Abstract
Background
Atopic diseases including asthma exacerbate type 2 immune responses and involve a number of immune cell types, including regulatory T cells (Tregs) and the emerging group 2 innate lymphoid cells (ILC2s). While ILC2s are potent producers of type 2 cytokines, the regulation of ILC2 activation and function is not well understood.
Objective
In the present study, we evaluate for the first time how Tregs interact with pulmonary ILC2s and control their function.
Methods
ILC2s and Tregs were evaluated using in vitro suppression assays, cell-contact assays, and gene expression panels. Also, human ILC2s and Tregs were adoptively transferred into NOD SCID gamma-C deficient (NSG) mice, which were given isotype or anti-ICOS-L antibodies, then challenged with IL-33 and assessed for AHR.
Results
We show that induced Tregs (iTregs), but not natural Tregs (nTregs), effectively suppress the production of ILC2-driven, pro-inflammatory cytokines IL-5 and IL-13, both in vitro and in vivo. Mechanistically, our data reveal the necessity of Inducible T cell Costimulator (ICOS):ICOS-Ligand cell contact for Treg-mediated ILC2 suppression, alongside suppressive cytokines TGF-β and IL-10. Using a translational approach, we then demonstrate that human iTregs suppress syngeneic human ILC2s via ICOS-L to control airway inflammation in a humanized ILC2 mouse model.
Conclusion
These findings suggest that peripheral expansion of induced Tregs may serve as a promising therapeutic target against ILC2-dependent asthma.
Keywords: ILC2, regulatory T cells, Tregs, IL-5, IL-13, asthma, airway hyperreactivity, AHR, ICOS, ICOS-L
Capsule summary
ICOS-L on ILC2s is required for Treg mediated suppression in asthma.
Introduction
Asthma is an immunological disease of chronic airway inflammation, characterized by airway hyperreactivity (AHR) and reversible airflow obstruction (1, 2), and involving a variety of cell types, including Tregs (3, 4) and ILC2s (5). Type 2 cytokines including IL-5 and IL-13 play a pivotal role in the disease pathogenesis. IL-5 promotes proliferation of lung eosinophils, while IL-13 increases mucus production and contraction of airway smooth muscle cells. These reactive phenomena lead to the development of AHR, a cardinal feature of asthma (6, 7).
Although type 2 helper (Th2) CD4 T cells have classically been considered to be the main source of type 2 cytokines such as IL-13 and IL-5, recent studies have shown that ILC2s also produce a type 2 immune response after exposure to stimuli known to induce AHR, such as IL-33, IL-25, or species from the ubiquitous fungal genus Alternaria (5, 8–10). Indeed, ILC2s are sufficient to induce hallmarks of asthma in immunodeficient mice. Our group has previously shown that both human and murine ILC2s express both ICOS and ICOS-L, and that ICOS:ICOS-L interaction is necessary for ILC2 function and survival to promote AHR (11). Conversely, we have shown ICOS expression on Tregs is necessary to inhibit AHR (4). We thus examined a potential ICOS:ICOS-L interaction among ILC2s and T cells to regulate AHR.
Regulatory T cells, a well-established subpopulation of CD4+ T cells, have the capacity to mediate suppression in a variety of autoimmune and inflammatory conditions, including asthma (4, 12). Depending on where they are generated, Tregs are divided into two subpopulations: natural Tregs (nTregs, also known as tTregs), which arise in the thymus, and extrathymic, or “induced” Tregs (iTregs, or pTregs), which are induced in the periphery (19). Mice deficient in iTregs exhibit allergic inflammation within mucosal sites, specifically leading to pathology characteristic of asthma (13). However, whether these T cells can modulate ILC2 effector number and function has remained to be explored.
Here, we found substantial suppressive effects of Tregs on ILC2s in a murine model of asthma. We discovered that direct cell-cell contact was essential to the Treg-ILC2 interaction. Specifically, we suggest that ICOS-L on ILC2s binds to ICOS on Tregs. This ICOS:ICOS-L interaction, alongside TGF-β and IL-10, is required for the regulation of ILC2s by Tregs. By utilizing humanized-ILC2 mice, we found conservation of ICOS-L-mediated ILC2 suppression by human Tregs in vivo. Together, the findings uncover Tregs as potent regulators of ILC2 activation.
Results
ITregs, but not nTregs, suppress production of cytokines by ILC2s in vitro
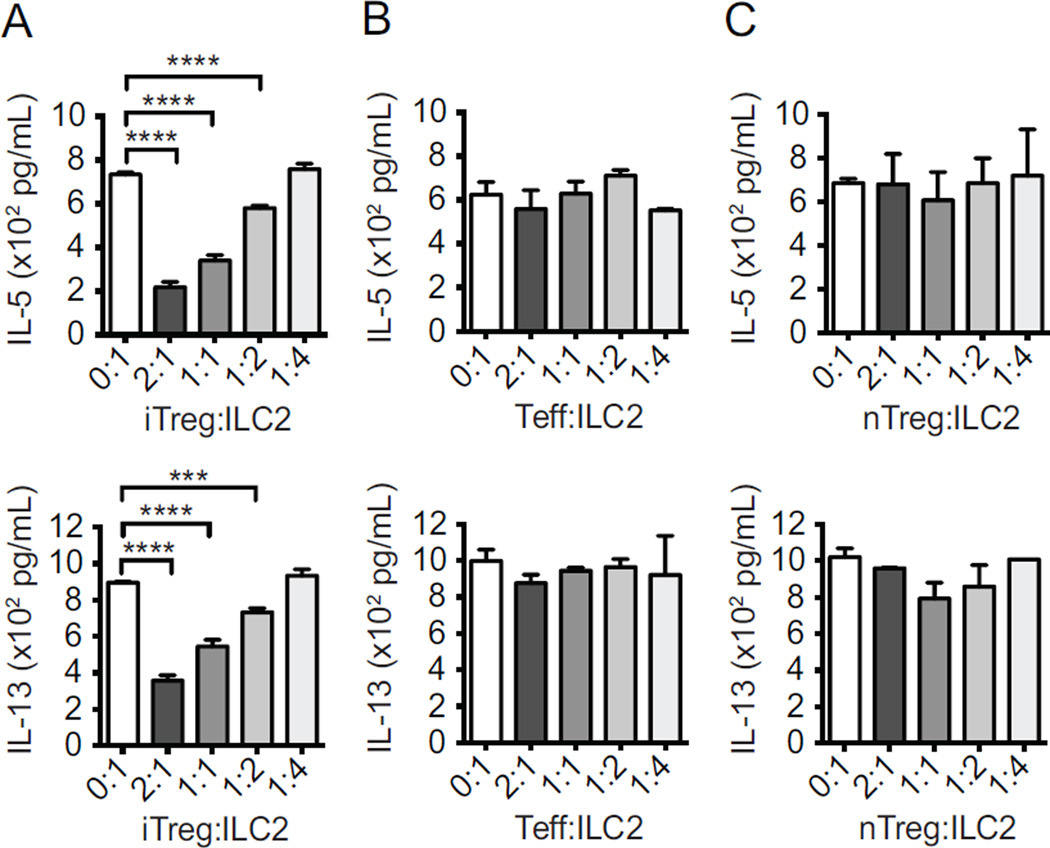
We initially evaluated the ability of Tregs to regulate ILC2s using an in vitro suppression assay. Purified iTregs were generated in vitro from naïve Foxp3− CD4+ T cells with TGF-β (see methods and Supplementary Fig 1, A) and co-cultured with purified ILC2s (lineage−CD45+CD127+ST2+; Supplementary Fig 1, B) ex vivo at different ratios. After two days in culture, ILC2s alone secreted large amounts of IL-5 and IL-13 as expected, as measured by ELISA. In contrast, adding increasing number of iTregs substantially reduced cytokine production; in the 2:1 iTreg:ILC2 group, IL-5 and IL-13 were reduced 70.3% and 60.1%, respectively, compared to the ILC2 group alone (Fig 1, A).
Figure 1. iTregs attenuate cytokine production.
ILC2s from rIL-33-treated mice were co-cultured for 48 hours with in vitro-induced iTregs (A), effector T (Teff) cells (B), and natural thymic nTregs (C) from FoxP3GFP+ mice, after which IL-5 and IL-13 cytokines were measured with ELISA. Data presented as means ± SEM (n=4, ***p<0.001, ****p<0.0001).
On the other hand, addition of neither nTregs from thymus, nor (non-Treg) effector T (Teff) cells from the spleen suppressed the production of IL-5 and IL-13 by ILC2s (Fig 1, B and C). These data suggest that the regulatory capacity exercised upon ILC2s is distinctly attributed to iTregs.
ITregs attenuate induction of ILC2-dependent AHR in vivo
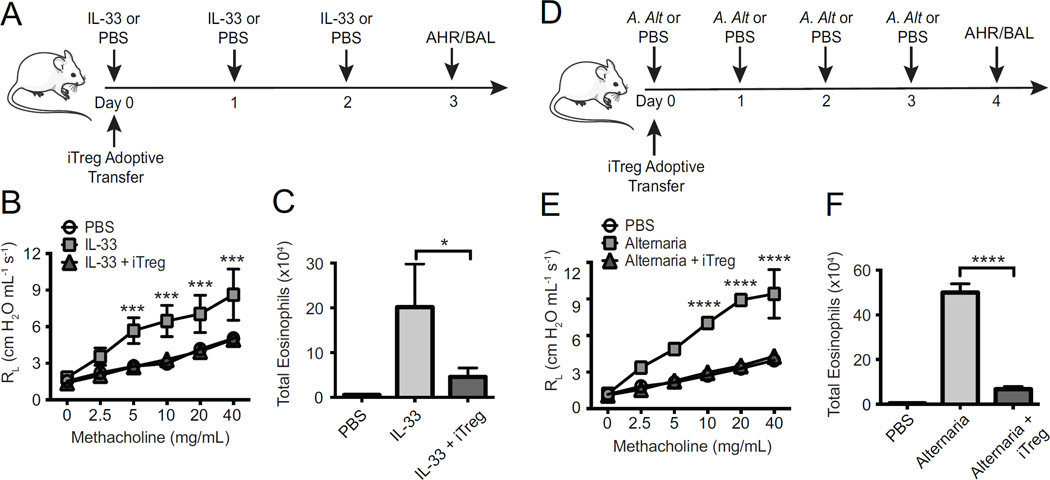
To assess the ability of iTregs to suppress ILC2s in vivo, we treated murine models of AHR, Rag2-deficient mice, intranasally (i.n.) with PBS, IL-33, or Alternaria alternata, a clinically relevant allergen that induces many inflammatory molecules including IL-33, IL-25 and TSLP (14). We then followed with intravenous (i.v.) administration of iTregs (schema in Fig 2, A and D). In mice treated with IL-33, adoptive transfer of iTregs reduced lung resistance as compared to mice receiving only IL-33 (Fig 2, B). The number of eosinophils in fluid obtained via bronchoalveolar lavage (BAL) was also decreased in the group that received the adoptive transfer of iTregs (Fig 2, C).
Figure 2. iTregs abrogate AHR induced by IL-33 or Alternaria.
Rag2−/− mice received PBS, IL-33 or Alternaria extract, and either iTregs or no Tregs, according to schema (A, D). AHR was assessed by measuring lung resistance (B, E) and BAL eosinophils were measured by flow cytometry (C, F). Data expressed as means ± SEM (n=5, *p< 0.05, **p<0.01, ***p< 0.001, ****p<0.0001).
Importantly, both AHR and eosinophilia in Alternaria alternata-sensitized mice were also reduced from iTreg transfer as compared to ILC2s alone (Fig 2, E and F). These findings show that iTregs are sufficient to suppress ILC2-dependent inflammation in vivo.
IL-10 and TGF-β are required for iTreg-induced suppression of ILC2s
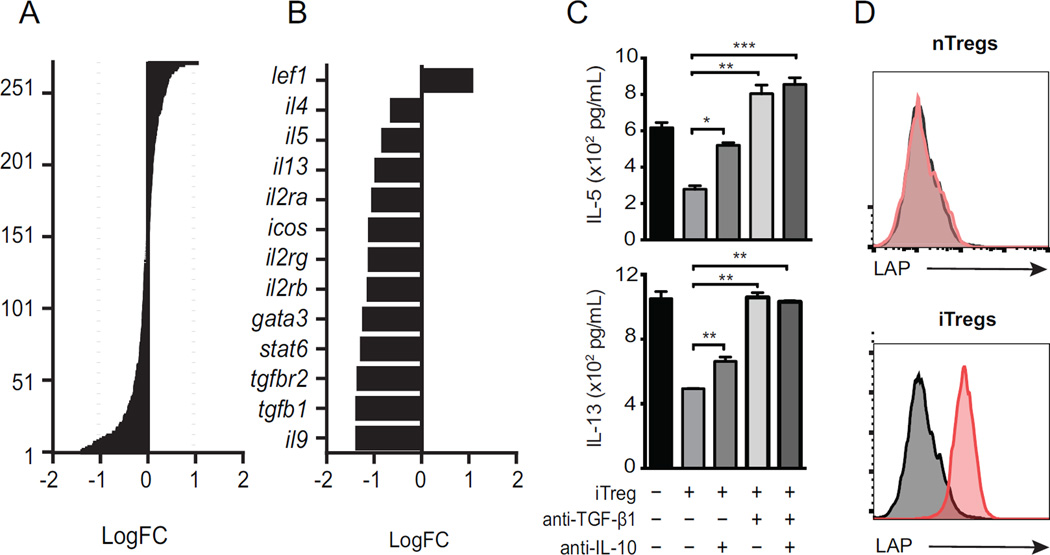
To gain insight into how Tregs were suppressing ILC2s, we re-isolated ILC2s by FACS after 24-hour co-culture with iTregs, then analyzed gene expression of a panel of immune related genes by NanoString® technology, as previously described (11). Of over 270 mRNAs measured in ILC2s, those that were modulated by iTreg addition are shown as Log fold change (LogFC, Fig 3, A). This included down-regulation of cytokines il-5 and il-13, as well as tgfb1 and surface receptors tgfbr1, icos and il-2ra (Fig 3, B). We also found reduced levels of gata3 in ILC2s co-cultured with iTregs, pertinent given that GATA3 drives ILC2 function (15).
Figure 3. iTregs suppress expression of type 2 cytokines by ILC2s.
Fold change (log2) of 270 mRNAs in ILC2s re-purified after 48-hour culture with iTregs (A). Significantly regulated genes (FC<2, p<0.05)(B). IL-5 and IL-13 production by ELISA in ILC2:iTreg co-cultures with anti-TGF-β or anti-IL-10 neutralizing antibodies (C). LAP expression on iTreg and nTreg by flow cytometry (D). Data shown as means ± SEM (n=3, *p<0.05, **p<0.01, ***p<0.001).
Since we found evidence of TGF-β1 signaling, and IL-10 and TGF-β are two canonical inhibitory cytokines produced by Tregs (4, 16), we questioned whether TGF-β was required for the suppressive effect of iTregs. First we co-cultured ILC2s and iTregs with neutralizing antibodies against IL-10 and TGF-β1. In these cultures, presence of neutralizing antibodies for IL-10 and TGF-β abrogated the iTreg-induced suppression of IL-5 and IL-13 production by ILC2s (Fig 3, C), confirming that suppression of ILC2s by iTregs is mediated by IL-10 and TGF-β. Some regulatory T cells express a form of membrane-bound TGF-β1 known as latency-associated peptide (LAP) (17). We asked if blocking ICOS:ICOS-L interaction could modulate the level of IL-10 expression or the percentage of LAP-expressing cells in co-cultures of iTregs and ILC2s. Adding anti-ICOS-L antibody to this system decreased both expression of IL-10, as measured by ELISA, and LAP, as measured by flow cytometry (Supplementary Fig 2, A).
In our preceding experiments, TGF-β contributed the majority of suppression by Tregs. To assess the expression of TGF-β by iTregs or nTregs, the presence of LAP on the cell surface was assessed by flow cytometry. While LAP was significantly expressed on iTregs, nTregs appeared to be LAP-negative (Fig 3, D), indicating that surface TGF-β is significantly produced by iTregs and may partially mediate the suppression exerted on ILC2s.
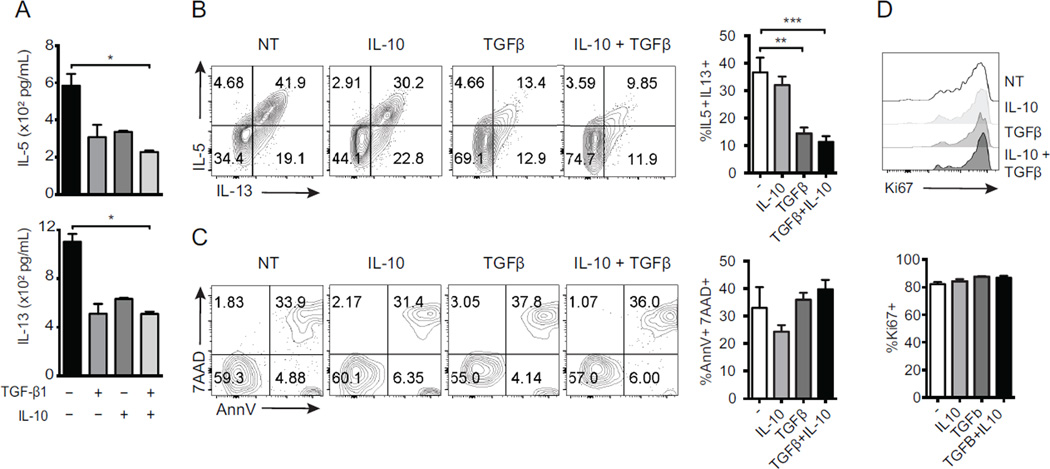
To further assess the role of TGF-β and IL-10 treatment, we cultured ILC2s in the presence of recombinant IL-10, recombinant TGF-β1, or both for 2 days, and subsequently measured IL-5 and IL-13 in the supernatant. Our results indicated that IL-10 and TGF-β1 significantly reduced cytokine secretion by ILC2s (Fig 4, A). We also performed the same experiments and assessed the proportion of IL-5 and IL-13 producing ILC2s by flow cytometry after re-stimulation with PMA and Ionomycin (Fig 4, B). This reduction in cytokine was not due to changes in apoptosis or proliferation as measured by AnnexinV/7AAD and Ki67, respectively (Fig 4, C and D). Our results further confirm that treatment with recombinant IL-10 and TGF-β1 significantly reduced cytokine secretion by ILC2s.
Figure 4. The suppressive effect of iTregs is mediated by inhibitory cytokines.
ILC2s were cultured in the presence of 10 ng/mL of rTGF-β1 and/ or rIL-10. IL-5 and IL-13 production was measured using ELISA (A–B). Cultured ILC2s were also assessed for the expression of 7AAD/AnnexinV and Ki67 by flow cytometry (C–D). Data presented as means ± SEM (n=4, *p<0.05, **p<0.005, ***p<0.0005).
The iTreg suppressive effect on ILC2s requires ICOS:ICOS ligand cell-cell contact
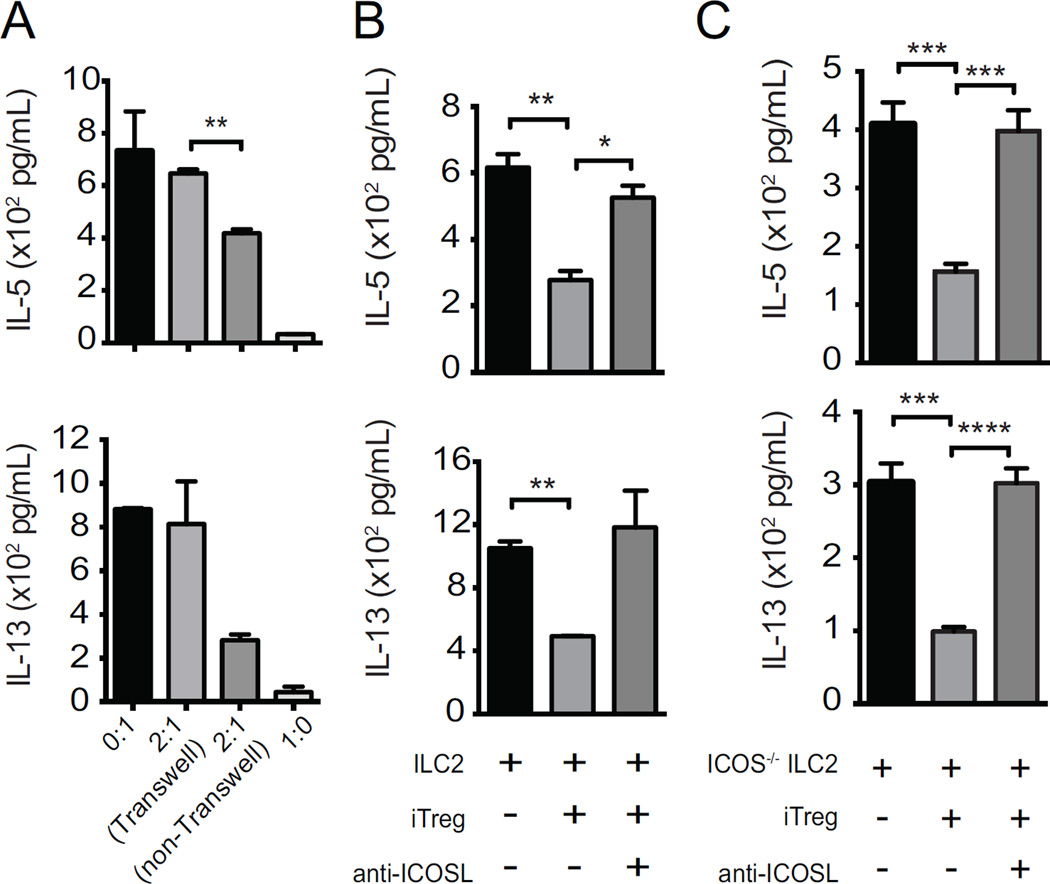
Prior studies suggested that cell-to-cell contact is required for Treg-mediated suppression (18). To analyze the contact dependency of the iTreg suppressive effect on ILC2s, we cultured both types of cells in a transwell stimulation assay. In conventional wells (allowing cell-cell contact), iTregs strongly suppressed the secretion of IL-5 and IL-13 by ILC2s. In transwells not allowing cell-cell contact, iTregs suppressive ability is abrogated, significantly with regards to IL-5 (Fig 5, A). Furthermore, we observed similar suppressive capacity of iTregs upon ILC2s after activation with IL-25 or TSLP (Supplementary Fig 2, B). These data suggest that cell-to-cell contact is required for suppression of ILC2s by iTregs.
Figure 5. iTreg-mediated suppression of ILC2s requires ICOS-L and is contact-dependent.
ILC2s were co-cultured with iTregs with or without transwell membrane (A). In the presence or absence of ICOS-L-neutralizing antibodies, iTregs were co-cultured with wild-type ILC2s (B) or ICOS−/− ILC2s (C). After 48 hours, IL-5 and IL-13 was measured with ELISA. Data presented as means ± SEM (n = 4, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
Given our recent finding that ICOS:ICOS-L interaction is necessary for ILC2 function (11), we next investigated whether the suppression of ILC2s by Tregs is mediated by an interaction between ICOS on Tregs and ICOS-L on ILC2s. Adding anti-ICOS-L (ICOS ligand blocking) antibody to co-cultures of iTregs and ILC2s abrogated suppression of cytokine production normally seen with iTregs (Fig 5, B). ICOS signaling has been suggested to be required for the suppressive activity of Tregs (19, 20), and that cell-cell contact boosts or potentiates Treg cell function (21). To further assess the importance of ICOS:ICOS-L interaction between Tregs and ILC2s, we co-cultured iTregs with ICOS−/− ILC2s in the presence or absence of ICOS-L blocking antibody and assessed IL-5 and IL-13 cytokine production by ILC2s after 48 hours (Fig 5, C). Given that ICOS−/− ILC2s are only capable of expressing ICOS-L, the only possible interaction between ICOS and ICOS-L in this system involves ICOS-L from ILC2s and ICOS on Tregs. As shown in Figure 5C, adding anti-ICOS-L blocking antibody to co-cultures of iTregs and ICOS−/− ILC2s abrogated suppression of cytokine production observed with iTregs. Thus, ICOS:ICOS-L interaction, specifically, ICOS on iTregs and ICOS-L on ILC2s, is necessary for contact dependent suppression of ILC2 activation by iTregs, which is consistent with previous findings of our group showing the necessity of ICOS:ICOS-L signaling for ILC2 survival and cytokine production (11). Overall these data suggest a model whereby Tregs use ICOS-L expression to find and then suppress ILC2s via membrane TGF-β.
Suppression of human ILC2s by human iTregs requires ICOS:ICOS ligand interaction
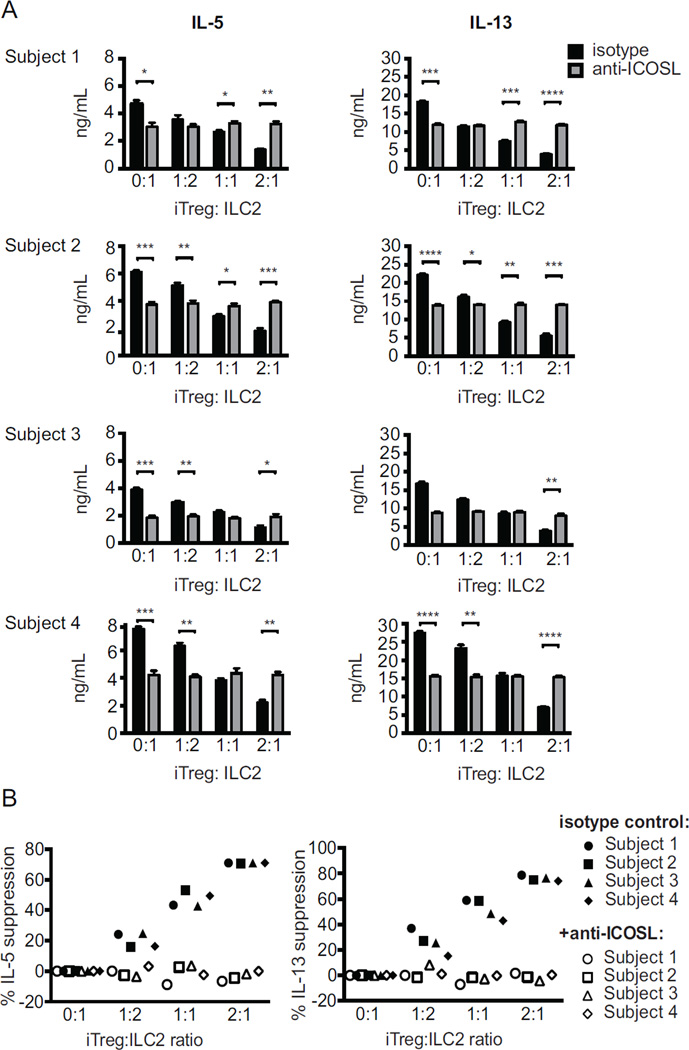
Similar to mouse ILC2s, 48-hour co-culture of human ILC2s with donor-matched human iTregs and isotype control antibody reduced production of IL-5 and IL-13, dependent on the number of Tregs added (Fig 6, A). In all four subjects, this suppressive effect was abolished in the presence of anti-ICOS-L antibodies, calculated as percent suppression of IL-5 or IL-13 production when compared to control (Fig 6, B). In the absence of Tregs, addition of anti-ICOS-L to ILC2s reduced IL-5 and IL-13 production compared to isotype control. As described by our group before, ILC2s express both ICOS and ICOS-L, and this interaction provides additional stimulation for ILC2s (4, 11). However, the suppressive effect of Tregs was abrogated in the presence of anti-ICOSL antibodies.
Figure 6. ICOS:ICOS-L interaction is required for the suppression of human ILC2s by iTregs.
Human CD4+ naïve cells were induced into iTregs, then purified and co-cultured with syngeneic ILC2s, in the presence of either isotype or anti-ICOS-L antibodies for 48 hours. Cytokine production determined by ELISA (A) and percent cytokine suppression are shown (B). Data presented as means ± SEM (n=4).
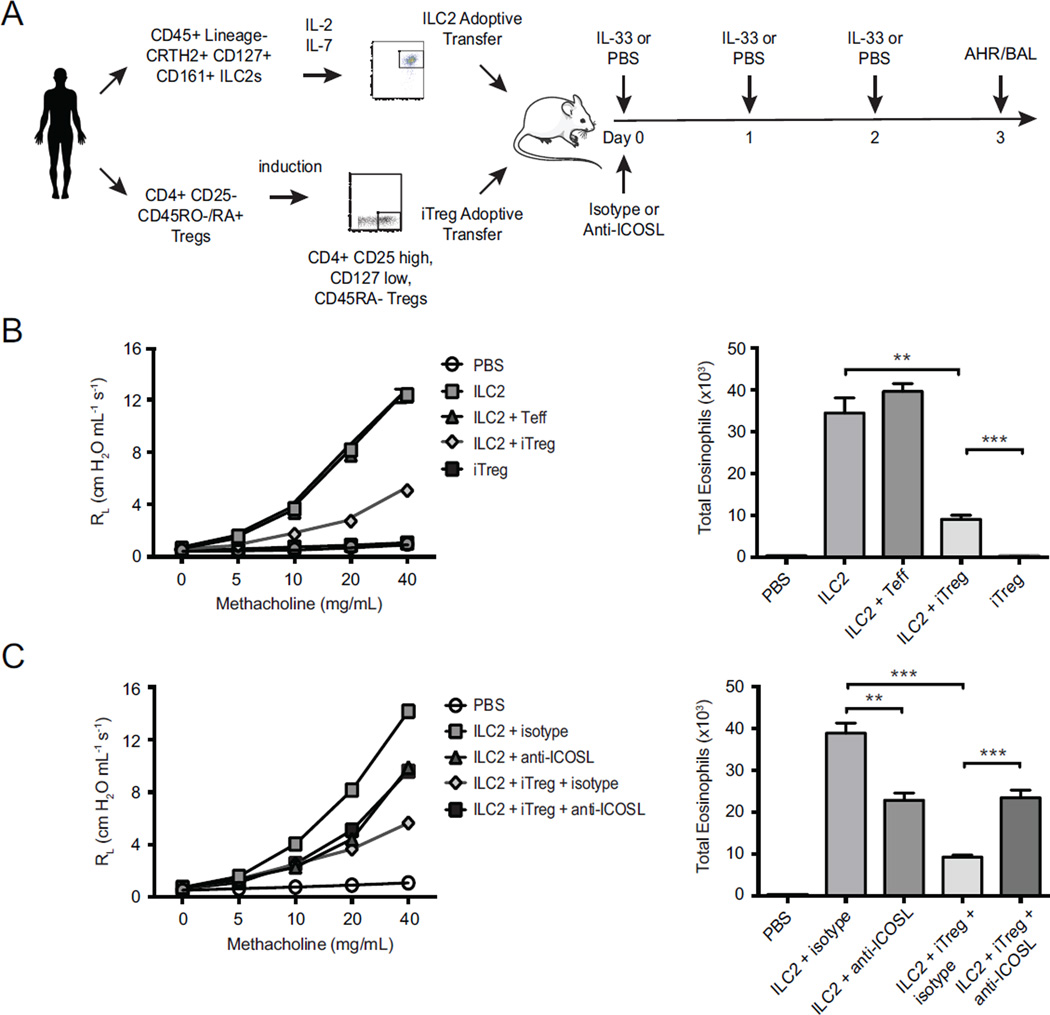
Finally, to substantiate the findings from our in vitro experiments we investigated the role of ICOS:ICOS-L interaction in the suppression of human ILC2s mediated induction of AHR by human iTregs in vivo. Using our recently-developed humanized model (11), we adoptively transferred human ILC2s and/or human iTregs into immunodeficient NSG mice prior to recombinant human IL-33 challenge to drive AHR (scheme in Fig 7, A). Transfer of ILC2s alone drove substantial airway constriction as expected, whereas additional co-transfer of Tregs substantially suppressed AHR (Fig 7, B, left panel). This effect was specific to iTregs, as transfer of other CD4+ T cells (Teff cells) alongside ILC2s did not affect AHR. Similarly, eosinophilia in BAL fluid was attenuated with iTreg co-transfer, but not CD4+ Teff co-transfer (Fig 7, B, right panel).
Figure 7. Suppression of syngeneic ILC2s by iTregs in humanized mice is dependent on ICOS-L.
Purified human ILC2s, human iTregs, or human Teff cells, alone or in homologous combination, were adoptively transferred to groups of NSG mice according to scheme (A). AHR and BAL eosinophils were assessed by measuring lung resistance on day 3. Data expressed as means ± SEM (n=5).
To determine the role of ICOS-L in our humanized murine model, we used human anti-ICOS-L blocking antibody treatment after adoptive transfer of human ILC2s and iTregs followed by IL-33 challenge in alymphoid recipients. We and others reported several years ago that blocking ICOS:ICOS-L interaction reduces airway inflammation and AHR (4, 22, 23). In confirmation of our previous results, here we also observed that blocking ICOS:ICOS-L interaction with anti-ICOS-L reduced the AHR and eosinophilia. However, the addition of iTregs suppresses the AHR and eosinophilia further, and the suppression effect of iTregs was abrogated when the recipients treated with anti-ICOS-L blocking antibody (Fig 7, C). These results confirm that suppression of human ILC2s by iTregs is indeed ICOS:ICOS-L-dependent in vivo and is sufficient to modulate airway inflammatory disease.
Discussion
In this study we describe the novel and marked ability of induced regulatory T cells (iTregs) to regulate type 2 immune lymphoid cells (ILC2s), both in vitro and in vivo. The balance between Treg and type 2 immunity can determine the severity of asthmatic disease (24), in which Tregs are known to dampen both dendritic and T cell responses (25). Here, we add ILC2s as direct Treg targets.
Tregs are divided into nTregs and iTregs based on the location of their development, and mice deficient in the peripherally-generated iTregs have been shown to develop allergic inflammation in their gastrointestinal tracts and lungs (13, 26). Our data show that iTregs, but not nTregs, have the ability to regulate lung ILC2s. Precise functional differences between Treg subtypes remain unclear; however, iTregs have large amounts of membrane bound TGF-β1 in comparison to nTregs as demonstrated the by expression of LAP in Figure 3D and described by others (27, 28), and iTregs may also be better positioned in peripheral tissues in vivo to suppress tissue resident ILCs.
Prior studies suggest iTregs, as opposed to nTregs, are important in suppressing T cell-driven responses in murine asthmatic models (29, 30). As potent producers of IL-5 and IL-13, ILC2s play a central role in the induction of airway hyperreactivity in the murine model of allergic inflammation in vivo (5, 31, 32). Here in one such model, Alternaria alternata-sensitized Rag2-deficient mice, transfer of iTregs reduced both AHR and eosinophilia, thus suppressing ILC2-dependent inflammation in vivo.
Tregs utilize multiple mechanisms to suppress target cells, including suppressive cytokine secretion, contact inhibition, and IL-2 sequestration (33, 34). We found that IL-10 and TGF-β1 were integral for iTreg-mediated ILC2 suppression, with the TGF-β1 effect dominating. This is unsurprising considering the high levels of surface TGF-β1 present on iTregs as compared to nTregs, and indeed oral and airway tolerance of OVA driven airway inflammation requires TGF-β1-mediated iTreg induction and function, not nTreg function (35, 36). However, it has also been shown that the suppressive abilities of iTregs, but not nTregs, in murine models of asthma, are sensitive to IL-10 neutralization when generated by IL-10 secreting dendritic cells (30). In these models, iTregs in peripheral tissues are recruited by and suppress targets with the same antigen specificity. Consistent with our data, several groups recently reported a high level of IL-10 receptor on murine ILC2s and that IL-10 significantly suppresses the levels of IL-5 and IL-13 in activated ILC2s upon IL33 stimulation (37, 38). Interestingly, IL-10 did not suppress IL-2 or IL-25-mediated stimulation of activated ILC2s in vitro, suggesting very specific cross-talk between activating and suppressive pathways that exist in ILC2s. Importantly, we showed that iTregs retain the ability to suppress ILC2s activated by IL-33, IL-25, or TSLP.
Interestingly, we found iTregs require cellular contact to suppress ILC2s. As ILC2s do not have specific T cell receptors, we suggest that the underlying mechanism could be ICOS:ICOS-L interaction. Antibody against ICOS-L resulted in decreased suppression of cytokines in co-cultures of iTregs and ILC2s. We have previously shown that ICOS-ICOSL interaction in-cis on ILC2s promotes survival and function of these cells and Tregs might disrupt this interaction. Furthermore, administration of blocking antibody against ICOS-L in the co-culture of ICOS-deficient ILC2s with iTregs resulted in decreased IL-5 and IL-13, confirming the trans ICOS-ICOS-L interaction between these cells does occur. The data also suggest the mechanism of suppression is not through withdrawal of ICOS survival signals on ILC2s, but rather through iTreg-TGF-β signaling. Therefore, the interaction of ICOS-L on ILC2s with ICOS on iTregs is necessary for iTreg-mediated suppression.
To extend the relevance of our findings towards human disease, we repeated experiments using human cells and human anti-ICOS-L in vitro and in vivo. Our data show evolutionary conservation of ICOS-L-dependent ILC2 suppression by iTregs in humans in vitro. By utilizing a humanized murine model, we validated ICOS-mediated iTreg suppression of human ILC2s in vivo.
ILC2s and Tregs are important in the study of asthma, as Tregs suppress ILC2s and their production of cytokines, which exacerbate atopic diseases. Indeed, therapeutic Treg expansion can limit T cell driven lung inflammation (39). The mechanism by which iTregs induce suppression of ILC2s is contact dependent and requires the ICOS:ICOS-L interaction, alongside canonical suppressive molecules IL-10 and TGF-β. Importantly, adoptive transfer of iTregs suppressed ILC2-dependent AHR in both murine and humanized murine models in vivo. In vivo blockade of ICOS diminished lung inflammation and reduced ex vivo production of type 2 cytokines. Our data suggest a mechanism by which Tregs regulate ILC2 function in vivo and open avenues for potential treatment of asthmatic disease.
Methods
Mice
BALB/cByJ, RAG2−/−, Foxp3+ GFP (BALB/cByJ), and NOD scid IL2Rg-null (NSG) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Dr. Arlene Sharpe (Harvard Medical School) generously provided mice deficient in ICOS. Six- to eight-week-old, age-matched female mice were used in accordance with University of Southern California Department of Animal Resource guidelines.
ILC2 Isolation
Lungs were aseptically removed, minced and incubated in 1.6 mg/ml of collagenase (CLS4, Worthington Biochemicals, Lakewood, NJ) at 37°C for 90 minutes and pressed through a 70µM filter mesh. ILC2s were identified as lineage negative (CD3ε, CD11c, CD11b, B220, Ter-119, Gr-1, FcεR1α, γδTCR), CD45+, CD127+, and ST2+ and purified by FACS ARIA flow cytometer (BD Biosciences, Mountain View CA) with a purity of greater than 93%.
Isolation of human ILC2s was performed as previously described (11). Briefly, peripheral blood mononuclear cells (PBMCs) were isolated from blood of four donors and then stained with lineage markers (CD3, CD14, CD16, CD19, CD20, CD56, CD235a, CD1a, CD123) and CRTH2, CD161, CD127 and CD45. Thereafter CD45+ Lineage− CRTH2+ CD127+ CD161+ population was purified by FACS ARIA flow cytometer with a purity of more than 95%.
Generation of Tregs
nTregs and effector T cells (Teff) from thymus and spleen of Foxp3+ GFP mice were purified by FACS ARIA flow cytometer as CD3+ CD4+ CD25+ Foxp3+ (thymic nTregs) and CD3+ CD4+ CD25+ Foxp3− (splenic Teff) cells. Induced Tregs (iTregs) were generated in vitro by first FACS sorting CD3+ CD4+ CD25− Foxp3− naive T cells from the spleens of Foxp3+ GFP mice, then culturing these cells with plate-bound anti-CD3 (10µg/ml), soluble anti-CD28 (2µg/ml), IL-2 (20ng/ml), rhTGF-β (10ng/ml, R+D systems), anti-IL-4 (5µg/ml) and anti-IFNγ (5µg/ml). All antibodies were purchased from eBioscience unless noted. Seven days later, live CD3+ CD4+ CD25+ Foxp3+ cells, now identified as iTregs, were purified and analyzed using the BD Cytofix/Cytoperm Fixation/Permeabilization kit and intracellular staining using FoxP3-PE (BioLegend). Cells were stained with target antibodies and isotype controls using standard flow cytometry procedures, and analyzed on a FACS Canto II (BD) using FlowJo 10.1r5 software (Tree Star).
For human iTregs, CD4+ PBMCs were enriched with magnetic sorting from the blood of four donors. From this population CD4+ CD25− CD45RO−/RA+ cells were sorted with a FACS ARIA flow cytometer. Sorted cells were cultured with plate-bound anti-CD3 (1µg/ml, OKT3), soluble anti-CD28 (5µg/ml), IL-2 (5ng/ml), TGF-β (5ng/ml). After 6–7 days, a CD4+, CD25-high, CD127-low, and CD45RA− population representing iTregs was purified using a BD FACS Aria cell sorter.
In vitro Suppression assay
Purified Teffs, nTregs and iTregs were co-cultured with ILC2s (5 × 103 cells/well) at indicated ratios: 2:1, 1:1, 1:2, and 1:4 for 48 hours in the presence of IL-2 (10ng/ml) and IL-7 (10ng/ml). IL-5 and IL-13 cytokine levels were measured in the supernatant by ELISA (eBioscience). For blocking experiments, iTregs (104104) and ILC2s (5 × 103) were co-cultured at 2:1 ratio in the presence or absence of neutralizing antibodies for IL-10 (10µg/ml), TGF-β (10µg/ml), IL-10+TGF-β, ICOS (10µg/ml) or ICOS ligand (10µg/ml) (all obtained from Bio × Cell, NH. USA). ILCs alone were cultured for 48 hours in the presence or absence of recombinant IL-10 (10ng/ml) and TGF-β (10ng/ml), and cytokines measured in supernatant. For intracellular flow cytometry cells were re-stimulated with PMA (5ng/mL) and Ionomycin (50ng/mL) with transport inhibitor Brefeldin A (BFA, 1ng/mL) 4 hours before staining with indicated antibody (eBioscience) with transcription factor staining kit (eBioscience) or Annexin V staining kit (BD Biosciences) per manufacturer’s instructions. For transwell experiments CD4+ CD3+ CD25+ Foxp3+ iTregs were co-cultured with ILC2s (5 × 103 cells/well) in a Corning HTS Transwell 96-well according to manufacturer’s instructions, in the presence of IL-2 (10ng/mL) and IL-7 (10ng/mL) for 48 hours. Staining antibody against ICOS-L was purchased from BioLegend for the assessment of ICOS-L expression on ILC2s.
For in vitro activation studies with rmICOS/Fc Chimera protein, activated pulmonary ILC2s were isolated as described above, and incubated on a plate coated with reagents (either ICOS-Ig or control Ig, 1µg/mL, R&D Systems, Minneapolis, MN) for 48 hours in the presence of IL-2/7. Culture Supernatants were taken for the measurement of cytokines by ELISA.
Bronchoalveolar lavage flow cytometry
Bronchoalveolar lavage (BAL) fluid was incubated with FITC CD19, PE Siglec-F, PerCP/Cy5 CD3, APC Gr-1, APC/Cy7 CD11c, PE/Cy7 CD45, eFluoro450 CD11b and Fc block (BioLegend, San Diego, CA). The cells were acquired on the FACS Canto and analyzed by using FlowJo software (Tree Star, Ashland, OR) as previously described (27). Neutrophils were gated as CD45+, Gr-1+, and CD11b+; macrophages as CD45+ and CD11c+; and Siglec-F+ cells and eosinophils as CD45+, Cd11c−, and Siglec-F−.
Asthma murine model and adoptive transfer of iTregs
iTregs (9 × 105 cells) were adoptively transferred through tail vein injection (i.v.) into six- to eight-week-old female Rag2−/− (BALB/cByJ) followed by three days of i.n. IL-33 (0.5µg/mouse), Alternaria alternata (25g/mouse; Greer Labs, Lenoir, NC), or PBS as indicated. Lung function was measured 24 hours after last injection by direct measurement of lung resistance and dynamic compliance in restrained, tracheostomized, mechanically ventilated mice via Finepointe RC system (Buxco Research System, Wilmington, NC) under general anesthesia as described previously (27).
Humanized mice
Purified human ILC2s were cultured with rh-IL-2 (20ng/ml) and rh-IL-7 (20ng/ml) for three days before adoptive transfer. ILC2s (2.5 × 104 cells/mouse), Teff (2.5 × 105 cells/mouse), and/ or iTregs (2.5 × 105 cells/mouse) were then injected intravenously into six- to eight-week-old female NOD SCID gamma-C deficient (NSG) mice as indicated, followed by i.n. treatment with isotype control or anti-ICOS-L antibodies (100µg/mouse). Human anti-ICOS-L antibodies were generated and provided by Dr. Gordon Freeman as described elsewhere (4). After 24 hours recombinant human (rh) IL-33 (0.5µg/mouse) was intranasally administered on three consecutive days and lung function was measured 24 hours after last treatment.
Gene expression analysis using NanoString® nCounter technology
Utilizing FACS sorting, ILC2s were purified from a cohort of WT mice after three serial i.n. IL-33 administrations. iTregs were generated as described above. The iTregs and ILC2s were co-cultured at a 2:1 ratio and after 24 hours, ILC2s were sorted from co-cultures. Total RNA was isolated using MicroRNAeasy (Qiagen, Valencia, CA) and analyzed for gene expression by NanoString nCounter system (Seattle, WA), as described by our group elsewhere (11). mRNA counts within samples were normalized to expression panel of 10 housekeeping genes and differences in gene expression were calculated as a logarithmic ratio between ILC2s and ILC2s/iTreg co-cultures using Bonferroni adjusted p-value.
Study Approval
Animal studies were approved by the University of Southern California (USC) Institutional Animal Care and Use Committee and conducted in accordance with the USC Department of Animal Resources’ guidelines. All human studies were approved by the USC institutional review board and conducted according to the principles of the Declaration of Helsinki. Participants gave written informed consent prior to their inclusion in the study, and were identified by number.
Supplementary Material
Clinical Implications.
Our data illustrate a mechanism by which Type 2 innate lymphoid cells (ILC2s) crosstalk with regulatory T cells (Tregs). This interaction is required for suppression of ILC2s, opening avenues for potential treatment of ILC2-associated airway inflammation and asthma.
Acknowledgments
Studies described in this article were financially supported by National Institutes of Health Public Health Service Grants: R01ES 021801, R01ES025786, R21AI109059 and R21 ES 024707 (to O.A.).
Abbreviations
- 7-AAD
7-Aminoactinomycin
- AHR
Airway hyperreactivity
- BAL
Bronchoalveolar lavage
- BFA
Brefeldin A
- CD
Cluster of differentiation
- CRTH2
Chemoattractant receptor-homologous molecule expressed on T cells
- ELISA
Enzyme-linked immunosorbent assay
- FACS
Fluorescence-activated cell sorting
- Foxp3
Forkhead box P3 protein (aka scurfin)
- ICOS
Inducible T-cell costimulator (aka CD278)
- ICOS-L
ICOS ligand (aka CD275)
- IL
Interleukin
- ILC
Innate lymphoid cell
- ILC2
Type 2 (Group 2) innate lymphoid cell
- iTreg
Induced regulatory T cell
- LAP
Latency-associated peptide
- LogFC
Logarithmic fold-change
- nTreg
Natural regulatory T cell
- PBMC
Peripheral blood mononuclear cell
- PMA
Phorbol 12-myristate 12-acetate
- RAG
Recombination-activating gene
- ST2
Interleukin 1 receptor-like 1 (aka IL1R1, IL33R) Teff Effector T cell
- TGF-β
Transforming growth factor beta
- Th2
Type 2 helper T cell
- Treg
Regulatory T cell
- TSLP
Thymic stromal lymphopoietin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
OA and PS devised the experimental plan; DR, BW, GL, HB, IS, HD, and RL conducted the experiments and acquired data; JLA and GL analyzed the data and computed statistics; JLA and GL created the figures; JLA and OA wrote the manuscript; and GJF and AHS critically reviewed the results and edited the manuscript.
References
- 1.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161(5):1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 2.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344(5):350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 3.Robinson DS, Larche M, Durham SR. Tregs and allergic disease. J Clin Invest. 2004;114(10):1389–1397. doi: 10.1172/JCI23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8(9):1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 5.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12(7):631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282(5397):2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 7.Webb DC, McKenzie AN, Koskinen AM, Yang M, Mattes J, Foster PS. Integrated signals between IL-13, IL-4, and IL-5 regulate airways hyperreactivity. J Immunol. 2000;165(1):108–113. doi: 10.4049/jimmunol.165.1.108. [DOI] [PubMed] [Google Scholar]
- 8.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463(7280):540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 10.Doherty TA, Khorram N, Chang JE, Kim HK, Rosenthal P, Croft M, et al. STAT6 regulates natural helper cell proliferation during lung inflammation initiated by Alternaria. Am J Physiol Lung Cell Mol Physiol. 2012;303(7):3. doi: 10.1152/ajplung.00174.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maazi H, Patel N, Sankaranarayanan I, Suzuki Y, Rigas D, Soroosh P, et al. ICOS:ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity. 2015;42(3):538–551. doi: 10.1016/j.immuni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, Kemeny DM, et al. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med. 2002;8(6):625–629. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]
- 13.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482(7385):395–399. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iijima K, Kobayashi T, Hara K, Kephart GM, Ziegler SF, McKenzie AN, et al. IL-33 and thymic stromal lymphopoietin mediate immune pathology in response to chronic airborne allergen exposure. J Immunol. 2014;193(4):1549–1559. doi: 10.4049/jimmunol.1302984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37(4):634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezende RM, da Cunha AP, Kuhn C, Rubino S, M'Hamdi H, Gabriely G, et al. Identification and characterization of latency-associated peptide-expressing gammadelta T cells. Nat Commun. 2015;6:8726. doi: 10.1038/ncomms9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199(11):1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kornete M, Sgouroudis E, Piccirillo CA. ICOS-dependent homeostasis and function of Foxp3+ regulatory T cells in islets of nonobese diabetic mice. J Immunol. 2012;188(3):1064–1074. doi: 10.4049/jimmunol.1101303. [DOI] [PubMed] [Google Scholar]
- 21.Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol. 2009;182(10):6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akbari O, Stock P, Meyer EH, Freeman GJ, Sharpe AH, Umetsu DT, et al. ICOS/ICOSL interaction is required for CD4+ invariant NKT cell function and homeostatic survival. J Immunol. 2008;180(8):5448–5456. doi: 10.4049/jimmunol.180.8.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalo JA, Tian J, Delaney T, Corcoran J, Rottman JB, Lora J, et al. ICOS is critical for T helper cell-mediated lung mucosal inflammatory responses. Nat Immunol. 2001;2(7):597–604. doi: 10.1038/89739. [DOI] [PubMed] [Google Scholar]
- 24.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199(11):1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akbari O, Stock P, DeKruyff RH, Umetsu DT. Role of regulatory T cells in allergy and asthma. Curr Opin Immunol. 2003;15(6):627–633. doi: 10.1016/j.coi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Xu W, Lan Q, Chen M, Chen H, Zhu N, Zhou X, et al. Adoptive transfer of induced-Treg cells effectively attenuates murine airway allergic inflammation. PLoS One. 2012;7(7):e40314. doi: 10.1371/journal.pone.0040314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerzerho J, Maazi H, Speak AO, Szely N, Lombardi V, Khoo B, et al. Programmed cell death ligand 2 regulates TH9 differentiation and induction of chronic airway hyperreactivity. J Allergy Clin Immunol. 2013;131(4):1048–1057. doi: 10.1016/j.jaci.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O'Shea JJ, et al. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J Exp Med. 2008;205(9):1975–1981. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29(1):114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Huang H, Ma Y, Dawicki W, Zhang X, Gordon JR. Comparison of induced versus natural regulatory T cells of the same TCR specificity for induction of tolerance to an environmental antigen. J Immunol. 2013;191(3):1136–1143. doi: 10.4049/jimmunol.1201899. [DOI] [PubMed] [Google Scholar]
- 31.Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, et al. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012;42(5):1106–1116. doi: 10.1002/eji.201142018. [DOI] [PubMed] [Google Scholar]
- 32.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188(3):1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 34.Tjota MY, Williams JW, Lu T, Clay BS, Byrd T, Hrusch CL, et al. IL-33-dependent induction of allergic lung inflammation by FcgammaRIII signaling. J Clin Invest. 2013;123(5):2287–2297. doi: 10.1172/JCI63802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005 Jul;115(7):1923–1933. doi: 10.1172/JCI24487. Epub 2005 Jun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hepworth MR, Fung TC, Masur SH, Kelsen JR, McConnell FM, Dubrot J, et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4(+) T cells. Science. 2015;348(6238):1031–1035. doi: 10.1126/science.aaa4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morita H, Arae K, Unno H, Miyauchi K, Toyama S, Nambu A, et al. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation by Promoting Regulatory T Cell Numbers. Immunity. 2015;43(1):175–186. doi: 10.1016/j.immuni.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moro K, Kabata H, Tanabe M, Koga S, Takeno N, Mochizuki M, et al. Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat Immunol. 2016;17(1):76–86. doi: 10.1038/ni.3309. [DOI] [PubMed] [Google Scholar]
- 39.Schreiber TH, Wolf D, Tsai MS, Chirinos J, Deyev VV, Gonzalez L, et al. Therapeutic Treg expansion in mice by TNFRSF25 prevents allergic lung inflammation. J Clin Invest. 2010;120(10):3629–3640. doi: 10.1172/JCI42933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.