Abstract
Objectives
Sleep disturbances and fatigue are common in prostate cancer patients undergoing radiotherapy. Prior research suggests mind-body techniques may improve these outcomes. We conducted a randomized-controlled trial of qigong/tai chi (QGTC) in men with prostate cancer undergoing radiotherapy.
Methods
Men with prostate cancer starting definitive radiation were randomized to one of three groups: (1) QGTC; (2) light exercise (LE); or (3) wait list control (WLC). Sleep disturbances (PSQI) and fatigue (BFI) were assessed at baseline, mid-radiotherapy (T2), during the last week of radiotherapy (T3) and at 1 (T4) and 3 months (T5) after the end of radiotherapy. Patients in the QGTC and LE groups attended three 40-minute classes per week throughout radiotherapy.
Results
Ninety patients were randomized to the three groups (QGTC=26; LE=26; WLC=24). QGTC group reported longer sleep duration at mid-XRT (QGTC=7.01 hours; LE=6.42; WL=6.50; p=0.05) but this difference did not persist over time. There were no group differences in other domains of sleep or fatigue. Exploratory analyses conducted to examine the effect of health-related QOL (EPIC and AUA score) on sleep and fatigue showed significant correlations across multiple domains.
Conclusions
QGTC during radiation for prostate cancer resulted in superior sleep duration midway through radiation, but this effect was not durable and there were no differences in other domains of sleep or fatigue. Exploratory analysis demonstrated that both sleep and fatigue were highly correlated with prostate cancer related physical symptoms. Future mind-body intervention studies should incorporate multi-modal therapy focused on improving physical symptoms in this population.
Keywords: Prostate cancer, Oncology, Qigong, Taichi, Sleep, Fatigue
BACKGROUND
Prostate cancer is the most common malignancy in men, with over 220,000 men diagnosed annually in the US.[1] Radiotherapy is an important curative modality in men with localized prostate cancer, and is often combined with androgen deprivation therapy (ADT) in patients with intermediate or high risk disease. Both radiation therapy and ADT have significant side effects including local toxicities such as bowel, bladder, and erectile dysfunction as well as more systemic effects such as loss of muscle mass, hormonal disruptions, and metabolic syndrome in the case of ADT.[2]
Fatigue is the most common complaint of patients undergoing radiotherapy, with a reported incidence of up to 80%,[3, 4] and this can be compounded with ADT.[5] Radiation[6] and ADT[7, 8] and their side effects may also result in significant sleep impairments. Fatigue and insomnia can influence QOL as well as physical and social functioning and in many cases become chronic issues impacting survivors. [5, 9]
Both aerobic[10, 11] and resistance[12–15] exercise programs have previously been shown to improve fatigue, physical functioning, and QOL in prostate cancer patients undergoing radiotherapy as well as in patients on ADT. Exercise may also mitigate the impact of ADT on metabolic parameters and muscle loss.[16]
Qigong/tai chi
Movement-based mind-body practices such as qigong, tai chi, and yoga combine physical postures or movements, focus on the breath, and mindfulness.[17] The ancient Chinese practices of qigong (“qi” energy and “gong” skill) and tai chi (“tai” supreme and “chi “ultimate”) originated in martial arts forms but the movements are slow and gentle, and this low-impact meditative exercise is widely practiced in China and is becoming increasingly popular in the West. In non-cancer populations, qigong/tai chi has been shown to decrease anxiety[18] and improve cognitive function[19] and sleep quality.[20–22] Previous work in breast cancer patients undergoing radiotherapy has shown that qigong practice can impact depressive symptoms, fatigue, and QOL.[23]
A small study recently reported promising results of improvement in fatigue in elderly prostate cancer survivors practicing qigong when compared to those randomized to a stretching intervention.[24] This is consistent with the findings of studies that have investigated the impact of other modalities combining physical movement with meditation, such as yoga, on depressive symptoms, sleep, fatigue, and QOL in cancer patients.[25–29] The effects of these multifaceted mind-body approaches on sleep and fatigue seems to be in part mediated by stress reduction and control of depression and anxiety.[23, 28]
The current study sought to examine the effects of incorporating a qigong/tai chi (QGTC) program alongside radiotherapy. We hypothesized that QGTC practice during radiation for prostate cancer would help mitigate treatment-related fatigue and sleep disturbances (primary outcomes) better than both a supervised light-exercise program and usual care through integrating physical movements with meditative stress reduction.
METHODS
Study Population
Patients with rectal, anal, or prostate cancer undergoing radiotherapy at the University of Texas MD Anderson Cancer Center were eligible. The trial was originally designed for rectal cancer patients and was expanded to include anal and then prostate cancer patients. Recruitment for the men with prostate cancer was more successful, so this more homogenous sample is reported on in this paper. Men with prostate cancer (Stage I-III) aged >18 years, able to read, write and speak English and scheduled to receive daily radiation to the prostate with or without androgen deprivation therapy (ADT) were eligible to participate. The radiation plan was at the treating physician’s discretion, but the institutional standard at the time was 75–76 Gy in 36 to 42 fxs with Intensity Modulated Radiation Therapy and image guidance, daily over 7 to 8 weeks time. Patients with physical disabilities resulting in an inability to walk unassisted, chronic pain while walking, or with difficulty walking for 20 minutes were excluded. Patients who reported practicing qigong or tai chi or taking qigong or tai chi classes within the prior year were also excluded. The protocol was approved by the MD Anderson Institutional Review Board.
Procedures
Eligible patients were identified through an institutional database or by referring physicians and were approached either by letter, phone call, or at their clinical visit with Radiation Oncology. After giving written informed consent, participants completed a 40-minute baseline assessment and provided a saliva sample for cortisol analysis (results reported elsewhere). Participants were then randomly assigned via an electronic database to one of three groups: (1) QGTC; (2) light exercise (LE); and (3) wait list control (WLC) using a form of adaptive randomization called minimization.[30] In minimization, treatment assignment for each patient is done sequentially to balance covariate characteristics among groups. In this case, patient characteristics for group assignment included stage, risk group, age, time since diagnosis, timing of radiotherapy, use of ADT, and baseline anxiety score. Follow-up assessments were conducted midway through radiotherapy (abbreviated assessment) (T2), during the last week of radiotherapy (T3) and at 1 (T4) and 3 months (T5) after the end of radiotherapy. Participants were given a gift certificate after completing each assessment ($20 value for full assessments, $10 for mid-therapy abbreviated assessment). The WLC group completed the same assessments and were given the option of participating in either QGTC or LE classes at the end of all assessments. Patients in the LE and WLC groups were asked to refrain from participating in any qigong or tai chi classes for the duration of the study.
Intervention Programs
Patients assigned to the QGTC and LE groups attended three 40-minute classes per week throughout radiotherapy. Classes were conducted one-on-one or with one or two other patients based on scheduling. However, the vast majority of classes were conducted one-on-one. Patients were given a DVD and printed instructional materials and encouraged to practice up to daily on their own.
Qigong/Tai Chi program
QGTC classes were taught by a trained qigong master. The program was based on one developed by Dr. Jerry Alan Johnson for cancer patients.[31] The program involved: (1) preparation exercises (6 minutes) consisting of guided breathing, the Great Tai Chi Circle to open the energy pathways, and grounding and centering exercises; (2) main exercises (20 minutes) including the classical 8-form Yang Style Tai Chi which is designed to take each major muscle system and joint through a range of motion, and Qigong forms specifically designed to reduce the side effects of cancer therapy through gentle movements[31] and; (3) ending exercises (9 minutes) including the Tai Chi Ball Form to rebalance energy, the Pat Down Form to remove unwanted energies, and Standing Meditation to quiet the mind.
Light Exercise program
An exercise physiologist led the light exercise classes. The program was focused on light resistance training and stretching exercises with a goal of maintaining muscle strength and range of motion. Although adding an aerobic component to the LE group may result in better outcomes, the level of exertion and movement was designed to match that of the QGTC group and provide an active control that also controls for attention and other non-specific effects. Resistance training employed a combination of 3 levels of resistance tubes and the focus is on lighter tension and more repetition (8–12 per set). Each participant was prescribed tailored resistance exercises using a combination of the three tubes targeting 8–12 repetitions per set. Participants were given individualized prescriptions based on baseline abilities detailing specific exercises for each day of the week, with muscle groups varying by day. Stretching exercises focused on major joints and were aligned with the muscle group being targeted by resistance training.
Outcome Measures
Patients completed a number of psychosocial questionnaires and here we report on the two primary outcomes measures (sleep and fatigue) and health-related QOL as an exploratory outcome.
Sleep disturbances were assessed using the Pittsburgh Sleep Quality Index (PSQI)[32], an 18-item self-rated questionnaire that assesses quality of sleep and sleep disturbances over the past one month. A total score is derived, as well as seven subscales: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction. Higher scores represent greater sleep disturbances.
Fatigue was assessed using the Brief Fatigue Inventory (BFI),[33] a 9-item questionnaire that asks participants to rate the severity of their fatigue at that moment and how much their fatigue has interfered with their lives over the previous 24 hours. Higher scores represent more fatigue.
Health-related quality of life
Health-related quality of life was assessed using the Expanded Prostate Cancer Index Composite (EPIC),[34] a 32- item questionnaire that asks participants about urinary, bowel, sexual and hormonal function. Higher scores represent better function and quality of life.
Data was tracked for patient interest in enrollment, study attrition, class attendance, and completion of questionnaires. Patient satisfaction with the QGTC and LE interventions was assessed in the middle and end of radiotherapy. Other secondary QOL and psychosocial measures were collected and will be reported elsewhere.
Data Analyses
The original goal of the trial was to recruit and retain 50 patients per group for a total sample size of 150 patients. Unfortunately, we had to stop the trial prior to achieving our recruitment goals due to slow recruitment. With 50 per group, and assuming a two-sided significance level of 0.05, we would have had at least 80% power to detect differences between any pair of group means of 0.57 standard deviation (SD) units across the three post intervention time points. With 25 patients per group our SD for detection was increased to 0.81.
To test for any baseline group differences in demographic, medical, or QOL characteristics we used chi-square tests for categorical factors and ANOVA for continuous measures. The main analyses used a mixed model procedure to analyze all repeated data to examine the treatment main effect and treatment by time interaction effect. In the mixed model, the intercept was treated as random effect and the covariance structure was unstructured.. Time effect was treated as categorical variable and we exam the post-hoc comparison at each assessment time point by using contrast statement in mixed model. We also calculated Cohen’s D using the statistics (least square means and SE) output from mixed model for the significant outcomes to estimate the magnitude of the effect.
Although there were no group differences between patients with and without missing data on demographic, medical, or the outcome variables at baseline, we imputed the missing data using multiple imputations (SAS V9.2 MI procedure) with Markov Chain Monte Carlo method and then used the MIANALYZE procedure to generate statistical inferences. All the analyses remained the same or resulted in similar p values.
To explore factors associated with sleep and fatigue, we used SAS CORR procedure to test the correlation between sleep and fatigue measures with prostate cancer specific quality of life at each time point. All the analyses were done with SAS version 9.2.
RESULTS
Baseline Characteristics of Sample
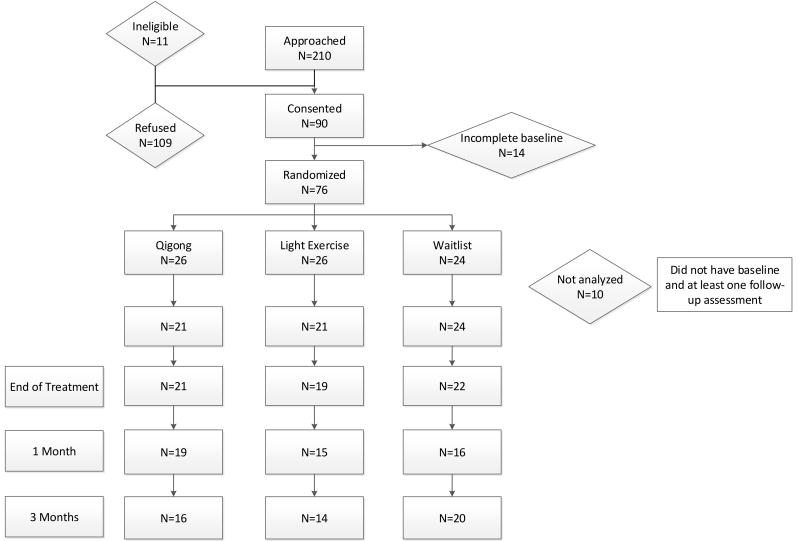
Between February 2009 and January 2012, we approached 210 eligible men, and 90 consented to participate in the study. Fourteen patients failed to complete the baseline assessment and thus 76 patients were randomized (26 QGTC, 26 LE, 24 WLC). Ninepatients did not provide any follow-up assessments and one patient did not have baseline data or follow-up assessments and therefore were not included in the primary analysis (Figure 1).
Figure 1.
Baseline characteristics of the participants assigned to each intervention were analyzed using chi-square tests for categorical variables and t-tests for continuous variables. There were no significant differences in demographic or medical characteristics between groups (Table 1). However, the LE group reported greater sleep time at baseline than the other two groups (QG 6.2 vs. LE 7.1 vs. WLC 6.5, p=0.04) (Table 2 and Figure 2). Eighty percent of the participants were of Caucasian ethnicity, with a mean age of 64.5 years.
Table 1.
Participant demographics and randomization factors.
| QGTC n=21) | LE n=21) | WLC n=24) | p | |
|---|---|---|---|---|
| Age (years: mean, SD) | 62.2 (7.4) | 65.0 (5.9) | 66.0 (8.4) | 0.212 |
| Race | 0.392 | |||
| White | 20 (95.2) | 18 (85.7) | 23 (95.8) | |
| Hispanic | 1 (4.8) | 2 (9.5) | - | |
| Black | - | - | 1 (4.2) | |
| Asian | - | 1 (4.8) | - | |
| Marital status (n=63) | 0.714 | |||
| Co-habitating (married, living with a partner) | 15 (71.4) | 19 (90.5) | 20 (83.3) | |
| Not cohabitating (not married, divorced, separated) | 5 (29.6) | 1 (9.5) | 3 (16.7) | |
| Education (n=65) | 0.709 | |||
| High school or technical school | 5 (23.8) | 3 (14.3) | 2 (8.7) | |
| Some college | 4 (19.0) | 4 (19.0) | 4 (17.4) | |
| Higher education | 12 (57.2) | 14 (66.7) | 17 (73.9) | |
| Time since diagnosis [months: mean (SD); n=65] | 8.0 (7.9) | 20.8 (32.4) | 27.7 (53.2) | 0.211 |
| Stage | 0.972 | |||
| T1–T2a | 10 (47.6) | 10 (47.6) | 13 (54.2) | |
| T2b-T2c | 6 (28.6) | 7 (33.3) | 6 (25.0) | |
| T3a and above | 5 (23.8) | 4 (19.1) | 5 (20.8) | |
| Gleason score (n=65) | 0.996 | |||
| ≤6 | 2 (10.0) | 2 (9.5) | 2 (8.3) | |
| 7 | 13 (65.0) | 14 (66.7) | 16 (66.7) | |
| 8–9 | 5 (25.0) | 5 (23.8) | 6 (25.0) | |
| PSA level (n=65) | 0.879 | |||
| ≤10 | 17 (81.0) | 17 (81.0) | 19 (82.6) | |
| 10.1–20 | 3 (14.3) | 3 (14.3) | 4 (17.4) | |
| >20 | 1 (4.7) | 1 (4.7) | - | |
| XRT timing | 0.602 | |||
| Definitive | 14 (66.7) | 14 (66.7) | 13 (54.2) | |
| Adjuvant | 7 (33.3) | 7 (33.3) | 11 (46.8) | |
| Type of radiation received | 0.750 | |||
| IMRT | 8 (38.1) | 10 (47.6) | 9 (37.5) | |
| Proton | 13 (61.9) | 11 (52.4) | 15 (62.5) | |
| Baseline anxiety levels (mean, SD) | 35.0 (11.1) | 32.6 (9.7) | 32.5 (11.7) | 0.698 |
| Concurrent androgen deprivation therapy | 0.934 | |||
| Yes | 16 (76.2) | 15 (71.4) | 18 (75.0) | |
| No | 5 (23.8) | 6 (29.6) | 6 (25.0) |
QGTC: Qigong/tai chi treatment group; LE: Light exercise; WLC: Waitlist control; PSA: Prostate-Specfic Antigen; XRT: radiation therapy; IMRT: Intensity-Modulated Radiation Therapy
Table 2.
Sleep and fatigue score means by group over time
| Baseline | Mid Treatment | End of Treatment | 1 month | 3 months | ||
|---|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | ||
| BFI | QGTC | 1.41 (0.36) | 1.96 (0.36) | 1.45 (0.35) | 2.97 (0.37) | 2.56 (0.39) |
| LE | 1.47 (0.36) | 1.57 (0.34) | 1.65 (0.38) | 2.11 (0.40) | 2.38 (0.42) | |
| WLC | 1.97 (0.34) | 1.16 (0.33) | 1.87 (0.33) | 1.64 (0.38) | 1.81 (0.35) | |
| PSQI total | QGTC | 6.85 (0.76) | 5.63 (0.54) | 5.16 (0.52) | 6.19 (0.56) | 6.29 (0.60) |
| LE | 5.58 (0.78) | 5.74 (0.55) | 5.33 (0.63) | 5.54(0.63) | 6.11 (0.65) | |
| WLC | 6.58 (0.69) | 6.41 (0.48) | 5.77 (0.50) | 5.32 (0.60) | 5.31 (0.52) | |
| Sleep Duration | QGTC | 6.24 (0.23)* | 7.00*+ (0.19) | 6.97 (0.19) | 6.72 (0.20) | 6.77 (0.21) |
| LE | 7.07 (0.23)* | 6.45* (0.19) | 6.66 (0.20) | 7.19 (0.21) | 6.96 (0.23) | |
| WLC | 6.54 (0.21) | 6.53+ (0.17) | 6.90 (0.18) | 6.99 (0.20) | 6.85 (0.19) |
Means at baseline are raw means. Means at follow-up are adjusted scores from Mixed models.
P<0.05 for 2 by 2 comparison from mixed model;
p=0.07 for 2 by 2 comparison from mixed model.
BFI: Brief Fatigue Inventory; PSQI: Pittsburg Sleep Quality Index; QGTC: Qigong/tai chi treatment group; LE: Light exercise; WLC: Waitlist control.
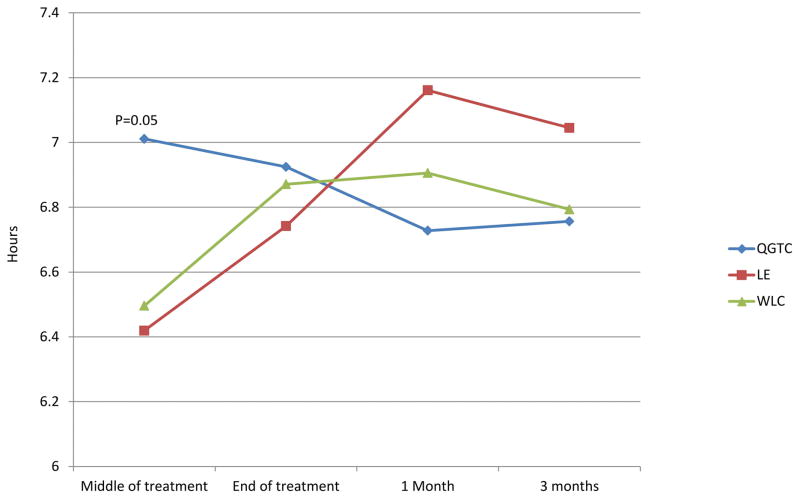
Figure 2.
Follow up means adjusted for baseline (least square means). QGTC: Qigong treatment group; LE: Light exercise; WLC: Waitlist control
Completion of interventions
Adherence to the interventions was high with 63.5% attending all sessions and 80.8% attending greater than 50% of the sessions. There were no differences between the QQTC and LE groups in class attendance.
Main effects of interventions
There was a statistically significant effect of time on sleep duration (p=0.03), with an increase in sleep duration over time. In the MIXED model analysis with repeated measures, there was no significant treatment main effect and there was a marginal group by time interaction for sleep duration (p=0.06). Post-hoc analyses from the MIXED model revealed that at T2 (mid-way through radiotherapy) the QGTC group slept significantly longer than those in the LE group (QGTC – 7.00 hours; LE – 6.45 hours, p=0.047; d = 0.56) and marginally significantly longer than WLC group. WLC – 6.53, p=0.07; d = 0.51) (Table 2 and Figure 2). However, this difference did not persist to the end of radiotherapy and there were also no group differences in sleep duration at the follow-up time points. No significant time, group, or group by time interactions were observed for the other sleep outcomes and all effect sizes were in the small range (d < 0.45) (Table 2). PSQI total scores were slightly above 5 for each group at each time point, indicating clinically significant sleep disturbance.[32] There was a main effect of time on fatigue (F=3.79, p=0.005), with increases over time. There were no significant group or group by time interactions for fatigue levels. However, fatigue remained in the “mild” range (≤ 3) in all groups at all time points.
Although there were no group differences between patients with and without missing data on demographic, medical, or the outcome variables at baseline, we imputed the missing data using multiple imputations (SAS V9.2 MI procedure) with Markov Chain Monte Carlo method and then used the MIANALYZE procedure to generate statistical inferences. All the analyses resulted in similar p values.
Exploratory Analyses
Due to the lack of group differences for both sleep outcomes and fatigue levels, we sought to explore factors that were associated with both variables to explore potential factors interfering with the effects of the intervention. We examined health-related QOL as assessed from the EPIC. We first sought to determine if there were any group differences on these measures. Mixed model and GLM analyses revealed no group differences on any of these variables at each time point. However, not surprisingly, urinary function, bowel function, and prostate symptom score (AUA score) were all significantly worse by the end of radiation therapy and improved by the 1 and 3 month follow-up (Table 3). There was no effect of time on hormonal function.
Table 3.
Prostate cancer related symptoms and quality of life by group over time
| Baseline | End of Treatment | 1 month | 3 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | p | Mean | p | Mean | p | Mean | p | ||
| Urinary function | QGTC | 90.39 (2.1) | 0.36 | 80.64 (3.36) | 0.18 | 83.91 (3.24) | 0.56 | 91.02 (2.74) | 0.91 |
| LE | 85.85 (2.35) | 84.08 (3.66) | 86.97 (3.6) | 89.47 (2.95) | |||||
| WLC | 88.54 (2.05) | 74.5 (3.19) | 82.14 (3.46) | 88.94 (2.38) | |||||
| Bowel function | QGTC | 93.42 (1.79) | 0.66 | 88.35 (2.61) | 0.89 | 89.58 (2.79) | 0.53 | 94.64 (1.36) | 0.86 |
| LE | 93.45 (2.01) | 89.29 (2.85) | 86.26 (3.28) | 95.33 (1.46) | |||||
| WLC | 95.45 (1.74) | 88.01 (2.48) | 92.53 (3.16) | 94.87 (1.21) | |||||
| Hormonal function | QGTC | 77.7 (3.27) | 0.53 | 80.5 (2.54) | 0.54 | 71.53 (4.17) | 0.76 | 75.15 (4.21) | 0.03 |
| LE | 82.27 (3.68) | 83.52 (2.76) | 72.59 (4.63) | 72.59 (4.52) | |||||
| WLC | 77.16 (3.19) | 76.73 (2.41) | 76.83 (4.46) | 83.85 (3.74) | |||||
| AUA score | QGTC | 6.1 (1.1) | 0.11 | 10.42 (1.61) | 0.39 | 8.22 (1.4) | 0.60 | 6.47 (1.08) | 0.65 |
| LE | 8.56 (1.23) | 9.81 (1.75) | 8.54 (1.65) | 8.15 (1.16) | |||||
| WLC | 5.14 (1.07) | 11.76 (1.53) | 6.57 (1.59) | 6.9 (0.93) | |||||
QGTC: Qigong/tai chi treatment group; LE: Light exercise; WLC: Waitlist control; AUA: American Urological Association Symptom Score
Exploratory analyses were then conducted to examine the effect of prostate cancer health-related quality of life (EPIC) and prostate-related symptoms (AUA score) on sleep and fatigue at each time point. We found significant and consistent correlations between sleep and fatigue with urinary, bowel, and hormonal function and AUA scores (Table 4). Table 4 indicates that worse prostate cancer health-related quality of life was associated with greater sleep disturbance and fatigue scores.
Table 4.
Correlations of prostate cancer related symptoms and quality of life with sleep and fatigue
| Domain | Baseline | End of Treatment | 1 month | 3 months | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PSQI | BFI | PSQI | BFI | PSQI | BFI | PSQI | BFI | |||||||||
| |r| | p | |r| | p | |r| | p | |r| | p | |r| | p | |r| | p | |r| | p | |r| | p | |
| Urinary function | −0.40 | 0.003 | −0.19 | 0.15 | −0.33 | 0.02 | −0.31 | 0.02 | −0.39 | 0.01 | −0.23 | 0.14 | −0.22 | 0.14 | −0.13 | 0.40 |
| Bowel function | 0.08 | 0.58 | 0.01 | 0.94 | −0.14 | 0.32 | −0.37 | 0.005 | −0.35 | 0.02 | −0.37 | 0.01 | −0.20 | 0.19 | −0.42 | 0.005 |
| Hormonal function | −0.58 | <0.0001 | −0.51 | <0.001 | −0.50 | 0.0002 | 0.55 | <0.001 | −0.33 | 0.04 | −0.49 | 0.0008 | −0.6 | <0.0001 | −0.66 | <0.0001 |
| aua score | 0.44 | 0.001 | 0.18 | 0.18 | 0.31 | 0.02 | 0.21 | 0.13 | 0.28 | 0.06 | 0.34 | 0.02 | 0.35 | 0.02 | 0.16 | 0.28 |
|r|: correlation coefficient; PSQI: Pittsburg Sleep Quality Index; BFI: Brief Fatigue Inventory
CONCLUSIONS
In this study, we found that a qigong/tai chi intervention during radiation for prostate cancer resulted in statistically superior self-reported sleep duration compared to both wait-control and a supervised light exercise intervention midway through radiation, but that this effect was not durable. Moreover, the clinical significance of an improvement in self-reported sleep duration from 6.4 to 7 hours is unclear, although 7 hours of sleep is the minimum recommended in many guidelines[35] based on data showing shorter sleep duration is associated with disease risk in many populations.[36, 37] Sleep duration of all three groups returned to baseline levels or in fact higher at subsequent time points, though the composite PSQI score remained above the clinical threshold for “sleep disturbance” for all groups at all timepoints other than the light exercise group at end of radiation. Within our limited sample, we did not detect any group differences in other domains of sleep, including sleep quality, sleep latency, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. There was no difference in self-reported fatigue scores either during or after radiation therapy between the groups. However, the overall level of fatigue in this population, though it did increase with time, was surprisingly mild.
The lack of detected durable benefit of the qi gong/tai chi intervention on improving sleep and fatigue in this population is likely driven by the fact that both sleep disturbances and fatigue in prostate cancer are mainly due to hot flashes and/or urinary symptoms which were not modified with the intervention.[7, 8, 38] Although there were group differences at mid-radiotherapy, all three groups returned to baseline levels or in fact higher than baseline at subsequent time points. This suggests that the negative effects of radiotherapy on sleep duration are short term and that qi gong/tai chi may be protective against these changes.
Indeed, in our study, urinary, bowel, and hormonal disturbances were reduced by the end of radiotherapy and significantly and consistently associated with sleep and fatigue. Prostate cancer specific QOL measures were consistent with previous published data and represent clinically meaningful levels of bother and dysfunction.[34, 39, 40] Future qi gong/tai chi intervention studies should incorporate movements targeting the pelvic floor focused on improving urinary symptoms to help improve sleep and fatigue. Mind-body therapies have previously been shown to improve hot flash symptoms and meditations targeting hormonal symptoms could also be incorporated into the qi gong/tai chi intervention.[41]
Unlike previous studies,[12, 13] we also did not find a difference in sleep and fatigue between the light exercise versus the control group. The exercise intervention in this study was designed to match the intensity of the QGTC intervention and thus differed from other exercise interventions and may not have met the threshold for benefit.
The feasibility of conducting a mind-body intervention in a prostate cancer population undergoing radiotherapy was demonstrated, though the acceptance rate was less than 50% and the trial was terminated early for slow recruitment. Participants in the QGTC arm shared that they found the classes enjoyable and beneficial, noting relaxation, stress reduction, and improved balance as benefits. The vast majority of mind-body intervention trials have been conducted in the breast cancer population, with a majority during the survivorship phase. This is likely a reflection of the higher intrinsic interest in and self-adoption of complementary medicine including mind-body approaches by younger breast cancer patients, a trend observed in multiple prior survey studies of complementary and alternative medicine (CAM) use in cancer patients.[42, 43]
Our study has several limitations, which must be acknowledged. Given that the sample size did not reach our target, we were underpowered to detect small differences in fatigue and sleep between groups. However, no consistent trends were observed towards superiority of the QGTC intervention group and small differences are likely not clinically meaningful. While the PSQI is a well-validated patient-reported measure of sleep disturbances, it is a measure of self-reported sleep habits over a one-month period of time and given that these patients were on active treatment, sleep may not have been highly homogenous over that one-month period. Furthermore, self-reported sleep and actual sleep duration measured by actigraphy have been found to be only modestly correlated in past studies.[44] Future studies should incorporate actigraphy as an objective sleep quality measure; however, self-reported sleep remains an important endpoint as a measure of satisfaction with sleep. As always, the inclusion of appropriate controls, as was done in this study, which included active and wait-list controls, are critical in research of self-reported outcomes.
In conclusion, we found that a qigong/tai chi intervention during radiation for prostate cancer resulted in statistically improved self-reported duration of sleep at mind-radiotherapy compared to both a light exercise and wait-control groups but that this effect was not sustained and other sleep and fatigue domains were not significantly improved. In this population, urinary and hormonal symptoms appear to drive sleep disturbances and qigong/tai chi did not modify these outcomes. Future mind-body intervention studies in this population should include multi-modal therapy to address physical symptoms the men are experiencing.
Acknowledgments
Funding/Support: NCI CA129201 (Cohen, PI); CA016672 and the Center for Energy Balance in Cancer Prevention and Survivorship, Duncan Family Institute. JLM is supported by an ASCO Young Investigator Award and a T32 Institutional Training Grant, T32 CA009666. Partial support for Lorenzo Cohen was provided by the Richard E. Haynes Distinguished Professorship in Clinical Cancer Prevention.
References
- 1.Howlader NNA, Krapcho M, Garshell J, Miller D, Altekruse Sf, Kosary Cl, Yu M, Ruhl J, Tatalovich Z, Mariotto a, Lewis Dr, Chen Hs, Feuer Ej, Cronin Ka, editors. Seer Cancer Statistics Review, 1975–2012. National Cancer Institute; Bethesda, Md: Apr, 2015. Http://Seer.Cancer.Gov/Csr/1975_2012/, Based on November 2014 Seer Data Submission, Posted to the Seer Web Site. [Google Scholar]
- 2.Gomella LG, Johannes J, Trabulsi EJ. Current prostate cancer treatments: effect on quality of life. Urology. 2009;73(5 Suppl):S28–35. doi: 10.1016/j.urology.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Hickok JT, Roscoe JA, Morrow GR, Mustian K, Okunieff P, Bole CW. Frequency, severity, clinical course, and correlates of fatigue in 372 patients during 5 weeks of radiotherapy for cancer. Cancer. 2005;104(8):1772–1778. doi: 10.1002/cncr.21364. [DOI] [PubMed] [Google Scholar]
- 4.Monga U, Kerrigan AJ, Thornby J, Monga TN, Zimmermann KP. Longitudinal study of quality of life in patients with localized prostate cancer undergoing radiotherapy. J Rehabil Res Dev. 2005;42(3):391–399. doi: 10.1682/jrrd.2004.06.0071. [DOI] [PubMed] [Google Scholar]
- 5.Lilleby W, Stensvold A, Dahl AA. Intensity-modulated radiotherapy to the pelvis and androgen deprivation in men with locally advanced prostate cancer: a study of adverse effects and their relation to quality of life. Prostate. 2013;73(10):1038–1047. doi: 10.1002/pros.22651. [DOI] [PubMed] [Google Scholar]
- 6.Miaskowski C, Paul SM, Cooper BA, et al. Predictors of the trajectories of self-reported sleep disturbance in men with prostate cancer during and following radiation therapy. Sleep. 2011;34(2):171–179. doi: 10.1093/sleep/34.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savard J, Hervouet S, Ivers H. Prostate cancer treatments and their side effects are associated with increased insomnia. Psychooncology. 2013;22(6):1381–1388. doi: 10.1002/pon.3150. [DOI] [PubMed] [Google Scholar]
- 8.Savard J, Ivers H, Savard MH, Morin CM. Cancer treatments and their side effects are associated with aggravation of insomnia: Results of a longitudinal study. Cancer. 2015;121(10):1703–1711. doi: 10.1002/cncr.29244. [DOI] [PubMed] [Google Scholar]
- 9.Drummond FJ, Kinnear H, O’leary E, Donnelly, Gavin A, Sharp L. Long-term health-related quality of life of prostate cancer survivors varies by primary treatment. Results from the PiCTure (Prostate Cancer Treatment, your experience) study. J Cancer Surviv. 2015 doi: 10.1007/s11764-014-0419-6. [DOI] [PubMed] [Google Scholar]
- 10.Monga U, Garber SL, Thornby J, et al. Exercise prevents fatigue and improves quality of life in prostate cancer patients undergoing radiotherapy. Arch Phys Med Rehabil. 2007;88(11):1416–1422. doi: 10.1016/j.apmr.2007.08.110. [DOI] [PubMed] [Google Scholar]
- 11.Windsor PM, Nicol KF, Potter J. A randomized, controlled trial of aerobic exercise for treatment-related fatigue in men receiving radical external beam radiotherapy for localized prostate carcinoma. Cancer. 2004;101(3):550–557. doi: 10.1002/cncr.20378. [DOI] [PubMed] [Google Scholar]
- 12.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21(9):1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 13.Segal RJ, Reid RD, Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27(3):344–351. doi: 10.1200/JCO.2007.15.4963. [DOI] [PubMed] [Google Scholar]
- 14.Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28(2):340–347. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 15.Bourke L, Gilbert S, Hooper R, et al. Lifestyle changes for improving disease-specific quality of life in sedentary men on long-term androgen-deprivation therapy for advanced prostate cancer: a randomised controlled trial. Eur Urol. 2014;65(5):865–872. doi: 10.1016/j.eururo.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen PL, Alibhai SM, Basaria S, et al. Adverse Effects of Androgen Deprivation Therapy and Strategies to Mitigate Them. Eur Urol. 2015;67(5):825–836. doi: 10.1016/j.eururo.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Chaoul A, Milbury K, Sood AK, Prinsloo S, Cohen L. Mind-body practices in cancer care. Curr Oncol Rep. 2014;16(12):417. doi: 10.1007/s11912-014-0417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, Lee EK, Wu T, et al. The effects of tai chi on depression, anxiety, and psychological well-being: a systematic review and meta-analysis. Int J Behav Med. 2014;21(4):605–617. doi: 10.1007/s12529-013-9351-9. [DOI] [PubMed] [Google Scholar]
- 19.Walsh JN, Manor B, Hausdorff J, et al. Impact of Short- and Long-term Tai Chi Mind-Body Exercise Training on Cognitive Function in Healthy Adults: Results From a Hybrid Observational Study and Randomized Trial. Glob Adv Health Med. 2015;4(4):38–48. doi: 10.7453/gahmj.2015.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li F, Fisher KJ, Harmer P, Irbe D, Tearse RG, Weimer C. Tai chi and self-rated quality of sleep and daytime sleepiness in older adults: a randomized controlled trial. J Am Geriatr Soc. 2004;52(6):892–900. doi: 10.1111/j.1532-5415.2004.52255.x. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen MH, Kruse A. A randomized controlled trial of Tai chi for balance, sleep quality and cognitive performance in elderly Vietnamese. Clin Interv Aging. 2012;7:185–190. doi: 10.2147/CIA.S32600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeh GY, Mietus JE, Peng CK, et al. Enhancement of sleep stability with Tai Chi exercise in chronic heart failure: preliminary findings using an ECG-based spectrogram method. Sleep Med. 2008;9(5):527–536. doi: 10.1016/j.sleep.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z, Meng Z, Milbury K, et al. Qigong improves quality of life in women undergoing radiotherapy for breast cancer: results of a randomized controlled trial. Cancer. 2013;119(9):1690–1698. doi: 10.1002/cncr.27904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campo RA, Agarwal N, Lastayo PC, et al. Levels of fatigue and distress in senior prostate cancer survivors enrolled in a 12-week randomized controlled trial of Qigong. J Cancer Surviv. 2014;8(1):60–69. doi: 10.1007/s11764-013-0315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer. 2012;118(15):3766–3775. doi: 10.1002/cncr.26702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31(26):3233–3241. doi: 10.1200/JCO.2012.43.7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sprod LK, Fernandez ID, Janelsins MC, et al. Effects of yoga on cancer-related fatigue and global side-effect burden in older cancer survivors. J Geriatr Oncol. 2015;6(1):8–14. doi: 10.1016/j.jgo.2014.09.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taso CJ, Lin HS, Lin WL, Chen SM, Huang WT, Chen SW. The effect of yoga exercise on improving depression, anxiety, and fatigue in women with breast cancer: a randomized controlled trial. J Nurs Res. 2014;22(3):155–164. doi: 10.1097/jnr.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 29.Cohen L, Warneke C, Fouladi RT, Rodriguez MA, Chaoul-Reich A. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100(10):2253–2260. doi: 10.1002/cncr.20236. [DOI] [PubMed] [Google Scholar]
- 30.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 31.Ja J. Chinese medical Qigong therapy: A comprehensive clinical guide. 1. International Institute of Medical Qigong; 2000. [Google Scholar]
- 32.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 33.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 34.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56(6):899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 35.Watson NF. Sleep duration: a consensus conference. J Clin Sleep Med. 2015;11(1):7–8. doi: 10.5664/jcsm.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shan Z, Ma H, Xie M, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38(3):529–537. doi: 10.2337/dc14-2073. [DOI] [PubMed] [Google Scholar]
- 37.Iftikhar IH, Donley MA, Mindel J, Pleister A, Soriano S, Magalang UJ. Sleep Duration and Metabolic Syndrome. An Updated Dose-Risk Metaanalysis. Ann Am Thorac Soc. 2015;12(9):1364–1372. doi: 10.1513/AnnalsATS.201504-190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Storey DJ, Mclaren DB, Atkinson MA, et al. Clinically relevant fatigue in men with hormone-sensitive prostate cancer on long-term androgen deprivation therapy. Ann Oncol. 2012;23(6):1542–1549. doi: 10.1093/annonc/mdr447. [DOI] [PubMed] [Google Scholar]
- 39.Bhattasali O, Chen LN, Woo J, et al. Patient-reported outcomes following stereotactic body radiation therapy for clinically localized prostate cancer. Radiat Oncol. 2014;9:52. doi: 10.1186/1748-717X-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20(2):557–566. doi: 10.1200/JCO.2002.20.2.557. [DOI] [PubMed] [Google Scholar]
- 41.Cramer H, Rabsilber S, Lauche R, Kummel S, Dobos G. Yoga and meditation for menopausal symptoms in breast cancer survivors-A randomized controlled trial. Cancer. 2015;121(13):2175–2184. doi: 10.1002/cncr.29330. [DOI] [PubMed] [Google Scholar]
- 42.Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol. 2000;18(13):2505–2514. doi: 10.1200/JCO.2000.18.13.2505. [DOI] [PubMed] [Google Scholar]
- 43.Molassiotis A, Fernadez-Ortega P, Pud D, et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol. 2005;16(4):655–663. doi: 10.1093/annonc/mdi110. [DOI] [PubMed] [Google Scholar]
- 44.Grandner MA, Kripke DF, Yoon IY, Youngstedt SD. Criterion validity of the Pittsburgh Sleep Quality Index: Investigation in a non-clinical sample. Sleep Biol Rhythms. 2006;4(2):129–139. doi: 10.1111/j.1479-8425.2006.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]