SUMMARY
Background
Antiplatelet therapy is a cornerstone of modern medical practice and is routinely employed to reduce the likelihood of myocardial infarction, thrombosis, and stroke. However, current antiplatelet therapies, such as aspirin, often have adverse side effects, including increased risk of bleeding, and some patients are relatively “aspirin-resistant”. Platelets are intimately involved in hemostasis and inflammation, and clinical consequences are associated with excessive or insufficient platelet activation.
Objectives
A major unmet need in the field of hematology is the development of new agents that safely prevent unwanted platelet activation in patients with underlying cardiovascular disease, while minimizing the risk of bleeding. Here, we investigate the potential of endogenously produced, specialized pro-resolving mediators (SPMs) as novel antiplatelet agents. SPMs are a recently discovered class of lipid-derived molecules that drive the resolution of inflammation, without being overtly immunosuppressive.
Methods
Human platelets were treated with lipoxin A4, resolvin D1, resolvin D2, 17-HDHA, or maresin 1 for 15 minutes, then were subjected to platelet function tests, including spreading, aggregation, and inflammatory mediator release.
Results
We show for the first time that human platelets express the SPM receptors, GPR32 and ALX. Furthermore, our data demonstrate that maresin 1 differentially regulates platelet hemostatic function, by enhancing platelet aggregation and spreading, while suppressing release of proinflammatory and pro-thrombotic mediators.
Conclusions
These data support the concept that SPMs differentially regulate platelet function and may represent a novel class of antiplatelet agents. SPMs also may play an important role in the resolution of inflammation in cardiovascular diseases.
Keywords: platelet activation, inflammation, antiplatelet agents, hemostasis, platelets
INTRODUCTION
Platelets are small anucleate blood cells derived from megakaryocytes. In addition to their pivotal roles in hemostasis, platelets are the smallest, yet most abundant, immune cell and regulate inflammation, immunity, and disease progression.[1] Platelets are key cellular mediators of cancer cell metastasis, atherosclerosis, type II diabetes, and even adaptive immune responses.[2] After disruption of vascular integrity, platelets are the first cells to arrive at the site of injury. Here, they activate to form a hemostatic plug to mitigate bleeding. Release of their granule contents recruit and activate immune cells, initiating a protective immune response.[1] Many mediators are known to regulate this process, including extracellular matrix proteins and bacterial products that serve to activate platelets[3, 4]. Additionally, endogenously produced prostaglandin I2 and nitric oxide act to keep platelets in a resting state until a threshold of activation signals has been reached.[5] Although anucleate and short-lived, platelets play crucial roles in promoting hemostasis, inflammation, and wound healing[6, 7].
Inflammation is a necessary and protective response to injury that allows the body to ward off infection and respond to external stimuli.[8] However, chronic inflammation can be damaging to host tissues, leading to chronic inflammatory diseases, like rheumatoid arthritis and atherosclerosis. For this reason, the resolution of inflammation is an active and dynamic process that involves down regulation of proinflammatory signals as well as up regulation of pro-resolution signals.[9] Determining how the resolution phase of inflammation occurs is a recent and novel area of study. A critical step in this process is the production of specialized pro-resolving mediators (SPMs).[10, 11] SPMs are generated in the later stages of inflammation and initiate the switch to resolution through reducing neutrophil recruitment and T cell cytokine production, and increasing recruitment of phagocytic monocytes.[12–14] SPMs are synthesized from dietary-derived omega-3 and omega-6 fatty acids and are a growing class of molecules that includes resolvins, protectins, maresins, and lipoxins.[11, 15]
Although numerous studies focus on the roles of SPMs in regulating immune responses to infection, wound repair, and other inflammatory diseases,[11, 16–19] little is known about whether or not SPMs regulate platelet function, despite the prevalence and involvement of platelets in these processes. Resolvin E1 is known to regulate ADP-induced activation of human platelets. It dampens P-selectin expression and reduces platelet aggregation.[20] The ability of resolvin E1 to regulate platelet function is mediated through the resolvin E1 receptor, ChemR23. To date, five SPM receptors have been identified: the resolvin E1 receptors, ChemR23 and BLT1; the resolvin D1 receptor, GPR32; the lipoxin A4 receptor, ALX; and the newly discovered RvD2 receptor, GPR18.[21, 22] However, the presence of GPR32 and ALX has not been investigated on platelets and the discovery of GPR18 as an SPM receptor occurred after these studies were completed. Here, we demonstrate that human platelets express both GPR32 and ALX. We hypothesized that the SPMs resolvin D1 and lipoxin A4 can regulate human platelet function through these SPM receptors, as these two molecules have been shown to signal through these receptors in other cell types. We also evaluated the ability of other SPMs namely, resolvin D2 and maresin 1, and the resolvin precursor 17-HDHA,[23] to regulate human platelet function.
METHODS
Reagents
Lipoxin A4, resolvin D1, resolvin D2, 17-HDHA, and maresin 1 (Cayman Chemical, Ann Arbor, MI) were resuspended in ethanol and stored in glass vials at −80°C under argon gas. SPMs were protected from heat and light to prevent oxidation. The chemical structures of the SPMs used can be found in supplemental figure 1.
Washed platelets
Human blood was obtained from consenting donors in accordance with the Declaration of Helsinki under an approved University of Rochester IRB protocol via venipuncture into vacutainer tubes containing 0.105 M sodium citrate (BD, Franklin Lakes, NJ) from healthy donors who had not taken aspirin or other non-steroidal anti-inflammatory drugs for 2 weeks prior to donation. Platelet rich plasma (PRP) was prepared by centrifugation (250 × g for 10 minutes at 20°C). PRP was centrifuged at 1000 × g for 10 minutes at 20°C with 1 μg/mL prostacyclin (Cayman Chemical, Ann Arbor, MI). Platelets were gently resuspended in Tyrode’s (Sigma-Aldrich, St Louis, MO) ACD solution (25/3, vol/vol) containing 0.1 μg/mL prostacyclin, then centrifuged at 1000 × g for 10 minutes. Platelets were resuspended in Tyrode’s and used within 3 hours of collection. Platelets were adjusted to 3 × 1010 platelets/L for spreading assays or 1 × 1011 platelets/L for all other assays with Tyrode’s. Washed platelets were treated with vehicle (0.1% ethanol) or SPMs (Cayman chemical, >95% pure) for 15 minutes at 20°C then either left unactivated or activated with 5 μM ADP (Chrono-Log Corp., Havertown, PA), 5 μg/mL collagen (Chrono-Log Corp., Havertown, PA), or 0.1 U/mL thrombin for 15–30 minutes at 20°C. Supernatants were generated by centrifugation at 1200 × g for 15 minutes and analyzed for mediator release.
Platelet spreading
Washed platelets (1 × 109) were spread on fibrinogen-coated coverslips (100 μg/mL; Sigma-Aldrich, St Louis, MO) for 45 minutes at 37°C, washed with PBS, then fixed with 4% paraformaldehyde. Spreading was visualized by differential interference contrast (DIC) optics using an Olympus BX51 microscope (Melville, NY) at 100X. The percentage of fully spread platelets was determined by manually counting four fields of view for each donor.
Immunoassays
Thromboxane B2 (TXB2) and prostaglandin E2 (PGE2) were assayed by enzyme immunoassay (EIA; Cayman Chemical, Ann Arbor, MI). Platelet factor 4 (PF4) was assayed by enzyme linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). Soluble CD40L (sCD40L) was assayed by a previously published ELISA.[24]
Aggregometry and dense granule release
Platelet lumi-aggregation was performed by the turbidimetric method using a Chrono-log Lumi-Aggregometer with AGGRO/LINK software (Chrono-Log Corp., Havertown, PA). PRP (450 μl) was placed in a silicone-coated cuvette with constant stirring at 1200 rpm using a siliconized stir bar. Chrono-lume reagent (50 μL) was added and allowed to incubate for 2 minutes prior to addition of agonist. The PPP from each sample was used as the reference sample denoting 100% light transmission. Aggregation was initiated using 5 μM ADP.
Flow cytometry
Platelets (1 × 109 platelets/L) were blocked with human Fc Receptor blocking reagent (Miltenyi Biotech, Bergisch Gladbach, Germany) for 15 minutes. Platelets were stained with mouse anti-human CD61-alexa fluor 647 (Biolegend, clone VI-PL2) and mouse anti-human CD62P-alexa fluor 488 (Biolegend clone AK4) or rabbit anti-human ALX-FITC (R&D Systems, clone 304405) or rabbit anti-human GPR32 (abcam, ab79516) or rabbit IgG isotype control (Cell signaling, clone DA1E) followed by rabbit Dylight 488 secondary (Jackson Immuno Research, 111-485-045) for 30 minutes at 20°C. Platelets were identified by forward and side scatter and CD61 positivity on an Accuri flow cytometer (Becton Dickinson).
Western Blotting
Total protein was quantified by BCA assay (Thermo-Sci, Waltham, MA) and 10 μg was separated using SDS-PAGE, transferred onto a PVDF membrane, blocked with 5% BSA, and probed with rabbit anti-human ALX (abcam, ab63022) or rabbit anti-human GPR32 ((abcam, ab79516).
Statistics
Results are expressed as mean +/− SEM. Significance was determined using a one-way or two-way repeated measures ANOVA with Dunnett’s multiple correction post-test. All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). Probability values of p ≤ 0.05 were considered statistically significant.
RESULTS
Human platelets and the megakaryocyte cell line, Meg01, express the SPM receptors, GPR32 and ALX
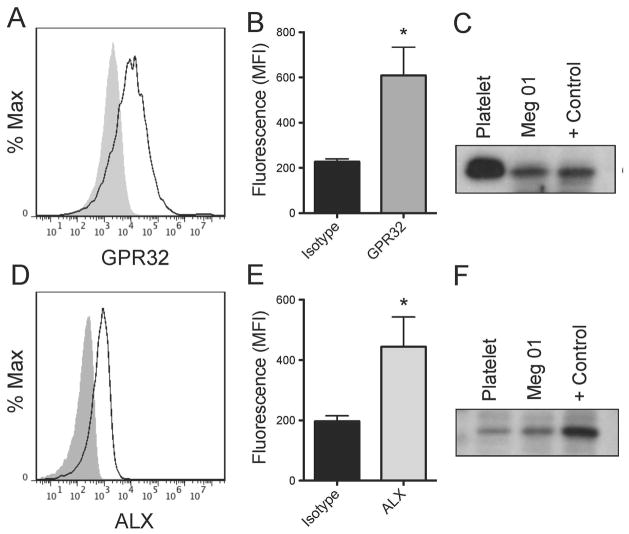
Human platelets express the Resolvin E1 receptor, ChemR23[20], but the presence of the other SPM receptors remains unknown. Here, we identified for the first time the presence of GPR32 and ALX on the surface of human platelets and the megakaryocyte cell line, Meg01, by flow cytometry (Fig 1A–B, D–E). This was confirmed using Western blotting (Fig 1C, F). By Western blotting and flow cytometry, GPR32 expression was found to be more highly expressed in unactivated platelets than ALX. Receptor expression was not altered by activation with ADP (data not shown).
Figure 1. Human platelets and the megakaryocyte cell line, Meg01, express the SPM receptors, GPR32 and ALX.
Washed human platelets were generated from leukoreduced whole blood from healthy human donors. Platelets were blocked with human Fc Receptor blocking reagent for 30 minutes, then stained with rabbit anti-human GPR32 followed by rabbit FITC secondary antibody (A–B) or rabbit anti-human fpr1-FITC (D–E) antibodies for one hour. Grey shaded histogram represents the isotype control. Mean +/− SEM n=3. The mean fluorescence intensity (MFI) for three independent human donors is shown for GPR32 (B) and ALX (E). The MFI for GPR32 was determined by subtracting the MFI from secondary only control. (C, F) Lysates were generated from washed human platelets, the megakaryocyte Meg01 cell line, A549 cells (GPR32 positive control), or HEK cells (ALX positive control). Proteins were separated on two separate 10% SDS-PAGE gels and probed for GPR32 or ALX. One representative donor is shown.
SPMs mitigate human platelet aggregation
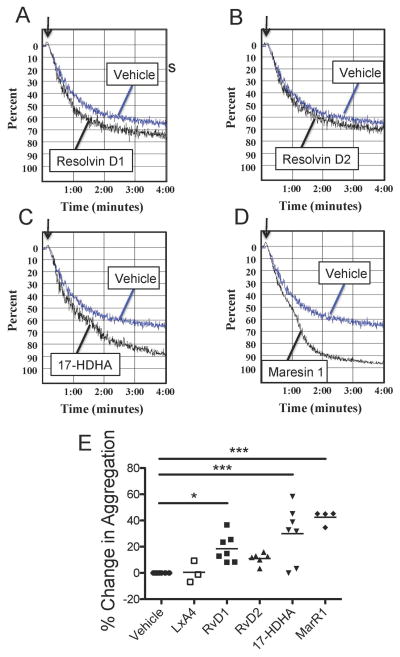
The ability of SPMs to mitigate aggregation of human platelets was investigated using PRP from healthy human donors. Aggregation is a measure of platelet-platelet interactions and is a crucial step in mediating thrombus formation. Platelets were treated with SPMs for 15 minutes prior to initiation of aggregation with ADP. As resolvin D1 can signal through both GPR32 and ALX, and lipoxin A4 signals through ALX, we hypothesized that these SPMs could alter platelet activation. Resolvin D1, but not resolvin D2 or lipoxin A4, enhanced platelet aggregation in response to ADP (Fig 2). Furthermore, the resolvin intermediate molecule, 17-HDHA, and maresin 1 potently enhanced platelet aggregation, although the receptors for these two SPMs have yet to be identified.
Figure 2. SPMs mitigate platelet aggregation.
PRP was generated from healthy human donors and treated with 100 nM SPMs (Lipoxin A4, LXA4; Resolvin D1, RvD1; Resolvin D2, RvD2; 17HDHA; and Maresin 1, Mar1) for 15 minutes prior to initiation of aggregation by 5 μM ADP (A–D). Aggregation was measured for five minutes and the percent change in maximum amplitude was calculated for each SPM relative to vehicle control (0.1% EtOH) (E). Statistical significance was determined by One-way ANOVA with Dunnett’s multiple comparison post-test. *p<0.05, ***p<0.001
SPMs mitigate platelet spreading
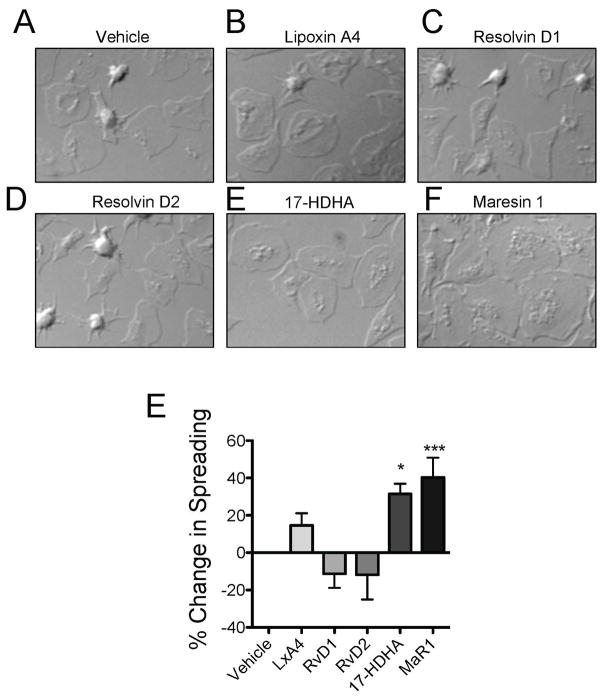
We next assessed the ability of SPMs to affect platelet spreading, a surrogate measure of hemostatic function. Washed platelets were treated with SPMs for 15 minutes then were spread on fibrinogen-coated coverslips for 30 minutes. While Lipoxin A4, resolvin D1, and resolvin D2 did not significantly alter platelet spreading, 17-HDHA and maresin 1 enhanced platelet spreading on fibrinogen (Fig 3). Notably, platelets treated with maresin 1 exhibited a larger, flatter appearance than vehicle treated platelets. In conjunction with the aggregation data, these data suggest that 17-HDHA and maresin 1 both enhanced the ability of platelets to activate.
Figure 3. SPMs mitigate platelet spreading.
Washed platelets were generated from healthy human donors and treated with vehicle or 100 nM SPMs for 15 minutes then were allowed to spread on fibrinogen-coated coverslips for 45 minutes. Coverslips were fixed and visualized by differential interference contrast (DIC) optics using an Olympus BX51 microscope at 100X and SPOT computer software. Scale bars = 10 microns. One representative donor of 5 is shown (A–F). The percentage of fully spread platelets per field of view was determined by manual counting four fields of view for 5 individual donors and the percent change in spreading was calculated for each SPM relative to vehicle control (0.1% EtOH). (E). Mean +/− SEM. n=5 Statistical significance determined by One-Way ANOVA with Dunnett’s multiple comparison post-test. *p<0.05, ***p<0.001
SPMs alter the ability of platelets to release inflammatory mediators from activated platelets
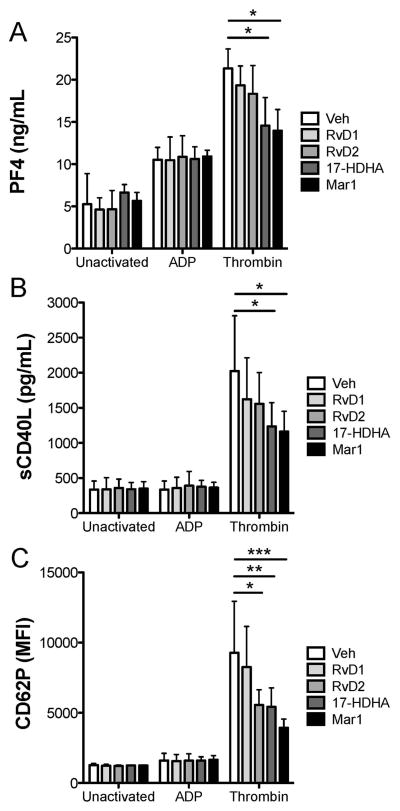
In addition to playing a crucial role in hemostasis, platelets are also important inflammatory cells that are capable of regulating many inflammatory processes through secretion of pro-inflammatory mediators. We next sought to investigate how SPMs could regulate this function of platelets. Platelet PF4 release was measured as a marker of alpha granule release. TXB2 and PGE2 are synthesized in platelets from arachidonic acid and are released upon activation. Measuring both TXB2 and PGE2 is a more comprehensive measure of lipid synthesis in platelets compared to the typical measurement of solely TXB2. CD40L is a costimulatory molecule for immune cells and can additionally activate vascular cells. We chose to evaluate its release from platelets, as it is an abundant inflammatory molecule that is released from platelets, independent of alpha and dense granules. CD62P expression was measured, as it is a crucial mediator of platelet-leukocyte interactions. Neither surface bound CD62P nor release of the inflammatory mediators, PF4 and sCD40L, was affected by SPMs in unactivated platelets (Fig 4). Surprisingly, ADP-mediated release of PF4, sCD40L, and CD62P were not altered by SPMs, despite the ability of resolvin D1, 17HDHA, and maresin 1 to enhance ADP-mediated platelet aggregation. However, thrombin-induced exposure of CD62P and release of sCD40L and PF4 were significantly dampened by pretreatment with 17HDHA or maresin 1, suggesting that these SPMs can negatively regulate platelet inflammatory mediator release.
Figure 4. SPMs differentially affect mediator release from unactivated and activated human platelets.
Freshly isolated washed platelets from healthy human donors were treated with vehicle (0.1% DMSO), SPMs for 30 minutes then were either left unactivated, or activated with 5 μM ADP or 0.1U/mL thrombin for 15 minutes. Supernatants were collected and analyzed for PF4 or sCD40L release by ELISA (A–B) or platelets were assessed for CD62P positivity by flow cytometry (C). Mean +/− SEM. n=4 Statistical significance was determined by Two-Way RM ANOVA with Dunnett’s multiple comparison post-test.
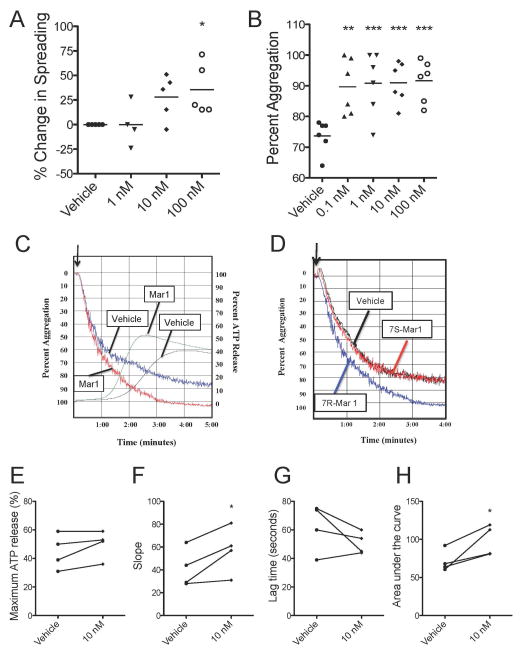
Maresin 1 dose-dependently enhances platelet spreading and aggregation
Due to the ability of maresin 1 to enhance platelet hemostatic function (measured by aggregation and spreading), while inhibiting platelet inflammatory mediator release, we sought to further investigate how maresin 1 alters platelet function. Here, we show that platelet spreading was dose-dependently enhanced by maresin 1 from 1 to 100 nM (Fig 5A). Interestingly, platelet aggregation was significantly enhanced by maresin 1 from 100 pM to 100 nM (Fig 5B).
Figure 5. Maresin 1 enhances platelet spreading, aggregation, and dense granule release.
PRP was generated from healthy human donors and treated with vehicle (0.1% EtOH) or maresin 1 for 15 minutes then were allowed to spread on fibrinogen-coated coverslips for 45 minutes. Coverslips were fixed and visualized by differential interference contrast (DIC) optics using an Olympus BX51 microscope at 100X and SPOT computer software. The percentage of fully spread platelets per field of view was determined by manual counting four fields of view for 5 individual donors and the percent change in spreading was relative to vehicle control (A). Aggregation was initiated by 5 μM ADP, then aggregation and ATP release were measured for five minutes. The maximum percent aggregation was calculated (B). One representative donor is shown for aggregation and ATP release in response to ADP (C). The inactive isomer of 7R-maresin 1, 7S-maresin 1, was added to platelets prior to aggregation with ADP (D). Maximum ATP release, the slope, lag time, and area under the curve were calculated for 4 independent donors (E–H). Statistical significance was determined by Kruskal-Wallis test with Dunns multiple comparison post-test. *p<0.05
Maresin 1 enhances platelet dense granule release
Platelet aggregation in response to ADP consists of a primary wave of aggregation, followed by dense granule release, which mediates the secondary wave of aggregation. Maresin 1 appeared to enhance the secondary wave of aggregation, while the primary wave remained similar to vehicle-treated platelets (Fig 5C). Therefore, we hypothesized that maresin 1 enhanced the secondary wave of aggregation via enhanced dense granule release. Although no differences were observed in the maximum release of ATP from dense granules, the rate of release and total area under the curve were increased compared to vehicle (Fig 5D–G). These data suggest that maresin 1 may enhance platelet aggregation through enhancing the rate of dense granule release. Similarly, it is possible that enhanced aggregation induced by maresin 1 could result in enhanced dense granule release.
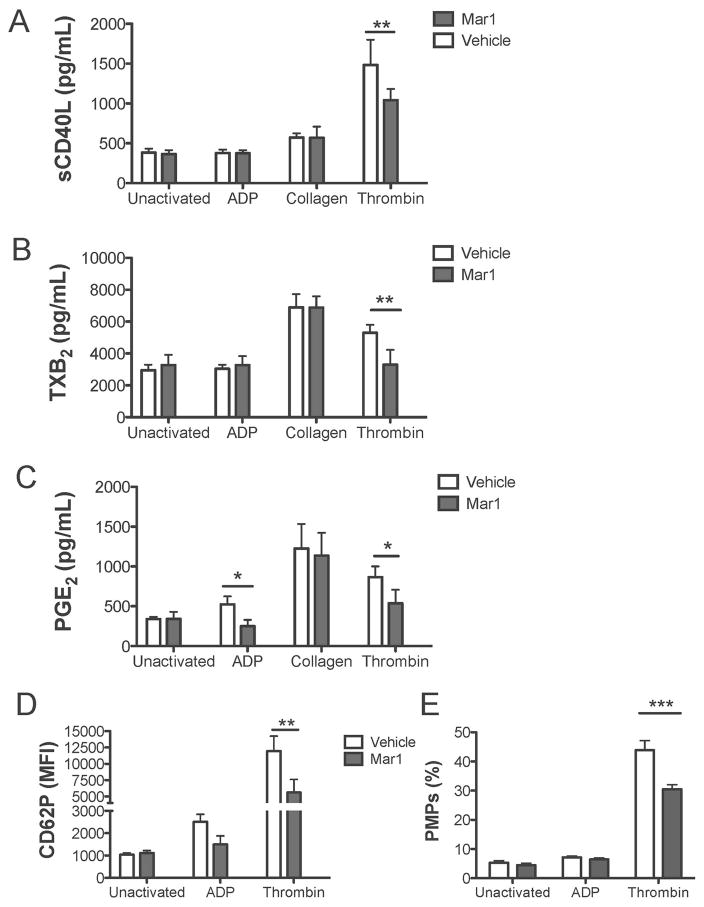
Maresin 1 differentially affects release of mediators from activated human platelets
Due to the ability of Maresin 1 to enhance ATP-mediated dense granule release (Fig 5) while dampening the release of CD62P, PF4, and sCD40L from thrombin activated platelets (Fig 4), we sought to more thoroughly investigate the platelet release reaction in response to Maresin 1. Human platelets were treated with Maresin 1 for 15 minutes then were either left unactivated or activated with ADP, collagen, or thrombin for 15 minutes. Release of soluble mediators was assayed using immunoassays (Fig 6) or a human cytokine and chemokine Luminex panel and a human cardiovascular Luminex panel (Fig 7). Maresin 1 reduced thrombin-induced release of CD62P, sCD40L, and TXB2, and additionally reduced PGE2 release from ADP and thrombin-stimulated platelets (Fig 6). Interestingly, maresin 1 did not affect the release of any mediators examined from collagen-activated platelets, suggesting agonist-specific regulation by maresin 1. Consistent with the data showing that maresin 1 dampened release of inflammatory mediators from thrombin-activated platelets, the release of platelet microparticles (PMPs) was similarly reduced by maresin 1 (Fig 6E).
Figure 6. Maresin 1 dampens inflammatory mediator release from activated platelets.
Freshly isolated PRP from healthy human donors was treated with vehicle (0.1% DMSO) or maresin 1 for 15 minutes then were either left unactivated, or activated with 5 μM ADP, 5 μg/mL Collagen, or 0.1 U/mL thrombin for 15 minutes. Supernatants were collected and analyzed for mediator release by ELISA (A–C) or platelets were assessed for CD62P positivity by flow cytometry (D). The percentage of platelet microparticles (PMPs) was determined by flow cytometry (E). Mean +/− SEM. n=4 Statistical significance was determined by Two-Way RM ANOVA with Dunnett’s multiple comparison post-test.
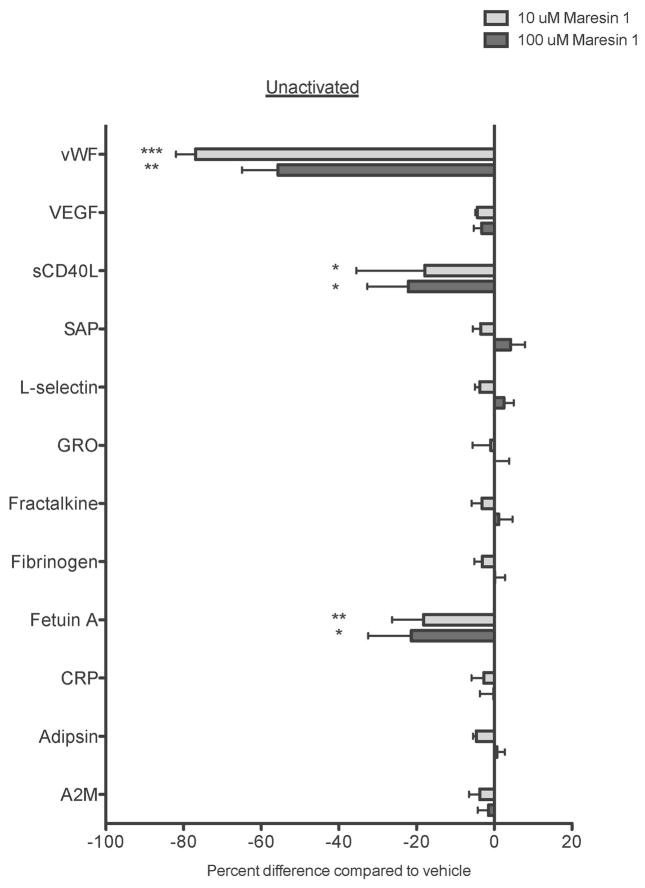
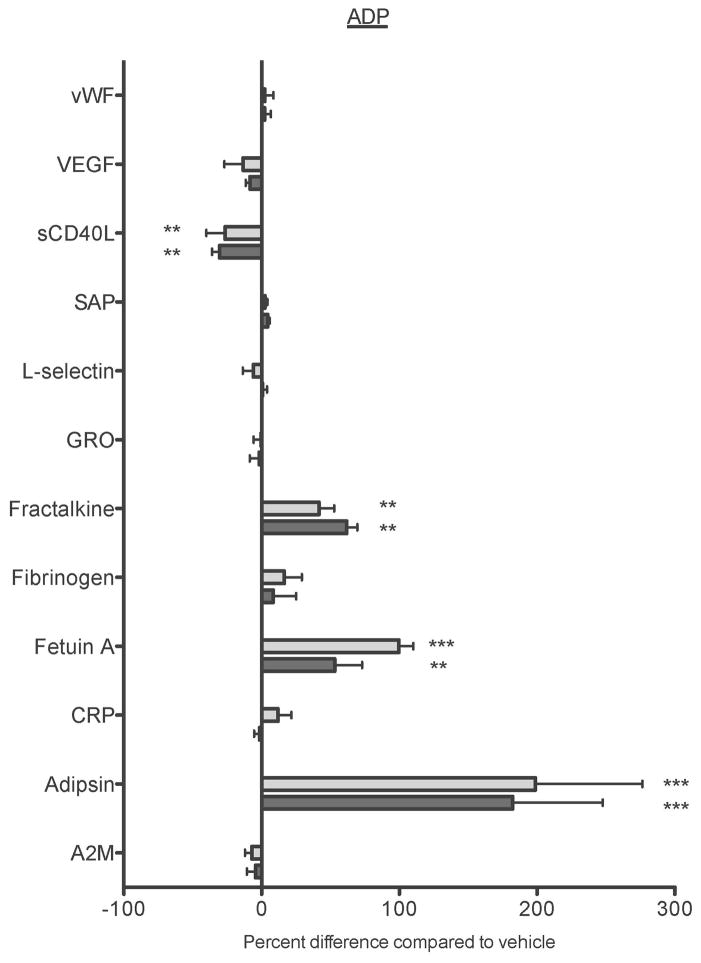
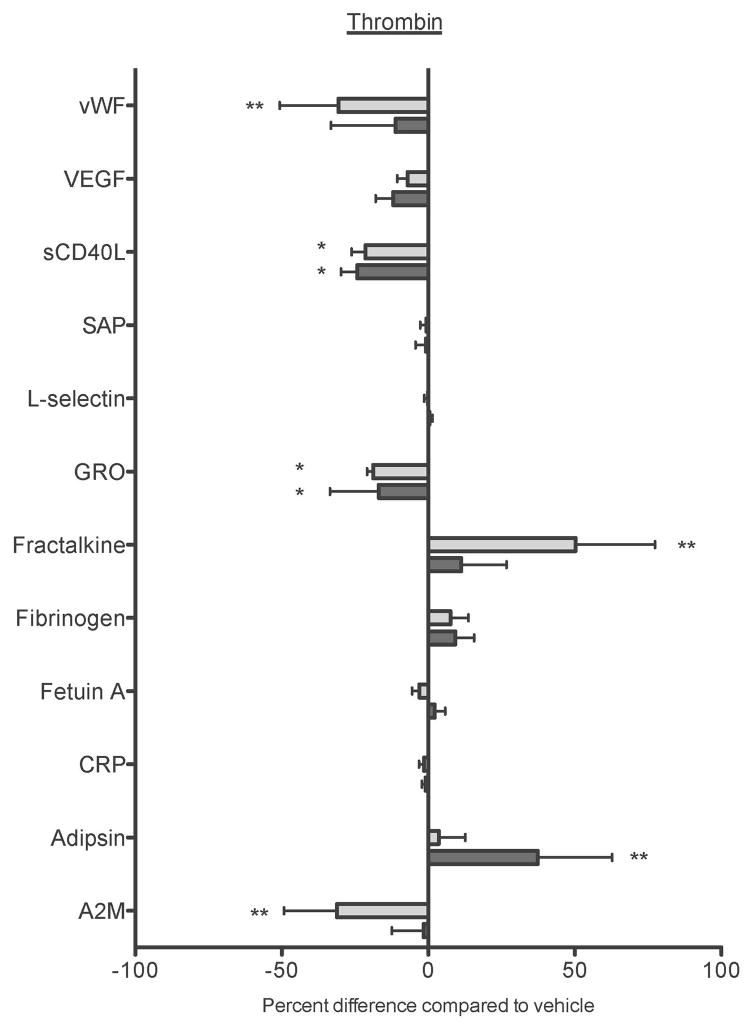
Figure 7. Maresin 1 differentially affects mediator release from activated platelets.
Freshly isolated washed platelets from healthy human donors were treated with vehicle (0.1% DMSO) or maresin 1 for 15 minutes then were either left unactivated (A), or activated with 5 μM ADP (B), or 0.1 U/mL thrombin (C) for 15 minutes. Supernatants were collected and analyzed for mediator release by Luminex Assay. Mean +/− SEM. n=4 Statistical significance was determined by One-Way RM ANOVA with Dunnett’s multiple comparison post-test.
As maresin 1 altered ADP and thrombin-induced platelet activation, but did not affect collagen-induced platelet activation, we chose to further evaluate only ADP and thrombin-induced activation in the Luminex panel. The Luminex panels revealed that while Maresin 1 dampened the release of sCD40L, vWF, and fetuin A from unactivated platelets, it differentially affected release of mediators from ADP or thrombin stimulated platelets (Fig 7). Maresin 1 dampened release of sCD40L from ADP-activated platelets, but enhanced the release of fracktalkine, fetuin A, and adipsin in the same release reaction (Fig 7B). Similarly, vWF, sCD40L, GRO, and A2M release were significantly reduced in thrombin-activated platelets, while release of fracktaline and adipsin were enhanced by Maresin 1 (Fig 7C). These data provide support that Maresin 1 differentially alters release of mediators from activated platelets.
DISCUSSION
Numerous clinical studies report beneficial effects of dietary omega-3 fatty acids in cardiovascular disease and other inflammatory diseases in which platelets are known to play a central role.[25] As SPMs are a recently discovered class of molecules derived from omega-3 fatty acids, we hypothesized that some of these beneficial actions are mediated by SPM-dependent regulation of platelet function. Here, we show for the first time that human platelets express the SPM receptors, GPR32 and ALX. Contrary to expectations, their ligands, resolvin D1 and lipoxin A4 did not dampen platelet activation, but rather resolvin D1 enhanced platelet aggregation. Although we did not test whether this effect was mediated through GPR32 or ALX, it is possible that signaling through these receptors elicits a different response than resolvin E1 signaling through ChemR23. Resolvin E1 dampens ADP-induced CD62P expression and aggregation in a receptor-dependent fashion, thus acting as a negative regulator of platelet function.[20] It is likely that different SPMs exert different actions on platelets, as they do in other cell types. The newly discovered class of proresolving mediators, termed SPMs, are comparable to proinflammatory cytokines in that each individual mediator exerts its unique action through a specific receptor to achieve a larger phenotype, such as inflammation or resolution. Therefore, in depth characterization of each individual SPM and its actions through its specific receptor in various cell types will be necessary to fully understand how each piece of this complex biological puzzle function in concert to achieve a larger goal. Just as cytokines have unique functions, we hypothesize that SPMs are similarly likely to exert unique actions on platelets, thus not all behaving in the same way. This study represents the first stepping-stone in this process.
It is possible that these SPMs do not regulate classical hemostatic activation of platelets as would be expected of a traditional anti-platelet agent. SPMs are generated during the later phases of inflammation, long after hemostatic activation of platelets has occurred.[26] At this point in the inflammatory response, it would be important to reduce inflammatory signals and promote the resolution of inflammation and wound healing. Early platelet activation at the onset of insult or injury acts to stop bleeding and recruit and activate immune cells. Dampening these crucial functions early on would be detrimental to the protective inflammatory response. However, once a hemostatic plug has been formed and the acute phase of inflammation is over, resolution of inflammation begins to take place. This process involves decreasing pro-inflammatory signals, promoting immune cell clearance, and wound healing.[27] Therefore, this lack of an SPM effect on hemostatic platelet activation may be due to differences in endogenous temporal regulation of SPM production.
Interestingly, our data reveal that maresin 1 and 17-HDHA enhanced hemostatic functions of platelets, while dampening inflammatory mediator release. This is a novel method of platelet regulation. Traditional modulators of platelet function typically either universally enhance or inhibit platelet function. This is the first report to our knowledge that exogenous treatment of platelets with a single molecule can dichotomously regulate platelet activation. Therefore, we chose to further evaluate how maresin 1 altered platelet activation in vitro. Most interestingly, in response to ADP, maresin-treated platelets had an enhanced ability to aggregate and released dense granules at a faster rate than control platelets, although maximal ATP release was not changed. However, under the same conditions, maresin-treated platelets released lower levels of prostaglandin E2, suggesting a novel method of regulation of platelet activation. Utilizing two Luminex panels, our data definitively demonstrate that maresin 1 differentially regulated the platelet release reaction in unactivated and ADP or thrombin-stimulated platelets. For example, maresin 1 dampened release of sCD40L from ADP-activated platelets, but enhanced the release of fracktalkine, fetuin A, and adipsin in the same release reaction. These data are consistent with the hypothesis that maresin 1 stimulates a pro-resolving phenotype in human platelets.
Furthermore, maresin 1 significantly impaired the ability of thrombin-activated platelets to release inflammatory mediators. This is consistent with data showing that maresin 1 treated mice had lower levels of circulating Thromboxane B2 in response to acute lung injury.[28] Similarly, maresin 1 inhibited CD62P expression on mouse platelets in vivo, consistent with our in vitro findings with human platelets. CD62P is an important mediator of platelet-leukocyte interactions, which are increased in inflammatory states.[29] New evidence suggests that platelets synthesize and transfer maresin precursor molecules to neutrophils and that this transcellular delivery is dependent on platelet-neutrophil interactions.[28] The ability of maresin 1 to decrease CD62P and inhibit platelet-neutrophil interactions could be an important switch from acute inflammation to resolution. Furthermore, reduced release of GRO, a neutrophil chemoattractant molecule, by maresin 1-treated platelets is consistent with data showing that maresin 1 reduced neutrophil recruitment in vivo. Our work suggests that in addition to disrupting inflammatory functions of platelets, maresin 1 promotes hemostatic activation of platelets. It is also possible that two distinct populations of platelet exist: one that is responsible for hemostasis, while the other functions primarily in inflammatory reactions. This is especially important in the context of wound healing, where platelets play important roles in promoting angiogenesis.[30]
Many SPMs, including maresin 1, induce proresolving phenotypes in treated cells, as well as in various mouse models. For example, maresin 1 decreases pain and stimulates tissue regeneration in a planeria model, demonstrating its anti-inflammatory and pro-resolving properties.[31] Maresin 1 consistently reduces inflammatory cell infiltration in response to various stimuli, and reduces inflammatory macrophage activation, while promoting a switch to a proresolving M2 macrophage phenotype.[32–36] Furthermore, maresin 1 attenuated neointimal hyperplasia in mice after carotid artery ligation, through reduced leukocyte recruitment, reduced MCP-1 expression, and macrophage phenotype skewing to M2 macrophages.[37] In vitro studies have confirmed direct effects of maresin 1 on vascular smooth muscle cells as well as endothelial cells, where maresin 1 potently reduced inflammatory cytokine production and decreased NFκB activation.[38] Taken together, these studies reveal the proresolving effects of maresin 1, focusing specifically on vascular and immune cells. However, our study is the first to demonstrate similar proresolving actions of maresin 1 on human platelets.
The mechanism of action of maresin 1 on human platelets is likely to be complex. Our data suggest that maresin 1 may differentially regulate platelet function in response to different agonists, as well as differentially regulate the release of different mediators simultaneously. For example, in ADP-activated platelets, maresin 1 enhanced the rate of dense granule release and enhanced aggregation, but also decreased PGE2 release without affecting TXB2 release. This could suggest a mechanism by which maresin 1 primes platelet activation so that dense granule release can occur faster upon ADP stimulation, resulting in enhanced aggregation. The ability of maresin 1 to dampen PGE2 release without affecting TXB2 release in ADP-activated platelets suggests regulation at the level of prostaglandin synthesis, rather than cyclooxygenase, which functions upstream, thus regulating both TXB2 and PGE2 synthesis. However, the ability of maresin 1 to dampen both TXB2, PGE2, and sCD40L release from thrombin-activated platelets suggests a more global mechanism. As our Luminex data show that maresin 1 enhanced the release of some mediators, while inhibiting the release of others within the same release reaction, it is likely that the mechanism by which maresin 1 acts is multifactorial and complex. There is a base of literature to suggest that platelet alpha granules can differentially release some, but not all, of their contents under different stimulation conditions. Similarly, our initial investigations demonstrated that RvD2 dampened CD62P expression, but did not affect any other markers of platelet activation that were tested in our studies, suggesting that it too may uniquely regulate platelet activation. Further investigating these potential mechanisms in the context of maresin 1 and other SPM treatment will be of vital importance to broaden our understanding of how platelet activation can be mitigated in this unique manner.
We suggest that maresin 1 may drive platelets to a novel pro-resolving phenotype, which would be crucial in mediating the resolution phase of inflammation. These ‘pro-resolving platelets’ not only exhibit dampened inflammatory functions, but are still able to perform basic hemostatic functions. This would be ideal in a wound healing setting, where the acute inflammatory phase has ceased, but platelets are still required to form a hemostatic plug. Additionally, platelets contain many pro-angiogenic and wound healing factors that can be released upon activation, which may be similarly regulated by SPMs.[30, 39] Further characterizing the mechanisms by which the platelet releasate is changed by maresin 1 will not only enhance our knowledge of the complexities of platelet activation pathways, but could also give insights into how platelet activation could be mitigated in a resolution-friendly manner.
Supplementary Material
ESSENTIALS.
Specialized proresolving mediators (SPMs) promote the resolution of inflammation.
This study sought to investigate the effects of SPMs on human platelet function.
The SPM, Maresin 1, enhanced hemostatic, but suppressed inflammatory functions of platelets.
SPMs uniquely regulate platelet function and may represent a new class of antiplatelet agents.
Acknowledgments
We would like to thank Catherine Burke for help with preliminary screening experiments. We would like to thank our study participants and the Strong Blood Bank and Ann Casey for phlebotomy. This work was supported in part by ES001247, HL095467, T32-AI007285, a University of Rochester grant from Howard Hughes Medical Institute through the Med into Grad Initiative, and UL1RR024160 and UL1TR000042 from the National Center For Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the views of NCRR or NIH.
Footnotes
CONFLICT OF INTEREST
N. Blumberg has received lecture honoraria and consulting fees from Antek, Inc., Fenwal, Pall BioMedical, and Caridian (Terumo), manufacturers of leukoreduction filters, blood component equipment, and cell washing devices. The other authors have nothing to disclose.
ADDENDUM
K. L. Lannan designed and performed research, analyzed data, and wrote the manuscript. S. L. Spinelli, N. Blumberg, and R. P. Phipps contributed to study design, supervised the project, and revised the manuscript.
References
- 1.Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123:2759–67. doi: 10.1182/blood-2013-11-462432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman LM, Aggrey AA, Field DJ, Srivastava K, Ture S, Yui K, Topham DJ, Baldwin WM, Morrell CN. Platelets present antigen in the context of MHC class I. J Immunol. 2012;189:916–23. doi: 10.4049/jimmunol.1200580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garraud O, Berthet J, Hamzeh-Cognasse H, Cognasse F. Pathogen sensing, subsequent signalling, and signalosome in human platelets. Thromb Res. 2011;127:283–6. doi: 10.1016/j.thromres.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Cognasse F, Hamzeh-Cognasse H, Garraud O. Platelets “Toll-like receptor” engagement stimulates the release of immunomodulating molecules. Transfus Clin Biol. 2008;15:139–47. doi: 10.1016/j.tracli.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Radomski MW, Palmer RM, Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987;92:181–7. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lannan KL, Phipps RP, White RJ. Thrombosis, platelets, microparticles and PAH: more than a clot. Drug Discov Today. 2014;19:1230–5. doi: 10.1016/j.drudis.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lannan KL, Refaai MA, Ture SK, Morrell CN, Blumberg N, Phipps RP, Spinelli SL. Resveratrol preserves the function of human platelets stored for transfusion. Br J Haematol. 2016;172:794–806. doi: 10.1111/bjh.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–7. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 9.Serhan C, Brain S, Buckley C, Gilroy D, Haslett C, O’Neill L, Perretti M, Rossi A, Wallace J. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–32. doi: 10.1096/fj.06-7227rev. 21/2/325 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–37. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 11.Serhan C, Chiang N, Van Dyke T. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. nri2294 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ariel A, Chiang N, Arita M, Petasis NA, Serhan CN. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J Immunol. 2003;170:6266–72. doi: 10.4049/jimmunol.170.12.6266. [DOI] [PubMed] [Google Scholar]
- 13.Lee TH, Horton CE, Kyan-Aung U, Haskard D, Crea AE, Spur BW. Lipoxin A4 and lipoxin B4 inhibit chemotactic responses of human neutrophils stimulated by leukotriene B4 and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Clin Sci (Lond) 1989;77:195–203. doi: 10.1042/cs0770195. [DOI] [PubMed] [Google Scholar]
- 14.Godson C, Mitchell S, Harvey K, Petasis N, Hogg N, Brady H. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164: 1663–7. doi: 10.4049/jimmunol.164.4.1663. ji_v164n4p1663 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Oh JY, Giles N, Landar A, Darley-Usmar V. Accumulation of 15-deoxy-delta(12,14)-prostaglandin J2 adduct formation with Keap1 over time: effects on potency for intracellular antioxidant defence induction. Biochem J. 2008;411:297–306. doi: 10.1042/bj20071189. BJ20071189 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramon S, Baker SF, Sahler JM, Kim N, Feldsott EA, Serhan CN, Martínez-Sobrido L, Topham DJ, Phipps RP. The specialized proresolving mediator 17-HDHA enhances the antibody-mediated immune response against influenza virus: a new class of adjuvant? J Immunol. 2014;193:6031–40. doi: 10.4049/jimmunol.1302795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croasdell A, Lacy SH, Thatcher TH, Sime PJ, Phipps RP. Resolvin D1 Dampens Pulmonary Inflammation and Promotes Clearance of Nontypeable Haemophilus influenzae. J Immunol. 2016;196:2742–52. doi: 10.4049/jimmunol.1502331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim N, Ramon S, Thatcher TH, Woeller CF, Sime PJ, Phipps RP. Specialized proresolving mediators (SPMs) inhibit human B-cell IgE production. Eur J Immunol. 2016;46:81–91. doi: 10.1002/eji.201545673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredman G, Van Dyke TE, Serhan CN. Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler Thromb Vasc Biol. 2010;30:2005–13. doi: 10.1161/ATVBAHA.110.209908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. Novel anti-inflammatory--pro-resolving mediators and their receptors. Curr Top Med Chem. 2011;11:629–47. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang N, Dalli J, Colas RA, Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med. 2015;212:1203–17. doi: 10.1084/jem.20150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102:7671–6. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufman J, Spinelli SL, Schultz E, Blumberg N, Phipps RP. Release of biologically active CD154 during collection and storage of platelet concentrates prepared for transfusion. J Thromb Haemost. 2007;5:788–96. doi: 10.1111/j.1538-7836.2007.02412.x. JTH2412 [pii] [DOI] [PubMed] [Google Scholar]
- 25.McEwen BJ. The influence of diet and nutrients on platelet function. Semin Thromb Hemost. 2014;40:214–26. doi: 10.1055/s-0034-1365839. [DOI] [PubMed] [Google Scholar]
- 26.Serhan C. A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Histochem Cell Biol. 2004;122:305–21. doi: 10.1007/s00418-004-0695-8. [DOI] [PubMed] [Google Scholar]
- 27.Ariel A, Serhan C. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–83. doi: 10.1016/j.it.2007.02.007. S1471-4906(07)00049-X [pii] [DOI] [PubMed] [Google Scholar]
- 28.Abdulnour RE, Dalli J, Colby JK, Krishnamoorthy N, Timmons JY, Tan SH, Colas RA, Petasis NA, Serhan CN, Levy BD. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc Natl Acad Sci U S A. 2014;111:16526–31. doi: 10.1073/pnas.1407123111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeo EL, Sheppard JA, Feuerstein IA. Role of P-selectin and leukocyte activation in polymorphonuclear cell adhesion to surface adherent activated platelets under physiologic shear conditions (an injury vessel wall model) Blood. 1994;83:2498–507. [PubMed] [Google Scholar]
- 30.Gawaz M, Vogel S. Platelets in tissue repair: control of apoptosis and interactions with regenerative cells. Blood. 2013;122:2550–4. doi: 10.1182/blood-2013-05-468694. [DOI] [PubMed] [Google Scholar]
- 31.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26:1755–65. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xian W, Wu Y, Xiong W, Li L, Li T, Pan S, Song L, Hu L, Pei L, Yao S, Shang Y. The pro-resolving lipid mediator Maresin 1 protects against cerebral ischemia/reperfusion injury by attenuating the pro-inflammatory response. Biochem Biophys Res Commun. 2016;472:175–81. doi: 10.1016/j.bbrc.2016.02.090. [DOI] [PubMed] [Google Scholar]
- 33.Nordgren TM, Heires AJ, Wyatt TA, Poole JA, LeVan TD, Cerutis DR, Romberger DJ. Maresin-1 reduces the pro-inflammatory response of bronchial epithelial cells to organic dust. Respir Res. 2013;14:51. doi: 10.1186/1465-9921-14-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nordgren TM, Bauer CD, Heires AJ, Poole JA, Wyatt TA, West WW, Romberger DJ. Maresin-1 reduces airway inflammation associated with acute and repetitive exposures to organic dust. Transl Res. 2015;166:57–69. doi: 10.1016/j.trsl.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalli J, Zhu M, Vlasenko NA, Deng B, Haeggström JZ, Petasis NA, Serhan CN. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013;27:2573–83. doi: 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishnamoorthy N, Burkett PR, Dalli J, Abdulnour RE, Colas R, Ramon S, Phipps RP, Petasis NA, Kuchroo VK, Serhan CN, Levy BD. Cutting edge: maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J Immunol. 2015;194:863–7. doi: 10.4049/jimmunol.1402534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akagi D, Chen M, Toy R, Chatterjee A, Conte MS. Systemic delivery of proresolving lipid mediators resolvin D2 and maresin 1 attenuates intimal hyperplasia in mice. FASEB J. 2015;29:2504–13. doi: 10.1096/fj.14-265363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chatterjee A, Sharma A, Chen M, Toy R, Mottola G, Conte MS. The pro-resolving lipid mediator maresin 1 (MaR1) attenuates inflammatory signaling pathways in vascular smooth muscle and endothelial cells. PLoS One. 2014;9:e113480. doi: 10.1371/journal.pone.0113480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Battinelli EM, Markens BA, Italiano JE. Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011;118:1359–69. doi: 10.1182/blood-2011-02-334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.