Abstract
Observational analyses have suggested that women using the injectable contraceptive depot medroxyprogesterone acetate (DMPA) may have heightened risk of acquiring HIV. However, those analyses were potentially confounded by sexual behavior, with possible differential condom use and reporting by women using DMPA versus no contraception. In a cross-sectional study, we measured the presence of a biomarker of recent condomless sex (Y chromosomal [Yc] DNA) in vaginal swabs from HIV-uninfected African women who had an HIV-infected partner and reported 100% condom use. Half of the samples tested were from women reporting DMPA and half were from women using no contraception. Among 428 specimens tested (213 from DMPA users and 215 from women using no contraception), 32.0% had Yc DNA detected, with a mean of 193 copies/10,000 human cells (range 0.1–8201). The frequency of detection did not differ by contraceptive use: 34.2% of DMPA users versus 29.8% of women using no contraception, adjusted odds ratio 1.3 (95% confidence interval 0.9–2.0). These results suggest that inaccurate reporting of condom use by DMPA users may not account for the heightened risk of HIV acquisition among DMPA users in some observational studies.
Keywords: condoms, biomarker, Y chromosomal DNA, DMPA, Africa, HIV
BACKGROUND
The greatest burden of the HIV epidemic is in sub-Saharan Africa, where heterosexual transmission continues to be the primary driver of incidence (1). To understand risk factors of heterosexual HIV transmission, studies must account for sex unprotected by male condoms (as well as other confounding factors) in order to fully interpret results since male condoms are an effective intervention for HIV prevention (2–5). Self-report is the most common method used to capture information about condom use, but data captured this way are often prone to inaccuracy due to difficulty recalling the frequency of condom use during a given period of time, desires to report condom use that aligns with socially accepted behavior, and misunderstood questions (6). Efforts to improve accuracy have used computer or cell phone-based data capture with mixed results (7–9). More recently, biomarkers, including the detection of prostate specific antigen (PSA) and Y chromosomal (Yc) DNA in female genital tract samples, have been used as a measure of semen exposure when condomless sex occurred within the past 7 days (9–11). PSA degrades in 24–48 hours (10,12,13) while Yc DNA has been shown to be detectable for at least 7 days (14,15), making Yc DNA testing potentially more sensitive to detecting condomless sex and a proxy measure of recent condomless sex.
In epidemiologic analyses of heterosexual HIV risk, inaccurate data on sex unprotected by male condoms have the potential to produce biased results depending on the magnitude of inaccuracy and how condom use relates to the primary exposure and outcome. Inaccurate condom use reporting has been a frequently discussed potential source of bias in studies assessing the possibility of heightened HIV risk among women using injectable contraception, particularly depot medroxyprogesterone acetate (DMPA) (4,5). Women who choose to use contraception may make different choices about their condom use than women who do not contracept (16,17). Since condom use is protective against HIV, observational analyses of DMPA and HIV acquisition that compare users to women using no effective contraception must account for sex unprotected by male condoms through statistical adjustment (3). In these analyses, inaccuracies in self-reported male condom use may have yielded results that are biased due to the presence of residual confounding. Most importantly, this bias would be more consequential if the magnitude of inaccuracy differed between women using DMPA and women using no effective contraception.
Most studies of DMPA and HIV have not incorporated data about sex unprotected by male condoms based on laboratory measures of biomarkers of semen exposure. Using archived specimens from a prospective study of HIV-uninfected women with an HIV-infected male partner in Kenya and Uganda, we estimated the degree to which women using DMPA and no effective contraception differentially misreported sex unprotected by male condoms by assessing the presence of Yc DNA in vaginal swab samples from women reporting 100% condom use.
METHODS
Study Population
Data for this analysis were from HIV-uninfected women participating in the Partners PrEP Study, a randomized, placebo controlled trial of daily oral pre-exposure prophylaxis for HIV prevention among 4,747 HIV serodiscordant couples in Kenya and Uganda (18,19). Study participants were recruited through study-initiated community outreach activities and referrals from HIV-1 testing and care centres, antenatal clinics, and non-governmental organizations. Study visits for the clinical trial took place between 2008 and 2012 and included couples-based HIV prevention counseling with encouragement to use condoms and free condom provision. HIV-uninfected partners were seen monthly for HIV testing and study drug refills. At each visit, participants completed interviewer-administered standardized questionnaires to assess demographic, medical, and sexual behavior characteristics. Sexual behavior was assessed by asking each participant to report the number of sex acts with their study partner and additional partners in the last month, the number of times condoms were used with each type of partner, and the timing of their last sex act. All women were offered contraception on-site but contraceptive use was not a study requirement. In Kenya and Uganda, DMPA was the only type of injectable contraception available during the study period. The study protocol was approved by the Human Subjects Division at the University of Washington and all study sites. All participants provided written informed consent, including consent for archived genital swab specimens to be used for additional analyses related to HIV, HIV-related diseases, and sexually transmitted infections (STI).
Study design
Data and specimens included in this study were from HIV-uninfected women who did not seroconvert and were not pregnant during the study period. For the present study, vaginal swab specimens were eligible for selection from visits when HIV-uninfected women reported: 1) 100% of sex acts protected by male condoms during the last month with all partners and 2) sex protected by a condom at the last vaginal sex act with their HIV-infected male study partner and 3) that the last vaginal sex act with their HIV-infected male study partner was <7 days ago. 220 specimens were randomly selected from women reporting DMPA use and an additional 220 from women reporting no use of any contraceptive method except condoms. Only one sample was selected per participant to remove the need to control for correlation between multiple samples from the same woman. This sample size provided 80% power to detect a 2-fold difference in Yc DNA detection if the background rate of detection was 10% among women not using contraception. Our prior work found a 2-fold increase in HIV risk with injectable contraception (20). Subsequent mathematical modeling suggested that at least a 2-fold difference in misreporting between injectable users and non-contracepting women would be necessary to create an injectable-HIV point estimate of this magnitude when in fact there is no increased risk (21). An additional set of 40 samples were also selected from women who reported: 1) no condom use during the last month with all partners and 2) that the last sex act was <7 days (n=20), 7 to 14 days (n=10), or >14 days ago (n=10). These samples were used to better understand the sensitivity of the Yc DNA assay in relation to time of sample collection following sex acts unprotected by a condom.
Sample collection
Archival vaginal swabs were collected from women by a study clinician during annual follow up visits and at study exit using a Copan nylon flocked swab that was inserted 2 inches into the vagina and rolled along the lateral vaginal wall for 15 seconds. All archival swabs were stored at −80°C and shipped to the University of Pittsburgh in 2014 on dry ice.
Laboratory testing
Total nucleic acid was isolated directly from vaginal swab samples by lysing the cells directly on the vaginal swab using a NAO (Nucleic Acid Optimizer) basket (NOVA Biostorage Plus), spinning the lysed material through the basket at 10,000×g for 5 minutes, then precipitating the nucleic acid with isopropanol. Precipitated DNA was washed with 70% ethanol and dried for 10 min. Pellets were then re-suspended in 5 mM Tris at pH 8.0, 1 µM DTT, and 1,000 units/mL of recombinant RNasin and stored at −80°C prior to testing (22).
A quantitative PCR assay (qPCR), Quantifiler Duo (Applied Biosystems), was used to detect the presence and quantity of semen in vaginal samples by measuring Yc and total human DNA as described previously (11). Samples were run in triplicate at 50 ng/ul total DNA input and considered to have detectible Yc DNA if 2 out of 3 wells measured one or more copies per reaction. Our prior study using this same technique observed no false positive samples (11). Due to the variability of sample collection, Yc DNA was normalized to a theoretical sample size of 10,000 cells based on the total copies of human DNA measured as part of the multiplex qPCR assay. Laboratory staff were blinded to the type of contraception women used and their report of sex unprotected by male condoms.
Statistical analysis
We used descriptive methods to summarize the frequency with which Yc DNA was detected in specimens from women using DMPA and no effective contraception as well as the quantity of Yc DNA recovered. Logistic regression models were used to calculate univariate odds ratios to determine correlates of having detectable Yc DNA. A multivariate logistic regression model estimated the association of using DMPA and having Yc DNA present with the adjustment factors (age, number of children and sexual frequency) determined a priori due to their known associations with contraceptive use and sexual behavior (16,23). SAS 9.4 (SAS Institute, Cary, NC) was used for all analysis.
RESULTS
The median age of women randomly selected for this study was 34 years (interquartile range [IQR] 29–39, Table I). Nearly all women were married (99%) and most had at least one child (96.9%). Two-thirds of the women in the sample had been randomized to an active PrEP agent and 5% were treated for an STI (gonorrhea, chlamydia, trichomonas, genital ulcer disease, bacterial vaginosis, or vaginal candidiasis) at the visit when their vaginal swab sample was collected. Women reported a median of 3 sex acts with their study partner (IQR 2–5) and only one woman reported having a partner in addition to her study partner during the month prior to swab collection. These characteristics were not different among women using DMPA and women using no contraception and are representative of the entire population of HIV-uninfected women in the Partners PrEP Study (18,19).
Table I.
Characteristics of women with vaginal swabs tested for Y chromosomal DNA and correlates of Y chromosomal DNA detection
| N (%) among women using DMPA |
N (%) among women using no hormonal contraception |
N (%) with Y chromosomal detected |
OR (95% CI) | p-value | Adjusted OR (95% CI) |
p-value | |
|---|---|---|---|---|---|---|---|
| Contraception | |||||||
| DMPA | 213 | -- | 73 (34.3) | 1.2 (0.8–1.9) | 0.3 | 1.3 (0.9–2.0) | 0.2 |
| No hormonal contraception | -- | 215 | 64 (29.8) | 1.00 | |||
| Age, years | |||||||
| Age <25 | 23 (10.8) | 22 (10.2) | 16 (35.6) | 1.00 | |||
| Age ≥25 | 190 (89.2) | 193 (89.8) | 121 (31.6) | 0.8 (0.4–1.6) | 0.6 | 1.2 (0.6–2.5) | 0.6 |
| Marital status* | |||||||
| Married | 212 (99.5) | 215 (100) | 137 (32.1) | ||||
| Not married | 1 (0.5) | 0 (0) | 0 (0.0) | ||||
| Total number of children | |||||||
| No children | 4 (1.9) | 9 (4.2) | 8 (61.5) | 3.8 (1.2–11.8) | 0.02 | 4.2 (1.3–13.5) | 0.02 |
| Has 1 child | 16 (7.5) | 23 (10.7) | 17 (43.6) | 1.8 (0.9–3.6) | 0.08 | 2.0 (1.0–4.2) | 0.06 |
| Has ≥2 children | 193 (90.6) | 183 (85.1) | 112 (29.8) | 1.00 | 1.00 | ||
| Study randomization arm | |||||||
| Active PrEP arm | 147 (69.0) | 161 (74.9) | 95 (30.8) | 0.8 (0.5–1.3) | 0.4 | ||
| Placebo arm | 66 (31.0) | 54 (25.1) | 42 (35.0) | 1.00 | |||
| Medical characteristics | |||||||
| STI treated at visit** | 11 (5.4) | 11 (5.2) | 9 (40.9) | 1.5 (0.6–3.6) | 0.4 | ||
| No STI treatment at visit | 194 (94.6) | 200 (94.8) | 125 (31.7) | 1.00 | |||
| Sexual frequency with study partner, past month | |||||||
| Reported 1–3 vaginal sex acts | 106 (49.7) | 111 (51.6) | 67 (30.9) | 1.00 | |||
| Reported 4–9 vaginal sex acts | 83 (39.0) | 85 (39.5) | 57 (33.9) | 1.2 (0.8–1.8) | 0.5 | 1.1 (0.7–1.7) | 0.6 |
| Reported ≥10 vaginal sex acts | 24 (11.3) | 19 (8.8) | 13 (30.2) | 1.0 (0.5–2.0) | 0.9 | 0.9 (0.4–1.9) | 0.8 |
| Additional partners, past month* | |||||||
| At least 1 additional partner | 1 (0.5) | 0 (0) | 1 (100.0) | ||||
| No additional partner | 212 (99.5) | 215 (100) | 136 (319.9) | ||||
There is not enough variation in marital status or reports of additional partners to run these in logistic regression models.
STI treatment includes treatment of syndromically or etiologically diagnosed genital ulcer disease, gonorrhea, chlamydia, trichomonas, bacterial vaginosis or vaginal candidiasis.
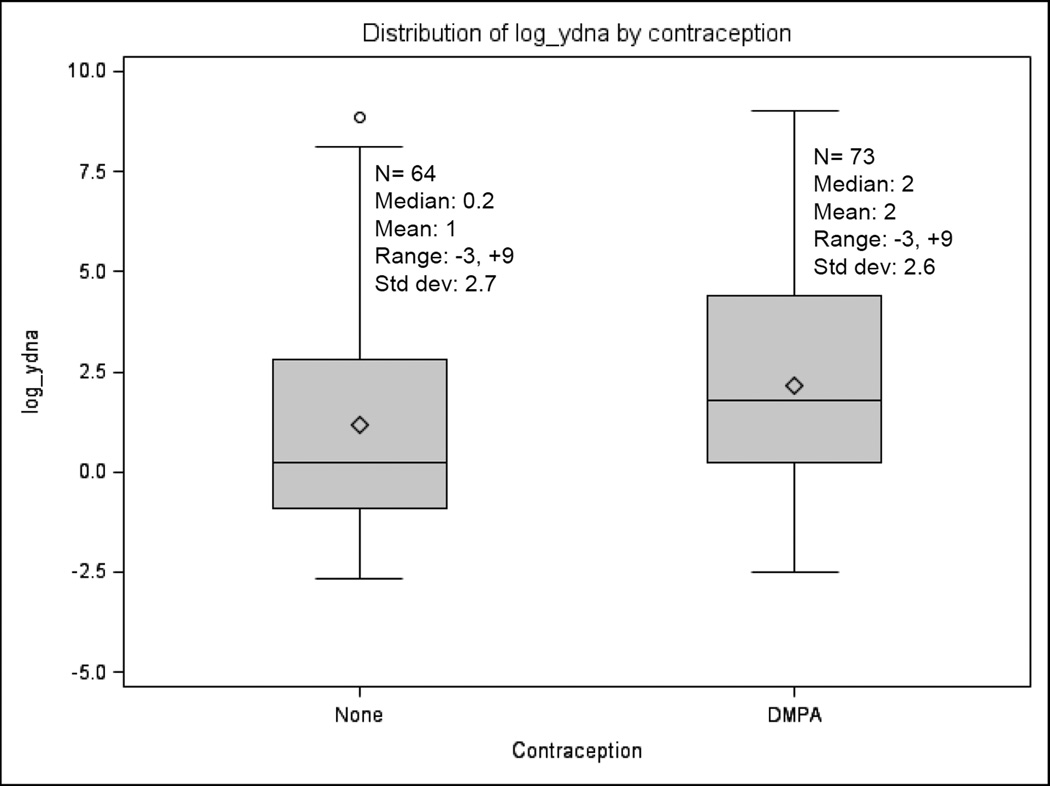
To better understand misreport of sex unprotected by male condoms and the potential bias in women using DMPA versus no contraception, the swabs analyzed were from women who reported using condoms with every sex act in the past month, including the most recent sex act that was within the past 7 days. Overall, 32.0% of women had Yc DNA detected in their vaginal swab sample. Among women with detectable Yc DNA, the median quantity detected was 193 copies/10,000 human cells (range 0.1–8201). 34.3% of DMPA users (73/213) and 29.8% (64/215) of women using no contraception had Yc DNA detected, a difference that was not statistically significant (adjusted odds ratio [OR] = 1.3, 95% confidence interval [CI] 0.9–2.0). One recent meta-analysis estimated that DMPA carried a 50% increase in HIV risk (24); given 29.8% of non-contracepting women in our population had Yc DNA detected, post hoc power calculations suggest we had 90% power to detect a 1.5-fold difference with statistical significance. Among women with Yc DNA detected, the distribution of Yc DNA quantities were also similar (Figure I). Women with fewer than 2 children were more likely to have Yc DNA detected than women with ≥2 children (adjusted OR=4.2, 95% CI 1.3–13.5 for women with no children and adjusted OR=2.0, 95% CI 1.0–4.2 for women with 1 child). No additional factors were significantly associated with Yc DNA detection.
Figure 1. Distribution of Y chromosomal DNA quantity (log10) recovered from vaginal swabs of women with Yc DNA detected, by contraceptive use status.
Non log-transformed values for women using DMPA: Median 6, Mean 180, Range 0.1–8200, Standard deviation 964. Non log-transformed values for women using no contraception: Median 1, Mean 207, Range 0.1–6997, Standard deviation 966.
In the swabs tested from women reporting no condom use, the assay detected Yc DNA in 80% (16/20) of samples collected <7 days after the last sex act, 60% (6/10) from samples collected 7–14 days after the last sex act, and 20% (2/10) when the sample was collected more than 14 days after the last sex act. These data indicate that Yc DNA detection from vaginal swabs is more reliable when the sample is collected within 7 days of the last sex act.
DISCUSSION
In this study of women self-reporting 100% condom use, DMPA users and women using no contraception did not have a statistically significant difference in the frequency of Yc DNA detection, a marker of sex unprotected by a male condom. Thus, for interpreting observational analyses comparing women using DMPA with women using no contraception, differential accuracy of self-reported sex unprotected by a male condom may not be a significant factor. Approximately one-third of women in this study potentially over-reported their condom use, a level consistent with prior studies that examined consistency of self-reported condom use and PSA or Yc DNA detection as biomarkers of semen exposure (9,11). Social desirability bias may account for much of this discrepancy, since study participants were receiving frequent HIV prevention counseling that emphasized the importance of condom use.
Observational analyses of HIV risk comparing women using hormonal contraception (DMPA, other injectable agents, or other formulations) versus no hormonal contraception have great potential for biased results when self-reported data are used to control for confounding by condom use. For women using no hormonal contraception, condoms offer protection from unintended pregnancy as well as HIV and STIs. Some have postulated that women using contraception, who do not need the pregnancy prevention benefit of condoms, have less impetus to use condoms but may report use because of social desirability, making self-reported condom use by these women potentially more inaccurate than reporting by women not using contraception (25–27). Preliminary data from mathematical modeling studies have suggested that such over-reporting by women using DMPA could produce spurious evidence of elevated HIV risk even if no difference in risk actually existed (21). However, one prior study of PSA levels found a non-significant trend for women using hormonal contraceptives to over-report condom use less frequently than women using non-hormonal contraceptives (28). Thus, our data add additional evidence that injectable contraceptive users do not substantially over-report condom use more frequently than non-contracepting women.
It is challenging to collect accurate data on sexual behavior, especially in the context of HIV prevention trials when participants are counseled regularly to use condoms and may not feel comfortable disclosing condomless sex (9). Male condom use is not the only important potential confounder to consider in final models; others such as sexual frequency, partner HIV status, and partner use of antiretrovirals are also important and may be subject to different degrees of reporting accuracy depending on study design. Methods to improve self-reported sexual behavior may include computer-assisted self-interview tools (CASI) but not all studies have seen improved accuracy with this tool (29,30). Biomarkers are a useful objective measure of sexual behavior and potential HIV exposure but they require invasive study procedures to collect samples, sample storage and processing that ensures specimen integrity, and time for testing which precludes immediate availability of results. Of the current methods, Yc DNA is the most sensitive for detecting semen exposure, with detection up to 15 days post-coitus under optimal conditions (14,15). In this study, semen exposure was consistently detected when sexual activity took place up to 7 days prior to swab collection (Table II). PSA and rapid stain identification of human semen (RSID) are less sensitive than quantitative PCR methods, with a detection threshold of up to 48 hours (31,32), but both assays detect specific markers of semen while condom breakage, condomless penile insertion without ejaculation, or potentially other exposures such as digital insertion could contribute to the Yc DNA signal. As the field of HIV prevention strives to understand factors that continue to drive the epidemic among women, including the potential for elevated risk due to DMPA use, biomarkers of semen exposure are an important measure of recent lack of consistent condom use to complement self-reported data.
Table II.
Detection of Yc DNA in women that report no condom use and relationship to time of sampling.
| Sample Collection Category |
Number of Days Since Last Sex Act Median (range) |
N | Detectable Yc DNA N (%) |
Yc DNA copies per 10,000 cells Median (range) |
|---|---|---|---|---|
| <7 days | 2 (0–5) | 20 | 16 (80%) | 4 (0–2147) |
| 7–14 days | 7 (7–14) | 10 | 6 (60%) | 0.3 (0–134) |
| >14 days | 21 (21–28) | 10 | 2 (20%) | 0 (0–480) |
One limitation of this study is that DMPA use was not associated with a statistically significant increase in HIV risk in the study population, potentially in part because of limited power due to relatively few HIV-1 seroconversions in the study because of effective PrEP use.(33,34) However, the study locations, recruitment approaches, and HIV prevention counseling mirrored those of our earlier studies with HIV serodiscordant couples (34,35), which demonstrated a 2-fold increase in HIV-1 risk associated with DMPA use and catalyzed recent global attention to the potential HIV risk of DMPA.
The World Health Organization has called for higher quality data to enable a definitive conclusion about any potential risk for HIV acquisition incurred by DMPA use and a randomized clinical trial of contraceptives has been launched (36,37). For analyses of observational data, it is unlikely that reliance on self-reported sex unprotected by male condoms can be avoided. Our study demonstrates that while there is likely to be some inaccuracy these self-reported data, the degree of inaccuracy is potentially similar among women using DMPA and women using no contraception. These results lend more credibility to the observational studies of DMPA and HIV infection that have already been published, particularly among populations similar to that studied in this analysis (20), and suggest that differential inaccuracy in reporting of condom use may not wholly account for associations observed between DMPA and HIV incidence. A definitive conclusion to the question about DMPA use and HIV acquisition remains one of the most important questions for women with high HIV risk. Novel prospective data collected to address this question would be enhanced by quantifying Yc DNA in vaginal samples to gauge exposure to semen.
Acknowledgments
The authors thank the couples who participated in this study and the teams at the study sites for work on data collection and management. The authors acknowledge the Director, KEMRI for support.
Funding: Eunice Kennedy Shriver National Institute of Child Health & Human Development of 3 the US National Institutes of Health (grants R21HD074439 and R00HD076679); Bill and Melinda Gates Foundation (grant OPP47674). The content is solely the responsibility of the authors and does not necessarily reflect the views of the study funders.
Partners PrEP Study Team
University of Washington Coordinating Center and Central Laboratories: Connie Celum (principal investigator, protocol co-chair), Jared M. Baeten (medical director, protocol co-chair), Deborah Donnell (protocol statistician), Robert W. Coombs, Lisa Frenkel, Craig W. Hendrix, Jairam Lingappa, M. Juliana McElrath.
Study sites and site principal investigators:
Eldoret, Kenya (Moi University, Indiana University): Kenneth Fife, Edwin Were; Kabwohe, Uganda (Kabwohe Clinical Research Center): Elioda Tumwesigye; Jinja, Uganda (Makerere University, University of Washington): Patrick Ndase, Elly Katabira; Kampala, Uganda (Makerere University): Elly Katabira, Allan Ronald; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig Cohen; Mbale, Uganda (The AIDS Support Organization, CDC-Uganda): Jonathan Wangisi, James Campbell, Jordan Tappero; Nairobi, Kenya (University of Nairobi, University of Washington): James Kiarie, Carey Farquhar, Grace John-Stewart; Kenya Medical Research Institute, Nairobi, Kenya: Nelly Rwamba Mugo; Tororo, Uganda (CDC-Uganda, The AIDS Support Organization): James Campbell, Jordan Tappero, Jonathan Wangisi.
Data management was provided by DF/Net Research, Inc. (Seattle, USA) and site laboratory oversight was provided by Contract Laboratory Services (University of the Witwatersrand, Johannesburg, South Africa).
Footnotes
Compliance with Ethical Standards
Research involving human participants and/or animals: All procedures performed were in accordance with the ethical standards of the University of Washington institutional review board, national research ethics committees for each study site, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Disclosure of potential conflicts of interest: All authors declare no conflicts of interest.
REFERENCES
- 1.UNAIDS. The Gap Report. Geneva, Switzerland: 2014. [Google Scholar]
- 2.Polis CB, Curtis KM, Hannaford PC, et al. Update on hormonal contraceptive methods and risk of HIV acquisition in women: a systematic review of epidemiological evidence, 2016. AIDS (London, England) 2016 Aug 5; doi: 10.1097/QAD.0000000000001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polis CB, Westreich D, Balkus JE, Heffron R Participants of the HCHIV Observational Analysis Meeting. Assessing the effect of hormonal contraception on HIV acquisition in observational data: challenges and recommended analytic approaches. AIDS (London, England) 2013 Oct;27(Suppl 1):S35–S43. doi: 10.1097/QAD.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. The Lancet infectious diseases. 2013 Sep;13(9):797–808. doi: 10.1016/S1473-3099(13)70155-5. [DOI] [PubMed] [Google Scholar]
- 5.Polis CB, Phillips SJ, Curtis KM, et al. Hormonal contraceptive methods and risk of HIV acquisition in women: a systematic review of epidemiological evidence. Contraception. 2014 Oct;90(4):360–390. doi: 10.1016/j.contraception.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Kelly CA, Soler-Hampejsek E, Mensch BS, Hewett PC. Social desirability bias in sexual behavior reporting: evidence from an interview mode experiment in rural Malawi. Int Perspect Sex Reprod Health. 2013 Mar;39(1):14–21. doi: 10.1363/3901413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mensch BS, Hewett PC, Gregory R, Helleringer S. Sexual behavior and STI/HIV status among adolescents in rural Malawi: an evaluation of the effect of interview mode on reporting. Studies in family planning. 2008 Dec;39(4):321–334. doi: 10.1111/j.1728-4465.2008.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curran K, Mugo NR, Kurth A, et al. Daily short message service surveys to measure sexual behavior and pre-exposure prophylaxis use among Kenyan men and women. AIDS and behavior. 2013 Nov;17(9):2977–2985. doi: 10.1007/s10461-013-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minnis AM, Steiner MJ, Gallo MF, et al. Biomarker validation of reports of recent sexual activity: results of a randomized controlled study in Zimbabwe. American journal of epidemiology. 2009 Oct 1;170(7):918–924. doi: 10.1093/aje/kwp219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jamshidi R, Penman-Aguilar A, Wiener J, et al. Detection of two biological markers of intercourse: prostate-specific antigen and Y-chromosomal DNA. Contraception. 2013 Dec;88(6):749–757. doi: 10.1016/j.contraception.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penrose KJ, Richardson BA, Besson G, et al. Y chromosome and HIV DNA detection in vaginal swabs as biomarkers of semen and HIV exposure in women. Sexually transmitted diseases. 2014 Nov;41(11):674–679. doi: 10.1097/OLQ.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahamondes L, Diaz J, Marchi NM, Castro S, Villarroel M, Macaluso M. Prostate-specific antigen in vaginal fluid after exposure to known amounts of semen and after condom use: comparison of self-collected and nurse-collected samples. Human reproduction (Oxford, England) 2008 Nov;23(11):2444–2451. doi: 10.1093/humrep/den283. [DOI] [PubMed] [Google Scholar]
- 13.Macaluso M, Lawson L, Akers R, et al. Prostate-specific antigen in vaginal fluid as a biologic marker of condom failure. Contraception. 1999 Mar;59(3):195–201. doi: 10.1016/s0010-7824(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 14.Brotman RM, Melendez JH, Smith TD, Galai N, Zenilman JM. Effect of menses on clearance of Y-chromosome in vaginal fluid: implications for a biomarker of recent sexual activity. Sexually transmitted diseases. 2010 Jan;37(1):1–4. doi: 10.1097/OLQ.0b013e3181b5f15d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zenilman JM, Yuenger J, Galai N, Turner CF, Rogers SM. Polymerase chain reaction detection of Y chromosome sequences in vaginal fluid: preliminary studies of a potential biomarker for sexual behavior. Sexually transmitted diseases. 2005 Feb;32(2):90–94. doi: 10.1097/01.olq.0000149668.08740.91. [DOI] [PubMed] [Google Scholar]
- 16.Heffron R, Were E, Celum C, et al. A prospective study of contraceptive use among African women in HIV-1 serodiscordant partnerships. Sexually transmitted diseases. 2010 Oct;37(10):621–628. doi: 10.1097/OLQ.0b013e3181e1a162. [DOI] [PubMed] [Google Scholar]
- 17.Cates W, Jr, Steiner MJ. Dual protection against unintended pregnancy and sexually transmitted infections: what is the best contraceptive approach? Sexually transmitted diseases. 2002 Mar;29(3):168–174. doi: 10.1097/00007435-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Mujugira A, Baeten JM, Donnell D, et al. Characteristics of HIV-1 serodiscordant couples enrolled in a clinical trial of antiretroviral pre-exposure prophylaxis for HIV-1 prevention. PLoS ONE. 2011;6(10):e25828. doi: 10.1371/journal.pone.0025828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. The New England journal of medicine. 2012 Aug 2;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heffron R, Donnell D, Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. The Lancet infectious diseases. 2012 Jan;12(1):19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler A, Smith S, Stanton D, Hallett D. Injectable Hormonal Contraception and the Spread of HIV: Implications for Global Health (Conference paper #1074). 19th Conference on Retroviruses and Opportunistic Infections; Seattle, WA. 2012. [Google Scholar]
- 22.Cillo AR, Sobolewski MD, Bosch RJ, et al. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proceedings of the National Academy of Sciences of the United States of America. 2014 May 13;111(19):7078–7083. doi: 10.1073/pnas.1402873111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin HL, Jr, Nyange PM, Richardson BA, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. The Journal of infectious diseases. 1998 Oct;178(4):1053–1059. doi: 10.1086/515654. [DOI] [PubMed] [Google Scholar]
- 24.Morrison CS, Chen PL, Kwok C, et al. Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis. PLoS medicine. 2015 Jan;12(1):e1001778. doi: 10.1371/journal.pmed.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray RH. Use of hormonal contraceptives and risk of HIV-1 transmission. The Lancet infectious diseases. 2012 Jul;12(7):507. doi: 10.1016/S1473-3099(12)70111-1. author reply 510-501. [DOI] [PubMed] [Google Scholar]
- 26.Hubacher D. Use of hormonal contraceptives and risk of HIV-1 transmission. The Lancet infectious diseases. 2012 Jul;12(7):508. doi: 10.1016/S1473-3099(12)70114-7. author reply 510-501. [DOI] [PubMed] [Google Scholar]
- 27.Shelton JD. Use of hormonal contraceptives and risk of HIV-1 transmission. The Lancet infectious diseases. 2012 Jul;12(7):507–508. doi: 10.1016/S1473-3099(12)70112-3. author reply 510-501. [DOI] [PubMed] [Google Scholar]
- 28.McCoy SI, Ralph LJ, Padian NS, Minnis AM. Are hormonal contraceptive users more likely to misreport unprotected sex? Evidence from a biomarker validation study in Zimbabwe. AIDS and behavior. 2014 Dec;18(12):2259–2264. doi: 10.1007/s10461-014-0741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Elst EM, Okuku HS, Nakamya P, et al. Is audio computer-assisted self-interview (ACASI) useful in risk behaviour assessment of female and male sex workers, Mombasa, Kenya? PLoS ONE. 2009;4(5):e5340. doi: 10.1371/journal.pone.0005340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorbach PM, Mensch BS, Husnik M, et al. Effect of computer-assisted interviewing on self-reported sexual behavior data in a microbicide clinical trial. AIDS and behavior. 2013 Feb;17(2):790–800. doi: 10.1007/s10461-012-0302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbott SA, Friedland BA, Sarna A, et al. An evaluation of methods to improve the reporting of adherence in a placebo gel trial in Andhra Pradesh, India. AIDS and behavior. 2013 Jul;17(6):2222–2236. doi: 10.1007/s10461-012-0402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oechsle CS, Haddad S, Sgueglia JB, Grgicak CM. Screening biological stains with qPCR versus lateral flow immunochromatographic test strips: a quantitative comparison using analytical figures of merit. Journal of forensic sciences. 2014 Jan;59(1):199–207. doi: 10.1111/1556-4029.12284. [DOI] [PubMed] [Google Scholar]
- 33.Heffron R, Mugo N, Were E, et al. Preexposure prophylaxis is efficacious for HIV-1 prevention among women using depot medroxyprogesterone acetate for contraception. AIDS (London, England) 2014 Nov 28;28(18):2771–2776. doi: 10.1097/QAD.0000000000000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lingappa JR, Petrovski S, Kahle E, et al. Genomewide association study for determinants of HIV-1 acquisition and viral set point in HIV-1 serodiscordant couples with quantified virus exposure. PLoS ONE. 2011;6(12):e28632. doi: 10.1371/journal.pone.0028632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. The New England journal of medicine. 2010 Feb 4;362(5):427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. Hormonal contraception and HIV: Technical statement. Vol. 2012. Geneva, Switzerland: World Health Organization; 2012. Department of Reproductive Health and Research. [PubMed] [Google Scholar]
- 37.ECHO Consortium. The Evidence for Contraceptive Options and HIV Outcomes (ECHO) Study. [Accessed 8/30/2016];2016 http://echo-consortium.com/ [Google Scholar]