Key Clinical Message
Therapeutic lipiodol lymphangiography for postoperative chyle leakage due to lymph duct damage has recently been attracting attention. Lymph duct puncture is technically complex and difficult. Lymphangiography and sclerotherapy can be easily applied by cannulation with a catheter for the neonatal central vein to the lymph duct under a microscope.
Keywords: Chyle leakage, lipiodol lymphangiography, microsurgery, the catheter for the neonatal central vein
Introduction
Chylous ascites is considered a relatively rare postoperative complication, but it cannot be disregarded because van der Gaag et al. 1 reported that the incidence after pancreaticoduodenectomy was 9%, and Malik et al. 2 reported that it was 6.7%. For treatment, there are conservative and surgical treatments. However, no reliable treatment method has been established, and treatment is difficult in many cases.
Therapeutic lipiodol contrast imaging has recently been attracting attention, but it is necessary to puncture a lymph duct to perform lymphangiography and sclerotherapy. Generally, a lymph duct is punctured with a 30‐G butterfly needle [3, 4], but lymph ducts are very thin and fragile, and cannulation may perforate the duct or its fixation is difficult. Thus, it is difficult to stably inject a drug.
We encountered a patient with chylous ascites after surgery for pancreatic cancer in whom a lymph duct was secured under a microscope and punctured with a catheter for the neonatal central vein. Lymphangiography and lipiodol injection could be stably applied, and a favorable outcome was achieved.
Case History
The patient was a 65‐year‐old man. He was diagnosed with pancreatic body cancer and underwent total pancreatectomy at the Department of Surgery. After surgery, ascites accumulation persisted, but the general condition was stable. Thus, the patient was discharged 2 months after surgery. However, abdominal distension gradually aggravated, and marked ascites was observed on CT (Fig. 1). The patient was diagnosed with chylous ascites and readmitted 6 months after surgery. In addition to fasting, high‐calorie infusion management, and several applications of abdominocentesis, internal medical treatment, such as octreotide and etilefrine administration, were performed, but the condition was not improved. The patient was referred to our department 9 months after surgery to perform a therapeutic lipiodol contrast imaging. We performed a new technique of lymph duct puncture to perform lipiodol contrast imaging and sclerotherapy.
Figure 1.
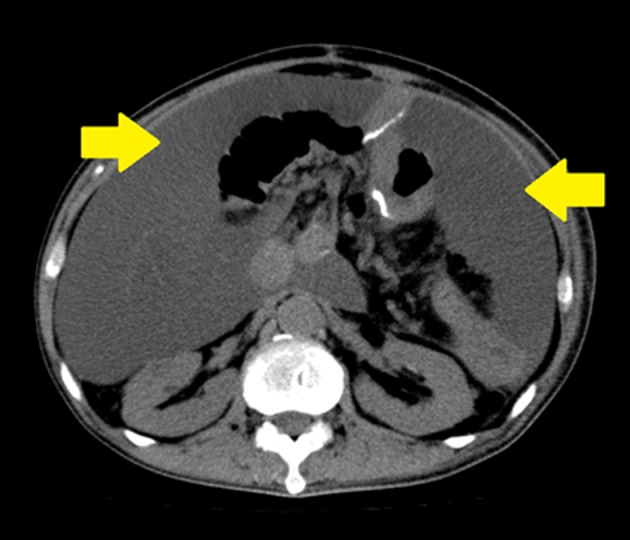
Plain CT image on readmission. The patient was a 65‐year‐old man who was diagnosed with chylous ascites caused by chyle leakage after total pancreatectomy for pancreatic cancer. Marked ascites accumulation was noted. Arrows: Ascites.
Surgical Findings
Surgery was performed under local anesthesia. Indocyanine green (ICG, DAIICHI SANKYO COMPANY, Tokyo, Japan) was intracutaneously injected into the right dorsum pedis, and the lymph duct distribution was observed using an infrared camera (Photo dynamic Eye (PDE), Hamamatsu Photonics, Japan). After applying local anesthesia with 1% xylocaine–epinephrine, a 12‐mm incision was made in the right lower leg using a scalpel (Fig. 2). A lymph duct was identified and secured under a microscope (Fig. 3). A 3/4 circumference incision was made in the lymph duct, and 8‐0 nylon thread was applied to the upper end as an auxiliary thread (Fig. 4). A 28‐G single‐lumen catheter for the neonatal central vein (Argyle™ PI catheter Kit, 0.42‐mm diameter × 20 cm, COVIDIEN Ltd. Tokyo, Japan) was used. Firstly, the guidewire was inserted into the lymph duct, the skin was punctured with a 23‐G needle on the distal side of the incision, and the outer catheter was subcutaneously passed through and advanced into the lymph duct using the guidewire (Fig. 4). The outer catheter was fixed to the lymph duct by ligation with 8‐0 nylon thread at three sites, and the guidewire was removed (Fig. 5A and B). The catheter was fixed to the skin with a surgical tape. Urografin (60%, Bayer Yakuhin, Ltd., Japan) was injected, and cannulation into the lymph duct was confirmed on radiography (Fig. 6). The skin was sutured with 5‐0 nylon, and surgery was completed. After returning to the ward, 12 mL of lipiodol (Guerbet Japan, Japan) was injected at a rate of 6 mL/h through the catheter.
Figure 2.
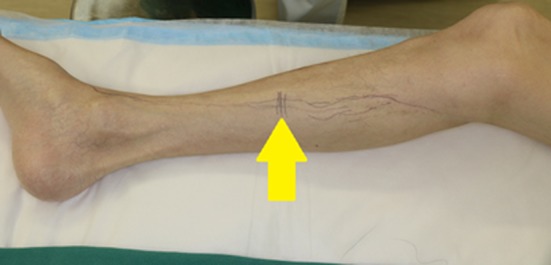
Incision in the right lower leg. After intracutaneous Indocyanine green injection, the lymph duct distribution was confirmed using an infrared camera and an incision above the duct was designed. Arrow: Incision line.
Figure 3.
A lymph duct was identified and secured under a microscope. The subcutaneous region was exposed under a microscope, and a lymph duct was identified and secured. Arrow: A lymph duct.
Figure 4.
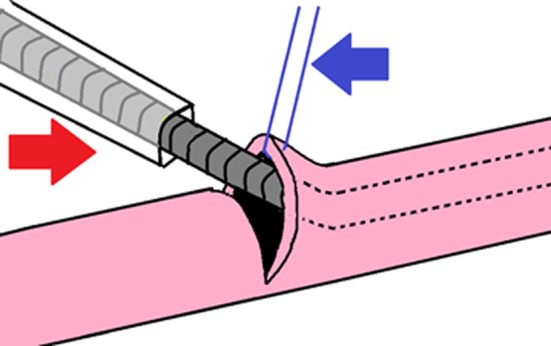
Schema. A 3/4 circumference incision was made in the lymph duct, and 8‐0 nylon thread (blue arrow) was applied to the upper end as an auxiliary thread to open the duct. The guidewire in the 28‐G catheter for the neonatal central vein (Red arrow) was inserted, followed by insertion of the outer catheter.
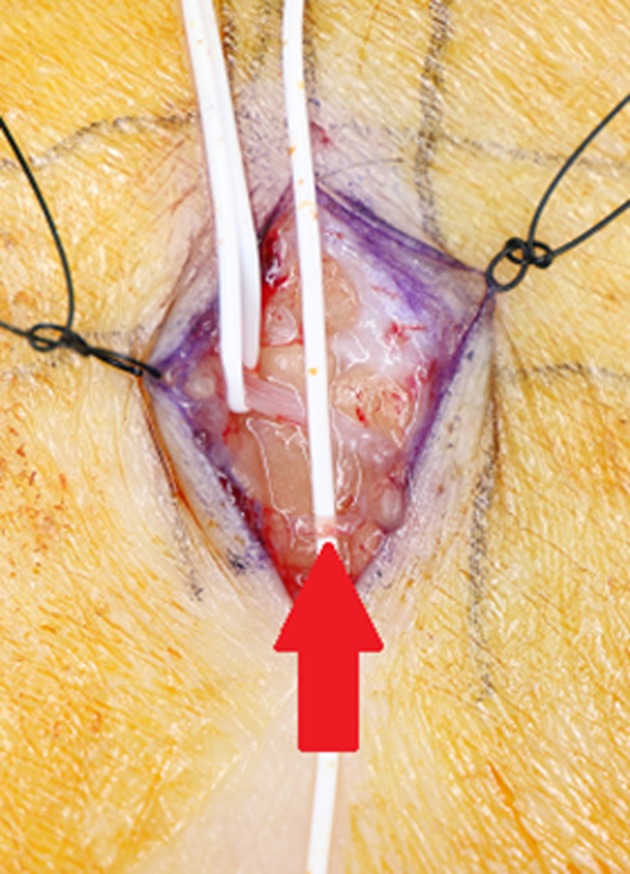
Figure 5.
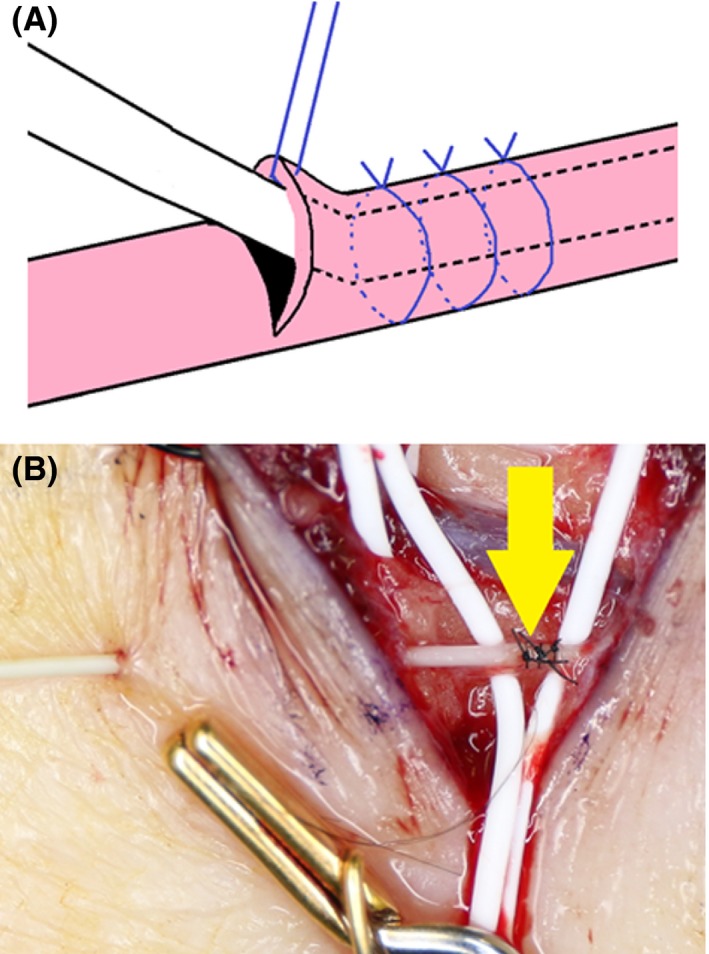
(A) Schema. The outer catheter was fixed to the lymph duct by ligation with 8‐0 nylon thread at three sites, which made the duct resistant to the pressure of drug injection. The lymph duct is not obstructed by slightly strong ligation because the guidewire is contained in the catheter. The guidewire was then removed. (B) Surgical field. The lymph duct was cannulated. The skin was then punctured with a 23‐G needle on the distal side of the incision, and the outer catheter was subcutaneously passed through. Arrow: Cannulated site.
Figure 6.
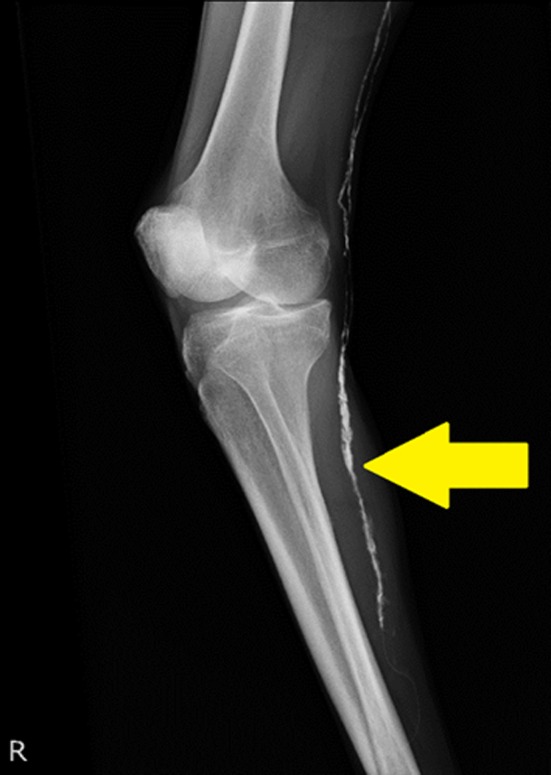
Confirmation by plain radiography after lymphangiography. Arrows: A lymph duct in the right knee was enhanced.
Outcome and Follow‐up
Plain CT was performed on the day following surgery. Lipiodol was noted around the aorta, confirming that it had reached the cistern of chyle (Fig. 7) Ascites production gradually decreased, with which abdominal circumference decreased. Recovery was favorable, and the patient was discharged 1 month after our surgery. One year has passed since the surgery, but recurrence has not been admitted (Fig. 8).
Figure 7.
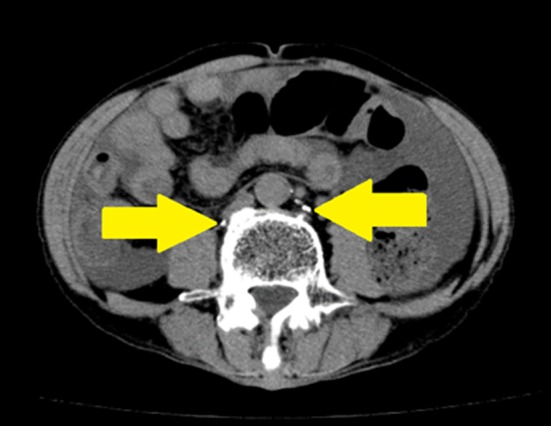
Plain CT image on the day following surgery. Lipiodol was noted around the aorta, confirming that it had reached the cistern of chyle. Arrows: Lymph duct and cistern of chyle enhanced by lipiodol.
Figure 8.
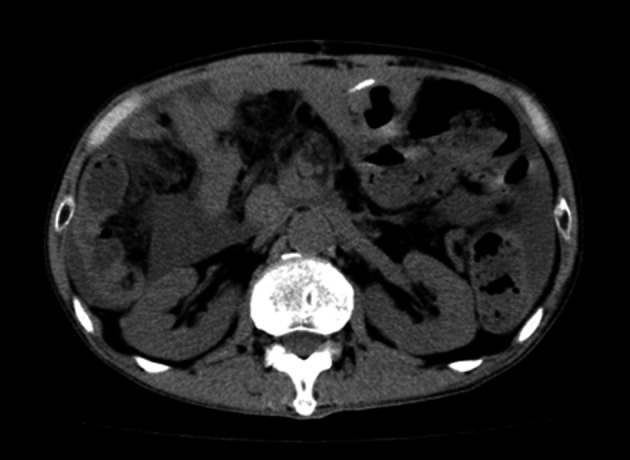
Plain CT image of 1 year after our surgery. No recurrence was observed.
Discussion
Postoperative chyle leakage due to lymph duct damage is a relatively rare complication. For treatment, there are conservative and surgical treatments. For conservative treatment, a low‐fat diet, fasting and nutrition management by central venous alimentation are basic 5. As Ulibarri et al. 6 reported the efficacy of somatostatin administration for the treatment of chyle leakage in 1992, the usefulness of a somatostatin derivative, octreotide, for intractable chylous ascites has been reported 7. In 1999, Guillem et al. reported that the combination of octreotide and etilefrine with sympathetic nerve α1 action improved lymphorrhea 8. On the other hand, surgical treatment includes low‐invasive drainage, a peritoneovenous shunt 9, and high‐invasive lymph duct ligation and clipping by laparotomy. However, no reliable treatment method has been established, and treatment is difficult in many cases.
Therapeutic lipiodol contrast imaging for postoperative chyle leakage has recently been attracting attention 10. In the present patient, all conservative treatments were applied, but remission could not be achieved, and surgical lymphangiography and lipiodol embolization were selected. The mechanism of healing lymphorrhea by lipiodol has not been clarified, but the promotion of granulation by lipiodol‐induced embolization and inflammation of the lymph duct damage site are considered 11. It is necessary to puncture a lymph duct to perform lymphangiography and sclerotherapy. In previous lymphangiography, isosulfan blue or indigo carmine was subcutaneously injected into the region between the toes, the dorsum of the foot was incised, a stained lymph duct was identified, and the lymph duct was cannulated with a 30‐G puncture needle for lymphangiography 3, 4. This method requires the cannulation technique and damages the lymph duct, or the puncture needle detaches while injecting contrast medium, being unreliable. Thus, we designed a new method which secures a lymph duct under a microscope using a catheter for the neonatal central vein and ensures cannulation into the lymph duct and stable injection of a drug.
This method has five advantages. First, it was performed under a microscope so that handling of a thin lymph duct was easy compared with that under direct vision. Second, using ICG and a PDE camera to confirm the distribution of lymph duct, it is possible to incise the center of the lower leg instead of the dorsum of the foot, thus securing thicker lymph duct. Third, a 3/4 circumference incision was made in the lymph duct, and 8‐0 nylon thread was applied to the upper end as an auxiliary thread. This is an important point because the lymph duct likely to escape or be smashed can be retained using this thread, and the application of cannulation becomes easy. Fourth, a 28‐G single‐lumen catheter for the neonatal central vein was used. Passing the guidewire through the incised lymph duct makes the subsequent advancement of the catheter easy. As the outer catheter was soft, it did not damage the lymph duct. Fifth, the outer catheter was fixed to the lymph duct by ligation with 8‐0 nylon thread at three sites, which made the duct resistant to the pressure of drug injection. The catheter may be manually removed after the completion of injection. Lymphorrhea from the punctured site may occur, but it did not occur in this patient.
This new method is advantageous in that the previous complex procedures of lymph duct puncture and drug injection can be easily and stably applied.
Conclusions
Lymphangiography and sclerotherapy can be easily applied by cannulation with a catheter for the neonatal central vein to the lymph duct under a microscope.
Authorship
TK, KH, and SM: managed the patient's case, contributed to the literature search, and wrote the manuscript. TK, KH, SM, KI, and KT: made substantial contributions to the concept and design of this surgical method. TE: also managed the patient. SE, FN, IO, MI, TH, MT, and MI: aggregated the data and helped draft the discussion of the manuscript.
Conflict of Interest
The authors declare that they have no conflict of interests.
References
- 1. van der Gaag, N. A. , Verhaar A. C., Haverkort E. B., Busch O. R., van Gulik T. M., and Gouma D. J.. 2008. Chylous ascites after pancreaticoduodenectomy: introduction of a grading system. J. Am. Coll. Surg. 207:751–757. [DOI] [PubMed] [Google Scholar]
- 2. Malik, H. Z. , Crozier J., Murray L., and Carter R.. 2007. Chyle leakage and early enteral feeding following pancreatico‐duodenectomy: management options. Dig. Surg. 24:418–422. [DOI] [PubMed] [Google Scholar]
- 3. Lee, E. W. , Shin J. H., Ko H. K., Park J., Kim S. H., and Sung K. B.. 2014. Lymphangiography to treat postoperative lymphatic leakage: a technical review. Korean J. Radiol. 15:724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Itkin, M. , Kucharczuk J. C., Kwak A., et al. 2010. Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients. J. Thorac. Cardiovasc. Surg. 139:584–589. [DOI] [PubMed] [Google Scholar]
- 5. Assumpcao, L. , Cameron J. L., Wolfgang C. L., Edil B., Choti M. A., Herman J. M., et al. 2008. Incidence and management of chyle leaks following pancreatic resection: a high volume single‐center institutional experience. J. Gantrointest. Surg. 12:1915–1923. [DOI] [PubMed] [Google Scholar]
- 6. Ulibarri, J. I. , Sanz Y., Fuentes C., Mancha A., Aramendia M., and Sánchez S.. 1992. Reduction of lymphorrhagia from ruptured thoracic duct by somatostatin. Lancet 339:491–492. [DOI] [PubMed] [Google Scholar]
- 7. Huang, Q. , Jiang Z.‐W., Jiang J., N. Li, Li J. S.. 2004. Chylous ascites: treated with total parenteral nutrition and somatostatin. World J. Gastroenterol. 10:2588–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guillem, P. , Billeret V., Houcke M. L., and Triboulet J. P.. 1999. Successful management of post‐esophagectomy chylothorax/chyloperitoneum by etilefrine. Dis. Esophagus 12:155–156. [DOI] [PubMed] [Google Scholar]
- 9. Kaas, R. , Rustman L. D., and Zoetmulder F. A.. 2001. Chylous ascites after oncological abdominal surgery: incidence and treatment. Eur. J. Surg. Oncol. 27:187–189. [DOI] [PubMed] [Google Scholar]
- 10. Kitahara, H. , Yoshitake A., Hachiya T., Inaba Y., Tamura K., Yashiro H.. et al. 2015. Management of aortic replacement‐induced chylothorax by lipiodol lymphography. Ann. Vasc. Dis. 8:110–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kos, S. , Haueisen H., Lachmund U., Roeren T.. 2007. Lymphangiography: forgotten tool or rising star in the diagnosis and therapy of postoperative lymphatic vessel leakage. Cardiovasc. Intervent. Radiol. 30:968–973. [DOI] [PubMed] [Google Scholar]


