Key Clinical Message
In selected cases, homozygosity mapping followed by direct sequencing of one or a few carefully selected candidate genes in a prenatal setting can be beneficial to obtain diagnosis in consanguineous families.
Keywords: CHST3, homozygosity mapping, prenatal, single nucleotide polymorphism array, skeletal dysplasia, spondyloephiphyseal dysplasia
Case Report
In 1987, Lander et al. published the first paper on homozygosity mapping for human genes that cause recessive traits. He describes the usefulness of the method to map recessive diseases for which it is impractical or impossible to collect adequate numbers of families with multiple affected offspring 1. Single nucleotide polymorphism (SNP) microarray has been documented to have clinical utility in the detection of regions of homozygosity 2. In recent years, homozygosity mapping has demonstrated its effectiveness in the detection of disease loci in affected children from consanguineous couples in the neonatal setting, as well as after pregnancy termination for prenatally diagnosed malformations 3, 4, 5, 6. To our knowledge, the use of homozygosity mapping has only been reported once in the prenatal setting (on DNA isolated from amniotic fluid or a chorion villi biopsy) 7, although prenatal homozygosity mapping shows additional value because it can help parents to reach an underpinned decision on whether or not to terminate the pregnancy.
The case presented here involved a G3P2 woman, originating from Afghanistan, at 17 weeks gestational age. Her first two pregnancies were uncomplicated and resulted in two healthy boys. The couple was consanguineous (first cousins). In the current pregnancy, the gynecologist referred the couple for a suspicion of fetal limb malformation on routine ultrasound. Advanced ultrasound showed an abnormal morphology of the fetal lower limbs, short femora and humeri, abnormal position of the knee joints, and bilateral pes equinovarus, suggestive of arthrogryposis (Fig. 1). Family history showed that the brother of the proband's mother had had clinical arthrogryposis and had died during pregnancy (Fig. 2). Based upon the combination of ultrasonographic anomalies, differential diagnosis included: autosomal dominant Larsen syndrome, caused by mutations in FLNB 8, autosomal recessive Larsen syndrome, caused by mutations in B3GAT3 9, diastrophic dysplasia, caused by homozygous or compound heterozygous mutations in SLC26A2 10, Desbuquois dysplasia, caused by homozygous or compound heterozygous mutations in CANT1 11, chondrodysplasia with joint dislocations, caused by homozygous mutations in IMPAD1 12, and spondyloepiphyseal dysplasia with congenital joint dislocations, caused by homozygous or compound heterozygous mutations in CHST3 13.
Figure 1.
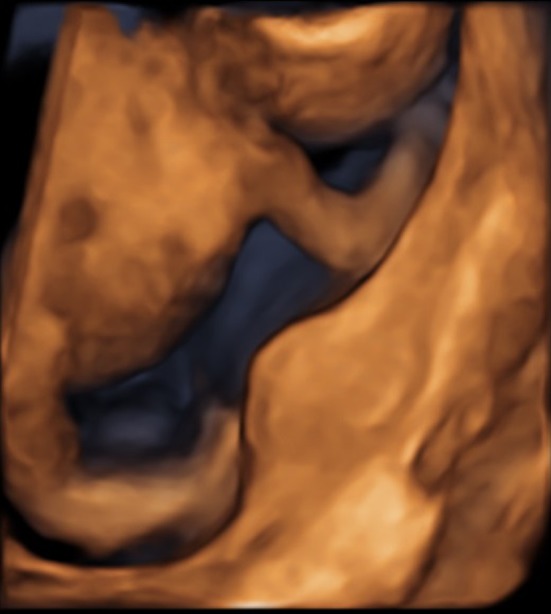
Advanced prenatal ultrasound showed an abnormal morphology of the fetal lower limbs. This figure shows the abnormal position of the knee joints and bilateral pes equinovarus on 3D ultrasound examination.
Figure 2.
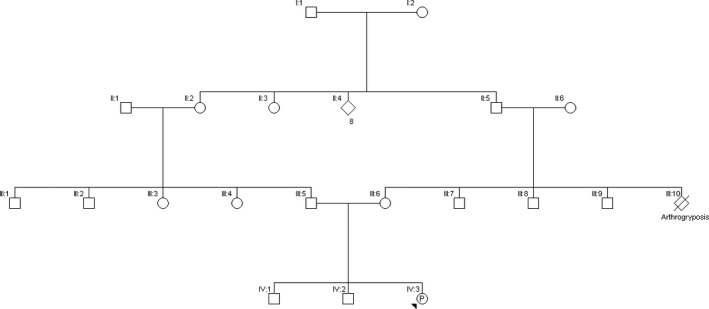
Family history. The unborn child is indicated as the proband (P‐IV:3). Note the consanguinity of the parents (III:5 and III:6) (first cousins). Family history shows that the brother of the proband's mother (III:10) had had clinical arthrogryposis and had died during pregnancy.
Given the anomalies found on prenatal ultrasound, amniocentesis was performed. Rapid aneuploidy testing (QF‐PCR) did not reveal any aneuploidies. SNP array analysis to detect deletions and duplications was performed using a HumanCyto‐SNP‐12 v2.1 BeadChip on an iScan system following standard protocols as provided by the manufacturer (Illumina, San Diego, CA, USA). Copy number variant (CNV) analysis was performed with CNV‐WebStore 14. SNP array analysis showed no pathogenic CNVs.
The pregnancy was terminated 2 weeks after the ultrasonographic findings because of the severity of the phenotype and after genetic counseling regarding future prospects for the unborn child. Postpartum clinical inspection of the fetus confirmed the joint and feet anomalies (Fig. 3). Radiographic examination confirmed the abnormal position of the left and right knee joints (Fig. 4). The ribs, upper limbs, and skull were normal. Autopsy report described a male fetus with biometry in the lower normal range for 17 weeks gestational age. Elbows showed a fixed flexion and knees had a fixed forward flexion as well. Macroscopically, there were no other remarkable skeletal anomalies. Facial features were normal. All organs in neck, thorax, abdomen, and pelvis were histologically and anatomically normal.
Figure 3.
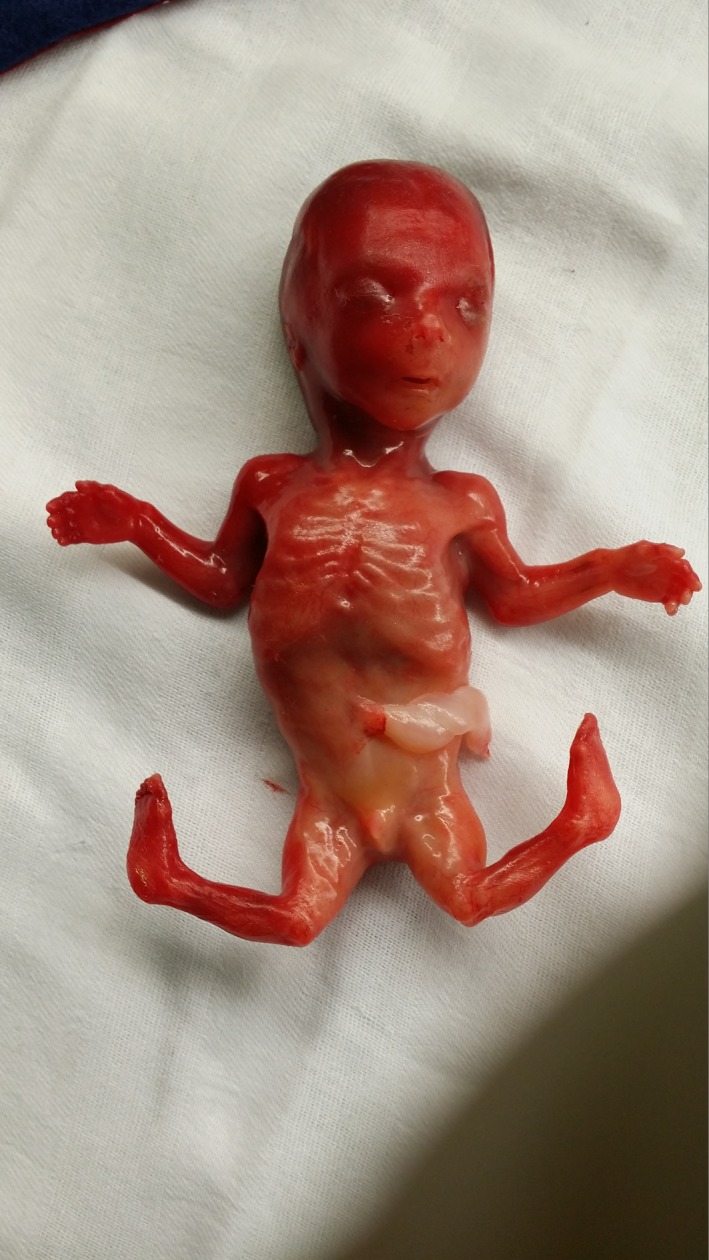
Postnatal clinical inspection and autopsy confirmed the joint and feet anomalies.
Figure 4.
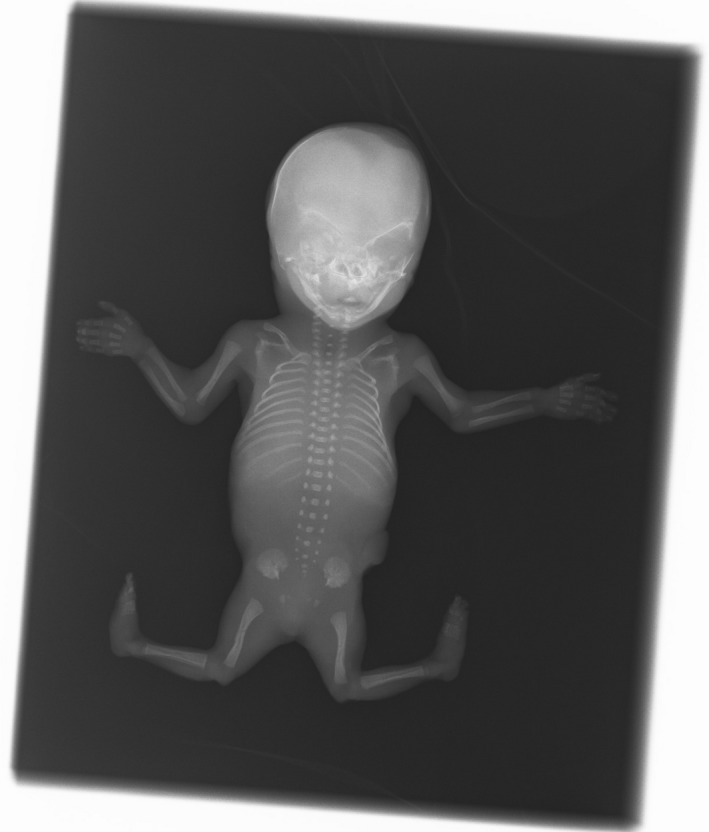
Radiographic examination confirmed the abnormal position of the left and right knee joints.
As the parents are first cousins, autozygosity, the presence of two alleles at a locus originating from a common ancestor as a result of inbreeding (identical by descent), might explain the phenotype; the fetus could have inherited the same mutant allele responsible for an autosomal recessive trait from both parents. To detect regions of autozygosity, possibly harboring a mutation, homozygosity mapping was performed on the sample obtained by amniocentesis using CNV‐WebStore for data analysis 14. Twenty seven homozygous regions larger than 1 Mb were identified, accounting for 8.43% of the genome (Table 1). Among the 2507 genes present in these regions, we carefully inspected all 362 MORBID genes. CHST3, located on chromosome 10q22.1, was considered to be the strongest candidate gene as homozygous and compound heterozygous mutations in this gene have been identified in spondyloepiphyseal dysplasia with congenital joint dislocations (OMIM #143095). This disorder is characterized by short stature (both prenatally and postnatally), a broad forehead, long philtrum, small ears, hypertelorism, high‐arched palate, variable cardiac anomalies, broad chest, joint dislocations in knee, hip and shoulder, limb malformations (e.g., fixed elbow flexion and knee dislocations), brachydactyly, camptodactyly, and feet malformations. Affected individuals have a normal intelligence, but a delayed gross motor development. Our case presented with several characteristics representative for this disorder. DNA of the fetus and his parents was analyzed via PCR amplification and direct sequencing of CHST3. A hitherto unknown variant, c.491C>T (p.P164L), in exon 3 of the CHST3 gene was detected in a homozygous state in the fetus. The proband's mother and father carry the mutation in a heterozygous state. This variant was classified as a likely pathogenic variant based on in silico predictions by Alamut (Alamut Visual version 2.7 [Interactive Biosoftware, Rouen, France]). This variant lies in the so‐called P‐loop containing nucleoside triphosphate hydrolase fold (amino acids 132–168). The Exome Aggregation Consortium (ExAc) 15 reports the heterozygous change of the same amino acid to Arg (Pro164Arg; rs771866012) in one of their 60700 individuals. The variant is classified as probably damaging by several in silico prediction programs, but no further studies have been performed. Mutations in three other amino acids in this domain have been described 16, 17. Functional analysis of one of these, T141M, shows it to have a dramatically decreased sulfotransferase activity 16.
Table 1.
Regions of homozygosity in the fetus
| Chromosome | Start | End | Size (bp) | Nr. Probes | % Hom | % Het | Nr. Genes |
|---|---|---|---|---|---|---|---|
| 1 | 213827795 | 216840371 | 3012577 | 407 | 0.995 | 0.002 | 9 |
| 2 | 86023398 | 87052934 | 1029537 | 163 | 0.994 | 0.006 | 17 |
| 2 | 206714121 | 221239894 | 14525774 | 1017 | 0.999 | 0.001 | 136 |
| 3 | 96162805 | 97346618 | 1183814 | 151 | 1.000 | 0.000 | 1 |
| 4 | 31661814 | 40255501 | 8593688 | 553 | 0.996 | 0.004 | 32 |
| 4 | 75771799 | 157116213 | 81344415 | 5134 | 1.000 | 0.000 | 362 |
| 5 | 171324289 | 175220428 | 3896140 | 603 | 0.998 | 0.002 | 27 |
| 8 | 47921405 | 49039681 | 1118277 | 141 | 0.986 | 0.014 | 6 |
| 8 | 140733440 | 142600093 | 1866654 | 290 | 1.000 | 0.000 | 10 |
| 9 | 101297144 | 102540372 | 1243229 | 199 | 0.995 | 0.005 | 8 |
| 10 | 3239252 | 19471726 | 16232475 | 2045 | 1.000 | 0.000 | 99 |
| 10 | 37608337 | 38685231 | 1076895 | 137 | 1.000 | 0.000 | 10 |
| 10 | 71056150 | 95747476 | 24691327 | 2845 | 1.000 | 0.000 | 201 |
| 10 | 121922699 | 135430043 | 13507345 | 1950 | 0.999 | 0.001 | 105 |
| 11 | 2348778 | 11644920 | 9296143 | 1277 | 0.998 | 0.001 | 190 |
| 11 | 46196053 | 47246397 | 1050345 | 151 | 0.987 | 0.013 | 22 |
| 12 | 87738065 | 88801020 | 1062956 | 159 | 1.000 | 0.000 | 5 |
| 12 | 111768973 | 113025901 | 1256929 | 170 | 0.982 | 0.018 | 17 |
| 12 | 120822453 | 121874019 | 1051567 | 160 | 0.981 | 0.019 | 25 |
| 16 | 30172627 | 31383304 | 1210678 | 241 | 1.000 | 0.000 | 67 |
| 16 | 82678897 | 87111021 | 4432125 | 697 | 0.997 | 0.003 | 43 |
| 17 | 7314216 | 18747176 | 11432961 | 1698 | 0.999 | 0.000 | 184 |
| 17 | 37265378 | 75271787 | 38006410 | 3837 | 0.999 | 0.001 | 650 |
| 17 | 77291311 | 81047565 | 3756255 | 555 | 0.998 | 0.002 | 94 |
| 18 | 18540853 | 19601717 | 1060865 | 179 | 0.989 | 0.011 | 9 |
| 19 | 9247389 | 13355633 | 4108245 | 565 | 0.995 | 0.004 | 149 |
| 22 | 40731134 | 42196467 | 1465334 | 208 | 0.995 | 0.005 | 29 |
Indicated in the table are number of the chromosome; start and stop position of the region of homozygosity; size of the region in base pairs (bp); the number of probes in this region; percentage of homozygosity (% Hom) and heterozygosity (% Het) as well as the number of genes involved, accounting in total for 8.43% of the genome. Genome Build: GRCh37 (hg19).
The proband's parents returned for further discussion 8 weeks after delivery. They received extensive genetic counseling and were informed about the possibility of preimplantation genetic diagnosis (PGD) or invasive testing in future pregnancies. The importance of further family testing was stressed. An informed consent for publication of the case was obtained after carefully informing the parents about the clinical significance of the case.
Since 2013, genomewide array – SNP array or array CGH (comparative genomic hybridization) – has replaced karyotyping in the prenatal setting in Belgium. At the time of introduction of this novel technology in the prenatal field, all Belgian genetic centers agreed on a resolution of 400 kb to maximize the detection of pathogenic CNVs while minimizing the detection of variants of unknown significance (VOUS) and on a list of susceptibility loci to report 18. The increase in resolution from 5 to 10 Mb to 400 kb has led to an average increase of 6.0% of diagnoses in fetuses with ultrasound anomalies 19, but smaller deletions/duplications and single point mutations stay well below the radar. The arrival of high‐density SNP array however 20, 21, as utilized in our center, allows the simultaneous determination of regions of homozygosity, which can lead to the detection of autosomal recessive mutations in offspring of consanguineous partners.
We could only identify one publication on the use of homozygosity mapping in the prenatal setting 7, making this report the second in its kind. The advantage of prenatal homozygosity mapping lies mainly in the short‐term interval between recognition of the problem (ultrasound anomalies) and the definite diagnosis (a recessive mutation). In several countries, termination of pregnancy can only be legally performed until 24 weeks gestation, as the fetus becomes viable around that age. With the work‐flow described here, complete results can be obtained within approximately 6 weeks: if amniocentesis is performed at 15 weeks gestational age, SNP array and homozygosity mapping results are known at 17 weeks gestational age and targeted analysis results at 21 weeks gestational age, well before 24 weeks. The result will guide gynecologists in counseling the parents correctly and can aid parents in reaching a well‐informed decision on whether or not to terminate the pregnancy. Moreover, as the parents can be screened immediately for carrier state of the recessive trait, they know whether or not they are at risk of having an affected fetus in a future pregnancy and can be counseled for the different options to avoid this, including PGD and invasive prenatal testing.
In this particular case, with the parents being first cousins, on average 6.25% of the genome is expected to be autozygous. Consequently, hundreds to thousands of putative candidate genes could be identified and prioritization of the genes, based on information regarding their function, is crucial. If the phenotype of the fetus can be clearly linked to one or a few of the genes, Sanger sequencing is the preferred method of choice. However, this approach precludes the identification of less obvious candidate genes. Therefore, in most cases successful autozygosity mapping relies on factors that further narrow the number of regions of interest like the investigation of multiple affected siblings. When no other affected family members are available, a combination of autozygosity mapping and next‐generation sequencing (NGS) is recommended, as it allows either massive parallel sequencing of all autozygous regions or filtering of variants found by whole‐exome or whole‐genome sequencing based on autozygosity mapping 22. One can argue that autozygosity mapping can be performed directly on the NGS data, but cost and technical limitations of NGS (e.g., low read depth in some positions) preclude this for the time being. Moreover, if, based on the examination of the autozygome, a strong candidate gene is identified and sequenced, as was the case here, this is much more cost‐effective than performing whole exome/whole‐genome sequencing.
We conclude that in selected cases, homozygosity mapping followed by direct sequencing of one or a few carefully selected candidate genes in a prenatal setting can be beneficial to obtain diagnosis in consanguineous families.
Ethics
This manuscript is a case report and therefore did not require approval from our institution's ethics committee.
Conflict of Interest
None declared.
Authorship
JM: main author of the paper, wrote the paper in close cooperation with KJ and gathered all information on the case and all reviewer's comments. BB: is the treating geneticist. She provided clinical insight in the case and reviewed the paper. YJ: is a gynecologist with expertise in prenatal diagnosis, provided clinical insight in the case, and reviewed the paper. KJ: is a main author of the paper and is the head of laboratory for prenatal genetics of the University of Antwerp.
Acknowledgments
We would like to thank Philip Loquet (University Hospital Antwerp and GZA Sint Augustinus Hospital Wilrijk), a gynecologist with expertise in prenatal ultrasound, for providing us with the ultrasound images.
References
- 1. Lander, E. S. , and Botstein D.. 1987. Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science 236:1567–1570. [DOI] [PubMed] [Google Scholar]
- 2. Sund, K. L. , Zimmerman S. L., Thomas C., Mitchell A. L., Prada C. E., Grote L., et al. 2013. Regions of homozygosity identified by SNP microarray analysis aid in the diagnosis of autosomal recessive disease and incidentally detect parental blood relationships. Genet. Med. 15:70–78. [DOI] [PubMed] [Google Scholar]
- 3. Capo‐Chichi, J. M. , Boissel S., Brustein E., Pickles S., Fallet‐Bianco C., Nassif C., et al. 2015. Disruption of CLPB is associated with congenital microcephaly, severe encephalopathy and 3‐methylglutaconic aciduria. J. Med. Genet. 52:303–311. [DOI] [PubMed] [Google Scholar]
- 4. Xin, B. , Puffenberger E., Tumbush J., Bockoven J. R. and Wang H.. 2007. Homozygosity for a novel splice site mutation in the cardiac myosin‐binding protein C gene causes severe neonatal hypertrophic cardiomyopathy. Am. J. Med. Genet. A 143A:2662–2667. [DOI] [PubMed] [Google Scholar]
- 5. Marshall, C. R. , Farrell S. A., Cushing D., Paton T., Stockley T. L., Stavropoulos D. J., et al. 2015. Whole‐exome analysis of foetal autopsy tissue reveals a frameshift mutation in OBSL1, consistent with a diagnosis fo 3‐M Syndrome. BMC Genom. 15(Suppl. 1):S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fiskerstrand, T. , Houge G., Sund S., Scheie D., Leh S., Boman H., et al. 2010. Identification of a gene for renal‐hepatic‐pancreatic dysplasia by microarray‐based homozygosity mapping. J. Mol. Diagn. 12:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abumansour, I. S. , Al Sulmi E., Chodirker B. N. and Hunt J. C.. 2015. Prenatal diagnosis of walker‐warburg syndrome using single nucleotide polymorphism array: a clinical experience from three related palestinian families with congenital hydrocephalus. AJP Rep. 5:e116–e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krakow, D. , Robertson S., King L., Morgan T., Sebald E. T., Bertolotto C., et al. 2004. Mutations in the gene encoding filamin B disrupt vertebral segmentation, joint formation and skeletogenesis. Nat. Genet. 36:405–410. [DOI] [PubMed] [Google Scholar]
- 9. Baasanjav, S. , Al‐Gazali L., Hashiguchi T., Mizumoto S., Fischer B., Horn D., et al. 2011. Faulty initiation of proteoglycan synthesis causes cardiac and joint defects. Am. J. Hum. Genet. 89:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hästbacka, J. , de la Chapelle A., Mahtani M., Clines G., Reeve‐Daly M.P., Daly M., et al. 1994. The diastrophic dysplasia gene encodes a novel sulfate transporter: positional cloning by fine‐structure linkage disequilibrium mapping. Cell 78:1073–1087. [DOI] [PubMed] [Google Scholar]
- 11. Faden, M. , Al‐Zahrani F., Arafah D., Alkuraya F. S.. 2010. Mutation of CANT1 causes Desbuquois dysplasia. Am. J. Med. Genet. 152A:1157–1160. [DOI] [PubMed] [Google Scholar]
- 12. Vissers, L. , Lausch E., Unger S., Campos‐Xavier A. B., Gilissen C., Rossi A., et al. 2011. Chondrodysplasia and abnormal joint development associated with mutations in IMPAD1, encoding the Golgi‐resident nucleotide phosphatase, gPAPP. Am. J. Hum. Genet. 88:608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rajab, A. , Kunze J., and Mundlos S.. 2014. Spondyloepiphyseal dysplasia Omani type: a new recessive type of SED with progressive spinal involvement. Am. J. Med. Genet. 126A:413–419. [DOI] [PubMed] [Google Scholar]
- 14. Vandeweyer, G. , Reyniers E., Wuyts W., Rooms L. and Kooy R. F.. 2011. CNV‐WebStore: online CNV analysis, storage and interpretation. BMC Bioinformatics 12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lek, M. , Karczewski K. J., Minikel E. V., Samocha K. E., Banks E., Fennell T., et al. 2016. The Exome Aggregation Consortium: Analysis of protein‐coding variation in 60706 humans. Nature. 536(7616):285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tuysuz, B. , Mizumoto S., Sugahara K., Celebi A., Mundlos S. and Turkmen S.. 2009. Omani‐type spondyloepiphyseal dysplasia with cardiac involvement caused by a missense mutation in CHST3. Clin. Genet. 75:375–383. [DOI] [PubMed] [Google Scholar]
- 17. Unger, S. , Lausch E., Rossi A., Mégarbané A., Sillence D., Alcausin M., et al. 2010. Phenotypic features of carbohydrate sulfotransferase 3 (CHST3) deficiency in 24 patients: congenital dislocations and vertebral changes as principal diagnostic features. Am. J. Med. Genet. A 152A:2543–2549. [DOI] [PubMed] [Google Scholar]
- 18. Vanakker, O. , Vilain C.., Janssens K., Van der Aa N., Smits G., Bandelier C., et al 2014. Implementation of genomic arrays in prenatal diagnosis: the Belgian approach to meet the challenges. Eur. J. Med. Genet. 57:151–156. [DOI] [PubMed] [Google Scholar]
- 19. Wapner, R. J. , Martin C. L., Levy B., Ballif B. C., Eng C. M., Zachary J. M., et al. 2012. Chromosomal microarray versus karyotyping for prenatal diagnosis. N. Engl. J. Med. 367:2175–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liao, C. , Fu F., Li R., Xie G. E., Zhang Y. L., Li J., et al. 2014. Implementation of high‐resolution SNP arrays in the investigation of fetuses with ultrasound malformations: 5 years of clinical experience. Clin. Genet. 86:264–269. [DOI] [PubMed] [Google Scholar]
- 21. Srebniak, M. I. , Boter M., Oudesluijs G. O., Cohen‐Overbeek T., Govaerts L. C., Diderich K. E., et al. 2012. Genomic SNP array as a gold standard for prenatal diagnosis of foetal ultrasound abnormalities. Mol. Cytogenet. 5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alkuraya, F. S. 2013. The application of next‐generation sequencing in the autozygosity mapping of human recessive diseases. Hum. Genet. 132:1197–1211. [DOI] [PubMed] [Google Scholar]


