 0.59 | |
| Aberrant Motor | 45 | 40.9 | 41.5 | 47.6 | 41.7 | 0.93 | |
| Sleep | 47 | 42.7 | 41.3 | 42.9 | 47.4 | 0.90 | |
| Appetite | 29 | 26.4 | 43.2 | 30 | 66.7 | 0.19 | |
| Randomization (Week=16) |
Hallucinations | 15 | 13.6 | 42.1 | 46.7 | 0 | 0.78 |
| Delusions | 36 | 32.7 | 45.9 | 39.4 | 0 | 0.32 | |
| Agitation/Aggression | 50 | 45.5 | 45 | 40.4 | 33.3 | 0.87 | |
| Depression/Dysphoria | 20 | 18.2 | 40 | 50 | 100 | 0.20 | |
| Anxiety | 33 | 30.0 | 40.3 | 46.9 | 100 | 0.39 | |
| Elation/Euphoria | 1 | 0.9 | 43.1 | 0 | 0 | 1.00 | |
| Apathy/Indifference | 42 | 38.2 | 42.6 | 48.3 | 30.8 | 0.58 | |
| Disinhibition** | 15 | 13.6 | 38.9 | 71.4 | 0 | 0.04 | |
| Irritability/Lability | 40 | 36.4 | 48.6 | 32.5 | 0 | 0.11 | |
| Aberrant Motor | 33 | 30.0 | 44.2 | 37.5 | 44.4 | 0.90 | |
| Sleep* | 20 | 18.2 | 47.8 | 17.6 | 33.3 | 0.04 | |
| Appetite | 18 | 16.4 | 41.3 | 41.7 | 66.7 | 0.61 | |
p <0.01,
p <0.05.
In additional analyses, a three-way contingency table was analyzed with the baseline hallucination domain and the variables of treatment and relapse during Weeks 16 to 32. Among patients with baseline hallucinations, 13 of 17 (76.5%) who switched to placebo relapsed, compared to 10 of 26 (38.5%) who continued risperidone treatment (Relative Risk=1.99, 95% CI=1.14 to 3.46, p<0.03). This difference was also observed for severe (77.8%) compared to mild (36%) hallucinations (Relative Risk=2.88, 95% CI=1.16 to 7.18, p<0.03). Within the subgroup of patients with severe baseline hallucinations, 10 of 11 (90.9%) who switched to placebo relapsed, compared to 4 of 7 (57.1%) who continued to receive risperidone (Relative Risk=1.591, 95% CI=0.82 to 3.10, p=0.17). In this analysis, the p-value did not reach statistical significance due to the small sample size of the subgroup with mild baseline hallucinations: 3 of 6 (50%) that switched to placebo relapsed compared to 6 of 19 (31.6%) on risperidone (Relative Risk=1.58, 95% CI=0.56 to 4.47, p=0.39).
In stratified Cox models that examined the interaction between baseline symptom severity for each NPI domain and treatment group after randomization (risperidone continuation versus discontinuation) in the prediction of time to relapse, only irritability/lability was significant (χ2=7.16, p<0.03). Among patients with severe irritability/lability at baseline, those who discontinued risperidone had a higher risk of relapse than those who continued risperidone (HR=7.78, 95% CI: 2.32–26.07, p < .01), while those with no irritability/lability or mild irritability/lability did not show a significantly increased risk of relapse (Table 2). Few patients demonstrated severe lability/irritability at baseline (Table 2).
Table 2.
Frequency of relapse by Neuropsychiatric Inventory (NPI) domain symptom severity at baseline and 16 weeks, and risperidone continuation versus discontinuation in the randomized phase (weeks 16 to 32).
| NPI Domains | Symptom Severity |
# of relapses by week 32 (n/total) |
p-value2 | ||
|---|---|---|---|---|---|
| Risperidone Continued |
Risperidone Discontinued |
||||
| At Baseline (Week 0) |
Hallucination | Absent | 13 / 44 | 11 / 23 | 0.35 |
| Mild | 6 / 19 | 3 / 6 | |||
| Severe | 4 / 7 | 10 / 11 | |||
| Delusions | Absent | 5 / 11 | 0 / 4 | 0.44 | |
| Mild | 6 / 28 | 7 / 9 | |||
| Severe | 12 / 31 | 17 / 27 | |||
| Agitation/Aggression | Absent | 2 / 3 | 4 / 5 | 0.15 | |
| Mild | 11 / 33 | 6 / 15 | |||
| Severe | 10 / 34 | 14 / 20 | |||
| Depression/Dysphoria | Absent | 13 / 41 | 11 / 21 | 0.14 | |
| Mild | 8 / 25 | 11 / 16 | |||
| Severe | 2 / 4 | 2 / 3 | |||
| Anxiety | Absent | 7 / 25 | 20 / 29 | 0.17 | |
| Mild | 10 / 32 | 3 / 8 | |||
| Severe | 6 / 13 | 1 / 3 | |||
| Elation/Euphoria1 | Absent | 22 / 64 | 23 / 38 | 0.88 | |
| Present | 1 / 6 | 1 / 2 | |||
| Apathy/Indifference | Absent | 15 / 44 | 14 / 22 | 0.07 | |
| Mild | 1 / 13 | 8 / 13 | |||
| Severe | 7 / 13 | 2 / 5 | |||
| Disinhibition | Absent | 14 / 38 | 10 / 19 | 0.38 | |
| Mild | 6 / 23 | 11 / 15 | |||
| Severe | 3 / 9 | 3 / 6 | |||
| Irritability/Lability* | Absent | 6 / 17 | 4 / 11 | 0.03 | |
| Mild | 13 / 32 | 7 / 15 | |||
| Severe | 4 / 21 | 13 / 14 | |||
| Aberrant Motor | Absent | 12 / 39 | 15 / 26 | 0.65 | |
| Mild | 6 / 15 | 4 / 6 | |||
| Severe | 5 / 16 | 5 / 8 | |||
| Sleep | Absent | 15 / 49 | 20 / 32 | 0.27 | |
| Mild | 3 / 14 | 3 / 6 | |||
| Severe | 5 / 7 | 1 / 2 | |||
| Appetite | Absent | 12 / 39 | 14 / 24 | 0.33 | |
| Mild | 8 / 19 | 4 / 9 | |||
| Severe | 3 / 12 | 6 / 7 | |||
| At Randomization (Week 16) |
Hallucination | Absent | 19 / 60 | 21 / 35 | 0.31 |
| Mild | 4 / 10 | 3 / 5 | |||
| Delusions1,* | Absent | 15 / 50 | 19 / 24 | 0.02 | |
| Present | 8 / 20 | 5 / 16 | |||
| Agitation/Aggression | Absent | 12 / 36 | 15 / 24 | 0.82 | |
| Mild | 11 / 32 | 8 / 15 | |||
| Severe | 0 / 2 | 1 / 1 | |||
| Depression/Dysphoria | Absent | 17 / 57 | 19 / 33 | 0.97 | |
| Mild | 4 / 11 | 5 / 7 | |||
| Severe | 2 / 2 | 0 / 0 | |||
| Anxiety | Absent | 12 / 47 | 19 / 30 | 0.10 | |
| Mild | 10 / 22 | 5 / 10 | |||
| Severe | 1 / 1 | 0 / 0 | |||
| Elation/Euphoria | Absent | 23 / 69 | 24 / 40 | NA | |
| Severe | 0 / 1 | 0 / 0 | |||
| Apathy/Indifference | Absent | 11 / 39 | 18 / 29 | 0.36 | |
| Mild | 9 / 20 | 5 / 9 | |||
| Severe | 3 / 11 | 1 / 2 | |||
| Disinhibition1 | Absent | 18 / 62 | 19 / 33 | 0.29 | |
| Present | 5 / 8 | 5 / 7 | |||
| Irritability/Lability | Absent | 17 / 44 | 17 / 26 | 0.89 | |
| Mild | 6 / 26 | 7 / 14 | |||
| Aberrant Motor | Absent | 14 / 46 | 20 / 31 | 0.68 | |
| Mild | 6 / 18 | 3 / 6 | |||
| Severe | 3 / 6 | 1 / 3 | |||
| Sleep | Absent | 18 / 59 | 20 / 33 | 0.56 | |
| Mild | 3 / 8 | 2 / 4 | |||
| Severe | 2 / 3 | 2 / 3 | |||
| Appetite | Absent | 21 / 56 | 22 / 34 | 0.40 | |
| Mild | 1 / 11 | 2 / 6 | |||
| Severe | 1 / 3 | 0 / 0 | |||
p<0.05
Due to the small number of patients with severe symptoms, severe and mild symptom groups were merged and are classified as Present.
P-values for the interaction between risperidone discontinuation status and symptom severity in the stratified Cox proportional hazard regression models. Stratification factors were nursing home status and presence of psychosis.
Pre-randomization Week 16 NPI domain scores and prediction of relapse
Each individual NPI domain was dichotomized as present or absent at Week 16 (time-point of randomization) and was compared for differences between relapsed and non-relapsed groups using Fisher’s exact test in the first 16 weeks after randomization (Weeks 16 to 32). The NPI domain score at Week 16 for absence of sleep problems (Fisher’s exact test, p=0.04; 4/20 with sleep problems relapsed compared to 43/90 without sleep problems) was significantly associated with relapse (Table 1). In stratified Cox regression analyses that examined time to relapse, absence of sleep problems at Week 16 was associated with increased risk of relapse (HR 0.28, 95% CI 0.10 to 0.79, p<0.02). For sleep problems at Week 16, analyses with the clinically relevant classification of no symptoms, mild symptoms and severe symptoms were not conducted because of small sample size in the subgroups that relapsed. Of 4 patients with sleep problems at Week 16 that relapsed, 3 with mild sleep problems and 1 with severe sleep problems relapsed.
At Week 16 (post-open treatment and pre-randomization), patients had improved markedly in symptoms of psychosis and agitation, e.g., 15/110 patients had hallucinations compared to 43/110 at baseline and 50/110 patients had agitation compared to 102/110 at baseline. The relapse rate in the 53 patients with baseline agitation that improved from baseline was 41.51% while the relapse rate in the 49 patients that continued to have agitation at week 16 was 38.78%.
In the stratified Cox models, there was no significant interaction between any of the twelve pre-randomization dichotomized NPI domain scores at week 16 and randomized group assignment (risperidone continuation or discontinuation) in the prediction of time to relapse, with the exception of delusions (χ2=5.65, p<0.02, Table 2). Patients who did not have delusions at the randomization timepoint had higher risk of relapse upon discontinuation of risperidone than patients who continued risperidone (HR 3.77, 95% CI 1.77 to 7.84, p < 0.001), while among patients with delusions at the randomization time-point there was no significant difference in relapse between patients who continued or discontinued risperidone (HR 0.70, 95% CI 0.22 to 2.23, p=0.55). The majority of patients with delusions at baseline (61 out of 95, or 64%) improved to the extent of not having delusions at randomization, and the number of relapses in this subgroup (47.54%, 29 out of 61) did not differ significantly (p=0.35) from the rest of the sample (37.73%, 18 out of 49).
Discussion
The majority of patients with hallucinations as a presenting symptom at baseline relapsed during the first 16 weeks after randomization. Severe hallucinations were associated with a three-fold increased risk of relapse compared to patients with mild or no hallucinations. Patients with severe baseline hallucinations were more likely to relapse in the entire randomized sample, and the risk of relapse was increased in patients randomized to placebo compared to continuation of risperidone. Patients with auditory hallucinations but not visual hallucinations at baseline were more likely to relapse, indicating that if patients with severe auditory hallucinations respond to treatment with antipsychotics, discontinuation should be approached with caution and close monitoring after discontinuation is advisable. It is unclear why auditory hallucinations and not visual hallucinations predicted relapse. One speculation is that illusions that can occur in patients with AD were rated as visual hallucinations, and since illusions may not improve with antipsychotic treatment the medication effect on visual hallucinations may have been diluted. Also, it is possible that some patients with AD and visual hallucinations had Lewy body dementia, a condition associated with low antipsychotic tolerability.
At Week 16, remission of delusions was associated with an increased risk of relapse, suggesting that the persistence of delusions after treatment response is not an indicator of increased relapse risk. However, the number of patients with delusions at week 16 was small (Table 2). Absence of sleep problems at Week 16 was associated with a decreased likelihood of relapse between Weeks 16 and 32, but the effect was marginally significant and its clinical relevance is unclear because of the small number of patients with sleep problems that relapsed. Overall, the results suggest that the initial severity of symptoms, particularly severity of hallucinations, rather than the residual severity of symptoms post-treatment response, is important to consider when evaluating the potential risk of relapse after antipsychotic discontinuation.
There have been few prior efforts to evaluate individual symptoms to predict treatment response to antipsychotics, or to predict relapse after antipsychotic discontinuation. In the DART-AD trial, in which discontinuation occurred after 3 months of antipsychotic treatment, patients with higher NPI scores (>14) worsened behaviorally and had worse outcomes when switched to placebo (12). If the total NPI score was > 14 at baseline, the placebo group manifested more neuropsychiatric symptoms than the antipsychotic treatment group by 12 months of follow-up (13). As previously reported in the ADAD trial, total NPI scores did not predict future relapse (6), and the current post hoc analyses indicate that hallucinations at baseline specifically rather than the total NPI score was predictive of relapse.
There are several hypothesized mechanisms for the occurrence of hallucinations in AD, including receptor changes, denervation, dysregulation of image perception/production, and a combination of attention and processing disturbances (14). One study used data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) with MRI brain and FDG-PET scans and found that AD patients with hallucinations had greater brain atrophy and more hypometabolism than AD patients without hallucinations (14). The areas of structural change included the left superior frontal gyrus and lingual gyri, with increased hallucination intensity associated with greater atrophy in the right precentral gyrus, right superior temporal gyrus, and left precuneus. Increased hallucination intensity was also associated with extensive hypometabolism on FDG-PET in the right frontal lobe, orbito-frontal region and insula, and in the left mid-cingulate gyrus. AD patients with hallucinations had overlapping regions of focal atrophy and metabolic changes in the right anterior insula and inferior frontal gyrus (14). These findings suggest that AD patients with hallucinations have a wide neuronal network of dysfunction in brain regions involved in sensory processing, judgment, and attention (14). We did not conduct functional brain imaging during the ADAD trial, and the magnitude and complexity of involvement of different brain regions in the prediction of relapse may need further investigation.
There were some limitations to this study. This was the largest prospective randomized controlled trial of antipsychotic discontinuation in AD, but the size of the study sample decreased over time because patients who relapsed immediately exited the study as per protocol design. This is a difficult group of patients to enroll and treat in a research protocol, and other antipsychotic withdrawal trials with smaller samples had higher dropout rates (15). The study inclusion criteria led to nearly all patients having agitation/aggression at baseline and as a result the comparison group of patients without agitation/aggression was very small. Therefore, the present/absent classification of NPI domains, particularly for agitation/aggression, became less useful in the prediction of relapse after antipsychotic withdrawal, and examination of severity of symptoms became more important. In the open-label treatment phase the average dose of risperidone was 0.97 mg daily that is similar to the average 1 mg dose used in the CATIE study where dosing was also based on doctor’s choice (16). Nonetheless, we cannot rule out the possibility that patients received lower or higher doses than optimal for each patient. We did not utilize a statistical correction for multiple comparisons, but the main finding of baseline severe hallucinations predicting an increased risk of relapse would have survived a Bonferroni correction (Table 2).
Prediction of relapse after treatment response is clinically important because concerns about antipsychotic side effects and increased mortality have led to increased scrutiny of antipsychotic use with the goal of further decreasing their use with new Federal regulations (5). Antipsychotic discontinuation should be considered in many patients in order to decrease morbidity and mortality due to side effects, but it is important to recognize that many patients respond well to antipsychotics without problematic side effects and maintaining these patients on antipsychotics may be advantageous (6). In line with the goals of precision medicine, it is essential for clinicians to identify the patients who are likely to demonstrate an advantageous benefit to risk ratio with antipsychotic treatment. The findings in this report indicate that the subgroup with hallucinations is at a high risk of relapse. In patients with dementia and severe psychotic symptoms, particularly auditory hallucinations, subsequent discontinuation after treatment response, if attempted, should be accompanied by close monitoring for symptom recurrence. Antipsychotic medication may need to be promptly reinstated if there is relapse.
Figure 2.
Kaplan-Meier Curves with the numbers of patients who did not relapse by symptom severity scores of hallucinations at baseline (week 0).
Acknowledgments
Supported by NIH grants R01 AG021488, R01 AG17761 and the Department of Veterans Affairs. Risperidone tablets (0.25, 0.5, 1, or 2 mg) and matching placebo were donated by Janssen, a division of Johnson & Johnson.
Dr. Gregory H. Pelton reports federal grants from NIMH, NIA, DOD and Avanir Pharmaceuticals; and support for travel to meetings from NIA and NIMH.
Dr. Susan K. Shultz reports research support from the Alzheimer’s Disease Cooperative Study in partnership with Toyama Chemical, and from the Alzheimer’s Therapeutic Research Institute in partnership with Eli Lilly and Astra Zeneca.
Dr. David L. Sultzer reports support from the Department of Veterans Affairs; research support from Eli Lilly, Avanir, Transition Therapeutics; and consulting for Otsuka, Lundbeck, Astellas, Insys, AbbVie.
Dr. Jacobo Mintzer reports that he is employed by Roper St. Francis Healthcare, Medical University of South Carolina, Ralph H. Johnson VA Medical Center, and NeuroQuest, and is founder of BioPharma Connex.
Dr. Danilo de la Pena reports research support from Genentech, Inc., Neurim Pharmaceuticals, Ltd., Avanir Pharmaceuticals, Inc., Biogen Idec MA, Inc., UCSD, Toyama Chemical Co., Ltd., H. Lundbeck A/S, Otsuka Pharmaceutical Development and Commercialization, Inc., TauRx Therapeutics Ltd., Merck Sharp & Dohme Corp., Eli Lilly and Company, Transition Therapeutics Ireland, Ltd., Hoffmann – La Roche Inc., EnVivo Pharmaceuticals, Inc., Takeda Development Center Americas, Inc., Pfizer, Inc.
Dr. Sanjay Gupta reports speaking engagements with Allergan, Sunovion, Takeda, Otsuka, Lundbeck, Alkemes, Avanir.
Dr. D.P. Devanand reports research support from Avanir; serving on scientific advisory boards for Lundbeck, AbbVie, Astellas; consulting for Intra-cellular Therapies.
Footnotes
Disclosures:
Dr. Anjali N. Patel reports no financial relationships with commercial interests.
Dr. Seonjoo Lee reports no financial relationships with commercial interests.
Dr. Howard F. Andrews reports no financial relationships with commercial interests.
Dr. Sylvia Colon reports no financial relationships with commercial interests.
Dr. Corbett Schimming reports no financial relationships with commercial interests.
Dr. Bruce Levin reports no financial relationships with commercial interests.
REFERENCES
- 1.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke A, Hall G, Tariot PN. The clinical problem of neuropsychiatric signs and symptoms in dementia. Continuum. 2013;19:382–396. doi: 10.1212/01.CON.0000429177.14354.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devanand DP, Jacobs DM, Tang M, et al. THe course of psychopathologic features in mild to moderate alzheimer disease. Archives of General Psychiatry. 1997;54:257–263. doi: 10.1001/archpsyc.1997.01830150083012. [DOI] [PubMed] [Google Scholar]
- 4.Jeste DV, Blazer D, Casey D, Meeks T, Salzman C, Schneider L, Tariot P, Yaffe K. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:957–970. doi: 10.1038/sj.npp.1301492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Website CfMMSCg. CMS Announces Partnership to Improve Dementia Care in Nursing Homes. 2012 [Google Scholar]
- 6.Devanand DP, Mintzer J, Schultz SK, Andrews HF, Sultzer DL, de la Pena D, Gupta S, Colon S, Schimming C, Pelton GH, Levin B. Relapse risk after discontinuation of risperidone in Alzheimer's disease. The New England journal of medicine. 2012;367:1497–1507. doi: 10.1056/NEJMoa1114058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 9.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 10.Efron B. The Efficiency of Cox's Likelihood Function for Censored Data. Journal of the American Statistical Association. 1977;72:557–565. [Google Scholar]
- 11.By the American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatrics Society. 2015;63:2227–2246. doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 12.Ballard CG, Thomas A, Fossey J, Lee L, Jacoby R, Lana MM, Bannister C, McShane R, Swann A, Juszczak E, O'Brien JT. A 3-month, randomized, placebo-controlled, neuroleptic discontinuation study in 100 people with dementia: the neuropsychiatric inventory median cutoff is a predictor of clinical outcome. The Journal of clinical psychiatry. 2004;65:114–119. doi: 10.4088/jcp.v65n0120. [DOI] [PubMed] [Google Scholar]
- 13.Ballard C, Lana MM, Theodoulou M, Douglas S, McShane R, Jacoby R, Kossakowski K, Yu L-M, Juszczak E on behalf of the Investigators DA. A Randomised, Blinded, Placebo-Controlled Trial in Dementia Patients Continuing or Stopping Neuroleptics (The DART-AD Trial) PLoS Medicine. 2008;5:e76. doi: 10.1371/journal.pmed.0050076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanc F, Noblet V, Philippi N, Cretin B, Foucher J, Armspach JP, Rousseau F Alzheimer's Disease Neuroimaging I. Right anterior insula: core region of hallucinations in cognitive neurodegenerative diseases. PloS one. 2014;9:e114774. doi: 10.1371/journal.pone.0114774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen-Mansfield J, Lipson S, Werner P, Billig N, Taylor L, Woosley R. Withdrawal of haloperidol, thioridazine, and lorazepam in the nursing home: A controlled, double-blind study. Archives of Internal Medicine. 1999;159:1733–1740. doi: 10.1001/archinte.159.15.1733. [DOI] [PubMed] [Google Scholar]
- 16.Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, Lebowitz BD, Lyketsos CG, Ryan JM, Stroup TS, Sultzer DL, Weintraub D, Lieberman JA Group C-AS. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. The New England Journal of Medicine. 2006;355:1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]