Summary
Background
Occlusion of vein grafts (VGs) after bypass surgery due to thrombosis and intimal hyperplasia (IH) is a major clinical problem. Apyrases are enzymes that scavenge extracellular ATP and ADP and promote adenosine formation at sites of vascular injury and hence have potential to inhibit vein graft pathology.
Objectives
We examined the effects of recombinant soluble human apyrase, APT102, on platelets, smooth muscle cells (SMCs), and endothelial cells (ECs) in vitro and thrombosis and IH in murine VGs.
Methods
SMC and EC proliferation and migration were studied in vitro. Inferior vena cava segments from donor mice were grafted into carotid arteries of recipient mice.
Results
While potently inhibiting ADP-induced platelet aggregation and VG thrombosis, APT102 did not impair surgical hemostasis. APT102 did not directly inhibit SMC or EC proliferation, but significantly attenuated the effects of ATP on SMC and EC proliferation. APT102 significantly inhibited SMC migration, but did not inhibit EC migration, which may be mediated, at least in part, by inhibition of SMC, but not EC, migration by adenosine. At 4 weeks after surgery, IH was significantly less in VGs of APT102-treated mice than in control VGs. APT102 significantly inhibited cell proliferation in VGs, but did not inhibit re-endothelialization.
Conclusions
Systemic administration of a recombinant human apyrase inhibits thrombosis and IH in VGs without increasing bleeding or compromising re-endothelialization. These results suggest that APT102 has the potential to become a novel, single-drug treatment strategy to prevent multiple pathological processes that drive early adverse remodeling and occlusion of VGs.
Keywords: adenine nucleotides, apyrase, coronary artery bypass, neointima, venous thrombosis
Approximately 400,000 coronary artery bypass graft (CABG) surgeries are performed annually in the United States. The saphenous vein is the most commonly used bypass graft. After CABG, vein grafts undergo significant structural and functional changes, driven by differences in blood pressure and sheer stress between the venous and arterial circulations. While some changes that occur in vein grafts represent adaptive responses, maladaptive responses are common and can lead to vein graft occlusion, which occurs in approximately 20% and 40% of vein grafts within 1 and 10 year after surgery, respectively [1]. Early graft failures are nearly always due to thrombosis. The process of intimal hyperplasia begins days to weeks after surgery. Excessive intimal hyperplasia produces vein graft stenosis and is an important component of atherosclerosis, a late complication of vein graft surgery. While substantial advances in the prevention and treatment of native coronary artery disease have been achieved over the past 20 years, through the development of antiplatelet and lipid-lowering drugs and drug-eluting stents, comparatively little progress has been made in improving the performance of saphenous vein grafts. Hence, there is a pressing need to develop new treatments to improve the durability of vein grafts [2].
Amongst the many molecular and cellular signaling pathways that drive vein graft remodeling, purinergic signaling pathways play important roles [3, 4]. Adenosine diphosphate (ADP) and adenosine triphosphate (ATP) are released from activated and damaged cells within vein grafts [5]. These extracellular nucleotides activate platelets, endothelial cells (ECs), vascular smooth muscle cells (SMCs), and immune cells, all of which contribute to vein graft pathology [6–8]. Human apyrases are a family of enzymes that hydrolyze extracellular ATP to ADP, as well as ADP to adenosine monophosphate (AMP), thereby inhibiting thrombotic, inflammatory, and proliferative pathways mediated by binding of ATP and ADP to P2Y receptors on blood platelets and vascular wall cells [9]. In addition to catabolizing ATP and ADP, apyrases promote formation of adenosine, which is generated from AMP by CD73. Adenosine inhibits inflammation and SMC proliferation [10, 11], while promoting vasodilation [12]. Together, these effects of apyrases would be expected to produce beneficial effects on vein grafts.
Ectonucleotide tri[di]phosphohydrolase-1 [ENTPD1], also known as ecto-ADPase or CD39, is an apyrase expressed on the surface of vascular ECs and some leukocytes [13]. APT102 is a recombinant, soluble form of CD39L3, which consists of the extracellular domain of the protein, into which amino acid substitutions have been introduced that increase enzyme activity and plasma half-life [14]. Given the antithrombotic, anti-proliferative, and other potential beneficial effects of apyrase, we hypothesized that APT102 would prove useful in preventing vein graft disease. We tested this hypothesis in a murine model of vein graft surgery.
Materials and Methods
Mice
C57BL/6J male mice from Envigo received normal chow (5008/LabDiet). Animal care and experimental procedures were approved by the University of Missouri Animal Care and Use Committee. A total of 129 mice were used to perform all the experiments.
Cells
Human coronary artery SMCs were from Invitrogen. Human aortic ECs were from ATCC. Passage number of SMCs and ECs was 3 or 4.
APT102 and other reagents
APT102 was expressed in CHO cells and purified to homogeneity, as described [14]. Protein purity was >99% by SDS-PAGE and HPLC analyses. Endotoxin level of recombinant protein was <1.0 EU/mg. APT102 used in all experiments was dissolved in 10 mM Tris-HCl, 150 mM NaCl, pH 7.4 (TBS). ATP, ADP, and adenosine were from Sigma-Aldrich.
Cell proliferation
Serum-starved SMCs and ECs, harvested by trypsin digestion, were suspended in Medium 231/SMGS and Medium 200/LVES, respectively (media were from Life Technologies). Cells (1.25×104) were placed in 24-well cell culture plates and incubated under standard conditions. After 24 hours, conditioned media were replaced with fresh media containing ATP (10 µM), APT102 (100 nM), both ATP and APT102, or an equal volume of vehicle control. After an additional 24–72 hours (during which media with added compounds were replaced daily), cells were harvested by trypsin digestion and counted in a hemocytometer. In each experiment 5–6 duplicates were performed for each set of experimental conditions and mean number of cells was calculated.
Cell migration
Cell migration was studied using a transwell system (Costar; 6.5 mm diameter wells, 8 µm diameter pores). Serum-starved cells were rinsed with PBS (11.9 mM phosphate, 137 mM NaCl, 2.7 mM KCl) and resuspended in DMEM F12 containing 0.2% FBS. Cells (5×104) lacking or containing APT 102 (100 nM) were added to transwell upper chambers, which were placed in lower chamber wells containing DMEM F12 with 2.5% FBS and different combinations of ATP (10 µM), APT102 (100 nM), and vehicle control. After 6 hours at 37°C, cells that migrated through pores to the lower chamber side of the porous membrane were fixed, stained with Diff-Quik stain set (Siemens), and counted. For experiments involving adenosine, 5×103 SMCs or 1.5×104 ECs were added to transwell upper chambers in media containing adenosine (10 or 100 µM) or an equal volume of vehicle control. In each experiment duplicates or triplicates were performed for each set of experimental conditions and mean number of migrating cells was calculated.
Pharmacokinetic studies
Acute pharmacokinetic properties of APT102 were assessed by administering a single intraperitoneal (IP) injection of APT102. Before APT102 administration, and 1, 3, 6, 12, 24, 48, and 72 hours thereafter, mice were placed without anesthesia or analgesia in a rodent restrainer device (Model 551-BSRR, Plas-Labs, Inc). An incision was made with a sterile scalpel over the lateral tail vein, approximately 2.5 cm proximal to the tail tip. Oozing blood (approximately 50 µL) was collected into 3.8% sodium citrate and platelet-poor plasma was prepared and stored at −80°C. Plasma concentration of APT102 antigen was determined by ELISA involving capture of APT102 with rabbit anti-APT102 antibody (Invitrogen) and detection of bound APT102 with horseradish peroxidase (HRP)-conjugated rabbit anti-APT102 antibody (Covance). Plasma ADPase activity was measured by passing plasma samples through buffer exchange columns (Econo-Pac 10DG, Bio-Rad) equilibrated with 50 mM Tris-HCl, 8 mM CaCl2, pH 7.4, after which ADP (0.5 mM) was added. The reaction mixture was incubated at 37°C for 30 min, after which generation of inorganic phosphate was measured by the Malachite green dye method [15]. For antigen and activity assays, a standard curve was generated by analysis of solutions of purified APT102. Chronic pharmacokinetic properties of APT102 were assessed by administering APT102 by IP injection every other day for 26 days, with the first injection occurring on day 0. Blood was collected, as described above, at baseline (1 hour before day 0 injection) and on day 7, 14, 21, and 28.
Vein graft surgery
Vein grafts were created by implanting a segment of inferior vena cava (IVC) from a donor mouse into the right common carotid artery of a recipient mouse [16]. Anesthesia for surgery was ketamine 93 mg/kg, xylazine 2 mg/kg, and acepromazine 2 mg/kg, administered by IP injection.
Histology
Intimal hyperplasia, % luminal stenosis, and total vessel area were measured in perfusion-fixed vein grafts, as described [17, 18]. Vascular ECs were immuno-stained using anti-CD31 primary antibody (ab28364, Abcam) and Texas-Red-conjugated goat anti-rabbit IgG (Vector), as described [19]. Cell proliferation was studied using anti-proliferating cell nuclear antigen (PCNA) antibody (PC10, Santa Cruz Biotechnology) and peroxidase-conjugated anti-mouse-IgG (Invitrogen), as described [17]. For all experiments, isotype non-immune primary antibodies were used as controls, and results confirmed the specificity of reaction conditions for the antigen of interest. Quantification of PCNA-positive immunostaining was performed by analyzing 4 pre-specified regions of interest (ROIs) within the neointima of each vein graft, assessed in computerized images of cross-sections of each vein graft by false-color segmentation analysis, as described [17]. Quantification of CD31-positive immunostaining was performed using similar methods, with the ROI drawn at the interface of the blood vessel wall and lumen (i.e. overlying ECs).
Vein graft thrombosis
Mice underwent vein graft surgery and received APT102 (1 mg/kg) or an equal volume of 0.9% NaCl by IP injection immediately after surgery and on days 2, 4, and 6 post-operatively. On day 6, 4 hours after administering APT102 or control, mice were anesthetized. The vein graft was exposed and a flow probe (Model 0.5 VB, Transonic Systems) interfacing with a Transonic Model T106 flowmeter and a computerized data acquisition program (Windaq, DATAQ Instruments) was applied to the vein graft. Thrombosis was induced by applying two pieces of filter paper (1×2 mm; Whatman No 1001 055) saturated with 10% FeCl3 (Fisher Scientific) on the vein graft, one on each left lateral aspect, for 3 minutes. Warmed saline was placed in the wound and vein graft blood flow was monitored for a maximum of 60 minutes. Occlusion time was defined as the time interval between initiation of FeCl3 injury and the fall of blood flow to 0 mL/min for 1 minute. Carstairs stain kit (American MasterTech Scientific, Lodi, CA) was used to stain thrombi in excised vein grafts.
Surgical bleeding
Mice received APT102 (1.0 mg/kg) or TBS control by IP injection. Four hours later mice were anesthetized by IP administration of ketamine, xylazine, and acepromazine and the right carotid artery was completely exposed by incising the skin and using blunt dissection to separate tissues surrounding the artery. Sterile 0.9% NaCl (75 µL) was gently infused into the surgical site, allowed to dwell for 2 minutes, and aspirated, after which the saline infusion was repeated twice, for a total of 3 washes of the surgical field. Blood in the saline washes was quantified by measuring the A560 of pooled saline samples. Operator was blinded to treatment group during surgery and while collecting saline washes.
Platelet aggregation
Mice used to study effects of APT102 on platelet aggregation were the same animals used to quantify surgical bleeding. Immediately after collecting saline washes from the carotid artery surgical field (which was approximately 4.5 hours after administration of APT102 or control, as described in the preceding paragraph), blood was collected from the surgically exposed abdominal IVC into 3.8% sodium citrate supplemented with lepirudin (Bayer, final plasma concentration 25 µg/mL). Platelet-rich plasma was prepared and ADP-induced platelet aggregation was studied by light transmission aggregometry, as described [20]. Operator was blinded to treatment group when collecting blood and studying platelet aggregation.
Prothrombin time
Prothrombin time (PT) was performed as described [20], except that mouse brain extract (prepared by homogenizing 200 mg of fresh brain tissue in 300 µL of PBS, centrifuging the suspension, and saving the supernatant) was used instead of commercial PT reagent.
Reverse-transcriptase (RT)-PCR
Real-time RT-PCR and quantification of gene expression were performed as described [17]. Primers for amplification of CD73 cDNA were GGGCGGAAGGTTCCTGTAG and GAGGAGCCATCCAGATAGACA. 18S ribosomal RNA gene expression was simultaneously assessed as an internal control.
Statistical Analyses
Results are expressed as mean ± standard error of the mean. Student’s t test, one-way ANOVA, and Kaplan-Meier survival analysis: log-rank test were used, as appropriate.
Results
APT102 exhibits favorable pharmacokinetic properties
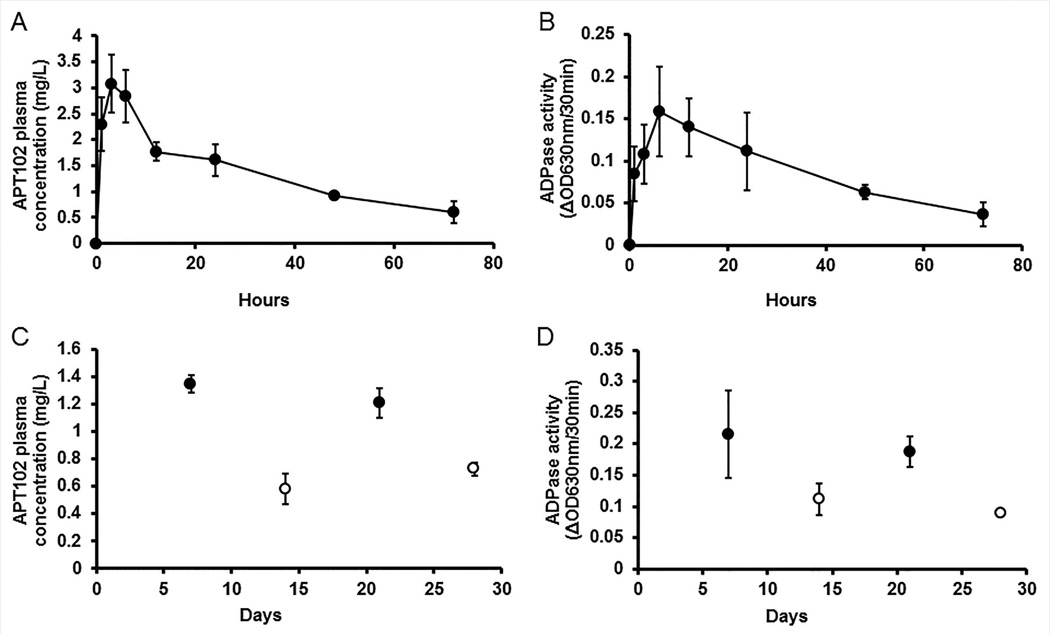
We studied the pharmacokinetic properties of APT102 in mice. APT102 antigen peaked in plasma 3 hours after a single IP injection and gradually declined over a 72 hour time course with an elimination-phase half-life of 36 ± 2.4 hours (Fig. 1A). Plasma ADPase activity followed a similar trajectory (Fig. 1B). In response to every-other-day injection of APT102 over 26 days, a steady-state plasma antigen concentration of approximately 1 µg/mL was achieved (Fig. 1C), with no significant decay of plasma ADPase activity over time (Fig. 1D).
Figure 1.
APT102 antigen concentration and ADPase activity achieved in plasma after intraperitoneal injections. (A–B) Single-dose (1.0 mg/kg) administration. Plasma concentrations of APT102 antigen (A) and ADPase activity (B) were measured at the indicated time points. (C–D) Multiple-dose administration. APT102 (1.0 mg/kg) was administered every other day for 26 days, with first injection designated as occurring on day 0. Plasma concentrations of APT102 antigen (C) and ADPase activity (D) were measured at the indicated time points, with blood being collected either 24 hours (black circles) or 48 hours (white circles) after the prior injection. All data points shown in panels A-D are derived from 4 mice.
APT102 inhibits vein graft thrombosis without increasing surgical bleeding
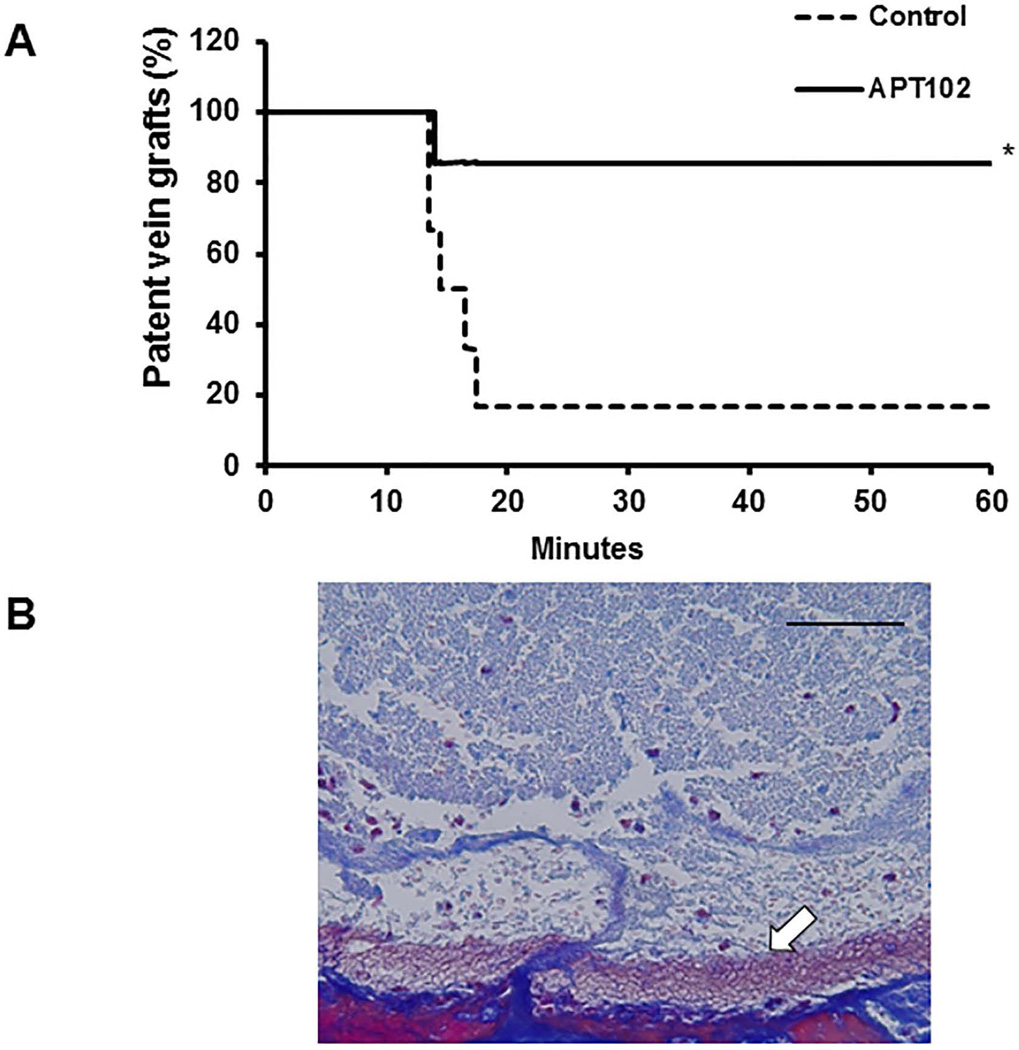
To determine if APT102 inhibits vein graft thrombosis, mice underwent vein graft surgery and were treated with APT102 or 0.9% NaCl control, administered once every other day for 6 days. On day 6, the vein graft was exposed 4 hours after administration of APT102 or control and subjected to FeCl3 injury. Within 60 minutes an occlusive thrombus formed in 1 of 7 mice (14%) treated with APT102 vs. 5 of 6 controls (83%; P<0.02; Fig. 2A). Histological analysis of a thrombus retrieved from a control animal revealed that it was platelet-rich in composition (Fig. 2B).
Figure 2.
APT102 inhibits vein graft thrombosis. (A) Mice underwent vein graft surgery, after which they received APT102 (n=7) or 0.9% NaCl control (n=6) by intraperitoneal injection, as described in Methods. On day 6 after surgery vein graft thrombosis was induced by topical application of ferric chloride. Time required to form an occlusive thrombus was measured. *P<0.02 vs. vehicle treated control group. (B) Image of thrombus induced by FeCl3 in a control (i.e. not treated with APT102) animal 6 days after surgery. Carstairs method, which stains platelets gray-blue, was used. Some leukocytes (larger, reddish cells) are also present in thrombus. White arrow indicates interface of vein graft wall and thrombus in lumen. Scale bar = 50µm.
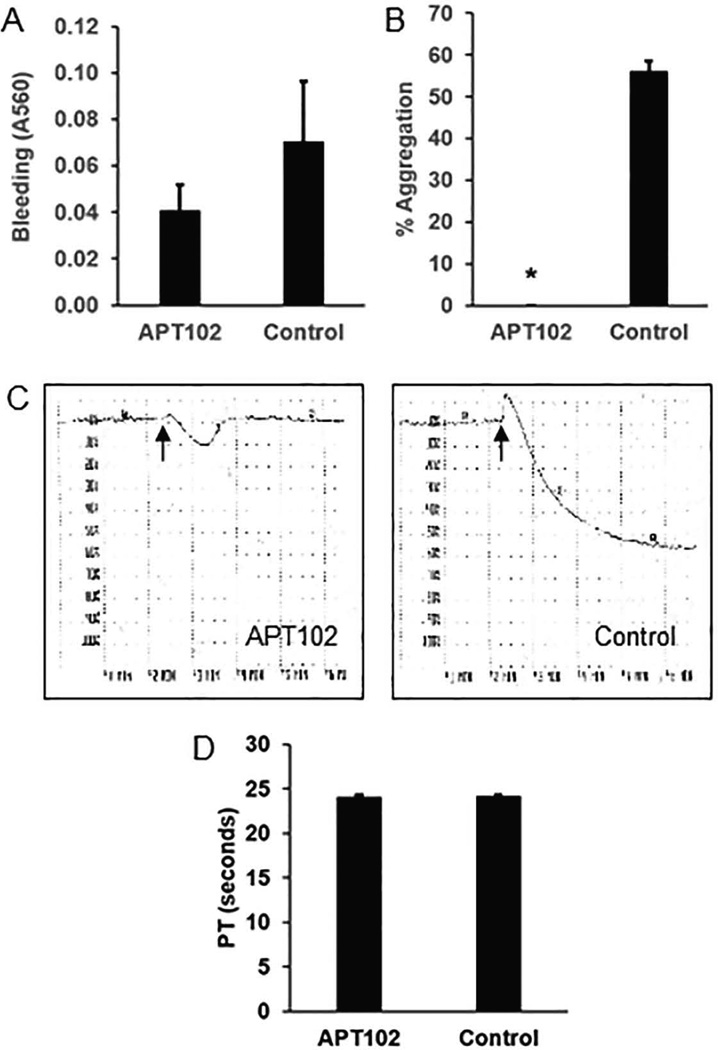
Inspection of the surgical field through the dissecting microscope during thrombosis and other experiments involving carotid artery surgery revealed that surgical bleeding was minor and suggested that hemostasis was not adversely affected by APT102. To confirm this observation, we performed an experiment in which mice received IP injections of APT102 (1 mg/kg) or TBS and 4 hours later underwent skin incision and tissue dissection to fully expose the carotid artery. Surgical bleeding, quantified by spectrophotometric analysis of saline rinses of the surgical wound, did not differ significantly between treatment groups (Fig. 3A). Immediately after assessing surgical bleeding, blood was collected from the IVC and platelet-rich plasma was prepared. ADP-induced aggregation was normal in control mice, but was essentially completely blocked in APT102-treated animals (Fig. 3B–C). APT102 had no discernable effect on the prothrombin time (Fig. 3D). Together, these results suggested that APT102 inhibits thrombosis in vein grafts by inhibiting ADP-induced platelet aggregation, while not adversely affecting hemostasis.
Figure 3.
APT102 inhibits platelet aggregation without adversely affecting surgical hemostasis. (A) Amount of bleeding into surgical wound, assessed by spectrophotometric analysis of surgical field rinses (higher absorbance indicates greater hemoglobin content), did not differ significantly between APT102 and control groups (P>0.4; n = 6/group). (B) % aggregation of platelets, assessed 5 min after addition of ADP (5 µM). *P<0.01; n = 3/group. (C) Representative platelet aggregation curves of platelet-rich plasma samples from a mouse treated with APT102 and a control mouse. Arrows denote addition of ADP. (D) APT102 does not affect the prothrombin time (PT). APT102 was added to pooled citrated plasma to achieve a concentration of 3 mg/L. An equal volume of TBS was added to control pooled plasma. Data shown represent results of 5 experiments.
APT102 exhibits no cellular toxicity and blocks the effects of ATP on SMC and EC proliferation
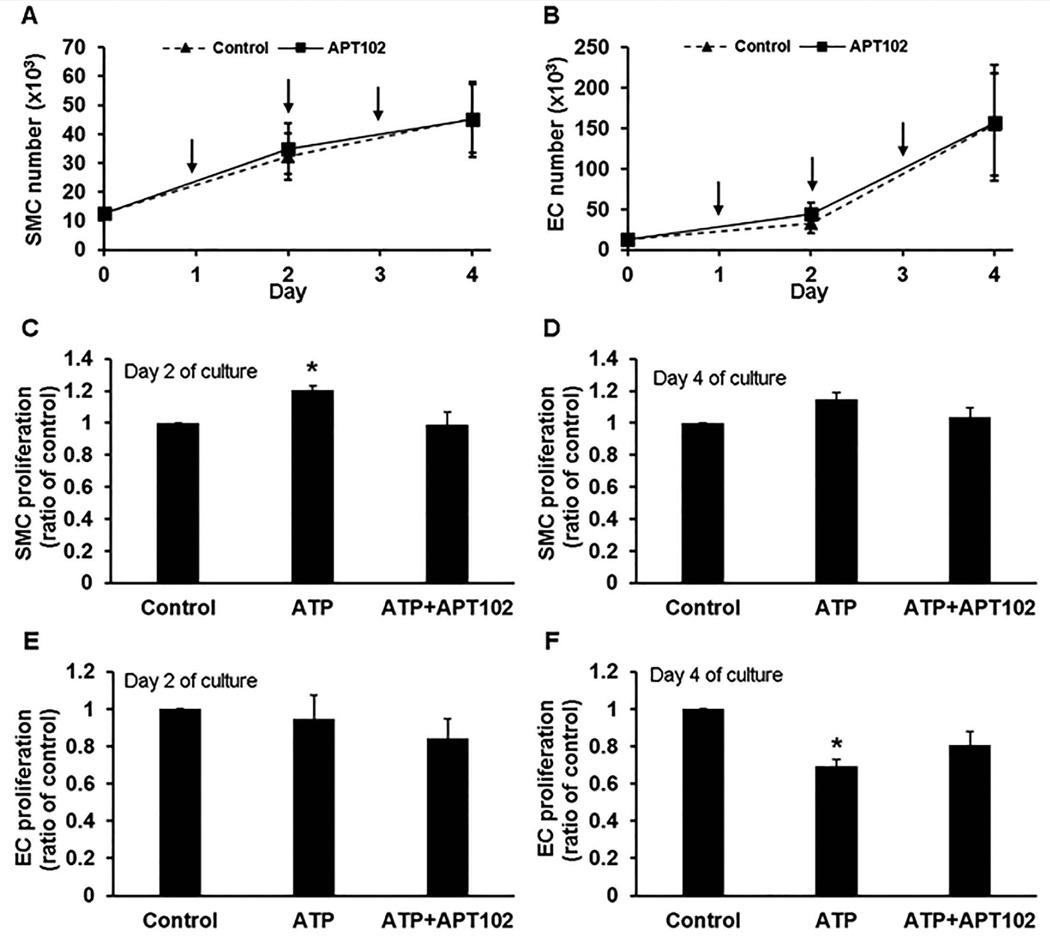
Vein graft remodeling is characterized by SMC and EC proliferation [21, 22]. APT102 had no significant effect on the growth kinetics of SMCs or ECs grown in vitro under standard cell culture conditions (Fig. 4A–B), suggesting that it was non-toxic to these cell types. ATP, which is released in response to cell necrosis and activation, regulates SMC and EC proliferation [23–25]. To determine if APT102 can block these effects, SMCs and ECs were incubated with ATP or vehicle control in the presence or absence of APT102. ATP produced a modest, statistically significant increase in SMC proliferation after 24 hours of treatment (i.e. on day 2 of culture) and this effect was completely blocked by co-administration of APT102 (Fig. 4C). After 72 hours of treatment (i.e. on day 4 of culture), there were no significant effects of ATP or APT102 on SMC proliferation (Fig. 4D). ATP had no significant effect on EC proliferation after 24 hours of treatment (Fig. 4E). However, a delayed effect of ATP was observed, with significant inhibition of EC proliferation after 72 hours of treatment, and this anti-proliferative effect was significantly attenuated by co-administration of APT102 (Fig. 4F). Together, these results suggest that APT102 in itself does not adversely affect SMC or EC proliferation, but can effectively attenuate the stimulatory effect of ATP on SMC proliferation and the inhibitory effect of ATP on EC proliferation.
Figure 4.
APT102 exhibits no cellular toxicity and blocks the effects of exogenous ATP on smooth muscle cell (SMC) and endothelial cell (EC) proliferation. (A–B) APT102 does not affect SMC or EC proliferation. Arrows denote time points at which cells were exposed to fresh preparations of APT102 (10 µM) during media exchanges. (C–D) Effects of ATP (10 µM) and ATP + APT102 (10 µM and 100 nM, respectively) on SMC proliferation, assessed after 2 days (C) and 4 days (D) of cell culture. (E–F) Effects of ATP (10 µM) and ATP + APT102 (10 µM and 100 nM, respectively) on EC proliferation, assessed after 2 days (E) and 4 days (F) of cell culture. *P<0.05 vs. control. Data are derived from quadruplicate experiments for SMCs and triplicate experiments for ECs.
APT102 inhibits SMC, but not EC, migration
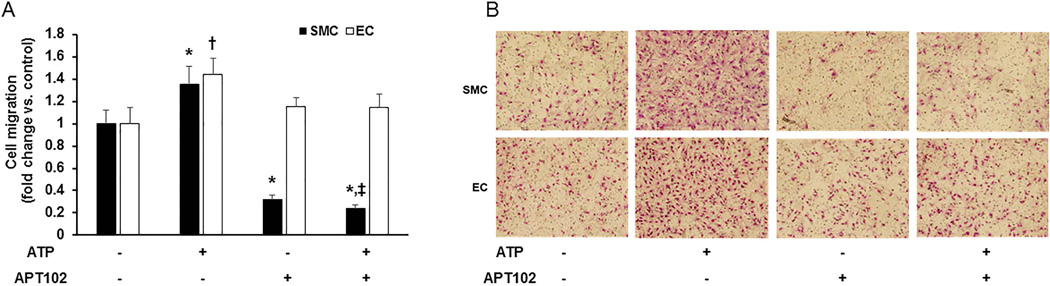
SMC migration promotes neointima formation in vein grafts [26], while EC migration is required for vein graft re-endothelialization [27]. Incubation of ECs with APT102 alone had no significant effect on their migration (Fig. 5A, 1st vs. 3rd white bars). In contrast, APT102 potently inhibited SMC migration (Fig. 5A, 1st vs. 3rd black bars). We also studied the capacity of APT102 to block the effects of ATP on EC and SMC migration. ATP significantly increased EC migration (Fig. 5A, 1st vs. 2nd white bars), and this effect was significantly attenuated by co-administration of APT102, restoring EC migration to a level that did not differ significantly from negative control (Fig. 5A, 1st vs. 4th white bars). ATP also stimulated SMC migration (Fig. 5A, 1st vs. second black bars). However, co-administration of ATP and APT102 inhibited SMC migration to a level that was significantly less than that of negative control (Fig. 5A, 1st vs. 4th black bars) and without significant difference from that of SMCs treated with APT102 alone (Fig. 5A, 3rd vs. 4th black bars). Together, these results suggest that APT102 inhibits SMC migration to a significantly greater extent than EC migration, and that this effect is not explained simply by scavenging of ATP.
Figure 5.
APT102 inhibits vascular smooth muscle cell (SMC) migration, but not endothelial cell (EC) migration. (A) Human coronary artery SMCs (black bars) or human aortic ECs (white bars) were seeded in upper chambers of transwells (5×104 cells/well). ATP (10 µM, +) or vehicle control (−) was added to lower chamber medium; APT102 (100 nM, +) or vehicle control (−) was added to both upper and lower chamber media. Cell migration was evaluated after 6 hours of incubation. Data are derived from 6 independent experiments for SMCs and 4 independent experiments for ECs and are expressed as relative cell migration (fold increase or decrease) vs. control (no APT102 or ATP = leftmost diad of bars). *P<0.05 vs. control SMCs; †P<0.05 vs. control ECs; ‡P>0.6 vs. SMCs treated with only APT102. (B) Representative images of migrated cells. Magnification 20x.
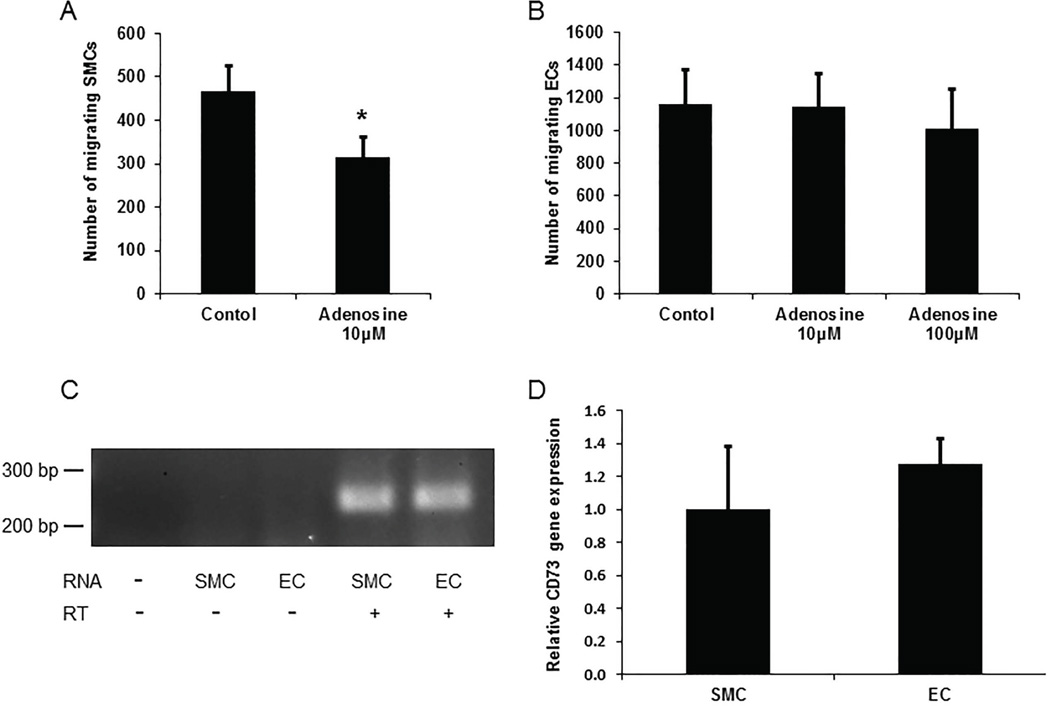
Adenosine inhibits SMC, but not EC, migration
SMCs and ECs express CD73 [28, 29], which converts AMP generated by CD39 to adenosine. Therefore, we performed experiments to examine the effects of adenosine on SMC and EC migration, reasoning that APT102 might regulate their migration not only by degrading ATP, but also by promoting adenosine formation. Adenosine inhibited SMC migration (Fig. 6A), but not EC migration (Fig. 6B). RT-PCR analysis demonstrated that SMCs and ECs express CD73 mRNA in similar amounts (Fig. 6C–D), providing a basis for adenosine formation in the experiments described above involving the addition of APT102 to cells, and suggesting a potential role for adenosine in the capacity of APT102 to inhibit SMC migration to a significantly greater extent than EC migration.
Figure 6.
Effects of adenosine on smooth muscle cell (SMC) and endothelial cell (EC) migration and expression of CD73 by SMCs and ECs. (A) Adenosine inhibits SMC migration. *P<0.02 vs. control. (B) Adenosine does not inhibit EC migration. (C) SMCs and ECs express CD73 mRNA. RT-PCR products are shown, with source of RNA (“-” denotes none) and presence (+) or absence (−) of reverse transcriptase (RT) in reaction mixtures shown. (D) Quantitative real-time RT-PCR data. Differences in CD73 gene expression between groups did not achieve statistical significance (P>0.5). Data represent triplicate experiments.
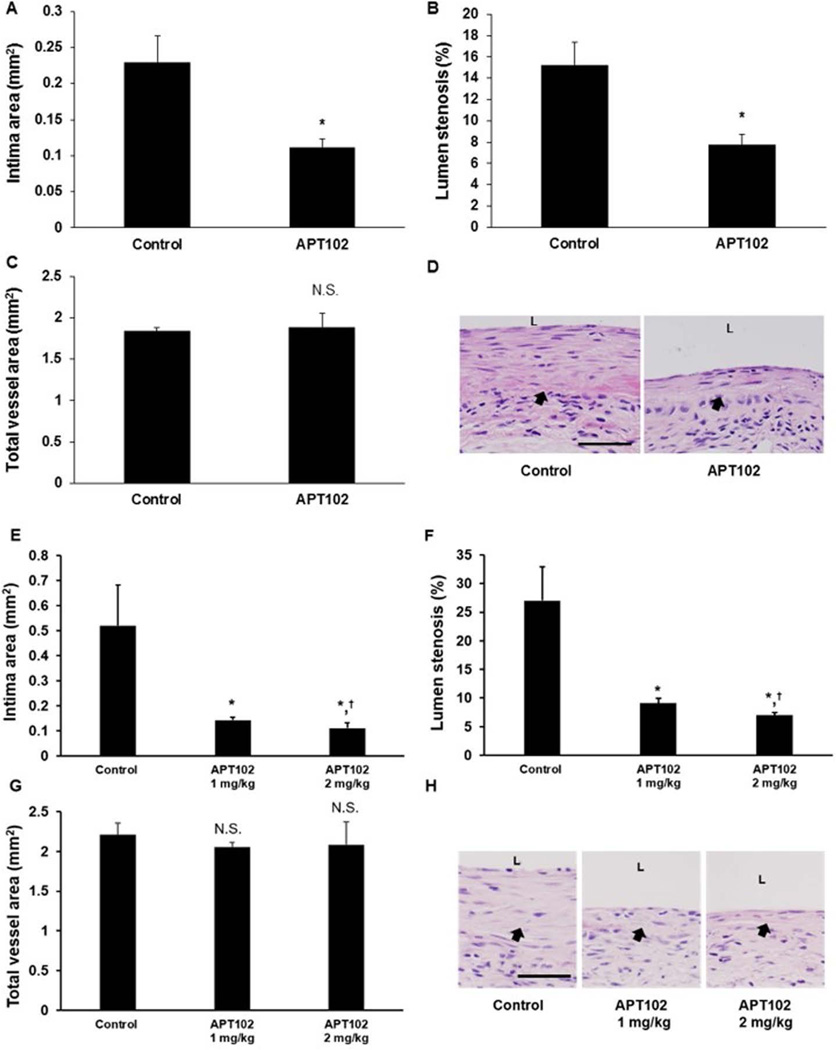
APT102 inhibits vein graft intimal hyperplasia
To examine the in vivo effects of APT102 on vein graft remodeling, mice were subjected to vein graft surgery, after which they received APT102 (1 mg/kg) or equal volumes of 0.9% NaCl, as a control, administered by IP injection every other day for 14 days. Vein grafts were harvested on day 28 day after surgery. Mean intima thickness was significantly less in vein grafts of APT102-treated mice than in controls (Fig. 7A–D). To exclude the small possibility that the minor differences in diluents between the APT102 and control group (i.e. TBS and 0.9% NaCl, respectively) may have affected results, and determine the effects of a higher dose of APT102 on intimal hyperplasia, we repeated the experiment, administering APT102 with the same frequency and duration as before (at doses of 1–2 mg/kg), with control animals receiving TBS injections. As in our initial experiment, APT102 significantly inhibited neointima formation, with no statistically significant difference in effect between the doses of 1 mg/kg and 2 mg/kg (Fig. 7E–H).
Figure 7.
APT102 inhibits vein graft intimal hyperplasia. Mice underwent vein graft surgery and were treated with APT102 or control for 2 weeks. Four weeks after surgery vein grafts were harvested and analyzed. (A–D) Initial experiment, in which APT102 (1 mg/kg/injection) was delivered in TBS (n=7 mice) and control injections consisted of 0.9% (n=8 mice). (A) Intima area; (B) lumen stenosis (%); (C) total vessel cross-sectional area; (D) representative images of vein grafts. (E–H) Follow-up experiment, involving identical methods, except that APT102 was delivered in TBS at doses of 1 and 2 mg/kg/injection (n=5 mice for each dose) and control injections consisted of TBS (n=5 mice). (E) Intima area; (F) lumen stenosis (%); (G) total vessel cross-sectional area; (H) representative images of vein grafts. *P<0.05 vs. control; N.S. = no significant difference vs. control; †P>0.6 vs. APT102 1 mg/kg. Scale bar = 50 µm; arrows indicate the boundary between intima and media; L = lumen.
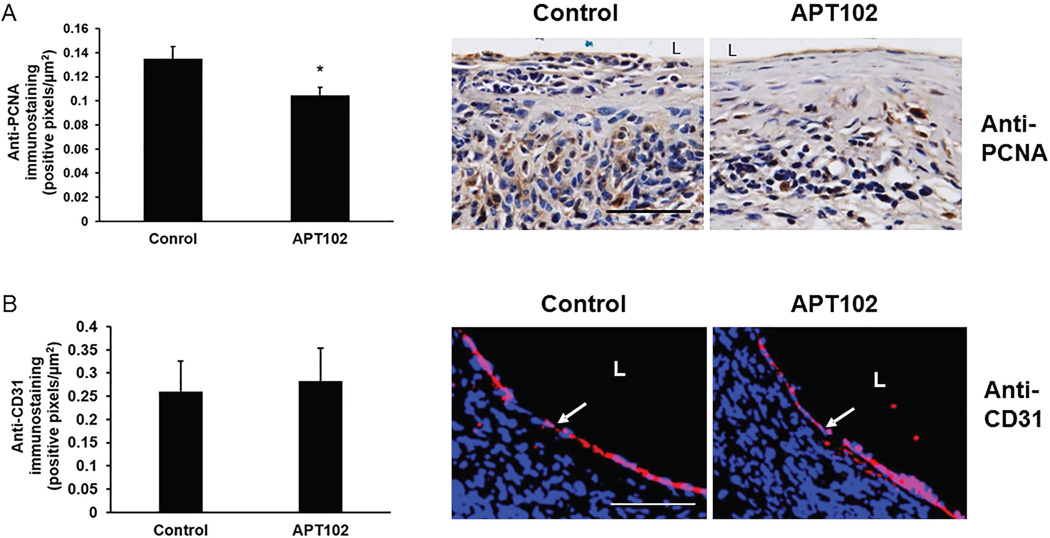
APT102 inhibits neointima cell proliferation in vein grafts without delaying re-endothelializaton
Consistent with our morphometric analysis of neointima formation, PCNA-positive immunostaining, a marker of cell proliferation, was significantly less in vein grafts 28 days after surgery in APT102-treated mice than in controls (Fig. 8A). We also performed CD31 immunostaining of vein grafts to study the effect of APT102 on recovery of an intact endothelium after surgery, which is an adaptive process in early vein graft remodeling [30]. There was no evidence of a delay of EC recovery in APT102-treated mice compared to controls (Fig. 8B). Together, these results suggested that APT102 inhibits cell proliferation within the wall of vein grafts without inhibiting re-endothelialization.
Figure 8.
APT102 inhibits cell proliferation in vein grafts without inhibiting re-endothelialization. (A) Cell proliferation data. Vein grafts retrieved 28 days after surgery from mice treated with APT102 (1 mg/kg/injection) or 0.9% NaCl control were immunostained for proliferating cell nuclear antigen (PCNA). Quantification of immunostaining is shown in graph (n=6 mice/group; *P<0.03 vs. control). Representative images from one mouse in each group are shown (brown = PCNA). (B) Re-endothelialization data. Vein grafts harvested 28 days after surgery were analyzed by immunofluorescence microscopy with anti-CD31 antibody to detect endothelial cells (ECs). Quantification of EC immunostaining is shown in graph (n=5 mice/group; difference between groups was not statistically significant, P>0.8). Representative images from one mouse in each group are shown. Arrows depict CD31-positive ECs (red). Cell nuclei for both PCNA and CD31 immunostaining are stained blue with 4’,6-diamidino-2-phenylindole (DAPI). L = Lumen. Scale bars = 50 µm.
Discussion
Thrombosis, mediated by platelet deposition and activation of the blood coagulation system in response to tissue injury, inflammation, and EC denudation, is a major threat to vein grafts early after surgery. In addition, intimal hyperplasia is a key early step in the remodeling response of vein grafts, which, when excessive, obstructs blood flow and produces ischemia. Currently, antiplatelet therapy, consisting of aspirin, either alone or in combination with a thienopyridine, is used to reduce vein graft thrombosis [1, 2]. Statins are the only drug class that has been shown to decrease vein graft intimal hyperplasia [31, 32]. New therapies are needed to inhibit thrombosis and intimal hyperplasia in vein grafts.
Herein we show that APT102, a soluble form of recombinant human apyrase, effectively inhibits both thrombosis and intimal hyperplasia in murine vein grafts early after surgery. APT102 exhibited a favorable pharmacological profile, with an elimination half-life of approximately 36 hours and no significant attenuation of plasma ADPase activity during repetitive administration over several weeks. Our results suggest that APT102 inhibits vein graft thrombosis by blocking ADP-induced aggregation. Of note, a dose of APT102 sufficient to completely block ADP-induced aggregation and markedly decrease thrombosis did not increase bleeding during surgical exposure of the carotid artery, suggesting that APT102 has the potential to inhibit thrombosis without compromising hemostasis. These results are consistent with previous studies [14, 33] and support the approach of degrading ADP, rather than blocking its receptor, P2Y12, as an antithrombotic strategy. Furthermore, APT102 inhibited vein graft intimal hyperplasia without inhibiting re-endothelialization, which would be expected to reduce thrombotic risk compared to other anti-proliferative strategies associated with impairment of EC recovery, such as drug-eluting stents. Overall, our results suggest that APT102 has considerable promise as a therapeutic strategy to prevent both thrombosis and intimal hyperplasia within vein graft thrombosis in the early post-operative period.
Several studies examined the effects of ATP and other nucleotides on the proliferation and migration of SMCs and ECs [23–25, 34–37]. Our data indicate that APT102 significantly attenuates ATP’s proliferative effect on SMCs and anti-proliferative effect on ECs, which together would be expected to have therapeutic value by reducing SMC proliferation and neointima formation, while blocking negative effects of ATP on re-endothelialization. An interesting finding of our in vitro experiments is the potent inhibitory effect of APT102 on SMC, but not EC, migration. This did not appear to be a cytotoxic effect, as treatment of SMCs with APT102 alone did not inhibit proliferation. Nor did APT102’s anti-migratory effect on SMCs appear to be mediated by degradation of ATP, as APT102 more than negated the increase in SMC migration induced by treating cells with ATP alone. In contrast, treatment of ECs with both ATP and APT102 restored cell migration to a level close to that of untreated controls, consistent with APT102’s effect on EC migration being mediated by catabolism of ATP. Given that APT102 promotes AMP formation, which is converted to adenosine by CD73 [8], we reasoned that the effects of APT102 on cell migration can be mediated not only by degradation of ATP and other nucleotides, but also by formation of adenosine. Our data suggest that adenosine inhibits SMC migration, which is consistent with a previous study [11]. However, adenosine did not inhibit EC migration. We hypothesize that the differential effects of adenosine on SMC vs. EC migration explain, at least in part, the capacity of APT102 to inhibit SMC migration to a significantly greater extent that EC migration. Overall, our data suggest that APT102 significantly attenuates SMC migration, a key process in the pathogenesis of intimal hyperplasia, while having a much less pronounced effect on EC migration, and potentially even enhancing EC proliferation, particularly in microenvironments with elevated ATP concentration, as can occur in vein grafts post-operatively, mediated by cell activation and death [1]. Consistent with our in vitro results, APT102 inhibited vein graft neointima formation without significantly attenuating re-endothelialization.
Drosopoulos et al. reported that soluble human CD39 inhibits intimal hyperplasia in injured femoral arteries of male mice, which was accompanied by reduced mural platelet deposition [38]. Our data suggest that the beneficial effects of soluble CD39 on intimal hyperplasia extend also to vein graft remodeling, which involves distinct molecular and cellular pathways compared to arterial remodeling. Our study also shows that soluble CD39 exerts a highly potent antithrombotic effect, sufficient not only to inhibit platelet deposition on injured vascular surfaces, but to effectively inhibit formation of completely occlusive thrombi. Imai et al. showed that adenovirus-mediated expression of human CD39 inhibited platelet deposition and inflammation in xenografts [39]. These findings are relevant to vein graft pathologies, which are also inflammation-dependent [40], and further support the use of recombinant CD39 to prevent vein graft disease. We used male mice in our experiments because they are larger than females, consistent with the original description of this model [16]. While coronary and other arterial bypass surgeries are performed significantly more in men than in women, the lack of female mice is a limitation of our experiments. We consider it unlikely that the effects of APT102 on thrombosis and vein graft remodeling would differ significantly between male and female mice; however, we cannot exclude this possibility, which should be addressed in future studies.
In summary, we have shown that APT102, a recombinant soluble form of human CD39L3, provides dual therapeutic benefit by effectively inhibiting thrombosis and intimal hyperplasia early after vein graft surgery, without any evidence of hemorrhagic or other side-effects, and without delaying re-endothelialization. Hence, APT102 may represent a new approach to target multiple pathways that promote vein graft failure. As an enzyme, APT102 requires intravenous or subcutaneous administration, which is feasible in the initial weeks to months following surgery, and could be facilitated by further modifications that prolong the compound’s plasma half-life. Our data support performing future experiments to determine if APT102 inhibits more advanced, obstructive vein graft remodeling, including in the presence of common metabolic disturbance, such as diabetes mellitus and hyperlipidemia.
Essentials.
New strategies are needed to inhibit thrombosis and intimal hyperplasia (IH) in vein grafts (VG).
We studied effects of apyrase (APT102) on VGs and smooth muscle and endothelial cells (SMC/EC).
APT102 inhibited thrombosis, SMC migration, and IH without impairing hemostasis or EC recovery.
Apyrase APT102 is a single-drug approach to inhibit multiple processes that cause VG failure.
Acknowledgments
This work was supported by NIH grants HL115860, HL57346, and HL095951.
Footnotes
Addendum
Y. Ji and O. Adeola designed and performed experiments, analyzed and discussed data and relevant literature, and prepared figures. T. L. Strawn and S. S. Jeong performed experiments and analyzed data. R. Chen designed experiments, discussed and analyzed data, and assisted with manuscript editing. W. P. Fay designed experiments, analyzed and discussed data, prepared figures, and wrote the manuscript.
Disclosures
R. Chen and S. S. Jeong have equity interests in APT Therapeutics Inc., which holds patents and commercial rights of APT102. The other authors declare no competing interests.
References
- 1.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–931. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 2.Parang P, Arora R. Coronary vein graft disease: Pathogenesis and prevention. Can J Cardiol. 2009;25:e57–e62. doi: 10.1016/s0828-282x(09)70486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ray FR, Huang W, Slater M, Barden JA. Purinergic receptor distribution in endothelial cells in blood vessels: a basis for selection of coronary artery grafts. Atherosclerosis. 2002;162:55–61. doi: 10.1016/s0021-9150(01)00681-5. [DOI] [PubMed] [Google Scholar]
- 4.Burnstock G. Purinergic regulation of vascular tone and remodelling. Autonomic and Autacoid Pharmacology. 2009;29:63–72. doi: 10.1111/j.1474-8673.2009.00435.x. [DOI] [PubMed] [Google Scholar]
- 5.Liu R, Ma S, Lu Z, Shen H, Sun L, Wei M. The ADP Antagonist MRS2179 Regulates the Phenotype of Smooth Muscle Cells to Limit Intimal Hyperplasia. Cardiovasc Drugs Ther. 2015;29:23–29. doi: 10.1007/s10557-014-6561-6. [DOI] [PubMed] [Google Scholar]
- 6.Marcus AJ, Broekman MJ, Drosopoulos JHF, Islam N, Pinsky DJ, Sesti C, Levi R. Metabolic Control of Excessive Extracellular Nucleotide Accumulation by CD39/Ecto-Nucleotidase-1: Implications for Ischemic Vascular Diseases. J Pharmacol Exp Therapeut. 2003;305:9–16. doi: 10.1124/jpet.102.043729. [DOI] [PubMed] [Google Scholar]
- 7.Idzko M, Ferrari D, Riegel A-K, Eltzschig HK. Extracellular nucleotide and nucleoside signaling in vascular and blood disease. Blood. 2014;124:1029–1037. doi: 10.1182/blood-2013-09-402560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnstock G, Ralevic V. Purinergic Signaling and Blood Vessels in Health and Disease. Pharmacolog Rev. 2014;66:102–192. doi: 10.1124/pr.113.008029. [DOI] [PubMed] [Google Scholar]
- 9.Deaglio S, Robson SC. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Advances Pharmacol. 2011;61:301–332. doi: 10.1016/B978-0-12-385526-8.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, Hilaire CS, Seldin DC, Toselli P, Lamperti E, Schreiber BM, Gavras H, Wagner DD, Ravid K. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Adenosine Inhibits Growth of Human Aortic Smooth Muscle Cells Via A2B Receptors. Hypertension. 1998;31:516–521. doi: 10.1161/01.hyp.31.1.516. [DOI] [PubMed] [Google Scholar]
- 12.Torssell L, Ekestrom S, Sollevi A. Adenosine-induced increase in graft flow during coronary bypass surgery. Scandinav J Thoracic Cardiovasc Surg. 1989;23:235–239. doi: 10.3109/14017438909106001. [DOI] [PubMed] [Google Scholar]
- 13.Marcus AJ, Broekman MJ, Drosopoulos JH, Islam N, Alyonycheva TN, Safier LB, Hajjar KA, Posnett DN, Schoenborn MA, Schooley KA, Gayle RB, Maliszewski CR. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J Clin Invest. 1997;99:1351–1360. doi: 10.1172/JCI119294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moeckel D, Jeong SS, Sun X, Broekman MJ, Nguyen A, Drosopoulos JHF, Marcus AJ, Robson SC, Chen R, Abendschein D. Optimizing human apyrase to treat arterial thrombosis and limit reperfusion injury without increasing bleeding risk. Science Translat Med. 2014;6:248ra105–248ra105. doi: 10.1126/scitranslmed.3009246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baykov AA, Evtushenko OA, Avaeva SM. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Analytical Biochem. 1988;171:266–270. doi: 10.1016/0003-2697(88)90484-8. [DOI] [PubMed] [Google Scholar]
- 16.Zou Y, Dietrich H, Hu Y, Metzler B, Wick G, Xu Q. Mouse model of venous bypass graft arteriosclerosis. Am J Pathol. 1998;153:1301–1310. doi: 10.1016/S0002-9440(10)65675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji Y, Strawn TL, Grunz EA, Stevenson MJ, Lohman AW, Lawrence DA, Fay WP. Multifaceted Role of Plasminogen Activator Inhibitor-1 in Regulating Early Remodeling of Vein Bypass Grafts. Arterioscler Thromb Vasc Biol. 2011;31:1781–1787. doi: 10.1161/ATVBAHA.111.228767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu P, Nguyen BT, Tao M, Bai Y, Ozaki CK. Mouse Vein Graft Hemodynamic Manipulations to Enhance Experimental Utility. Am J Pathol. 2011;178:2910–2919. doi: 10.1016/j.ajpath.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji Y, Fish PM, Strawn TL, Lohman AW, Wu J, Szalai AJ, Fay WP. C-reactive protein induces expression of tissue factor and plasminogen activator inhibitor-1 and promotes fibrin accumulation in vein grafts. J Thromb Haemost. 2014;12:1667–1677. doi: 10.1111/jth.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day SM, Reeve JL, Myers DD, Fay WP. Murine thrombosis models. Thromb Haemostas. 2004;92:486–494. [PubMed] [Google Scholar]
- 21.Kenagy RD, Fukai N, Min SK, Jalikis F, Kohler TR, Clowes AW. Proliferative capacity of vein graft smooth muscle cells and fibroblasts in vitro correlates with graft stenosis. J Vasc Surg. 2009;49:1282–1288. doi: 10.1016/j.jvs.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown MA, Zhang L, Levering VW, Wu J-H, Satterwhite LL, Brian L, Freedman NJ, Truskey GA. Human Umbilical Cord Blood-derived Endothelial Cells Re-endothelialize Vein Grafts and Prevent Thrombosis. Arterioscler Thromb Vasc Biol. 2010;30:2150–2155. doi: 10.1161/ATVBAHA.110.207076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D-J, Huang N-N, Heppel LA. Extracellular ATP and ADP stimulate proliferation of porcine aortic smooth muscle cells. J Cellular Physiol. 1992;153:221–233. doi: 10.1002/jcp.1041530202. [DOI] [PubMed] [Google Scholar]
- 24.Van Daele P, Van Coevorden A, Roger PP, Boeynaems JM. Effects of adenine nucleotides on the proliferation of aortic endothelial cells. Circ Res. 1992;70:82–90. doi: 10.1161/01.res.70.1.82. [DOI] [PubMed] [Google Scholar]
- 25.Xiao Z, Yang M, Lv Q, Wang W, Deng M, Liu X, He Q, Chen X, Chen M, Fang L, Xie X, Hu J. P2Y11 impairs cell proliferation by induction of cell cycle arrest and sensitizes endothelial cells to cisplatin-induced cell death. J Cellular Biochem. 2011;112:2257–2265. doi: 10.1002/jcb.23144. [DOI] [PubMed] [Google Scholar]
- 26.Kallenbach K, Salcher R, Heim A, Karck M, Mignatti P, Haverich A. Inhibition of smooth muscle cell migration and neointima formation in vein grafts by overexpression of matrix metalloproteinase-3. J Vasc Surg. 2009;49 doi: 10.1016/j.jvs.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dilley RJ, McGeachie JK, Prendergast FJ. Experimental vein grafts in the rat: Re-endothelialization and permeability to albumin. Brit J Surg. 1983;70:7–12. doi: 10.1002/bjs.1800700104. [DOI] [PubMed] [Google Scholar]
- 28.Tamajusuku ASK, Carrillo-Sepúlveda MA, Braganhol E, Wink MR, Sarkis JJF, Barreto-Chaves MLM, Battastini AMO. Activity and expression of ecto-5′-nucleotidase/CD73 are increased by thyroid hormones in vascular smooth muscle cells. Molec Cellular Biochem. 2006;289:65–72. doi: 10.1007/s11010-006-9148-0. [DOI] [PubMed] [Google Scholar]
- 29.Narravula S, Lennon PF, Mueller BU, Colgan SP. Regulation of Endothelial CD73 by Adenosine: Paracrine Pathway for Enhanced Endothelial Barrier Function. J Immunol. 2000;165:5262–5368. doi: 10.4049/jimmunol.165.9.5262. [DOI] [PubMed] [Google Scholar]
- 30.Tseng C-N, Karlöf E, Chang Y-T, Lengquist M, Rotzius P, Berggren P-O, Hedin U, Eriksson EE. Contribution of Endothelial Injury and Inflammation in Early Phase to Vein Graft Failure: The Causal Factors Impact on the Development of Intimal Hyperplasia in Murine Models. PLoS ONE. 2014;9:e98904. doi: 10.1371/journal.pone.0098904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamanouchi D, Banno H, Nakayama M, Sugimoto M, Fujita H, Kobayashi M, Kuwano H, Komori K. Hydrophilic statin suppresses vein graft intimal hyperplasia via endothelial cell-tropic Rho-kinase inhibition. J Vasc Surg. 2005;42:757–764. doi: 10.1016/j.jvs.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 32.Une D, Kulik A, Voisine P, Le May M, Ruel M. Correlates of Saphenous Vein Graft Hyperplasia and Occlusion 1 Year After Coronary Artery Bypass Grafting: Analysis From the CASCADE Randomized Trial. Circulation. 2013;128:S213–S218. doi: 10.1161/CIRCULATIONAHA.112.000328. [DOI] [PubMed] [Google Scholar]
- 33.Tan Z, Li X, Turner RC, Logsdon AF, Lucke-Wold B, DiPasquale K, Jeong SS, Chen R, Huber JD, Rosen CL. Combination treatment of r-tPA and an optimized human apyrase reduces mortality rate and hemorrhagic transformation 6h after ischemic stroke in aged female rats. Eur J Pharmacol. 2014;738:368–373. doi: 10.1016/j.ejphar.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaczmarek E, Erb L, Koziak K, Jarzyna R, Wink MR, Guckelberger O, Blusztajn JK, Trinkaus-Randall V, Weisman GA, Robson SC. Modulation of endothelial cell migration by extracellular nucleotides Involvement of focal adhesion kinase and phosphatidylinositol 3-kinase - mediated pathways. Thromb Haemost. 2005;93:735–742. doi: 10.1267/THRO05040735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaulet H, Desgranges C, Renault MA, Dupuch F, Ezan G, Peiretti F, Loirand G, Pacaud P, Gadeau AP. Extracellular Nucleotides Induce Arterial Smooth Muscle Cell Migration Via Osteopontin. Circ Res. 2001;89:772–778. doi: 10.1161/hh2101.098617. [DOI] [PubMed] [Google Scholar]
- 36.Meininger CJ, Schelling ME, Granger HJ. Adenosine and hypoxia stimulate proliferation and migration of endothelial cells. Am J Physiol - Heart Circ Physiology. 1988;255:H554–H562. doi: 10.1152/ajpheart.1988.255.3.H554. [DOI] [PubMed] [Google Scholar]
- 37.Ethier MF, Chander V, Dobson JG. Adenosine stimulates proliferation of human endothelial cells in culture. Am J Physiol - Heart and Circ Physiology. 1993;265:H131–H138. doi: 10.1152/ajpheart.1993.265.1.H131. [DOI] [PubMed] [Google Scholar]
- 38.Drosopoulos JHF, Kraemer R, Shen H, Upmacis RK, Marcus AJ, Musi E. Human solCD39 inhibits injury-induced development of neointimal hyperplasia. Thromb Haemost. 2010;103:426–434. doi: 10.1160/TH09-05-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imai M, Takigami K, Guckelberger O, Lin Y, Sevigny J, Kaczmarek E, Goepfert C, Enjyoji K, Bach FH, Rosenberg RD, Robson SC. CD39/vascular ATP diphosphohydrolase modulates xenograft survival. Transplantation Proceedings. 2000;32:969. doi: 10.1016/s0041-1345(00)01065-4. [DOI] [PubMed] [Google Scholar]
- 40.Hoch JR, Stark VK, van Rooijen N, Kim JL, Nutt MP, Warner TF. Macrophage depletion alters vein graft intimal hyperplasia. Surgery. 1999;126:428–437. [PubMed] [Google Scholar]