Abstract
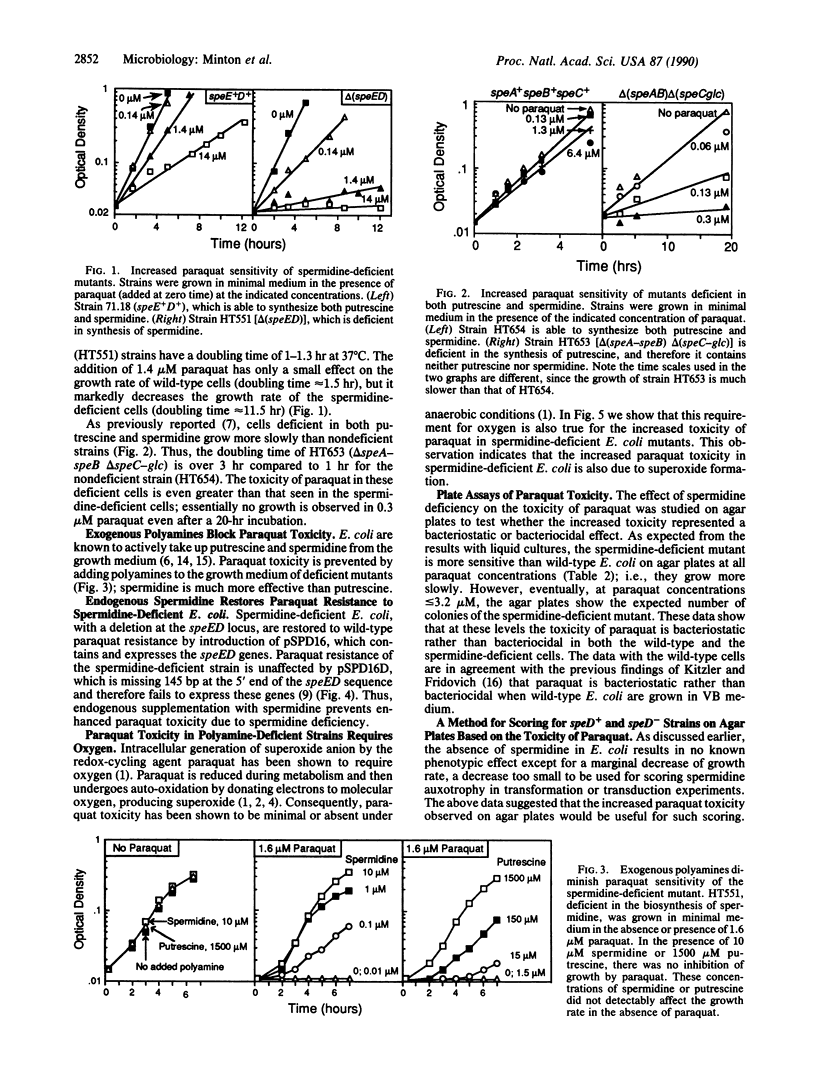
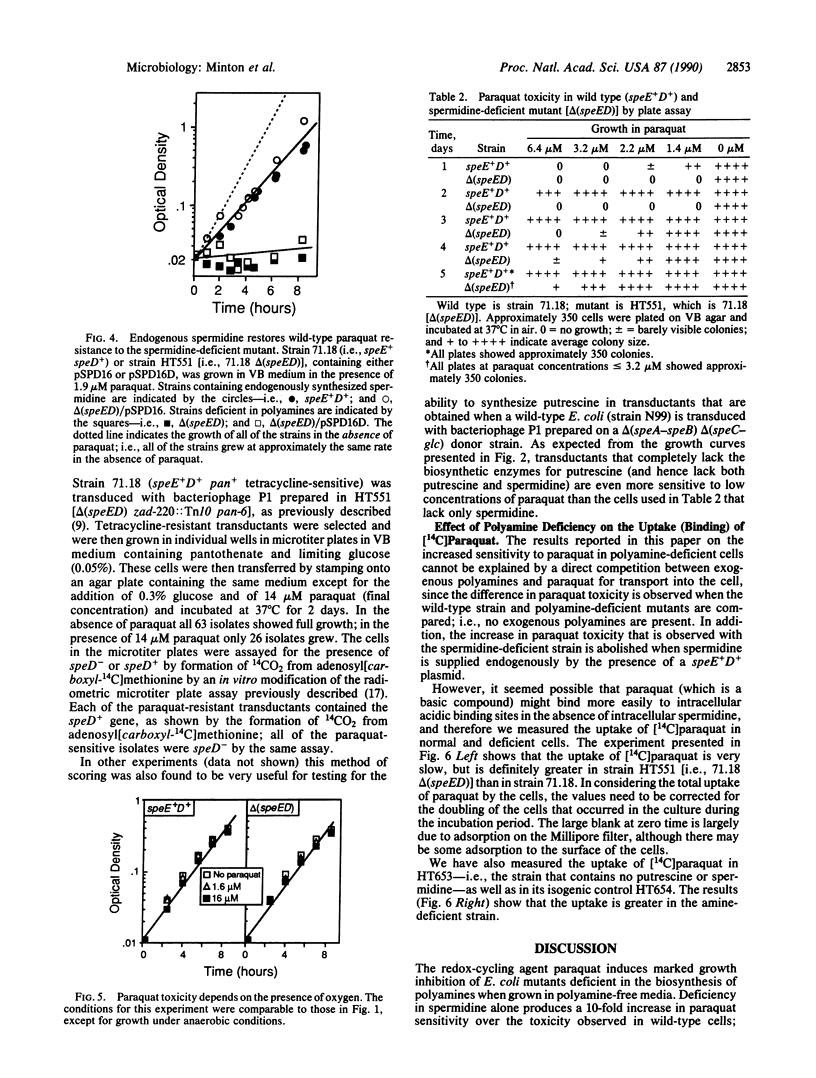
We have shown that toxicity of paraquat for Escherichia coli is increased over 10-fold in strains defective in the biosynthesis of spermidine compared to isogenic strains containing spermidine. The increased sensitivity of these spermidine-deficient mutants to paraquat is eliminated by growth in medium containing spermidine or by endogenous supplementation of spermidine by the use of a speE+D+ plasmid. No paraquat toxicity is seen in the absence of oxygen, even in amine-deficient strains, indicating that superoxide is the agent responsible for the increased toxicity. However, the specific mechanisms responsible for the increased paraquat toxicity in the spermidine-deficient mutants remain to be determined. The marked sensitivity to paraquat of E. coli deficient in spermidine is of particular interest, since such mutants have no other phenotypic properties that can be easily assayed. This increased sensitivity has been used as the basis of a convenient method for scoring for mutants in polyamine biosynthesis and for the detection of plasmids containing the biosynthetic genes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byers T. L., Kameji R., Rannels D. E., Pegg A. E. Multiple pathways for uptake of paraquat, methylglyoxal bis(guanylhydrazone), and polyamines. Am J Physiol. 1987 Jun;252(6 Pt 1):C663–C669. doi: 10.1152/ajpcell.1987.252.6.C663. [DOI] [PubMed] [Google Scholar]
- Fairlamb A. H., Blackburn P., Ulrich P., Chait B. T., Cerami A. Trypanothione: a novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science. 1985 Mar 22;227(4693):1485–1487. doi: 10.1126/science.3883489. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem. 1989 May 15;264(14):7761–7764. [PubMed] [Google Scholar]
- Greenberg J. T., Demple B. Glutathione in Escherichia coli is dispensable for resistance to H2O2 and gamma radiation. J Bacteriol. 1986 Nov;168(2):1026–1029. doi: 10.1128/jb.168.2.1026-1029.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner E. W., Tabor C. W., Tabor H. Mutants of Escherichia coli that do not contain 1,4-diaminobutane (putrescine) or spermidine. J Biol Chem. 1979 Dec 25;254(24):12419–12426. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Paraquat and Escherichia coli. Mechanism of production of extracellular superoxide radical. J Biol Chem. 1979 Nov 10;254(21):10846–10852. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J Biol Chem. 1978 Nov 25;253(22):8143–8148. [PubMed] [Google Scholar]
- Imlay J. A., Linn S. DNA damage and oxygen radical toxicity. Science. 1988 Jun 3;240(4857):1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Kao S. M., Hassan H. M. Biochemical characterization of a paraquat-tolerant mutant of Escherichia coli. J Biol Chem. 1985 Sep 5;260(19):10478–10481. [PubMed] [Google Scholar]
- Kashiwagi K., Kobayashi H., Igarashi K. Apparently unidirectional polyamine transport by proton motive force in polyamine-deficient Escherichia coli. J Bacteriol. 1986 Mar;165(3):972–977. doi: 10.1128/jb.165.3.972-977.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzler J., Fridovich I. Effects of salts on the lethality of paraquat. J Bacteriol. 1986 Jul;167(1):346–349. doi: 10.1128/jb.167.1.346-349.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall J., Bagley A. C., Mullenbach G. T., Hallewell R. A., Lynch R. E. Superoxide mediates the toxicity of paraquat for cultured mammalian cells. J Biol Chem. 1988 Feb 5;263(4):1910–1914. [PubMed] [Google Scholar]
- Minchin R. F. Evidence for the reversible binding of paraquat to deoxyribonucleic acid. Chem Biol Interact. 1987 Feb;61(2):139–149. doi: 10.1016/0009-2797(87)90035-4. [DOI] [PubMed] [Google Scholar]
- Minchin R. F., Martin R. L., Ilett K. F. Paraquat is not accumulated in B16 tumor cells by the polyamine transport system. Life Sci. 1989;45(1):63–69. doi: 10.1016/0024-3205(89)90436-0. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H., Hafner E. H. Mass screening for mutants in the biosynthetic pathway for polyamines in Escherichia coli: a general method for mutants in enzymatic reactions producing CO2. Methods Enzymol. 1983;94:83–91. doi: 10.1016/s0076-6879(83)94014-4. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H., Hafner E. W. Escherichia coli mutants completely deficient in adenosylmethionine decarboxylase and in spermidine biosynthesis. J Biol Chem. 1978 May 25;253(10):3671–3676. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985 Mar;49(1):81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. The speEspeD operon of Escherichia coli. Formation and processing of a proenzyme form of S-adenosylmethionine decarboxylase. J Biol Chem. 1987 Nov 25;262(33):16037–16040. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Transport systems for 1,4-diaminobutane, spermidine, and spermine in Escherichia coli. J Biol Chem. 1966 Aug 25;241(16):3714–3723. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H., Xie Q. W. Spermidine synthase of Escherichia coli: localization of the speE gene. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6040–6044. doi: 10.1073/pnas.83.16.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Isolation, characterization, and turnover of glutathionylspermidine from Escherichia coli. J Biol Chem. 1975 Apr 10;250(7):2648–2654. [PubMed] [Google Scholar]
- Tadolini B., Cabrini L., Landi L., Varani E., Pasquali P. Polyamine binding to phospholipid vesicles and inhibition of lipid peroxidation. Biochem Biophys Res Commun. 1984 Jul 31;122(2):550–555. doi: 10.1016/s0006-291x(84)80068-6. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]



