Abstract
Objective
Major Depressive Disorder is characterized by reduced reward-related striatal activation and dysfunctional reward learning, putatively reflecting decreased dopaminergic signaling. The goal of this study was to test whether a pharmacological challenge designed to facilitate dopaminergic transmission can enhance striatal responses to reward and improve reward learning among depressed individuals.
Method
In a double-blind placebo-controlled design, 46 unmedicated depressed participants and 43 healthy controls were randomized to receive either placebo or a single low dose (50 mg) of the D2/D3 antagonist amisulpride, which was believed to increase dopamine signaling through presynaptic autoreceptor blockade. To investigate the effects of increased dopaminergic transmission on reward-related striatal function and behavior, a monetary incentive delay task (in conjunction with functional magnetic resonance imaging) and a probabilistic reward learning task were administered at absorption peaks of amisulpride.
Results
Depressed participants selected previously rewarded stimuli less frequently than controls, indicating reduced reward learning, but this effect was not modulated by amisulpride. Relative to depressed participants receiving placebo (and controls receiving amisulpride), depressed participants receiving amisulpride exhibited increased striatal activation and potentiated corticostriatal functional connectivity between the nucleus accumbens and midcingulate cortex in response to monetary rewards. Stronger corticostriatal connectivity in response to rewards predicted better reward learning among depressed individuals receiving amisulpride as well as among controls receiving placebo.
Conclusions
Acute enhancement of dopaminergic transmission potentiated reward-related striatal activation and corticostriatal functional connectivity in depressed individuals (but had no behavioral effects), suggesting that targeted pharmacological treatments may normalize neural correlates of reward processing in depression. Thus, despite acute effects on neural function, behavioral modification may require more chronic exposure, fitting prior reports that antidepressant effects of amisulpride in depression emerged after sustained administration.
ClinicalTrials.gov Protocol
2010-P001568
Keywords: Depression, Dopamine, Reward, Striatum, fMRI, Pharmacology
Introduction
Major Depressive Disorder is a highly prevalent psychiatric condition characterized by blunted reward processing and diminished positive affect (1). Preclinical research has shown that phasic dopamine signaling, particularly in the striatum, constitutes an important neural mediator of reward-related behaviors, including reinforcement learning (2, 3) and incentive motivation (4). Functional magnetic resonance imaging (fMRI) studies in humans have corroborated the central role of striatal function in reinforcement learning (5) and reward processing (6), and demonstrated that these striatal functions are disrupted in depression (7, 8). Accordingly, reduced striatal dopamine functioning is believed to play a key role in the pathophysiology of depression, particularly in the context of impaired reward processing and reward learning (9–11). Functional MRI studies have further suggested that reward dysfunction in depression is related to disrupted corticostriatal functional connectivity (12, 13), consistent with the notion that altered communication among dopamine-rich striatal regions and cortical regulatory systems is an important substrate of depression (14).
Despite theories implicating striatal dopamine dysfunction in depression, it is currently unknown whether an acute manipulation thought to transiently increase dopamine signaling might normalize reward processing in depression. Among healthy individuals, studies combining fMRI with acute pharmacologically-induced dopaminergic enhancements have shown increased reward-related striatal responses and improved reward learning, relative to placebo administration (15–17). For instance, acute administration of amisulpride (200 mg) improved healthy participants’ ability to select the better of two rewarding options, purportedly by enhancing reinforcement learning signals in the striatum and ventromedial prefrontal cortex (vmPFC) (15). However, no study to date has tested whether pharmacologically-induced enhancement of dopaminergic transmission can improve reward learning or striatal activity and corticostriatal connectivity in response to reward in depression.
To address these important gaps in the literature, we conducted a double-blind, randomized, placebo-controlled study integrating neural and behavioral measures of reward processing in conjunction with a dopamine pharmacological challenge. To this end, 46 unmedicated depressed individuals and 43 healthy controls were randomized to receive either placebo or a single low dose (50 mg) of the D2/D3 antagonist amisulpride, which has a particularly high affinity for mesolimbic pathways and is believed to increase dopaminergic transmission by means of presynaptic D2/D3 autoreceptor blockade (see (18, 19) and supplementary methods). Following administration of amisulpride or placebo, participants underwent fMRI scanning during a Monetary Incentive Delay task involving anticipation and receipt of monetary rewards and penalties (7). After the scan, participants completed a Probabilistic Selection Task that separately measured the ability to learn from rewards or penalties (20). We selected a 50 mg dose in light of prior reports that a (sustained) 50 mg dosage of amisulpride has anti-depressant and anti-anhedonic effects in depressive disorders (21, 22) and in order to avoid post-synaptic blockade (23), with the goal of maximizing the likelihood of autoreceptor effects. We therefore hypothesized that the current pharmacological manipulation would be associated with increased striatal response to reward and improved reward learning, and furthermore, that such effects would be largest among depressed individuals.
Method
Participants
Participants were recruited from the Boston metropolitan community. The depressed and control groups were matched for age, gender, ethnicity, and years of education (Table 1). Inclusion criteria restricted recruitment to right-handed individuals ages 18 to 45 with no MRI contraindications, no lifetime substance dependence or substance abuse within the last 12 months, and no serious medical conditions. For the depression group, inclusion required a diagnosis of Major Depressive Disorder according to the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I/NP) (24). Exclusion criteria for depressed participants were use of any psychotropic medication in the past two weeks (six weeks for fluoxetine, six months for dopaminergic drugs or neuroleptics) and psychiatric history of other major Axis I disorders. For the control group, inclusion criteria included medication-free status for at least three weeks, absence of current or past psychiatric illnesses (SCID-I/NP), and absence of first-degree familial psychiatric illness. Participants received $15/hr in compensation plus earnings in the fMRI task and provided written informed consent to a protocol approved by Partners Human Research Committee.
Table 1.
Demographic and clinical characteristics by group.
| Depressed + Amisulpride (N=23) | Depressed + Placebo (N=23) | Controls + Amisulpride (N=23) | Controls + Placebo (N=20) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (S.D.) | ||||||||
|
| ||||||||
| Age (years) | 27.7 | (8.2) | 26.3 | (5.2) | 26.5 | (6.8) | 25.3 | (5.6) |
| Education (years) | 10.9 | (5.1) | 11.4 | (6.1) | 13.0 | (5.0) | 12.9 | (5.2) |
| a Beck Depression Inventory, 2nd Ed. (BDI-II) | 26.6 | (8.1) | 28.0 | (9.4) | 1.3 | (2.1) | 1.7 | (2.6) |
| a Mood and Anxiety Symptom Questionnaire (MASQ) total score | 170.3 | (15.0) | 176.6 | (25.0) | 91.0 | (13.5) | 91.3 | (14.2) |
| a MASQ General Distress Depression (GDD) sub-scale score | 39.3 | (7.9) | 39.3 | (8.9) | 14.3 | (3.1) | 14.4 | (2.7) |
| a MASQ Anhedonic Depression (AD) sub-scale score | 86.7 | (9.3) | 85.2 | (9.3) | 43.4 | (7.9) | 46.7 | (9.9) |
| a MASQ General Distress Anxiety (GDA) sub-scale score | 22.2 | (5.6) | 25.8 | (6.9) | 13.8 | (2.8) | 12.3 | (1.8) |
| a MASQ Anxious Arousal (AA) sub-scale score | 22.1 | (4.2) | 26.3 | (8.7) | 19.4 | (3.1) | 17.9 | (1.3) |
| a Snaith–Hamilton Pleasure Scale (SHAPS) | 32.9 | (4.5) | 32.8 | (6.2) | 21.5 | (6.0) | 22.6 | (6.8) |
| Length of the current MDE (months) | 18.1 | (16.4) | 21.3 | (42.2) | -- | -- | -- | -- |
| Numbers of past depressive episodes | 3.9 | (3.0) | 4.6 | (3.2) | -- | -- | -- | -- |
|
| ||||||||
| n (%) | ||||||||
|
| ||||||||
| Female | 21 | (91.3) | 16 | (69.6) | 18 | (78.3) | 15 | (75.0) |
| Caucasian | 10 | (43.5) | 10 | (43.5) | 10 | (43.5) | 5 | (25.0) |
| Handedness (right) | 23 | (100) | 23 | (100) | 23 | (100) | 20 | (100) |
| Current comorbid anxiety disorders | 3 | (13.0) | 2 | (8.7) | -- | -- | -- | -- |
| Past comorbid anxiety disorders | 3 | (13.0) | 2 | (8.7) | -- | -- | -- | -- |
Main effect of Diagnosis in a factorial ANOVA with Diagnosis (Depressed vs. Controls) and Drug (Amisulpride vs. Placebo) as between-subjects variables. No effects of Drug, or Diagnosis by Drug interactions, were significant.
Procedure
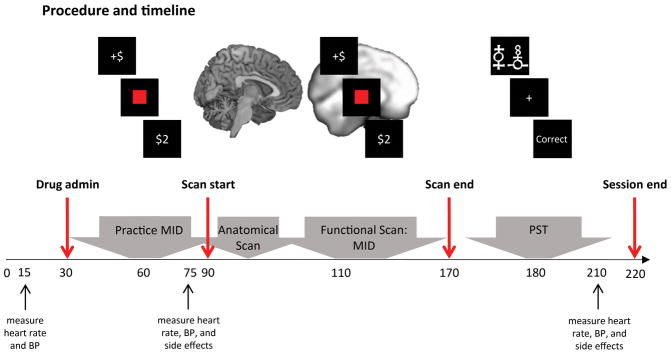
Participants first completed a clinical evaluation to determine eligibility (SCID-I/NP), and self-report measures of depression and anhedonia (Table 1 and supplementary methods). Eligible participants were invited to take part in the neuroimaging session; at the beginning of this session the study physician administered either amisulpride or placebo. Participants were randomly selected to receive amisulpride or placebo under double-blind conditions. Pharmacokinetic data indicate that plasma concentration of amisulpride has two peaks, at approximately 1–1.5 hours, and 2.5 hours, after administration (18, 19). Therefore, fMRI scanning of the Monetary Incentive Delay task started 1 hour after drug (or placebo) administration. The Probabilistic Selection Task was administered after scan completion, approximately 2.5 hours post amisulpride or placebo administration, to coincide with the second plasma concentration peak. Heart rate, blood pressure, and side effects were assessed by the study physician throughout the session (Figure 1).
Figure 1.
Procedure and timeline. Upon arrival to the scanning session participants completed a pre-MRI safety screening form and provided a urine sample for drug and pregnancy (if applicable) testing. Participants’ heart rate and blood pressure were then examined by the study physician. Next, the study physician administered a capsule of either amisulpride or placebo to participants. Participants were randomly selected to receive amisulpride or placebo, and the study physician and members of the research team were blind to the assignment of participants to active or placebo conditions. Participants then waited for one hour in a quiet room to allow amisulpride plasma concentration to peak (18, 19). During the waiting period participants practiced the Monetary Incentive Delay Task. Forty-five minutes post-drug administration, the study physician measured participants’ heart rate and blood pressure for the second time, and participants were asked to complete a drug side-effects questionnaire. Next, participants completed an (approximately 1.5 hour long) MRI scan that included structural scans and a functional scan while completing the Monetary Incentive Delay Task. Following the scan, and approximately 2.5 hours post-drug administration, participants completed the Probabilistic Selection Task (administered to coincide with the second peak in amisulpride plasma concentration (18, 19)). After completing the Probabilistic Selection Task, participants’ heart rate, blood pressure and side-effects were reexamined by the study physician and then, upon the physician’s approval, participants were debriefed, paid, and discharged. BP - Blood Pressure; MID - Monetary Incentive Delay; PST - Probabilistic Selection Task.
Functional MRI task
The Monetary Incentive Delay task involves anticipation and receipt of monetary rewards and penalties, which have been shown to elicit robust striatal response in healthy individuals (25). Previous studies with this task revealed reduced striatal activation and functional connectivity in depressed versus healthy adults during anticipation and receipt of monetary reward (7, 26), making it well-suited for the present study (supplementary methods).
Behavioral task
A Probabilistic Selection Task was used to probe learning from positive and negative feedback (20). In the learning phase, participants repeatedly viewed three pairs of stimuli (AB, CD, and EF), and had to integrate feedback over several trials to learn which stimulus in each pair was rewarded most consistently. In the test phase, the most reliably rewarded (A) and penalized (B) stimuli were presented in conjunction with all other stimuli (e.g., AC, AD, AE, AF); participants’ ability to “Choose A” or to “Avoid B” were used as measures of reward or penalty learning, respectively (supplementary methods).
MRI acquisition parameters
fMRI data analysis
fMRI data were preprocessed using Statistical Parametric Mapping (SPM12; Wellcome Department of Cognitive Neurology, London, UK). Preprocessing included coregistration of functional and anatomical images, segmentation, nonlinear volume-based spatial normalization (MNI), and spatial smoothing with a Gaussian filter (6mm FWHM).
Hemodynamic responses were modeled using a canonical hemodynamic response function that was convolved with the onset times of task regressors in order to compute a general linear model (GLM) at the single subject level. The GLM included nine task-related regressors: three cues (Reward, Penalty, No-incentive), the target, and five outcomes (Win [reward outcome following reward cue], No-Win [no-change outcome following reward cue], Loss [penalty outcome following penalty cue], No-Loss [no-change outcome following penalty cue], and No-Change [no-change outcome following no-incentive cue]). The GLM also included high-pass temporal filtering (0.008 Hz), seven rigid-body movement parameters, nuisance regressors accounting for no-response trials, and outlier time points (supplementary methods).
To test a priori hypotheses regarding striatal responses to reward (7), we conducted a region of interest (ROI) analysis in which activations (beta weights) were extracted from anatomical masks of the caudate, Nucleus Accumbens (Nacc) and putamen for each participant and for each task regressor (relative to baseline). To avoid any biases, masks were defined using a manually segmented MNI-152 brain and implemented as overlays on the SPM12 canonical brain (see supplementary Figure 1 as well as (27) for anatomically defined masks). Activations reported throughout the text were quantified by averaging beta weights from all voxels within a mask. Exploratory whole brain analyses were also conducted (supplementary methods and results).
Psychophysiological interaction (PPI) analyses were performed to examine the effects of reward and penalty outcomes on striatal functional connectivity. Because hemispheric effects on task activation were non-significant (see Results), striatal masks were collapsed across hemispheres, yielding three bilateral seeds (caudate, Nacc, putamen). Analyses retained the subject-level GLMs described above, adding regressors corresponding to the seed timecourse and the interaction of the seed timecourse with the task condition of interest (separately for reward and penalty outcome). Single subject connectivity maps for the interaction between each seed time-course and the regressor of interest were entered into second-level whole brain random effects analysis. Effects were thresholded at peak p<0.001, whole brain family-wise error (FWE) corrected to p<0.05 at the cluster-level.
Statistical analyses
Results
Behavioral results
Accuracy in “Choose A” and “Avoid B” trials of the Probabilistic Selection Task test phase were used as measures of reward and penalty learning, respectively. A repeated-measures ANOVA with learning Type (“Choose A” and “Avoid B” accuracy) as the within-subject variable, and Diagnosis (Depressed vs. Controls) and Drug (Amisulpride vs. Placebo) as between-subject variables, revealed no signification main effects or interactions (supplementary Figure 2A). Because the primary focus of this work was reward processing, we also performed analyses that separately probed group differences in reward learning (which may be driven by a mixture of reward responsiveness and learning ability) and penalty learning (which may be driven by both penalty sensitivity and learning ability). Factorial ANOVAs were conducted separately with either reward or penalty learning (i.e., accuracy in “Choose A” and “Avoid B” trials, respectively) as the dependent variable, and with Diagnosis (Depressed vs. Controls) and Drug (Amisulpride vs. Placebo) as between-subject variables. For reward learning, there was a main effect of Diagnosis (F(1,75) = 6.28, p=0.014), due to reduced reward learning in depressed compared to control individuals. No significant group differences in penalty learning were observed. Thus, depressed participants exhibited impaired reward learning, but not penalty learning, relative to controls, and this impairment was not affected by drug administration. Nevertheless, the lack of a significant Type (“Choose A” and “Avoid B” accuracy) by Diagnosis (Depressed vs. Controls) interaction in the repeated-measures ANOVA precludes us from drawing strong inference about the specificity of these findings. No other significant effects of Diagnosis or Drug emerged across behavioral analyses of either experimental task (supplementary results).
Striatal response to cues
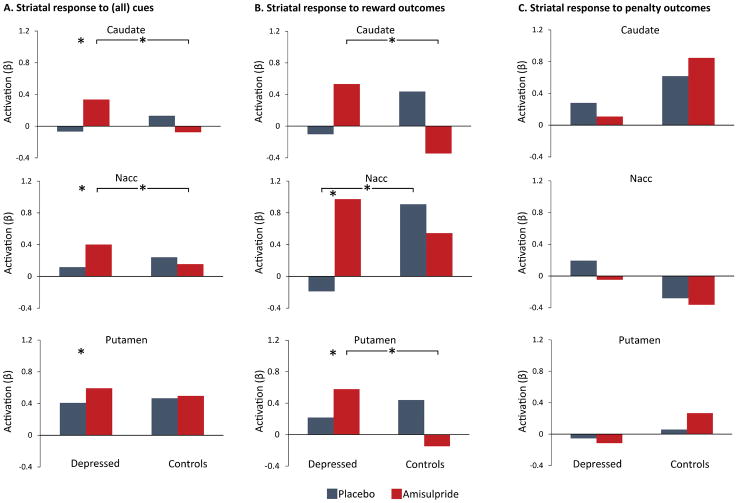
A repeated measures ANOVA was performed for each striatal region with the following factors: Hemisphere (Left vs. Right) and Cue (Reward, Penalty, No-incentive) as within-subject variables, and Diagnosis (Depressed vs. Controls) and Drug (Amisulpride vs. Placebo) as between-subject variables. These analyses revealed a main effect of Cue in all three regions (caudate: F(2,170) = 56.55, p < 0.001; Nacc: F(2,170) = 61.33, p < 0.001; putamen: F(2,170) = 40.31, p < 0.001). Consistent with prior studies (7), post-hoc analyses indicated that this effect was driven by increased striatal responses to reward cues, followed by penalty cues, followed by no-incentive cues (supplementary Figure 3). Relevant to the study hypotheses, a Diagnosis by Drug interaction also emerged for all regions (caudate: F(1,85) = 9.65, p = 0.003; Nacc (trend): F(1,85) = 3.35, p = 0.071; putamen: F(1,85) = 5.84, p = 0.018). These effects were driven by increased striatal response to cues (regardless of cue type) in depressed participants receiving amisulpride relative to depressed participants receiving placebo (Caudate: p = 0.022; Nacc: p = 0.036; Putamen: p = 0.049), and relative to control participants receiving amisulpride (Caudate: p = 0.017; Nacc (trend): p = 0.063). Together, these results indicate that amisulpride enhanced striatal responses to cues, regardless of cue valance, in depressed but not healthy participants (Figure 2A).
Figure 2.
Striatal response to cues and outcomes. (A) Striatal response to cues, across all type, was greater in depressed participants receiving amisulpride compared to depressed participants receiving placebo (Caudate: p = 0.022; Nacc: p = 0.036; Putamen: p = 0.049), as well as compared to healthy control participants receiving amisulpride (Caudate: p = 0.017; Nacc (trend): p = 0.063). (B) Striatal response to reward outcomes was greater in depressed participants receiving amisulpride compared to depressed participants receiving placebo (Nacc, p = 0.007; Putamen, p = 0.050), as well as compared to control participants receiving amisulpride (Caudate, p = 0.044; Putamen, p = 0.003). Nacc activation in response to reward outcome was also higher in controls receiving placebo relative to depressed participants receiving placebo (p = 0.026). (C) Striatal response to penalty outcomes did not differ across groups and there was no consistent pattern across regions in the penalty condition.
Striatal response to outcomes
A repeated measures ANOVA was performed for each striatal region with the following factors: Hemisphere (Left vs. Right) and Outcome (Reward Outcome vs. Penalty Outcome) as within-subject variables, and Diagnosis (Depressed vs. Controls) and Drug (Amisulpride vs. Placebo) as between-subject variables. These analyses revealed a main effect of Outcome in the Nacc (F(1,85) = 11.30, p = 0.001) related to greater Nacc activation to rewards than penalties across participants. Critically, all three striatal regions showed an Outcome by Diagnosis by Drug interaction (caudate: F(1,85) = 4.64, p = 0.034; Nacc (trend): F(1,85) = 3.17, p = 0.078; putamen: F(1,85) = 6.73, p = 0.011). As shown in Figure 2B, amisulpride administration in depressed individuals enhanced striatal response to reward outcomes relative to placebo administration (Nacc, p = 0.007; Putamen, p = 0.050), and relative to amisulpride administration in controls (Caudate, p = 0.044; Putamen, p = 0.003). Nacc response to reward outcome was also greater in controls receiving placebo than in depressed participants receiving placebo (p = 0.026). In contrast, no significant group differences emerged in striatal response to penalty outcome (Figure 2C). In sum, amisulpride selectively enhanced striatal response to reward outcomes, but not penalty outcomes, in depressed (but not healthy) participants.
Striatal connectivity in response to outcomes
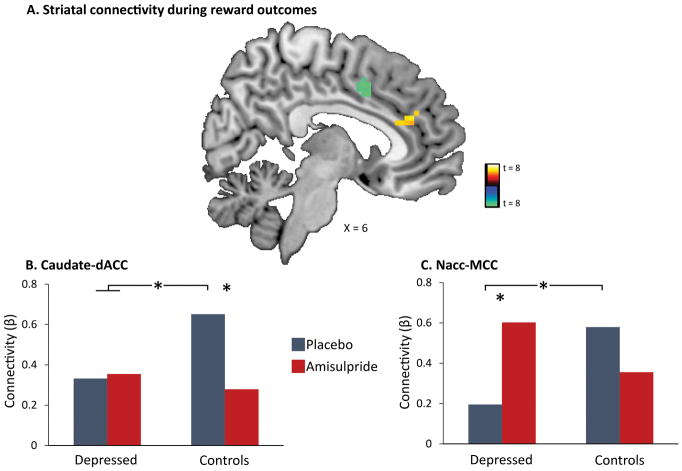
Whole-brain PPI analyses were conducted to separately investigate the effects of reward and penalty outcomes on striatal functional connectivity. A whole brain Diagnosis (Depressed vs. Controls) by Drug (Amisulpride vs. Placebo) ANOVA revealed no significant group differences for striatal connectivity in response to reward or penalty outcomes at peak p<0.001, whole brain FWE p<0.05. Next, striatal connectivity at the whole brain level was investigated across the entire sample (n = 89). These analyses revealed that in response to reward, but not penalty, outcomes, participants exhibited increased functional connectivity between bilateral caudate and a region (k=22 voxels) of the dorsal anterior cingulate cortex (dACC), as well as between bilateral Nacc and a region (k=13 voxels ) of the midcingulate cortex (MCC) (Figure 3A and supplementary Table 1). Post-hoc analyses were conducted to investigate whether depression or amisulpride moderated these reward-related corticostriatal connectivity patterns. To this end, caudate-dACC and Nacc-MCC connectivity values were extracted and used as the dependent variables in mixed-effect ANOVAs with Diagnosis (Depressed vs. Controls) and Drug (Amisulpride vs. Placebo) as between-subject variables. For both analyses investigating caudate-dACC as well as Nacc-MCC connectivity, significant Diagnosis by Drug interactions emerged (F(1,85) = 4.26, p = 0.043; F(1,85) = 6.25, p = 0.015, respectively). Post-hoc analyses revealed that control participants receiving placebo exhibited stronger reward-related caudate-dACC functional connectivity relative to all other three groups (all p’s < 0.033; Figure 3B). With regard to Nacc-MCC functional connectivity, both control participants receiving placebo and depressed participants receiving amisulpride showed stronger connectivity than depressed participants receiving placebo (p = 0.037 and p = 0.022, respectively, Figure 3C).
Figure 3.
Striatal connectivity in response to reward outcomes. (A) Whole-brain psychophysiological interaction (PPI) analyses revealed increased functional connectivity between the bilateral caudate and regions of the dorsal anterior cingulate cortex (dACC) (yellow), and between the bilateral nucleus accumbens (Nacc) and regions of the midcingulate cortex (MCC) (green) in response to reward outcomes across the entire sample at peak p<0.001, FWE p<0.05. No changes in striatal connectivity were found in response to penalty outcomes. (B) Caudate-dACC functional connectivity was significantly higher in control participants receiving placebo relative to all other groups (all p’s < 0.033). (C) Nacc-MCC functional connectivity was significantly higher in both controls receiving placebo and depressed participants receiving amisulpride than in depressed participants receiving placebo (p = 0.037; p = 0.022, respectively).
Striatal connectivity during reward outcomes and reward learning
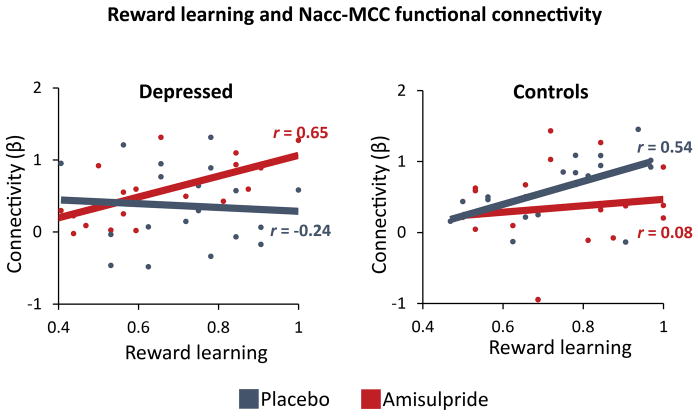
Given the observed effects of amisulpride on Nacc-MCC functional connectivity in depressed participants, multiple regression analyses were conducted to investigate the relationship between reward-related Nacc-MCC connectivity and reward learning. Specifically, Diagnosis (Depressed coded as +1, Controls coded as −1), Drug (Amisulpride coded as +1, Placebo coded as −1), Reward Learning (“Choose A” accuracy from the Probabilistic Selection Task), and their interactions, were regressed on reward-related Nacc-MCC functional connectivity. Results revealed a significant Diagnosis by Drug by Reward Learning interaction (F(1,67) = 5.76, p = 0.019). Post-hoc simple regression analyses within each group revealed positive relationships between reward learning and reward-related Nacc-MCC functional connectivity in depressed participants receiving amisulpride (β = 0.65, p = 0.003), and control participants receiving placebo (β = 0.54, p = 0.029), but not for depressed participants receiving placebo (β = −0.24, p = 0.35) or controls receiving amisulpride (β = 0.08, p = 0.74) (Figure 4). These results indicate that amisulpride administration enhanced Nacc-MCC functional connectivity in response to reward in depressed individuals to a level comparable to that exhibited by healthy controls receiving placebo. Furthermore, the magnitude of Nacc-MCC functional connectivity for both depressed individuals receiving amisulpride and controls receiving placebo was positively associated with reward learning in the Probabilistic Selection Task.
Figure 4.
Reward learning and Nacc-MCC functional connectivity. Regression analyses revealed positive relationships between reward learning and reward-related nucleus accumbens to midcingulate (Nacc-MCC) functional connectivity in depressed participants receiving amisulpride (β = 0.65, p = 0.003) and healthy control participants receiving placebo (β = 0.54, p = 0.029), but not in depressed participants receiving placebo (β = −0.24, p = 0.354) or controls participants receiving amisulpride (β = 0.08, p = 0.740). Reward learning - “Choose A” accuracy from the Probabilistic Selection Task.
Discussion
Major depression is a debilitating psychiatric disorder characterized by high rates of relapse and recurrence. Discovering treatment tools that target putative mechanisms of illness in depression – such as blunted response to reward – is therefore a key clinical priority. Findings from the current proof-of-mechanism study suggest that an acute pharmacological challenge transiently increased striatal response to reward among adults with Major Depressive Disorder, putatively via enhancement of dopaminergic transmission owing to autoreceptor blockade. Specifically, depressed individuals receiving amisulpride exhibited increased striatal activity in response to cues, and increased striatal activity and corticostriatal functional connectivity in response to reward outcomes. Furthermore, stronger corticostriatal functional connectivity between the nucleus accumbens and midcingulate cortex in depressed individuals who received amisulpride was associated with better reward learning performance, a pattern similar to that observed in healthy controls receiving placebo. Together, these results provide converging evidence for abnormalities in neural reward systems in depression, and highlight the potential of targeted pharmacological treatments to normalize reward processing in depression.
Extensive preclinical research has emphasized the key role of striatal dopamine signaling in mediating reward-related behaviors (2–4), and postulated links between reduced striatal dopamine function and blunted reward processing and reinforcement learning in depression (9, 10). Interestingly, previous research indicates that dopamine differentially mediates anticipatory and consummatory phases of reward processing (28), and thus may uniquely impact their putative dysfunction in anhedonia and depression (29). In support of this idea, we observed that acute amisulpride administration enhanced striatal response to cues regardless of valence (e.g., signaling potential rewards, penalties, or null outcomes), yet in response to outcomes, striatal enhancement was selective to reward.
In addition to increasing striatal activity in response to rewards, enhancement of dopamine signaling in depressed individuals was also associated with amplified functional connectivity between the striatum and areas of midcingulate cortex. This finding is consistent with a model in which abnormal coordinated activity among large-scale brain circuits, including corticostriatal pathways, is central to the pathophysiology of depression (30, 31). Critically, those depressed individuals who exhibited the strongest Nacc-MCC connectivity in response to rewards after amisulpride administration also exhibited better reward learning in an independent behavioral task, and this pattern was not found among depressed individuals who received placebo. Of relevance to the present findings, increased functional connectivity has been observed between midcingulate and striatal regions (and insula) during learning (32), supporting the importance of this corticostriatal sub-circuit in dopamine-mediated functioning. Coordination between dopamine-rich areas of striatum and midline regions involved in processing behavioral salience may therefore be an important dimension of healthy reinforcement learning, and dopamine enhancement may help to regulate this functional circuit in depression. In fact, given preclinical evidence that amisulpride has a particularly high affinity for mesolimbic pathways (18, 19), one may speculate that amisulpride may enhance striatal function by affecting regulatory mechanisms beyond the striatum, and in particular in regions of the mesocorticolimbic pathway that communicate with the striatum via dopaminergic signaling to enable reward motivation and reinforcement learning (29). Thus, while in the present study we investigated the effects of amisulpride on striatal functioning, other brain systems that have exhibited abnormal activity or functional connectivity in depression (e.g., prefrontal cortex) may be important targets of dopamine manipulation.
Several additional questions remain open for future investigation. First, evidence from preclinical studies linking reinforcement learning and motivation with phasic dopamine signaling in the striatum suggests that amisulpride enhancement of reward processing among depressed individuals most likely occurs via increased phasic dopamine signaling (2–4). Nevertheless, the mechanisms by which amisulpride may act to enhance striatal response to reward are complex, and may involve modifications of phasic and tonic levels of dopamine, as well as of additional neurotransmitters (33, 34). Future research, especially in humans, investigating the effects of amisulpride on tonic and phasic dopamine release is needed. A second area for future investigation is motivated by differences between our findings and the results of prior investigations in which dopaminergic manipulation in healthy individuals resulted in better reward learning and increased striatal activity. The modest amisulpride dosage of the present study (50 mg as opposed to 200 mg in (15), or 400 mg in (35)) may have contributed to these discrepancies. Here we selected a 50 mg dosage based on animal work showing that low doses of amisulpride potentiate striatal dopamine release, have strong hedonic effects, and increase the incentive value of environmental cues (18, 19). In humans, a 50 mg dosage of amisulpride has been associated with reduced blockade of postsynaptic D2/D3 receptor in comparison to higher doses of 200–400 mg (23), increasing the likelihood of presynaptic effects. Perhaps more importantly, (sustained) 50 mg amisulpride dosing has been shown to have anti-depressant and anti-anhedonic effects in depressive disorders (21, 22), suggesting that the present pharmacological manipulation may preferentially benefit depressed individuals as compared with their healthy peers. Nevertheless, while the pharmacological manipulation enhanced striatal function among depressed individuals, it had no such effect on behavior (i.e., reward learning). One potential reason for this could relate to the fact that we only implemented a single administration of the drug. Thus, while the drug may have an immediate effect on neural function, modifying behavior may require longer and more chronic exposure. In support of this idea, antidepressant effects of amisulpride among depressed individuals have been observed following sustained (but not acute) administration (21, 22).
In conclusion, among depressed individuals (but not controls), acute pharmacological challenge transiently increased striatal activity and corticostriatal functional connectivity in response to rewards, putatively via enhancement of dopaminergic transmission. These findings suggest that an acute pharmacological manipulation believed to increase dopamine transmission may help normalize reward processing in depressed individuals through the enhancement of key corticostriatal mechanisms.
Supplementary Material
Acknowledgments
This research was made possible by NIMH R01 grants MH068376 and MH101521 awarded to Dr. Pizzagalli. Dr. Admon was supported by a Brain & Behavior Research Foundation Young Investigator Award and The Adam Corneel Young Investigator Award. Dr. Kaiser and Dr. Dillon were supported by F32MH106262 and K99MH094438 grants, respectively. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIMH, or the National Institutes of Health.
Footnotes
Previous presentations:
Annual Convention of the Anxiety and Depression Association of America (ADAA), Miami, FL: April 2015.
Location of work and address for reprints:
Center for Depression, Anxiety and Stress Research, McLean Hospital, 115 Mill Street, Belmont, MA 02478
Disclosures
All authors contributed substantially to the content of this manuscript and none have conflicts of interest, financial or otherwise, that may have influenced the way data was collected, analyzed, or reported. In the past three years, Dr. Pizzagalli has received honoraria/consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Otsuka America Pharmaceutical, and Pfizer for activities unrelated to this project.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: 2013. [Google Scholar]
- 2.Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Wise RA. Dopamine, learning and motivation. Nature Reviews Neuroscience. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 4.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 5.Chase HW, Kumar P, Eickhoff SB, Dombrovski AY. Reinforcement learning models and their neural correlates: An activation likelihood estimation meta-analysis. Cognitive, affective & behavioral neuroscience. 2015;15:435–459. doi: 10.3758/s13415-015-0338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. The American Journal of Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C, Takahashi T, Nakagawa S, Inoue T, Kusumi I. Reinforcement learning in depression: A review of computational research. Neuroscience and Biobehavioral Reviews. 2015;55:247–267. doi: 10.1016/j.neubiorev.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Archives of General Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 10.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biological Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Admon R, Pizzagalli DA. Dysfunctional Reward Processing in Depression. Current Opinion in Psychology. 2015;4:114–118. doi: 10.1016/j.copsyc.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Admon R, Pizzagalli DA. Cortico-striatal pathways contribute to the natural time course of positive mood. Nature Communications. 2015 doi: 10.1038/ncomms10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature reviews Neuroscience. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jocham G, Klein TA, Ullsperger M. Dopamine-mediated reinforcement learning signals in the striatum and ventromedial prefrontal cortex underlie value-based choices. The Journal of Neuroscience. 2011;31:1606–1613. doi: 10.1523/JNEUROSCI.3904-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhury R, Guitart-Masip M, Lambert C, Dayan P, Huys Q, Duzel E, Dolan RJ. Dopamine restores reward prediction errors in old age. Nature Neuroscience. 2013;16:648–653. doi: 10.1038/nn.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoemaker H, Claustre Y, Fage D, Rouquier L, Chergui K, Curet O, Oblin A, Gonon F, Carter C, Benavides J, Scatton B. Neurochemical characteristics of amisulpride, an atypical dopamine D2/D3 receptor antagonist with both presynaptic and limbic selectivity. The Journal of Pharmacology and Experimental Therapeutics. 1997;280:83–97. [PubMed] [Google Scholar]
- 19.Coukell AJ, Spencer CM, Benfield P. Amisulpride: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of schizophrenia. CNS Drugs. 1996;6:237–256. [Google Scholar]
- 20.Frank MJ, Seeberger LC, O’Reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- 21.Amore M, Jori MC Investigators A. Faster response on amisulpride 50 mg versus sertraline 50–100 mg in patients with dysthymia or double depression: a randomized, double-blind, parallel group study. International Clinical Psychopharmacology. 2001;16:317–324. doi: 10.1097/00004850-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Cassano GB, Jori MC, Group A. Efficacy and safety of amisulpride 50 mg versus paroxetine 20 mg in major depression: a randomized, double-blind, parallel group study. International Clinical Psychopharmacology. 2002;17:27–32. doi: 10.1097/00004850-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 23.la Fougere C, Meisenzahl E, Schmitt G, Stauss J, Frodl T, Tatsch K, Hahn K, Moller HJ, Dresel S. D2 receptor occupancy during high- and low-dose therapy with the atypical antipsychotic amisulpride: a 123I-iodobenzamide SPECT study. Journal of Nuclear Medicine. 2005;46:1028–1033. [PubMed] [Google Scholar]
- 24.First BM, Spitzer LR, Gibbon M, Williams BWJ. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 25.Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- 26.Admon R, Nickerson LD, Dillon DG, Holmes AJ, Bogdan R, Kumar P, Dougherty DD, Iosifescu DV, Mischoulon D, Fava M, Pizzagalli DA. Dissociable cortico-striatal connectivity abnormalities in major depression in response to monetary gains and penalties. Psychological Medicine. 2015;45:121–131. doi: 10.1017/S0033291714001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Admon R, Holsen LM, Aizley H, Remington A, Whitfield-Gabrieli S, Goldstein JM, Pizzagalli DA. Striatal Hypersensitivity During Stress in Remitted Individuals with Recurrent Depression. Biological Psychiatry. 2015;78:67–76. doi: 10.1016/j.biopsych.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berridge KC, Robinson TE. Parsing reward. Trends in neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 29.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neuroscience and Biobehavioral Reviews. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchetti I, Koster EH, Sonuga-Barke EJ, De Raedt R. The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychology Review. 2012;22:229–251. doi: 10.1007/s11065-012-9199-9. [DOI] [PubMed] [Google Scholar]
- 31.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Limongi R, Sutherland SC, Zhu J, Young ME, Habib R. Temporal prediction errors modulate cingulate-insular coupling. NeuroImage. 2013;71:147–157. doi: 10.1016/j.neuroimage.2012.12.078. [DOI] [PubMed] [Google Scholar]
- 33.Nikiforuk A, Popik P. Amisulpride promotes cognitive flexibility in rats: the role of 5-HT7 receptors. Behavioural Brain Research. 2013;248:136–140. doi: 10.1016/j.bbr.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Meneses A, Perez-Garcia G, Liy-Salmeron G, Ponce-Lopez T, Lacivita E, Leopoldo M. 5-HT7 receptor activation: procognitive and antiamnesic effects. Psychopharmacology. 2015;232:595–603. doi: 10.1007/s00213-014-3693-0. [DOI] [PubMed] [Google Scholar]
- 35.Jocham G, Klein TA, Ullsperger M. Differential modulation of reinforcement learning by D2 dopamine and NMDA glutamate receptor antagonism. The Journal of Neuroscience. 2014;34:13151–13162. doi: 10.1523/JNEUROSCI.0757-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.