Key Clinical Message
The mechanism of abscopal effect is becoming clear by the progress of cancer immunology. A 54‐year‐old male with recurrent gastric cancer presented an abscopal effect after radiotherapy with concurrent adoptive T‐cell immunotherapy (immunoradiotherapy). Immunoradiotherapy has potential to induce abscopal effect by strengthening systemic antitumor immunity even in recurrent cancer.
Keywords: Abscopal effect, adoptive T‐cell immunotherapy, gastric cancer, peritoneal dissemination, radiotherapy
Introduction
Radiotherapy is one of the treatments used for most types of cancer, regardless of the clinical stage. It is generally accepted that radiotherapy induces cancer cell death via apoptosis and/or necrosis by damaging (either directly or indirectly) the DNA. If irradiation could induce cell death just by damaging the DNA, the effect would be seen only within the irradiated field. However, the cytoreductive effects on the distant metastatic lesions outside the irradiated field, known as “abscopal effect”, have been observed in patients with melanoma, lung cancer, and renal cancer, among others 1. This phenomenon might be caused by the antitumor immunity induced by radiotherapy 2. The emerging evidence suggests that the immunogenic cell death caused by irradiation might be another important effect of radiotherapy, in addition to the DNA damage 3. Radiation‐induced cell death elicits the damage signals, such as high‐mobility group box 1 (HMGB1), which trigger the activation of antigen presentation cells (APCs). Subsequently, APCs present the tumor‐derived antigens to cytotoxic T lymphocytes (CTLs), which then kill the target cells. Radiotherapy also increases the expression of HLA class I molecules, which also can activate CTLs by presenting tumor‐derived antigen, on the surface of tumor cells 4. Enhanced antigen presentation and activated CTLs have potential for an immune‐mediated abscopal effect.
The abscopal effect has been reported several times over the years, reviving the interest in the progress of cancer immunology 5. Accumulating evidence shows that the combination of radio‐ and immunotherapy (e.g., using immune checkpoint inhibitors) induces the abscopal effect in several types of cancers 2, 6, 7, 8. These reports suggest that the combined radio‐ and immunotherapy can be an effective strategy for advanced or recurrent cancers. Here, we report a case of postoperative, locally and peritoneally disseminated, recurrent gastric cancer treated using a concomitant radiotherapy and adoptive T‐cell immunotherapy. The treatment had an abscopal effect and achieved a complete remission of the irradiated tumor.
Case Report
A 54‐year‐old Japanese male was diagnosed with gastric cancer and underwent distal gastrectomy with Billroth‐I. The histology and pathological stage of the tumor were poorly differentiated adenocarcinoma and pT4aN3aM0 (stage IIIC) with no residual tumor, respectively. The adjuvant chemotherapy TS‐1 was prescribed. However, 10 months after the surgery, a local recurrence (invasion of the pancreas and the left kidney) and peritoneal dissemination with cancerous peritonitis were diagnosed using computed tomography (CT) and endoscopic biopsy. Then, another chemotherapy treatment (paclitaxel) was prescribed. The chemotherapy had to be stopped after two courses because of a hematological adverse effect (neutrophil count decreased to <1000/mm3). The patient was referred to the outpatient clinic to undergo the adoptive T‐cell immunotherapy and dendritic cell (DC) therapy; details of the therapies are described elsewhere 9. Briefly, 3–10 × 109 adoptive T cells and 1–10 × 106 DCs were infused intravenously and intratumorally, respectively, at intervals of approximately 2 weeks. However, after four courses of the therapies, a progression of local recurrence and peritoneal dissemination was observed.
Subsequently, the patient received 48 Gy in 24 fractions of radiotherapy using the intensity‐modulated radiotherapy (IMRT) method (Fig. 1) with concurrent adoptive T‐cell immunotherapy (immunoradiotherapy). Figure 2 shows changes in local recurrence and peritoneal dissemination before, during, and after immunoradiotherapy. During immunoradiotherapy, shrinkage of the locally reoccurred tumor was observed, although the peritoneal dissemination progressed (Fig. 2A and B). Two months after the initiation of radiotherapy, the performance status was improved from 4 to 2, and CT images showed a complete remission of the locally recurrent tumor. Furthermore, the shrinkage of metastatic peritoneal tumor (i.e., the abscopal effect) was observed (Fig. 2C). However, the abscopal effect lesions could not be measured because they consisted of small (<5 mm) multiple nodular peritoneal dissemination: lesions <10 mm are categorized into “nonmeasurable” by the RECIST (version 1.1). After completion of the immunoradiotherapy, the adoptive T‐cell immunotherapy was continued (11 courses). However, 5 months after the immunoradiotherapy, the patient died because of new multiple distant metastases in the peritoneum. He did not experience significant discomfort until just before his death. Figure 3 shows the clinical time course and the corresponding changes in the serum tumor marker (CEA, CA19‐9) after postoperative recurrence.
Figure 1.
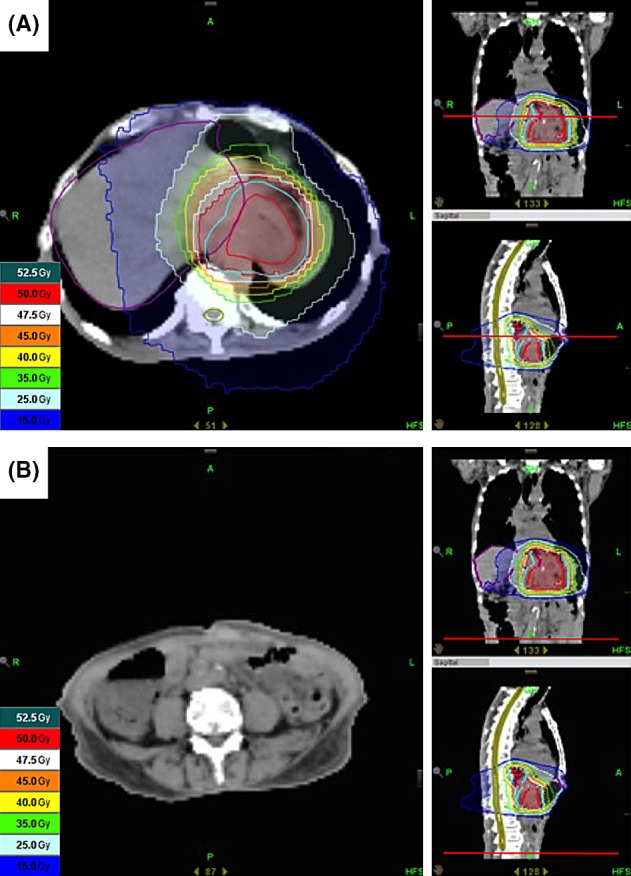
Dose distribution of radiotherapy. (A) Radiotherapy (48 Gy in 24 fractions) using the IMRT method was prescribed for local recurrent tumor, which includes the pancreas and the left kidney. (B) The metastatic peritoneal tumor in which the abscopal effect was observed was not included in the irradiated field.
Figure 2.
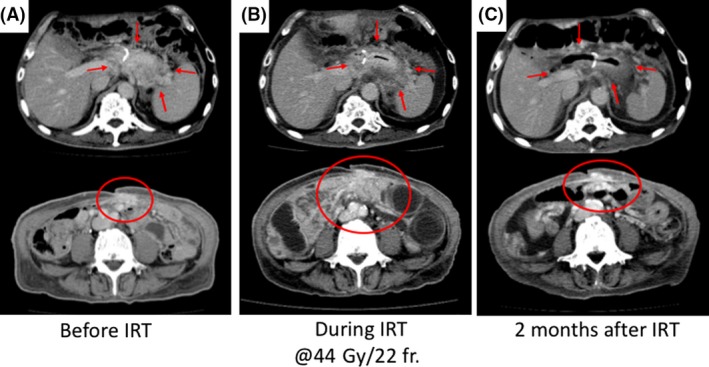
CT images of local recurrence and peritoneal dissemination before, during, and after immunoradiotherapy. (A) CT images before immunoradiotherapy: 2 months after the first paclitaxel treatment followed by immunotherapy. Progression of the local recurrence lesion and peritoneal dissemination was confirmed. (B) CT image was acquired during immunoradiotherapy at 44 Gy in 22 fractions point. Shrinkage of the locally reoccurred tumor (red arrow) and progression in peritoneal dissemination (red circle) were observed. (C) CT image was taken 2 months after the start of the therapy. A complete remission of the locally recurrent tumor and an abscopal effect were observed. IRT, immunoradiotherapy.
Figure 3.
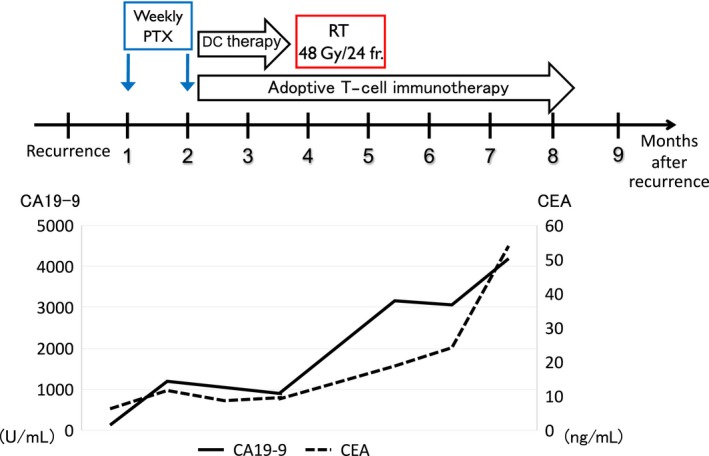
The clinical time course and corresponding changes in the serum tumor marker after postoperative recurrence. CA19‐9 temporally decreased in response to complete remission of the locally recurrent tumor and abscopal effect after immunoradiotherapy, whereas CEA steadily increased. However, both of tumor markers were eventually uncontrolled. PTX, paclitaxel; DC, dendritic cell; RT, radiotherapy; IRT, immunoradiotherapy.
Discussion
In this case, we observed an abscopal effect and a complete local response of the recurrent gastric cancer to a multimodal therapy. The treatment consisted of 48 Gy in 24 fractions of radiotherapy and concurrent T‐cell immunotherapy. To date, the optimal irradiation dose for recurrent gastric cancer is unknown. Previous studies have demonstrated that neoadjuvant chemoradiotherapy (using 45 Gy in 25 fractions) showed a pathological complete response rate of 16–30% 10. From these data, 48 Gy in 24 fractions seemed to be an insufficient dose to achieve a complete response on its own. It suggested that the combination therapy not only caused the abscopal effect but also affected the locally irradiated tumor. To confirm the mechanism and obtain a detailed validation of the immune response, the immune status and tumor environment should be examined by analyzing the tumor‐infiltrating T lymphocytes and the expression of HLA class I molecules. However, this was ethically unfeasible because of the worsening general health of the patient.
Clinical reports of abscopal effect are scarce. Reynders et al. have reviewed five recent clinical case reports showing an abscopal effect after radiotherapy combined with immunotherapy 11. The studies have used various radiation doses (24 Gy in 3 fractions, 28.5 Gy in 3 fractions, 30 Gy in 5 fractions, 54 Gy in 3 fractions, and 58 Gy in 29 fractions), examined varied histology (melanoma and lung adenocarcinoma), and employed different types of immunotherapy (ipilimumab and BCG vaccine). Hence, the optimal dose and number of fractions to achieve the abscopal effect has remained unclear. Interestingly, the abscopal effect was observed after the shrinkage of the locally reoccurred tumor suggesting that the activation of systemic immune‐stimulation through elicited damage signals followed by radiation‐induced cell death plays a critical role in the abscopal effect.
Accumulating evidence suggests that a dysfunction in the host systemic immunity and/or low immunogenic characteristics of the tumor may favor the progression of cancer 12. Therefore, the therapies restoring or strengthening the immune functions might constitute an effective treatment. Kim et al. have reported that T‐cell‐based immunotherapy destroys the human gastric cancer cells (MKN74) in vitro and inhibits the growth of these cells in the nude mouse 13. Recent studies have shown that radiotherapy can act as an immunostimulant, causing the immunogenic cell death and releasing the danger signals such as HMGB1 14, 15. We have also shown that chemoradiotherapy releases HMGB1 and induces tumor antigen‐specific T‐cell response in esophageal cancer patients 16, and that hyperthermo‐chemoradiotherapy enhances the HLA class I expression in rectal cancer patients 16, 17. These results suggest that radiotherapy could be profitably combined with immunotherapy.
With the recent advances in diagnostic and treatment modalities, the prognosis of gastric cancer has become more favorable, particularly in the early stages. However, the postoperative recurrence is one of the most crucial factors associated with the cancer‐related deaths of gastric cancer patients 18. Spolverato et al. have reported such recurrence (local, peritoneal, and hematogenous) in 29.9% of patients, including those who had undergone radiotherapy and/or chemotherapy as adjuvant treatment 19. They observed a rate of 24.2% and 19.3% for local and peritoneal recurrence, respectively, with the median survival period of only 9.1 and 2.7 months after local and peritoneal recurrence, respectively. Recently, Yang et al. have reported that the combined intravenous and intraperitoneal chemotherapy effectively prevents peritoneal recurrence. A meta‐analysis has shown that this therapy prolongs the survival period in comparison with intravenous chemotherapy alone 20. However, there is still no standard treatment for postoperative recurrence.
Recently, immunotherapy has been attracting increasing attention. Nivolumab, a PD‐1 immune checkpoint inhibitor antibody, has shown a significant superiority over chemotherapy in phase III trials for melanoma and squamous cell carcinoma of the lung 21, 22. However, despite prolonged survival, the disease progression rate has remained around 30%. These results suggest that immunotherapy should be combined with an effective local treatment, such as radiotherapy. Hence, immunoradiotherapy is a promising treatment for postoperative recurrent gastric cancer, although further investigation is warranted.
Informed Consent
Written informed consent was obtained from the patient, agreeing to the publication of this case report and any accompanying images.
Conflict of Interest
The authors declare that they have no competing interests.
Authorship
HS, YS, and YT: summarized case information and drafted the manuscript. YS, YY, SN, TS, and TN: coordinated the clinics, prescribed and carried out the immunotherapy, and participated in the follow‐up of the patient. KM: carried out the contouring of the patient and simulated the radiotherapy dose. KM, AO, and TS: participated in patient care in the hospital. All authors read and approved the final manuscript.
References
- 1. Siva, S. , MacManus M. P., Martin R. F., and Martin O. A.. 2015. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett. 356:82–90. [DOI] [PubMed] [Google Scholar]
- 2. Postow, M. A. , Callahan M. K., Barker C. A., Yamada Y., Yuan J., Kitano S., et al. 2012. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 366:925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee, Y. , Auh S. L., Wang Y., Burnette B., Wang Y., Meng Y., et al. 2009. Therapeutic effects of ablative radiation on local tumor require CD8 + T cells: changing strategies for cancer treatment. Blood 114:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reits, E. A. , Hodge J. W., Herberts C. A., Groothuis T. A., Chakraborty M., Wansley E. K., et al. 2006. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 203:1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teng, F. , Kong L., Meng X., Yang J., and Yu J.. 2015. Radiotherapy combined with immune checkpoint blockade immunotherapy: achievements and challenges. Cancer Lett. 365:23–29. [DOI] [PubMed] [Google Scholar]
- 6. Grimaldi, A. M. , Simeone E., Giannarelli D., Muto P., Falivene S., Borzillo V., et al. 2014. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology 3:e28780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Golden, E. B. , Demaria S., Schiff P. B., Chachoua A., and Formenti S. C.. 2013. An abscopal response to radiation and ipilimumab in a patient with metastatic non‐small cell lung cancer. Cancer Immunol. Res. 1:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Golden, E. B. , Chhabra A., Chachoua A., Adams S., Donach M., Fenton‐Kerimian M., et al. 2015. Local radiotherapy and granulocyte‐macrophage colony‐stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof‐of‐principle trial. Lancet Oncol. 16:795–803. [DOI] [PubMed] [Google Scholar]
- 9. Goto, S. , Kaneko T., Miyamoto Y., Eriguchi M., Kato A., Akeyama T., et al. 2005. Combined immunocell therapy using activated lymphocytes and monocyte‐derived dendritic cells for malignant melanoma. Anticancer Res. 25:3741–3746. [PubMed] [Google Scholar]
- 10. Newton, A. D. , Datta J., Loaiza‐Bonilla A., Karakousis G. C., and Roses R. E.. 2015. Neoadjuvant therapy for gastric cancer: current evidence and future directions. J. Gastrointest. Oncol. 6:534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reynders, K. , Illidge T., Siva S., Chang J. Y., and De Ruysscher D.. 2015. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat. Rev. 41:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dunn, G. P. , Bruce A. T., Ikeda H., Old L. J., and Schreiber R. D.. 2002. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3:991–998. [DOI] [PubMed] [Google Scholar]
- 13. Kim, Y. J. , Lim J., Kang J. S., Kim H. M., Lee H. K., Ryu H. S., et al. 2010. Adoptive immunotherapy of human gastric cancer with ex vivo expanded T cells. Arch. Pharmacal Res. 33:1789–1795. [DOI] [PubMed] [Google Scholar]
- 14. Perez, C. A. , Fu A., Onishko H., Hallahan D. E., and Geng L.. 2009. Radiation induces an antitumour immune response to mouse melanoma. Int. J. Radiat. Biol. 85:1126–1136. [DOI] [PubMed] [Google Scholar]
- 15. Gameiro, S. R. , Jammeh M. L., Wattenberg M. M., Tsang K. Y., Ferrone S., and Hodge J. W.. 2014. Radiation‐induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T‐cell killing. Oncotarget 5:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suzuki, Y. , Mimura K., Yoshimoto Y., Watanabe M., Ohkubo Y., Izawa S., et al. 2012. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res. 72:3967–3976. [DOI] [PubMed] [Google Scholar]
- 17. Sato, H. , Suzuki Y., Ide M., Katoh T., Noda S. E., Ando K., et al. 2014. HLA class I expression and its alteration by preoperative hyperthermo‐chemoradiotherapy in patients with rectal cancer. PLoS ONE 9:e108122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deng, J. , Liang H., Wang D., Sun D., Pan Y., and Liu Y.. 2011. Investigation of the recurrence patterns of gastric cancer following a curative resection. Surg. Today 41:210–215. [DOI] [PubMed] [Google Scholar]
- 19. Spolverato, G. , Ejaz A., Kim Y., Squires M. H., Poultsides G. A., Fields R. C., et al. 2014. Rates and patterns of recurrence after curative intent resection for gastric cancer: a United States multi‐institutional analysis. J. Am. Coll. Surg. 219:664–675. [DOI] [PubMed] [Google Scholar]
- 20. Yang, S. , Feng R., Pan Z. C., Jiang T., Xu Q., and Chen Q.. 2015. A comparison of intravenous plus intraperitoneal chemotherapy with intravenous chemotherapy alone for the treatment of gastric cancer: a meta‐analysis. Sci. Rep. 5:12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robert, C. , Long G. V., Brady B., Dutriaux C., Maio M., Mortier L., et al. 2015. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372:320–330. [DOI] [PubMed] [Google Scholar]
- 22. Brahmer, J. , Reckamp K. L., Baas P., Crino L., Eberhardt W. E., Poddubskaya E., et al. 2015. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell Lung cancer. N. Engl. J. Med. 373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]


