Abstract
Many antidepressants stimulate adult hippocampal neurogenesis, but the mechanisms by which they increase neurogenesis and modulate behavior are incompletely understood. Here we show that hippocampal bone morphogenetic protein (BMP) signaling is modulated by antidepressant treatment, and that the changes in BMP signaling mediate effects of antidepressant treatment on neural progenitor cell proliferation and behavior. Treatment with the selective serotonin reuptake inhibitor fluoxetine suppressed BMP signaling in the adult mouse hippocampus both by decreasing levels of BMP4 ligand and increasing production of the BMP inhibitor noggin. Increasing BMP signaling in the hippocampus via viral overexpression of BMP4 blocked the effects of fluoxetine on proliferation in the dentate gyrus and on depressive behavior. Conversely, inhibiting BMP signaling via viral overexpression of noggin in the hippocampus or infusion of noggin into the ventricles exerted antidepressant and anxiolytic activity along with an increase in hippocampal neurogenesis. Similarly, conditional genetic deletion of the type II BMP receptor in Ascl1-expressing cells promoted neurogenesis and reduced anxiety- and depression-like behaviors, suggesting that neural progenitor cells contribute to the effects of BMP signaling on affective behavior. These observations indicate that BMP signaling in the hippocampus regulates depressive behavior, and that decreasing BMP signaling may be required for the effects of some antidepressants. Thus BMP signaling is a new and powerful potential target for the treatment of depression.
Introduction
Major depressive disorder (MDD) is a leading cause of disability1, with an estimated lifetime prevalence of approximately 10–20%.2, 3 Despite recent advances in our understanding of MDD, nearly one third of patients are refractory to antidepressant treatment.4 Thus, new molecular targets and treatment strategies are needed to address this disorder.
Antidepressant treatment in rodents and non-human primates increases proliferation and neurogenesis in the dentate gyrus (DG)5–8 and inhibition of hippocampal neurogenesis can prevent the behavioral effects of antidepressant treatment.9, 10 This suggests a causal link between increased hippocampal neurogenesis and antidepressant efficacy. Studies in humans have found that patients with MDD have reduced hippocampal volume.11–13 Moreover, patients with MDD treated with antidepressants have an increased number of neural progenitor cells in the DG compared to untreated patients,14 further supporting a link between hippocampal neurogenesis and depression.
In the adult hippocampus, BMP signaling regulates multiple phases of neurogenesis.15 BMPs are extracellular ligands which bind to heterotetramers of type I and type II BMP receptor (BMPR) subunits16 leading to phosphorylation of Smads 1, 5, and 8 which then translocate to the nucleus to induce changes in gene expression.17 In the adult DG, BMP signaling promotes quiescence of neural stem cells (NSCs) and neural progenitor cells (NPCs), and inhibition of BMP signaling results in NSC activation and increased neurogenesis.15, 18 Inhibition of BMP signaling in mice results in improved performance on hippocampus-dependent cognitive tasks19 and can reverse some cognitive deficits associated with aging.20 While many exogenous factors in the DG stimulate hippocampal neurogenesis,21 BMP signaling conversely diminishes neurogenesis and provides a mechanism for allostatic control of the neurogenic process.
Given the critical role of BMP signaling in regulating adult hippocampal neurogenesis, we hypothesized that it might also regulate anxiety- and depression-like behaviors, and that neurogenic effects of antidepressants might be mediated, at least in part, by changes in BMP signaling. Here we show that antidepressant treatment reduces levels of BMP signaling in the adult DG and that overexpression of BMP4 blocks the effects of antidepressant treatment on behavior and hippocampal NPC proliferation. Further, inhibition of BMP signaling, either via overexpression of the BMP inhibitor noggin or conditional deletion of the BMP type II receptor in NPCs, reduces anxiety- and depression-like behaviors concurrent with an increase in levels of hippocampal neurogenesis.
Materials and Methods
Animals
All animal procedures were performed in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and were approved by the Northwestern University Institutional Animal Care and Use Committee. Eight to ten week old C57Bl/6 male and female mice (Jackson Laboratory) were used for antidepressant treatment experiments as well as DG virus injection experiments. For antidepressant and virus injection experiments, animals were allocated to experimental groups by randomly assigning each cage to an experimental treatment. BMPRII floxed mice (BMPRIIfx/fx)22 were mated to mice containing tamoxifen-inducible Cre recombinase under the control of the Ascl1 promoter (Ascl1-CreER™)23. To monitor Cre expression, these mice were crossed with Rosa-CAG-LSL-ZsGreen1 mice (RosaZG/ZG, Jackson Laboratory)24. Tamoxifen-induced ablation of BMPRII was performed at 8–10 weeks of age in BMPRIIfx/fx; Ascl1-CreER™; RosaZG/ZG mice. BMPRII+/+; Ascl1-CreER™; RosaZG/ZG littermates were used as controls. All mice were housed 3–5 per cage under a 14:10 hour light:dark cycle with ad libitum access to food and water.
Drug administration
Fluoxetine (Sigma Aldrich) was dissolved in saline and administered at a dose of 10 mg/kg via intraperitoneal (i.p.) injection daily for 14 consecutive days. For bromodeoxyuridine (BrdU) labeling, mice received four consecutive i.p. injections of 50 mg/kg BrdU (Sigma Aldrich) at two-hour intervals and were sacrificed 24 hours after the first injection. For conditional deletion of BMPRII, tamoxifen was administered via i.p. injection at 180 mg/kg for five consecutive days.
Viral vector production and stereotaxic viral injection
The lentiviral vectors were produced as previously described.15 See Supplementary Materials and Methods for detailed procedures. Briefly, pBOB-IRES2-mCherry (LV-Control), pBOB-secNoggin-IRES2-mCherry (LV-Noggin), or pBOB-secBMP4-IRES2-mCherry (LV-BMP4) lentiviral vectors were generated and lentiviruses were used at a titer of 108 cfu/ml. Stereotaxic virus injections were performed using a stereotaxic frame (Kopf Model 900), a Micro 4 Microsyringe Pump Controller (World Precision Instruments), and a microsyringe (Hamilton) with a pulled glass pipette. Mice were anesthetized via isofluorane inhalation. 2µl of lentivirus were injected at a rate of 0.5µl per minute into the bilateral DG. Lentiviral infection was confirmed by immunohistochemistry, and mice lacking successful virus infection in the bilateral DG were excluded from analysis.
Intraventricular infusion
Infusion experiments were performed with an Alzet pump as previously described.19 The pump delivered 50 ng/µl BMP4 (R&D), 50 ng/µl Noggin (R&D), or vehicle for 15 days at a rate of 0.25µl/hr.
Behavioral analysis
For all behavioral analyses, mice were transferred to the testing room at least one hour prior to testing for acclimation to the test environment. All behavioral apparatus were wiped with 70% ethanol prior to each trial. The elevated zero maze (EZM) and open field (OF) tests were performed at the Northwestern University Behavioral Phenotyping Core Facility. Behavioral analyses were performed with the experimenter blinded to the experimental condition.
EZM
The EZM consisted of a 56 cm diameter round track divided into two closed quadrants separated by 180 degrees and two open quadrants separated by 180 degrees. The closed quadrants were enclosed by 15 cm high walls and the open quadrants had no walls. The maze was elevated 46 cm above the ground and testing was conducted under white light. The mouse was placed into a closed quadrant and behavior was video recorded for 5 minutes. LimeLight software (Actimetrics) measured the percentage of time the mouse spent in open quadrants.
OF
The OF apparatus consisted of a 56 × 56 cm open arena with 30 cm high walls. The mouse was placed into the center of the arena and behavior was video recorded for 5 minutes. LimeLight software (Actimetrics) measured the total distance traveled and the percentage distance traveled in the periphery and the 28 × 28 cm central area of the OF.
Tail Suspension Test (TST)
Tape was affixed to the mouse’s tail 2 cm from the tip and the mouse was suspended from a horizontal bar at a height of 30 cm. Cylindrical plastic tubes were placed at the base of the tail to prevent tail climbing. The mouse was suspended for 6 minutes and video recordings of the test were quantified by an observer blinded to experimental condition. The total time spent in an immobile posture and the latency to the first bout of immobility were measured.
Immunohistochemistry
Mice were transcardially perfused with PBS followed by 4% paraformaldehyde. Brains were fixed overnight in 4% paraformaldehyde and then transferred to 30% sucrose for 24 hours. 40µm-thick floating sections were obtained and immunohistochemical staining was performed using standard techniques. Primary antibodies used were: mouse anti-Ascl1 (1:500, BD Pharmingen, 556604), mouse anti-BrdU (1:200, BD Pharmingen, 347580), goat anti-Dcx (1:500, Santa Cruz, sc-8066), mouse anti-NeuN (1:500, Millipore, MAB377), and goat anti-Sox2 (1:500, Santa Cruz, sc-17320). See Supplementary Materials and Methods for detailed procedures.
Confocal imaging and quantification
Images were acquired using a Leica TCS SP5 Confocal Microscope. Z-stacks of the DG were obtained (step size: 1 µm) using sequential scanning to prevent bleed-through between fluorophores. Six or more Z-stacks of equal thickness and equivalent rostrocaudal position were quantified for each sample. For viral injection experiments, only sections with confirmed infection in the DG were quantified. Stereological cell counting was performed using ImageJ software. For per volume quantification, cell counts were normalized to the volume of the DG granule cell layer. All imaging and quantification was performed blinded to experimental condition.
Protein extraction and western blotting
The DG was micro-dissected from adult mice as previously described.25 The tissue was mechanically homogenized on ice in T-per protein extraction reagent (Thermo Fisher) with Halt protease and phosphatase inhibitors (Thermo Fisher). Lysates were centrifuged at 10,000 rpm and the supernatant was collected for western blot analysis. Western blotting was performed using standard techniques. Primary antibodies used were: mouse anti-BMP4 (1:1000, OriGene, UM500038), mouse anti-GAPDH (1:4000, Millipore, MAB374), rabbit ant-Id2 (1:200, Santa Cruz, sc-489), rabbit anti-noggin (1:2000, Millipore, AB5729), rabbit anti-phospho-Smad1/5/8 (1:1000, Cell Signaling, CS9511), and rabbit anti-Smad1/5/8 (1:1000, Santa Cruz, sc-6031-R). See Supplementary Materials and Methods for detailed procedures.
Statistical analysis
Statistical analyses were performed via two-tailed, unpaired Student’s t-test or two-way ANOVA with Bonferroni’s paired comparisons test for correction for multiple comparisons, as indicated in the figures and text. GraphPad Prism 6 software was used for the analyses. Normality was assessed using the Shapiro-Wilk and Kolmogorov-Smirnov tests and equality of variances was verified using the F-test of equality of variance. The significance threshold used was p<0.05. Sample sizes were chosen based on results from preliminary experiments. The exact sample size for each experimental condition is indicated in the results section, with n representing the number of individual animals analyzed. All data are reported as mean ± SEM.
Results
Fluoxetine treatment decreases BMP signaling in the adult DG
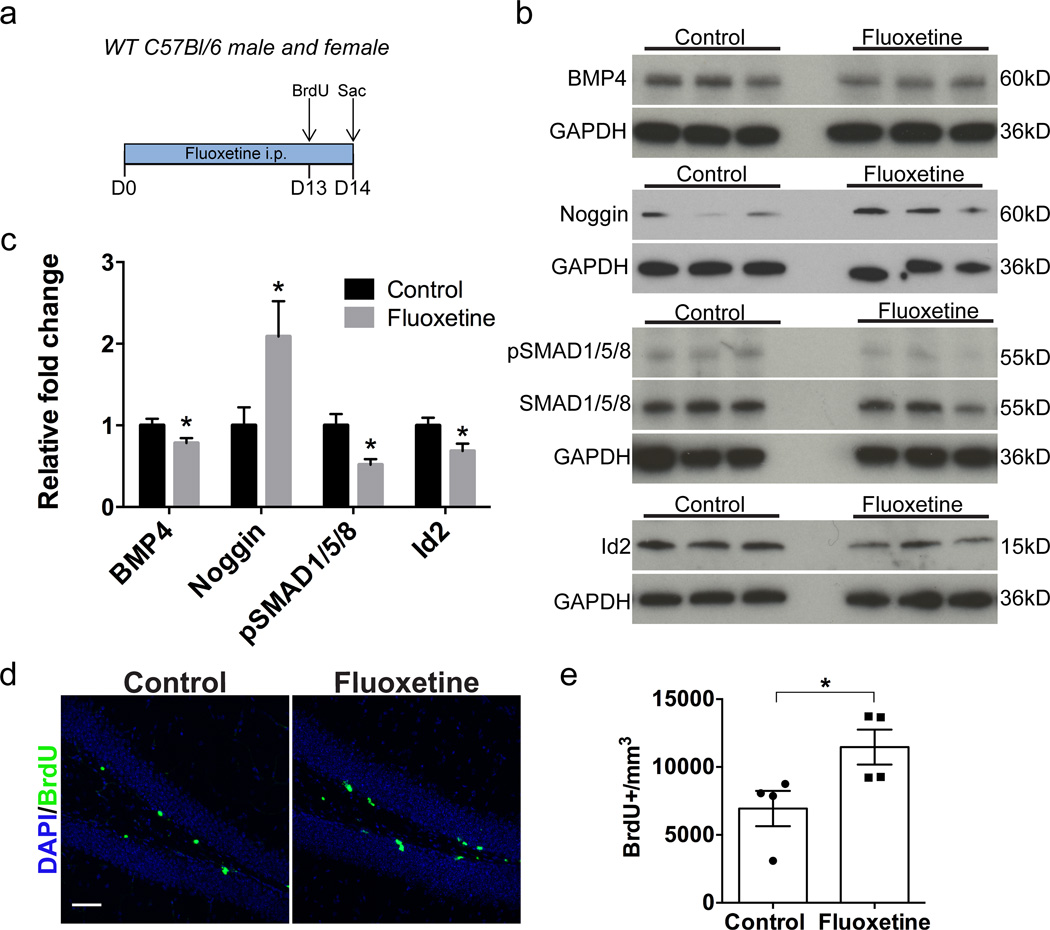
Given the critical role of BMP signaling in modulating hippocampal neurogenesis and the link between neurogenesis and antidepressant efficacy, we asked whether antidepressant treatment alters BMP signaling in the adult DG. To address this question, we treated C57Bl/6 mice with fluoxetine for 14 days and performed western blot analysis to quantify levels of BMP signaling molecules in micro-dissected DG (Figure 1a). BMP4 expression was significantly decreased in the DG of fluoxetine-treated mice (Figure 1b, c; Control 1.0±0.08 n=8, Fluoxetine 0.78±0.06 n=8, p<0.05), and there was an increase in levels of the BMP signaling inhibitor noggin following fluoxetine treatment (Figure 1b, c; Control 1.0±0.22 n=10, Fluoxetine 2.09±0.43 n=10, p<0.05). We observed a similar decrease in BMP4 expression in mice treated with imipramine (data not shown). The observed changes in BMP4 and noggin levels suggested that fluoxetine reduces BMP signaling in the adult DG. In fact, there was a nearly 50% reduction in levels of phospho-Smad1/5/8 in the fluoxetine-treated group (Figure 1b, c; Control 1.0±0.14 n=4, Fluoxetine 0.52±0.07 n=4, p<0.05) as well as reduction in expression of the BMP target gene Id2 (Figure 1b, c; Control 1.0±0.09 n=8, Fluoxetine 0.69±0.09 n=8, p<0.05). Immunohistochemical analysis showed that fluoxetine treatment for 14 days significantly increased the total number of BrdU+ cells in the DG (Figure 1d, e; Control 6948±1299 n=4, Fluoxetine 11472±1284 n=4, p<0.05), consistent with previous observations.5, 6 Taken together, these data indicate that fluoxetine reduces BMP signaling in the adult DG with an associated increase in proliferation.
Figure 1. Fluoxetine treatment decreases levels of BMP signaling in the dentate gyrus.
(a) Experimental paradigm: Mice received intraperitoneal fluoxetine or saline once daily for 14 days. On day 13 BrdU was administered to acutely mark the proliferating cell population. On day 14 mice were sacrificed (Sac) and tissue was collected for western blot or immunohistochemical analysis. (b) Western blots of DG tissue show that there is a decrease in expression of the BMP4 ligand and an increase in levels of the BMP inhibitor noggin following fluoxetine treatment. Levels of phospho-Smad1/5/8 and the BMP target gene Id2 are reduced following fluoxetine treatment. GAPDH was used as a loading control. (c) Quantification of western blots by densitometric analysis indicates that there is a decrease in BMP signaling in the dentate gyrus of fluoxetine-treated mice. Values are normalized to loading control and expressed as relative fold compared to saline-treated control mice. n=4–10 per group. (d) Immunostaining for DAPI (blue) and BrdU (green) shows that there is an increase in proliferation in the dentate gyrus following fluoxetine treatment. (e) Quantification of the number of BrdU+ cells per mm3 in the dentate gyrus indicates that there is a 65% increase in the number of BrdU+ cells in fluoxetine-treated mice compared to controls. n=4 per group.
Data are presented as mean±SEM. Unpaired student’s t-test: *p<0.05. Scale bar: 50µm
BMP4 overexpression blocks the proliferative and behavioral effects of antidepressants
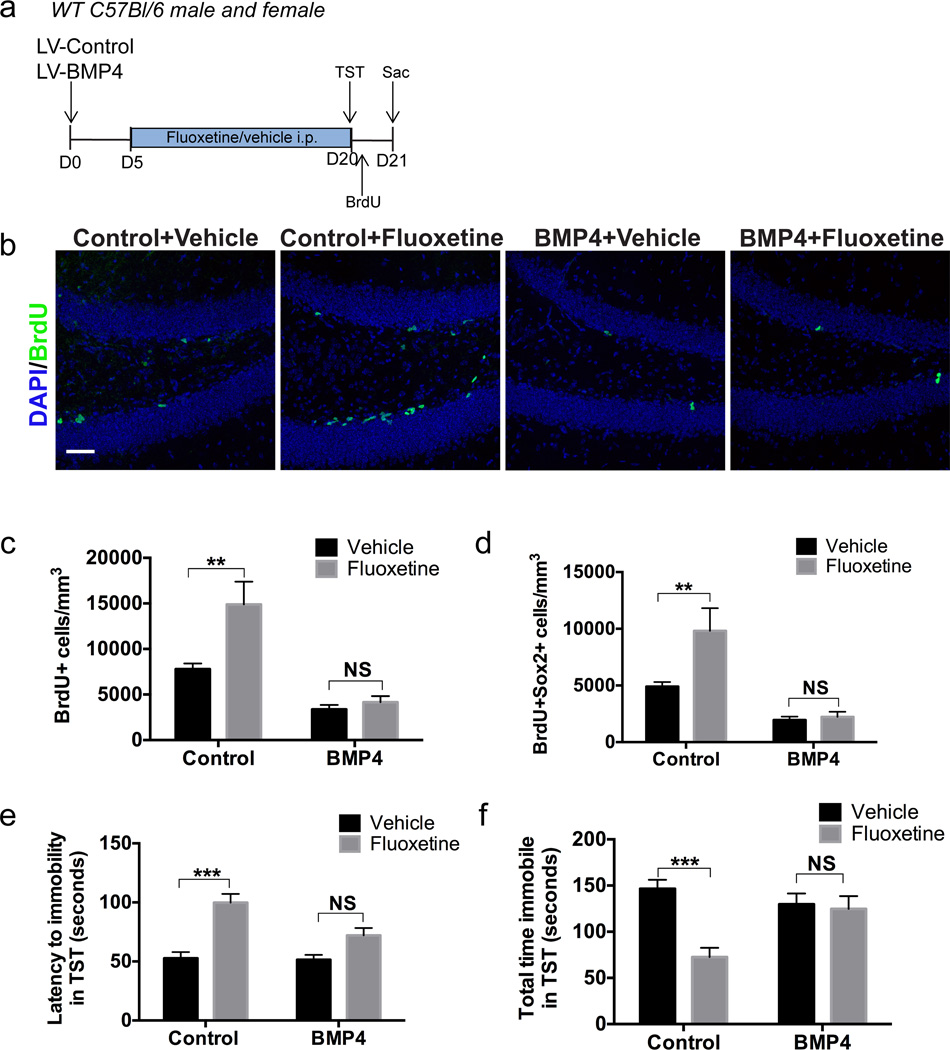
We next asked whether augmentation of BMP signaling in the DG would attenuate neurogenic and behavioral effects of fluoxetine treatment. We stereotaxically injected a lentivirus overexpressing the BMP ligand, BMP4, (LV-BMP4) or a control lentivirus (LV-Control) into the DG and subsequently treated mice with fluoxetine or vehicle (Figure 2a). Affective behavior was assessed on the tail suspension test (TST) at day 20 post infection and mice received BrdU 24 hours prior to sacrifice to assess proliferation in the DG. The lentiviruses contained an mCherry fluorescent reporter, which allowed for verification of virus infection in the DG (See Supplementary Figure S1). BrdU+ cells were first quantified to analyze the effects on overall proliferation. Two-way ANOVA demonstrated a significant effect of virus treatment (Figure 2c; F(1,30)=27.72, p<0.001), antidepressant treatment (Figure 2c; F(1,30)=7.545, p<0.05), and a significant interaction effect (Figure 2c; F(1,30)=4.815, p<0.05). Fluoxetine treatment significantly increased the number of BrdU+ cells in mice infected with LV-Control (Figure 2b, c; LV-Control+Vehicle 7785±623.9 n=10, LV-Control+Fluoxetine 14892±2496 n=9, Bonferroni’s test p<0.01). However, overexpression of BMP4 prevented a fluoxetine-induced increase in BrdU+ cells (Figure 2b, c; LV-BMP4+Vehicle 3369±487.4 n=7, LV-BMP4+Fluoxetine 4164±663.3 n=8, Bonferroni’s test p>0.05). We next quantified the number of BrdU+Sox2+ cells in the DG to assess effects on NPC proliferation. Two-way ANOVA demonstrated a significant effect of virus treatment (Figure 2d; F(1,30)=21.94, p<0.001) and antidepressant treatment (Figure 2d; F(1,30)=5.325, p<0.05) on the number of BrdU+Sox2+ cells, and a significant interaction effect was also observed (Figure 2d; F(1,30)=4.247, p<0.05). Fluoxetine treatment increased the number of BrdU+Sox2+ cells in mice infected with LV-Control (Figure 2d; LV-Control+Vehicle 4907±401 n=10, LV-Control+Fluoxetine 9817±1997 n=9, Bonferroni’s test p<0.01), but no such effect was observed in BMP4-overexpressing mice (Figure 2d; LV-BMP4+Vehicle 1959±796.3 n=7, LV-BMP4+Fluoxetine 2236±447 n=8, Bonferroni’s test p>0.05), suggesting that BMP4 blocked the effects of fluoxetine on NPC proliferation. Furthermore, analysis of doublecortin-expressing (Dcx+) neuroblasts demonstrated a trend towards an increase in the number of neuroblasts following fluoxetine treatment in mice infected with control lentivirus. Viral overexpression of BMP4 in fluoxetine-treated mice reduced the number of Dcx+ cells below levels observed in fluoxetine-treated mice exposed to control lentivirus (Supplementary Figure S2).
Figure 2. Overexpression of BMP4 blocks proliferative and behavioral effects of antidepressant treatment.
(a) Experimental paradigm: Control lentivirus (LV-Control) or a BMP4-overexpressing lentivirus (LV-BMP4) was stereotaxically injected into the dentate gyrus of wild type C57Bl/6 mice. Daily fluoxetine (or vehicle) treatment began on day 5 post-injection. Behavioral performance on the tail suspension test (TST) was performed on day 20 post-injection. BrdU was administered 24 hours prior to sacrifice (Sac) to acutely mark the proliferating cell population and tissue was collected for immunohistochemical analysis on day 21. (b) Immunostaining for DAPI (blue) and BrdU (green) shows that there is an increase in proliferation in the dentate gyrus following fluoxetine treatment in mice infected with LV-Control (Control), but overexpression of BMP4 prevents the fluoxetine-induced increase in BrdU. (c) Quantification of the number of BrdU+ cells per mm3 in the dentate gyrus shows that there is over a two-fold increase in the number of BrdU+ cells following fluoxetine treatment but the effect of fluoxetine is blocked by overexpression of BMP4. n=7–10 per group. (d) Quantification of the number of BrdU+Sox2+ cells per mm3 in the dentate gyrus shows that the number of proliferating progenitor cells is elevated in fluoxetine-treated mice but the effect of fluoxetine is reduced by overexpression of BMP4. n=7–10 per group. (e) Fluoxetine treatment increased the latency to immobility in the TST and the effect of fluoxetine was blocked by overexpression of BMP4. n=7–10 per group. (f) Fluoxetine treatment decreased the total immobility time in the TST and the effect of fluoxetine was blocked by overexpression of BMP4. n=7–10 per group.
Data are presented as mean±SEM. Two-way ANOVA with Bonferroni’s paired comparisons test: *p<0.05, **p<0.01, ***p<0.001. NS=not significant. Scale bar: 50µm
In the TST, two-way ANOVA demonstrated a significant interaction between fluoxetine treatment and virus treatment in both latency to immobility (Figure 2e; F(1,30)=4.789, p<0.05) and total time immobile (Figure 2f; F(1,30)=9.286, p<0.01). In mice infected with LV-Control, fluoxetine treatment resulted in an increased latency to immobility (Figure 2e; LV-Control+Vehicle 52.7±5.2 n=10, LV-Control+Fluoxetine 99.8±7.3 n=9, Bonferroni’s test p<0.001) and a decrease in total immobility time in the TST (Figure 2f; LV-Control+Vehicle 146.5±9.7 n=10, LV-Control+Fluoxetine 72.5±10.1 n=9, Bonferroni’s test p<0.001), demonstrating the anticipated antidepressant effect. However, fluoxetine treatment had no statistically significant effect on BMP4-overexpressing mice in either the latency to immobility in the TST (Figure 2e; LV-BMP4+Vehicle 51.5±4.5 n=7, LV-BMP4+Fluoxetine 72.1±6.3 n=8, Bonferroni’s test p>0.05) or the total immobility time in the TST (Figure 2f; LV-BMP4+Vehicle 129.8±30.5 n=7, LV-BMP4+Fluoxetine 124.7±13.8 n=8, Bonferroni’s test p>0.05), indicating that BMP4 blocked the behavioral effects of fluoxetine. No differences were observed between males and females in the effects of fluoxetine or BMP4 on proliferation and affective behavior. (Supplementary Figure S3). Intraventricular infusion of BMP4 also inhibited the effects of fluoxetine on the TST (Supplementary Figure S4). Taken together, these results indicate that increased BMP signaling blocks the effects of fluoxetine both on proliferation in the DG and on behavioral performance in a test of depressive activity.
The BMP inhibitor noggin promotes neurogenesis and alters affective behavior
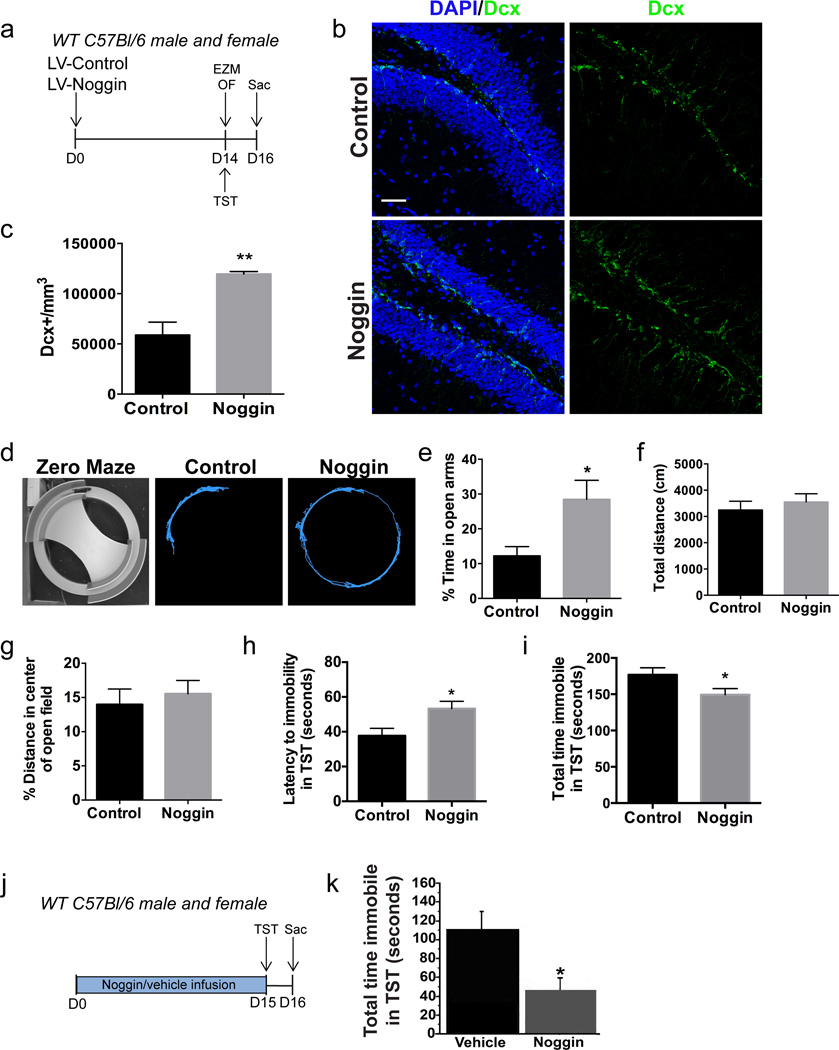
In view of these findings, we next hypothesized that inhibition of BMP signaling in the DG would both increase neurogenesis and alter affective behavior. To test this hypothesis, we stereotaxically injected a lentivirus overexpressing the BMP inhibitor noggin (LV-Noggin) into the DG (Figure 3a). One cohort of mice was subsequently tested on the elevated zero maze (EZM) and the open field (OF), while a second cohort of mice was tested on the TST. Noggin overexpression led to a greater than two-fold increase in the number of doublecortin positive (Dcx+) neuroblasts (Figure 3b, c; LV-Control 58931±12726 n=5, LV-Noggin 119743±2309 n=4, p<0.01), consistent with previous findings demonstrating an increased rate of neurogenesis in response to hippocampal noggin overexpression.15 Furthermore, noggin overexpression produced a comparable increase in neuroblast generation in both the dorsal and ventral DG (Supplementary Figure S1e). Behavioral performance on the elevated zero maze (EZM) was assessed at 14 days post infection. Noggin overexpression significantly increased the percentage of time spent in the open arms of the EZM (Figure 3d, e; LV-Control 12.24±2.65 n=8, LV-Noggin 28.49±5.47 n=6, p<0.05), suggesting a reduction in anxiety-like behavior. Examination of total distance traveled in the open field (OF) showed no difference in general locomotor activity between groups (Figure 3f; LV-Control 3247±334.1 n=9, LV-Noggin 3553±313.3 n=6, p=0.54). There was also no significant difference observed in the percentage distance traveled in the center of the open field (Figure 3g; LV-Control 13.97±2.28% n=9, LV-Noggin 15.54±1.94 n=6, p>0.05). Performance on the TST was assessed at 14 days post injection in a separate cohort of mice in order to assess depressive behavior. Noggin overexpression increased the latency to immobility in the TST (Figure 3h; LV-Control 37.7±4.3 n=13, LV-Noggin 53.3±4.2 n=12, p<0.05) and decreased the total immobility time (Figure 3i; LV-Control 176.8±9.6 n=13, LV-Noggin 149.3±8.4 n=12, p<0.05), suggestive of an antidepressant-like effect. As an alternative approach, we next performed intraventricular infusion of noggin, which promotes neurogenesis and improves hippocampus-dependent cognition.19 Noggin or vehicle was infused into the lateral ventricles for 15 days and performance on the TST was assessed on day 15 (Figure 3j). Noggin infusion led to a reduction in the total time spent immobile in the TST (Figure 3k; Vehicle 111±19, Noggin 47.5±12), consistent with the viral overexpression results. Taken together, these results indicate that inhibition of BMP signaling in the DG increases neurogenesis and produces an associated reduction in anxiety- and depression-like behaviors.
Figure 3. The BMP inhibitor noggin increases neurogenesis and alters affective behavior.
(a) Experimental paradigm: Control lentivirus (LV-Control) or a noggin-overexpressing lentivirus (LV-Noggin) was stereotaxically injected into the dentate gyrus of wild type C57Bl/6 mice. In one cohort of mice, behavior was assessed on the elevated zero maze (EZM) and open field (OF) tests on day 14 post injection. In a second cohort of mice, behavior was assessed on the tail suspension test (TST) on day 14 post injection. On day 16 mice were sacrificed (Sac) and tissue was harvested for immunohistochemical analysis. (b) Immunostaining for DAPI (blue) and doublecortin (Dcx, green) shows that there is an increase in the generation of neuroblasts following noggin administration. (c) Quantification of the number of Dcx+ cells per mm3 in the dentate gyrus shows a greater than two-fold increase in neuroblasts in noggin-treated mice compared to controls. n=4–5 per group. (d) Representative path traces from the EZM demonstrate that there is a decrease in anxiety-like behavior in noggin-treated mice. (e) The percentage of time spent in the open arms of the EZM is higher in noggin-treated mice compared to controls. n=6–8 per group. (f) In the open field, no difference was observed in the total distance traveled by control and noggin-treated mice. n=6–9 per group. (g) In the open field, no difference was observed in the percentage of distance traveled in the center of the arena. n=6–9 per group. (h) The latency to immobility in the TST was increased in noggin-treated mice. n=12–13 per group. (i) The total immobility time in the TST was decreased in noggin-treated mice compared to controls. n=12–13 per group. (j) Experimental paradigm: Mice were administered noggin or vehicle for 15 days via intraventricular infusion. Performance on the tail suspension test (TST) was assessed on day 15 and mice were sacrificed (Sac) on day 16. (k) Noggin infusion led to a reduction in the total time immobile in the TST. n=6–8 per group.
Data are presented as mean±SEM. Unpaired Student’s t-test: *p<0.05, **p<0.01. Scale bar: 50µm
Inhibition of BMP signaling in NPCs promotes neurogenesis and reduces anxiety- and depression-like behavior
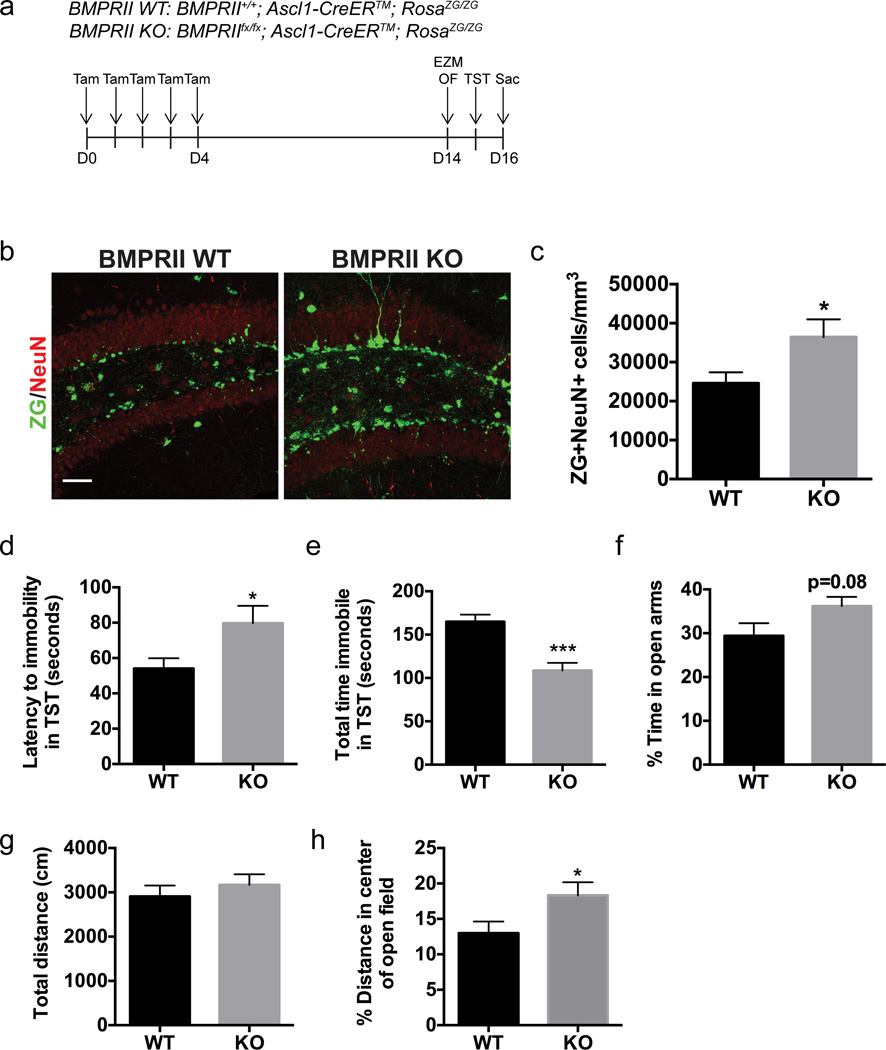
To assess whether inhibition of BMP signaling within the NPC population produces anxiolytic and antidepressant behavioral effects, we utilized a BMPRIIfx/fx; Ascl1-CreER™; RosaZG/ZG mouse line. This allowed for conditional ablation of the type II BMP receptor in Ascl1-expressing cells, which includes intermediate NPCs as well as a small subset of actively dividing NSCs in the DG and subventricular zone.26 These mice have a Cre-responsive ZsGreen (ZG) reporter in the Rosa locus which allowed for fluorescent labeling of the recombined cells in which BMPRII was ablated. BMPRII was ablated via treatment with tamoxifen (Figure 4a). We have previously reported reduction of BMPRII expression in recombined cells in the DG using this protocol.15 To address the specificity of this Cre line, we evaluated ZG recombination in the DG and other brain regions. In addition to a high level of recombination in the subgranular zone of the DG, ZG+ cells were also observed in the subventricular zone neurogenic niche, and scattered ZG+ cells were observed in other brain regions, including the cortex27 (Supplementary Figure S5). Following short-term ablation, the majority of the ZG+ cells in the DG were progenitors of the granule cell neuronal lineage (Supplementary Figure S5b). Furthermore, the majority of both Ascl1-expressing NPCs in the SGZ and total proliferating cells in the SGZ were ZG+, indicating successful targeting of the NPC population (Supplementary Figure S5c, d). At 16 days post injection, BMPRII ablation significantly increased the number of newborn neurons as assessed by the number of ZG+ cells expressing the neuronal marker NeuN (Figure 4b, c; WT 24600±2791 n=8, BMPRII KO 36424±4547 n=8, p<0.05), consistent with previous studies demonstrating enhanced maturation of NPCs into neurons in the absence of BMP signaling.15, 20 Behavioral analysis on the TST demonstrated that ablation of BMPRII increased latency to immobility (Figure 4d; WT 54.08±5.84 n=8, BMPRII KO 79.73±9.78 n=8, p<0.05) and decreased the total time immobile (Figure 4e; WT 165±8.2 n=8, BMPRII KO 108.6±8.8 n=8, p<0.001). In the EZM, BMPRII ablation produced a trend towards an increase in the percentage of time spent in the open arms (Figure 4f; WT 29.42±2.88 n=8, BMPRII KO 36.17±2.12 n=8, p=0.08). Examination of total distance traveled in the OF showed no differences in general locomotor activity between groups (Figure 4g; WT 2908±245.9 n=8, BMPRII KO 3170±237.1 n=8, p=0.46). Furthermore, BMPRII ablation increased the percentage distance traveled in the center of the open field (Figure 4h; WT 13.0±1.6 n=8, BMPRII KO 18.3±1.9 n=8, p<0.05), which is suggestive of reduced anxiety-like behavior. Lastly, performance on the TST was not affected by prior exposure to the EZM and OF, as demonstrated by the finding that ablation of BMPRII in a cohort of mice exposed only to the TST (Supplementary Figure S6) had similar behavioral effects on the TST as that observed in Figure 4. Taken together, these results indicate that inhibition of BMP signaling in NPCs increases neurogenesis with an associated reduction in anxiety-and depression-like behavior.
Figure 4. Ablation of BMPRII in neural progenitor cells promotes hippocampal neurogenesis and reduces anxiety- and depression-like behaviors.
(a) Experimental paradigm: BMPRIIfx/fx; Ascl1-CreER™; RosaZG/ZG mice (KO) were administered tamoxifen for five consecutive days in order to ablate the BMP type II receptor in neural progenitor cells. BMPRII+/+; Ascl1-CreER™; RosaZG/ZG littermates were used as wild type (WT) controls. Behavior was assessed on the elevated zero maze (EZM) and open field (OF) tests on day 14 and on the tail suspension test (TST) on day 15. On day 16 mice were sacrificed (Sac) and tissue was harvested for immunohistochemical analysis. (b) Immunostaining for the neuronal marker NeuN demonstrates that there is an increase in the number of recombined cells (ZG+) that have differentiated into neurons in the BMPRII KO mice compared to WT controls. (c) Quantification of the number of ZG+NeuN+ cells per mm3 in the dentate gyrus shows that there is an increase in neurogenesis in the BMPRII KO mice. n=8 per group. (d) Knockout of BMPRII increased the latency to immobility in the TST. n=8 per group. (e) Knockout of BMPRII decreased the total time spent immobile in the TST. n=8 per group. (f) The percentage of time spent in the open arms of the EZM is higher in BMPRII KO mice compared to BMPRII WT controls. n=8 per group. (g) No difference was observed between WT and BMPRII KO groups in the total distance traveled in the open field. n=8 per group. (h) In the open field, BMPRII knockout led to an increase in the percentage of distance traveled in the center of the arena rather than the periphery. n=8 per group.
Data are presented as mean±SEM. Unpaired Student’s t-test: *p<0.05, **p<0.01, ***p<0.001. Scale bar: 50µm
Discussion
MDD is associated with reductions in hippocampal volume in humans11–13 and in hippocampal neurogenesis in animal models of this disorder.5–7 Hippocampal neurogenesis is decreased by stress and increased by chronic antidepressant treatment, raising the possibility that this process may be involved both in the pathogenesis and the treatment of MDD.8 Overexpression of the BMP antagonist, noggin, significantly increases neurogenesis in the DG whereas overexpression of BMP4 suppresses it.15 Further, noggin-overexpressing mice perform markedly better on tests of hippocampus-dependent cognition.19, 20 Since BMP signaling has such a major regulatory influence on neurogenesis and cognition, we asked whether BMP signaling regulates antidepressant activity. Here we demonstrate that BMP signaling is inhibited by fluoxetine, and prevention of this decrease via overexpression of BMP4 blocks effects of fluoxetine treatment on hippocampal neural progenitor cell proliferation and behavior. Further, inhibition of BMP signaling in the hippocampus produces an increase in hippocampal neurogenesis concurrent with behavioral reductions in anxiety- and depression-like behavior. Lastly, targeted inhibition of BMP signaling in Ascl1-expressing cells is sufficient to produce similar neurogenic and behavioral effects, suggesting that neural progenitor cells may play a key role in mediating the effects of reduced BMP signaling. The downstream targets of the BMP pathway that are required for the anxiolytic and antidepressant-like effects of inhibition of BMP signaling are not yet known. However, the cyclin-dependent kinase inhibitor p21 is a BMP target gene that modulates neurogenesis by regulating the proliferation of neural progenitor cells, and expression of p21 is reduced by antidepressants.28, 29 This suggests that p21 may be one of the key BMP target genes regulated by antidepressant treatment.
Hippocampal neurogenesis is increased by multiple different classes of pharmacologic antidepressants as well as non-pharmacologic treatments such as exercise, electroconvulsive treatment, and deep brain stimulation.5–7, 30, 31 This suggests that stimulation of neurogenesis may be a general mechanism that contributes to antidepressant efficacy. Notably, various signaling molecules stimulate hippocampal neurogenesis and produce antidepressant-like behavioral effects.32–35 However, the signaling pathways that provide negative feedback to maintain stability of the neurogenic process are less well described. Our observations suggest that BMP signaling provides a mechanism for allostatic control of neurogenesis and affective behavior. We observed a strong association between changes in neurogenesis and affective behavior following manipulation of hippocampal BMP signaling, suggesting that the behavioral effects of modulating BMP signaling are mediated through its effects on hippocampal neurogenesis. This is supported by the finding that inhibition of BMP signaling within Ascl1-expressing NPCs produces changes in affective behavior. A recent study showed that ablation of BMP signaling in post-mitotic neurons in the hippocampus and forebrain produced changes in exploratory behavior but did not alter depressive behavior,36 supporting the conclusion that NPCs are critical for the antidepressant-like effects of altered BMP signaling. However, causality in our study, as well as others that have linked neurogenesis and depression, remains to be fully ascertained. Furthermore, given that the Ascl1-CreER™ transgene expression is not exclusively restricted to the hippocampal NPC population, we cannot rule out the possibility that the behavioral effects of BMPRII ablation occurred secondary to blockade of BMP signaling in other cell populations. Furthermore, the anxiolytic and antidepressant effects of BMPRII ablation were significant, but the degree of the effect was not as large as that observed with intraventricular infusion or viral overexpression of noggin. We hypothesize that this reflects the fact that intraventricular infusion and viral overexpression of noggin produce a profound increase in noggin expression in the DG and therefore theoretically reduce BMP signaling in all progenitor cells in the subgranular zone, whereas the tamoxifen-induced BMPRII ablation protocol only affects a subset of progenitors. It is alternatively possible that other cell populations in the DG besides the NPCs contribute to the behavioral effects of noggin overexpression. The heterogeneity of MDD implies that many neuronal circuits and signaling systems may contribute to its etiology.37 It is therefore likely that not all antidepressants produce comparable effects on BMP signaling and neurogenesis, but our findings support the conclusion that BMP signaling-mediated changes in hippocampal neurogenesis may be one potential mechanism of antidepressant activity.
Our data also demonstrated that overexpression of BMP4 alone markedly reduced hippocampal neurogenesis, but did not have an appreciable effect on depression-like behavior. BMP4 overexpression significantly reduced the total number of proliferating cells as well as the number of proliferating progenitor cells in the DG compared to control mice, consistent with previous studies showing that BMP signaling causes neural stem and progenitor cells to exit cell cycle.15, 20 Despite the reduction in hippocampal proliferation in BMP4-overexpressing mice, we did not observe an increase in depression-like behavior in these mice, which is consistent with previous studies which have shown that inhibiting hippocampal neurogenesis is not sufficient to induce a depression-like phenotype.9, 38–40 Chronic stress produces a decrease in hippocampal neurogenesis,41–43 raising the possibility that ablation of neurogenesis may increase the susceptibility to the behavioral effects of stress. Some studies have observed such an increase in stress susceptibility,44, 45 whereas others have reported that ablation of neurogenesis on its own does not produce an increased sensitivity to the behavioral effects of stress.6, 46 Our results on the overexpression of BMP4 support the conclusion that stimulation of neurogenesis plays a key role in antidepressant efficacy, but that a reduction in adult hippocampal neurogenesis is not, by itself, sufficient to induce the onset of depressive symptoms.
In the dentate gyrus, functional differences have been observed along the dorsoventral axis. In particular, the ventral DG has been implicated in the mood regulatory function of the hippocampus, and studies have demonstrated a preferential decrease in neurogenesis in the ventral DG following stress.47 Furthermore, the ventral hippocampus displays enhanced connectivity with brain regions involved in mood regulation.48–50 In the conditional BMPRII ablation experiments, BMPRII was knocked out in both the dorsal and ventral DG. In the noggin and BMP4 virus overexpression experiments, virus infection was observed in the dorsal DG with extension into the ventral DG. Furthermore, no differences were observed in the degree of neurogenesis induction in the dorsal versus ventral DG following inhibition of BMP signaling. Therefore, the relative contribution of different regions of the DG to the behavioral effects of BMP signaling modulation cannot be delineated from these studies. In the lentiviral experiments, virus infection was observed in the ventral DG, but the virus did not consistently extend to span the entire length of the ventral DG. Therefore, future experiments to more specifically target the ventral DG could be performed to address the contribution of this subregion. Given prior evidence on functional dissociation along the dorsoventral axis, it is possible that inhibition of BMP signaling specifically in the ventral DG would be sufficient to produce antidepressant or anxiolytic effects. Alternately, it has previously been shown that neurogenesis in both the ventral and dorsal DG is required for behavioral effects of antidepressant treatment in specific stress paradigms,51 suggesting that both subregions of the DG may contribute to affective behavior in specific behavioral contexts. Future studies with targeted inhibition of BMP signaling in the ventral DG will therefore be necessary to address these questions.
Given that SSRIs increase serotonin levels via inhibition of serotonin reuptake, we hypothesize that 5-HT receptor activation may be responsible for the observed decrease in hippocampal BMP signaling following fluoxetine treatment. There are multiple serotonin receptors expressed in the adult dentate gyrus, including 5HT1AR, 5HT2CR, and 5HT4R.52–54 In particular, 5-HT1AR has been implicated in the mechanism of action of SSRIs.9 Recent data has demonstrated that ablation of 5-HT1AR from dentate granule cells prevents fluoxetine-induced increases in expression of brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF) in the DG,53 suggesting that this receptor modulates gene expression and secretion from granule cells in response to SSRI treatment. We therefore hypothesize that fluoxetine-induced changes in BMP4 and noggin expression could be mediated by similar 5HTR-mediated modulation of gene expression from dentate granule cells or other cell populations in the hippocampal niche. Further, we hypothesize that targeting multiple signaling pathways could produce additive effects on both neurogenesis and depressive behavior.
Antidepressant treatment stimulates BDNF expression in the hippocampus55, 56 and modulation of BDNF or TrkB, a BDNF receptor, is sufficient to alter depression-like behavior.33, 57, 58 In the adult DG, BDNF increases survival of newly generated neurons.59 Conversely, inhibition of BMP signaling causes hippocampal neural stem and progenitor cells to exit quiescence, leading to increased proliferation and a resulting increase in the number of neurons generated.15, 18 Therefore, inhibition of BMP signaling and BDNF stimulation could have additive effects on neurogenesis, with inhibition of BMP signaling increasing neuron generation and BDNF promoting survival of these neurons. In fact, concurrent overexpression of BNDF and noggin in the adult rat brain has an additive effect on the number of neurons generated from the subventricular zone60 and allows for sustained neuronal generation with long-term survival of newborn neurons.61 Similarly, the effects of noggin on hippocampal neurogenesis can be sustained long-term via environmental enrichment, an intervention that increases BDNF expression.20 Therefore, we hypothesize that targeting both the BDNF and BMP pathways may have additive effects on affective behavior, leading to improved antidepressant-like responses compared to either manipulation alone.
Data from the STAR*D trial has demonstrated that approximately two-thirds of patients with MDD don’t achieve remission of symptoms with first-line antidepressant treatment and nearly one-third of patients are refractory to all treatment strategies,4 indicating that there is substantial need for improved therapies. Here we show that the BMP signaling pathway is a potential new therapeutic target for MDD. Interestingly, the neurogenic effects of fluoxetine treatment decline with age,62 which parallels an age-related increase in BMP signaling.20 However, inhibition of BMP signaling in aging mice both increases neurogenesis and prevents aging-related cognitive decline,20 raising the possibility that targeting BMP signaling may be a particularly effective antidepressant strategy for aged subjects.
In summary, hippocampal BMP signaling is reduced by treatment with the antidepressant fluoxetine. Overexpression of BMP4 specifically in the DG prevents both neurogenic and behavioral effects of fluoxetine treatment. Inhibition of BMP signaling, either via overexpression of the BMP inhibitor noggin or conditional genetic ablation of the type II BMP receptor in NPCs, both promotes adult hippocampal neurogenesis and leads to a reduction in anxiety- and depression-like behaviors. These studies highlight the importance of the BMP signaling pathway as a potential new target for antidepressant activity.
Supplementary Material
Control lentivirus, noggin-overexpressing lentivirus, or BMP4-overexpressing lentivirus was stereotaxically injected into the dentate gyrus of C57Bl/6 wild type mice as shown in figures 2 and 3. Virus localization was visualized via immunostaining for the mcherry fluorescent tag. (a) Representative low magnification image of mcherry immunostaining in the hippocampus demonstrates that lentivirus infection localized to the dentate gyrus (DG). (b) Representative images demonstrate that lentivirus infection is localized to the DG, particularly in the subgranular zone. Virus infection is strong in the dorsal DG and extends into the ventral DG. However, mCherry fluorescence was not typically observed at bregma −3.6mm. (c) mCherry immunofluorescence was largely absent from the CA3 region of the hippocampus, indicating lack of virus infection in this region. (d) mCherry immunofluorescence was largely absent from the CA1 region of the hippocampus, indicating lack of virus infection. (e) Control lentivirus (Control) or a noggin-overexpressing lentivirus (Noggin) were injected into the DG as shown in Figure 3. Quantification of the number of Dcx+ cells per mm3 shows an approximately two-fold increase in neuroblasts in both the dorsal and ventral DG in noggin-overexpressing mice at day 16 post injection, suggesting that the neurogenic effects of noggin virus are comparable in the dorsal and ventral subregions of the DG.
Data are presented as mean±SEM. Unpaired Student’s t-test: *p<0.05, **p<0.01. Scale bars: 100µm
Mice received stereotaxic injection of control lentivirus or a BMP4-overexpressing lentivirus and were subsequently treated with vehicle or fluoxetine, as shown in figure 2a. (a) Two-way ANOVA analysis demonstrated a significant main effect of BMP4 (F(1,29)=16.72, p<0.001) on the number of doublecortin-expressing (Dcx+) neuroblasts, but there was not a significant main effect of fluoxetine treatment (F(1,29)=1.145, p>0.05) or a significant interaction effect (F(1,29)=1.129, p>0.05). Fluoxetine did produce a trend towards an increase in Dcx+ neuroblasts in mice treated with control lentivirus. In BMP4-overexpressing mice treated with fluoxetine, the number of neuroblasts is reduced relative to fluoxetine-treated mice exposed to control lentivirus.
Data are presented as mean±SEM. Two-way ANOVA with Bonferroni’s paired comparisons test. NS=not significant.
Mice received stereotaxic injection of control lentivirus or a BMP4-overexpressing lentivirus and were subsequently treated with vehicle or fluoxetine, as shown in figure 2a. (a) No differences were observed between males and females in the numbering of proliferating BrdU+ cells in the DG in response to fluoxetine treatment or BMP4 overexpression. (b) No differences were observed between males and females in the total time immobile on the TST following BMP4 overexpression and fluoxetine treatment.
Data are presented as mean±SEM. ANOVA with Bonferroni’s paired comparisons test. *p<0.05.
(a) Experimental paradigm: Wild type mice were administered BMP4 or vehicle for 15 days via intraventricular infusion. Concurrently, mice were treated with fluoxetine or vehicle for the duration of the experiment. Performance on the tail suspension test (TST) was assessed on day 15. (b) Fluoxetine treatment led to a reduction in the total time immobile in the TST, suggesting a decrease in depression-like behavior. Infusion of BMP4 blocked the effects of fluoxetine on performance in the TST. n=6–10 per group.
Data are presented as mean±SEM. ANOVA with Tukey’s post-hoc test: *p<0.05.
BMPRIIfx/fx; Ascl1-CreER™; RosaZG/ZG mice (KO) were administered tamoxifen for five consecutive days in order to ablate the BMP type II receptor and recombination was assessed by ZG immunofluorescence on day 7 or day 16. BMPRIIfx/+; Ascl1-CreER™; RosaZG/ZG littermates were used as controls. (a) Representative images demonstrating areas of Zgreen (ZG) recombination within the brain on day 16. ZG+ cells are observed in the dentate gyrus, predominantly in the subgranular zone and granule cell layer. ZG+ cells are also observed in the subventricular zone, in which neural progenitor cells reside. Scattered ZG+ cells are additionally observed in non-neurogenic brain regions including the cortex. (b) Quantification of ZG+ cells in the DG on day 7 demonstrates that the majority of ZG+ cells express the progenitor markers Sox2 or NeuroD. (c) Quantification of ZG-immunoreactivity in Ascl1-expressing cells in the DG on day 7 demonstrates that over 70% of Ascl1-expressing cells have undergone Cre-mediated recombination. (d) EdU was administered on day 6 to mark proliferating cells. Quantification of ZG-immunoreactivity in EdU+ cells in the DG on day 7 shows that over 75% of proliferating cells have undergone Cre-mediated recombination.
Scale bars: 50µm
(a) Experimental paradigm: BMPRIIfx/fx; Ascl1-CreER™; RosaZG/ZG mice (KO) were administered tamoxifen for five consecutive days in order to ablate the BMP type II receptor in neural progenitor cells. BMPRIIfx/+; Ascl1-CreER™; RosaZG/ZG littermates were used as controls. Behavior was assessed on the tail suspension test (TST) on day 15. On day 16 mice were sacrificed (Sac). (b) BMPRII ablation led to an increase in the latency to immobility in the TST, suggesting a decrease in depression-like behavior. n=8–9 per group. (c) BMPRII ablation led to a reduction in the total time immobile in the TST, suggesting a decrease in depression-like behavior. n=8–9 per group.
Data are presented as mean±SEM. Unpaired Student’s t-test: *p<0.05, **p<0.01.
Acknowledgments
This research was supported by funding from the National Institute of Neurological Disorders and Stroke (R01NS020778, F31NS089154) and the National Institute of General Medical Sciences (T32GM008152). We acknowledge the assistance of the Northwestern University Behavioral Phenotyping Core facility.
Footnotes
Conflict of Interest statement:
The authors declare no conflict of interest
Supplementary information is available at Molecular Psychiatry’s website
References
- 1.Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119–138. doi: 10.1146/annurev-publhealth-031912-114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 5.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64(4):293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27(18):4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs BL, van Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry. 2000;5(3):262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- 9.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 10.Perera TD, Dwork AJ, Keegan KA, Thirumangalakudi L, Lipira CM, Joyce N, et al. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS One. 2011;6(4):e17600. doi: 10.1371/journal.pone.0017600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161(11):1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 12.Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161(4):598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 13.Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, et al. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology. 2013;38(6):1068–1077. doi: 10.1038/npp.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34(11):2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bond AM, Peng CY, Meyers EA, McGuire T, Ewaleifoh O, Kessler JA. BMP signaling regulates the tempo of adult hippocampal progenitor maturation at multiple stages of the lineage. Stem Cells. 2014;32(8):2201–2214. doi: 10.1002/stem.1688. [DOI] [PubMed] [Google Scholar]
- 16.Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, et al. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci U S A. 1995;92(17):7632–7636. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wrana JL. Regulation of Smad activity. Cell. 2000;100(2):189–192. doi: 10.1016/s0092-8674(00)81556-1. [DOI] [PubMed] [Google Scholar]
- 18.Bonaguidi MA, Peng CY, McGuire T, Falciglia G, Gobeske KT, Czeisler C, et al. Noggin expands neural stem cells in the adult hippocampus. J Neurosci. 2008;28(37):9194–9204. doi: 10.1523/JNEUROSCI.3314-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gobeske KT, Das S, Bonaguidi MA, Weiss C, Radulovic J, Disterhoft JF, et al. BMP signaling mediates effects of exercise on hippocampal neurogenesis and cognition in mice. PLoS One. 2009;4(10):e7506. doi: 10.1371/journal.pone.0007506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyers EA, Gobeske KT, Bond AM, Jarrett JC, Peng CY, Kessler JA. Increased bone morphogenetic protein signaling contributes to age-related declines in neurogenesis and cognition. Neurobiol Aging. 2016;38:164–175. doi: 10.1016/j.neurobiolaging.2015.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faigle R, Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim Biophys Acta. 2013;1830(2):2435–2448. doi: 10.1016/j.bbagen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beppu H, Lei H, Bloch KD, Li E. Generation of a floxed allele of the mouse BMP type II receptor gene. Genesis. 2005;41(3):133–137. doi: 10.1002/gene.20099. [DOI] [PubMed] [Google Scholar]
- 23.Battiste J, Helms AW, Kim EJ, Savage TK, Lagace DC, Mandyam CD, et al. Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development. 2007;134(2):285–293. doi: 10.1242/dev.02727. [DOI] [PubMed] [Google Scholar]
- 24.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagihara H, Toyama K, Yamasaki N, Miyakawa T. Dissection of hippocampal dentate gyrus from adult mouse. J Vis Exp. 2009;(33) doi: 10.3791/1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim EJ, Ables JL, Dickel LK, Eisch AJ, Johnson JE. Ascl1 (Mash1) defines cells with long-term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PLoS One. 2011;6(3):e18472. doi: 10.1371/journal.pone.0018472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim EJ, Leung CT, Reed RR, Johnson JE. In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J Neurosci. 2007;27(47):12764–12774. doi: 10.1523/JNEUROSCI.3178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pechnick RN, Zonis S, Wawrowsky K, Pourmorady J, Chesnokova V. p21Cip1 restricts neuronal proliferation in the subgranular zone of the dentate gyrus of the hippocampus. Proc Natl Acad Sci U S A. 2008;105(4):1358–1363. doi: 10.1073/pnas.0711030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pechnick RN, Zonis S, Wawrowsky K, Cosgayon R, Farrokhi C, Lacayo L, et al. Antidepressants stimulate hippocampal neurogenesis by inhibiting p21 expression in the subgranular zone of the hipppocampus. PLoS One. 2011;6(11):e27290. doi: 10.1371/journal.pone.0027290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 31.Schmuckermair C, Gaburro S, Sah A, Landgraf R, Sartori SB, Singewald N. Behavioral and neurobiological effects of deep brain stimulation in a mouse model of high anxiety- and depression-like behavior. Neuropsychopharmacology. 2013;38(7):1234–1244. doi: 10.1038/npp.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A. 2007;104(11):4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22(8):3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng Y, Rodriguiz RM, Murthy SR, Senatorov V, Thouennon E, Cawley NX, et al. Neurotrophic factor-alpha1 prevents stress-induced depression through enhancement of neurogenesis and is activated by rosiglitazone. Mol Psychiatry. 2015;20(6):744–754. doi: 10.1038/mp.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yau SY, Li A, Hoo RL, Ching YP, Christie BR, Lee TM, et al. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc Natl Acad Sci U S A. 2014;111(44):15810–15815. doi: 10.1073/pnas.1415219111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McBrayer ZL, Dimova J, Pisansky MT, Sun M, Beppu H, Gewirtz JC, et al. Forebrain-Specific Loss of BMPRII in Mice Reduces Anxiety and Increases Object Exploration. PLoS One. 2015;10(10):e0139860. doi: 10.1371/journal.pone.0139860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 38.David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62(4):479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zou J, Wang W, Pan YW, Abel GM, Storm DR, Xia Z. Conditional Inhibition of Adult Neurogenesis by Inducible and Targeted Deletion of ERK5 MAP Kinase Is Not Associated with Anxiety/Depression-Like Behaviors(1,2) eNeuro. 2015;2(2) doi: 10.1523/ENEURO.0014-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol Psychiatry. 2010;15(12):1152–1163. doi: 10.1038/mp.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hellsten J, Wennstrom M, Mohapel P, Ekdahl CT, Bengzon J, Tingstrom A. Electroconvulsive seizures increase hippocampal neurogenesis after chronic corticosterone treatment. Eur J Neurosci. 2002;16(2):283–290. doi: 10.1046/j.1460-9568.2002.02093.x. [DOI] [PubMed] [Google Scholar]
- 42.de Andrade JS, Cespedes IC, Abrao RO, Dos Santos TB, Diniz L, Britto LR, et al. Chronic unpredictable mild stress alters an anxiety-related defensive response, Fos immunoreactivity and hippocampal adult neurogenesis. Behav Brain Res. 2013;250:81–90. doi: 10.1016/j.bbr.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 43.Garza JC, Guo M, Zhang W, Lu XY. Leptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of GSK-3beta/beta-catenin signaling. Mol Psychiatry. 2012;17(8):790–808. doi: 10.1038/mp.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476(7361):458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mateus-Pinheiro A, Pinto L, Bessa JM, Morais M, Alves ND, Monteiro S, et al. Sustained remission from depressive-like behavior depends on hippocampal neurogenesis. Transl Psychiatry. 2013;3:e210. doi: 10.1038/tp.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, et al. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry. 2011;16(12):1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanti A, Belzung C. Neurogenesis along the septo-temporal axis of the hippocampus: are depression and the action of antidepressants region-specific? Neuroscience. 2013;252:234–252. doi: 10.1016/j.neuroscience.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15(10):655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- 49.Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- 50.Bagot RC, Parise EM, Pena CJ, Zhang HX, Maze I, Chaudhury D, et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun. 2015;6:7062. doi: 10.1038/ncomms8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu MV, Hen R. Functional dissociation of adult-born neurons along the dorsoventral axis of the dentate gyrus. Hippocampus. 2014;24(7):751–761. doi: 10.1002/hipo.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klempin F, Babu H, De Pietri Tonelli D, Alarcon E, Fabel K, Kempermann G. Oppositional effects of serotonin receptors 5-HT1a, 2, and 2c in the regulation of adult hippocampal neurogenesis. Front Mol Neurosci. 2010:3. doi: 10.3389/fnmol.2010.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samuels BA, Anacker C, Hu A, Levinstein MR, Pickenhagen A, Tsetsenis T, et al. 5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat Neurosci. 2015;18(11):1606–1616. doi: 10.1038/nn.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vilaro MT, Cortes R, Mengod G. Serotonin 5-HT4 receptors and their mRNAs in rat and guinea pig brain: distribution and effects of neurotoxic lesions. J Comp Neurol. 2005;484(4):418–439. doi: 10.1002/cne.20447. [DOI] [PubMed] [Google Scholar]
- 55.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15(11):7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russo-Neustadt A, Beard RC, Cotman CW. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology. 1999;21(5):679–682. doi: 10.1016/S0893-133X(99)00059-7. [DOI] [PubMed] [Google Scholar]
- 57.Koponen E, Rantamaki T, Voikar V, Saarelainen T, MacDonald E, Castren E. Enhanced BDNF signaling is associated with an antidepressant-like behavioral response and changes in brain monoamines. Cell Mol Neurobiol. 2005;25(6):973–980. doi: 10.1007/s10571-005-8468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry. 2010;15(1):80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi SH, Li Y, Parada LF, Sisodia SS. Regulation of hippocampal progenitor cell survival, proliferation and dendritic development by BDNF. Mol Neurodegener. 2009;4:52. doi: 10.1186/1750-1326-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chmielnicki E, Benraiss A, Economides AN, Goldman SA. Adenovirally expressed noggin and brain-derived neurotrophic factor cooperate to induce new medium spiny neurons from resident progenitor cells in the adult striatal ventricular zone. J Neurosci. 2004;24(9):2133–2142. doi: 10.1523/JNEUROSCI.1554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benraiss A, Bruel-Jungerman E, Lu G, Economides AN, Davidson B, Goldman SA. Sustained induction of neuronal addition to the adult rat neostriatum by AAV4-delivered noggin and BDNF. Gene Ther. 2012;19(5):483–493. doi: 10.1038/gt.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Couillard-Despres S, Wuertinger C, Kandasamy M, Caioni M, Stadler K, Aigner R, et al. Ageing abolishes the effects of fluoxetine on neurogenesis. Mol Psychiatry. 2009;14(9):856–864. doi: 10.1038/mp.2008.147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Control lentivirus, noggin-overexpressing lentivirus, or BMP4-overexpressing lentivirus was stereotaxically injected into the dentate gyrus of C57Bl/6 wild type mice as shown in figures 2 and 3. Virus localization was visualized via immunostaining for the mcherry fluorescent tag. (a) Representative low magnification image of mcherry immunostaining in the hippocampus demonstrates that lentivirus infection localized to the dentate gyrus (DG). (b) Representative images demonstrate that lentivirus infection is localized to the DG, particularly in the subgranular zone. Virus infection is strong in the dorsal DG and extends into the ventral DG. However, mCherry fluorescence was not typically observed at bregma −3.6mm. (c) mCherry immunofluorescence was largely absent from the CA3 region of the hippocampus, indicating lack of virus infection in this region. (d) mCherry immunofluorescence was largely absent from the CA1 region of the hippocampus, indicating lack of virus infection. (e) Control lentivirus (Control) or a noggin-overexpressing lentivirus (Noggin) were injected into the DG as shown in Figure 3. Quantification of the number of Dcx+ cells per mm3 shows an approximately two-fold increase in neuroblasts in both the dorsal and ventral DG in noggin-overexpressing mice at day 16 post injection, suggesting that the neurogenic effects of noggin virus are comparable in the dorsal and ventral subregions of the DG.
Data are presented as mean±SEM. Unpaired Student’s t-test: *p<0.05, **p<0.01. Scale bars: 100µm
Mice received stereotaxic injection of control lentivirus or a BMP4-overexpressing lentivirus and were subsequently treated with vehicle or fluoxetine, as shown in figure 2a. (a) Two-way ANOVA analysis demonstrated a significant main effect of BMP4 (F(1,29)=16.72, p<0.001) on the number of doublecortin-expressing (Dcx+) neuroblasts, but there was not a significant main effect of fluoxetine treatment (F(1,29)=1.145, p>0.05) or a significant interaction effect (F(1,29)=1.129, p>0.05). Fluoxetine did produce a trend towards an increase in Dcx+ neuroblasts in mice treated with control lentivirus. In BMP4-overexpressing mice treated with fluoxetine, the number of neuroblasts is reduced relative to fluoxetine-treated mice exposed to control lentivirus.
Data are presented as mean±SEM. Two-way ANOVA with Bonferroni’s paired comparisons test. NS=not significant.
Mice received stereotaxic injection of control lentivirus or a BMP4-overexpressing lentivirus and were subsequently treated with vehicle or fluoxetine, as shown in figure 2a. (a) No differences were observed between males and females in the numbering of proliferating BrdU+ cells in the DG in response to fluoxetine treatment or BMP4 overexpression. (b) No differences were observed between males and females in the total time immobile on the TST following BMP4 overexpression and fluoxetine treatment.
Data are presented as mean±SEM. ANOVA with Bonferroni’s paired comparisons test. *p<0.05.
(a) Experimental paradigm: Wild type mice were administered BMP4 or vehicle for 15 days via intraventricular infusion. Concurrently, mice were treated with fluoxetine or vehicle for the duration of the experiment. Performance on the tail suspension test (TST) was assessed on day 15. (b) Fluoxetine treatment led to a reduction in the total time immobile in the TST, suggesting a decrease in depression-like behavior. Infusion of BMP4 blocked the effects of fluoxetine on performance in the TST. n=6–10 per group.
Data are presented as mean±SEM. ANOVA with Tukey’s post-hoc test: *p<0.05.
BMPRIIfx/fx; Ascl1-CreER™; RosaZG/ZG mice (KO) were administered tamoxifen for five consecutive days in order to ablate the BMP type II receptor and recombination was assessed by ZG immunofluorescence on day 7 or day 16. BMPRIIfx/+; Ascl1-CreER™; RosaZG/ZG littermates were used as controls. (a) Representative images demonstrating areas of Zgreen (ZG) recombination within the brain on day 16. ZG+ cells are observed in the dentate gyrus, predominantly in the subgranular zone and granule cell layer. ZG+ cells are also observed in the subventricular zone, in which neural progenitor cells reside. Scattered ZG+ cells are additionally observed in non-neurogenic brain regions including the cortex. (b) Quantification of ZG+ cells in the DG on day 7 demonstrates that the majority of ZG+ cells express the progenitor markers Sox2 or NeuroD. (c) Quantification of ZG-immunoreactivity in Ascl1-expressing cells in the DG on day 7 demonstrates that over 70% of Ascl1-expressing cells have undergone Cre-mediated recombination. (d) EdU was administered on day 6 to mark proliferating cells. Quantification of ZG-immunoreactivity in EdU+ cells in the DG on day 7 shows that over 75% of proliferating cells have undergone Cre-mediated recombination.
Scale bars: 50µm
(a) Experimental paradigm: BMPRIIfx/fx; Ascl1-CreER™; RosaZG/ZG mice (KO) were administered tamoxifen for five consecutive days in order to ablate the BMP type II receptor in neural progenitor cells. BMPRIIfx/+; Ascl1-CreER™; RosaZG/ZG littermates were used as controls. Behavior was assessed on the tail suspension test (TST) on day 15. On day 16 mice were sacrificed (Sac). (b) BMPRII ablation led to an increase in the latency to immobility in the TST, suggesting a decrease in depression-like behavior. n=8–9 per group. (c) BMPRII ablation led to a reduction in the total time immobile in the TST, suggesting a decrease in depression-like behavior. n=8–9 per group.
Data are presented as mean±SEM. Unpaired Student’s t-test: *p<0.05, **p<0.01.