Abstract
Objective(s):
Pregabalin (PGB) is a new antiepileptic drug that has received FDA approval for patient who suffers from central neuropathic pain, partial seizures, generalized anxiety disorder, fibromyalgia and sleep disorders. This study was undertaken to evaluate the possible adverse effects of PGB on the muscular system of mice.
Materials and Methods:
To evaluate the effect of PGB on skeletal muscle, the animals were exposed to a single dose of 1, 2 or 5 g /kg or daily doses of 20, 40 or 80 mg/kg for 21 days, intraperitoneally (IP). Twaenty-four hr after the last drug administration, all animals were sacrificed. The level of fast-twitch skeletal muscle troponin I and CK-MM activity were evaluated in blood as an indicator of muscle injury. Skeletal muscle pathological findings were also reported as scores ranging from 1 to 3 based on the observed lesion.
Results:
In the acute and sub-acute toxicity assay IP injection of PGB significantly increased the activity and levels of CK-MM and fsTnI compared to the control group. Sub-acute exposure to PGB caused damages that include muscle atrophy, infiltration of inflammatory cells and cell degeneration.
Conclusion:
PGB administration especially in long term care causes muscle atrophy with infiltration of inflammatory cells and cell degeneration. The fsTnI and CK-MM are reliable markers in PGB-related muscle injury. The exact mechanisms behind the muscular damage are unclear and necessitate further investigations.
Keywords: Acute, Muscle injury, Pregabalin, Skeletal muscle, Subacute
Introduction
Pregabalin(PGB)[S-(+)-3-isobutyl-GABA] is derived from gamma- aminobutyricacid (GABA) that exerts its anticonvulsant activity through binding to alpha 2 delta 1 auxiliary subunit of the voltage-gated calcium channel, although it was originally designed to target the GABA system (1). PGB has a chemical structure similar to gabapentin but PGB is absorbed faster and has pharmacokinetic advantages. PGB has received FDA approval for patients who suffer from central neuropathic pain, partial seizures, generalized anxiety disorder, fibromyalgia and sleep disorders (2, 3). The common and rare side effects listed in patient leaflets for PGB include dizziness, somnolence, headache, blurred vision and weight gain, as well as muscle pain and tenderness. FDA prescribing information states that PGB (Lyrica®) treatment is associated with elevation in creatine kinase. It is also mentioned that PGB induces rhabdomyolysis in premarketing clinical trials (Lyrica®, Pfizer, New York City, USA). We did not find any studies evaluating the skeletal muscle side effects associated with PGB use. The result of our previous study on PGB-Induced limb defects in mouse embryos revealed that PGB administration during organogenesis has side effect on muscle, especially mesenchymal tissue that lead to limb deformities (1, 4). This study was undertaken to evaluate the possible adverse effects of PGB on the muscular system of mice.
Materials and Methods
Animal and materials
Male BALB/c mice, aged 10–12 weeks, weighing 25-30 g were obtained from the Animal Center, School of Pharmacy, Mashhad University of Medical Sciences. Animals were maintained in an animal house with a 12-hr light/12-hr dark cycle at 18-22 °C and had free access to water and standard laboratory pellet chow (Khorasan Javan Co, Mashhad, Iran). All experiments were approved by the Animal Care Committee of Mashhad University of Medical Sciences. PGB was purchased from Pfizer Inc, and alizarin red and alcian blue was provided from Merck (Germany). Skeletal muscle troponin-I (SkM-TnI) was measured by a mouse skeletal muscle troponin-I Elisa kit (Life Diagnostics, Inc., Catalog Number: STNI-1). Levels of creatine kinase MM isoenzyme (CK-MM) were tested using a CK-MM ELISA Kit (MBS705327).
Acute and sub-acute toxicities
Acute toxicity testing was performed on twenty four mice that were randomly distributed into one control and three treated groups. The treated groups received different single doses of PGB (1, 2 and 5 g/kg body weight) via intraperitoneal injection (IP). Any sign of toxicity and mortality during 24 hrs was noted. For the sub-acute study, twenty four animals were divided into four groups and received PGB at doses of 0, 20, 40 or 80 mg/kg IP for 21 consecutive days. Body weight was recorded weekly. 24 hrs after the last administration, all animals were sacrificed. Approximately 2 ml of whole blood from each mouse was taken by cardiac puncture for biochemical analysis.
Troponin I measurement
The serum was isolated and kept frozen until used to assess the fast-twitch isoform of troponin-I by ELISA method. Briefly, samples or standards were dispensed into the anti-skeletal muscle troponin-I coated wells. HRP (Horseradish peroxides) conjugate antibody was then used for detection. After adding the reagent, the optical densities were read at 450 nm. The detection limit of fast-twitch skeletal muscle troponin I (fsTnI) in mouse serum was ≤ 4 ng/ml.
Measurement of CK-MM activity
Quantitative sandwich enzyme immunoassay was used to detect CK-MM in serum. Samples were pipetted into the wells that had been pre-coated with CK-MM. HRP conjugated antibody and substrate solution were then added to the microplate. At the end of the procedure the color intensity was measured with a microplate reader at 450 nm.
Histopathological examination
The skeletal muscle of the hind legs of the mice were dissected and fixed in 10% neutral buffered formalin and processed for paraffin embedding. All paraffin blocks were cut in 5 µm thick tissue sections and stained with hematoxylin and eosin. The skeletal muscle lesions were scored semi-quantitatively as following: 0: No lesion, 1: Lesion less than 25% in the microscopic field of view, 2: Lesion in about 25 to 75% in the microscopic field of view, 3: Lesion more than 75% in the microscopic field of view.
Statistical analysis
All data were presented as mean ± SEM. Statistical analyses were performed with one way analysis of variance (ANOVA) followed by a Tukey–Kramer test. SPSS 18.0 was used for all statistical analyses. P<0.05 was considered statistically significant.
Results
Acute and sub-acute toxicity evaluation
In the acute toxicity assay, single IP injections of PGB at doses of 1, 2 and 5 g/kg did not cause any death or signs of toxicity. This means that the PGB had an LD50 of greater than 5 g/kg. According to the classification of toxic agents based on their LD50 values, PGB is assumed to be practically low-toxic in acute exposure. In sub-acute study, no mortality or behavioral changes was observed. There were no significant differences in mean body weight or food and water intake between control and treated groups (data not shown).
Skeletal muscle troponin-I
The single administration of PGB at high doses up to 5 g/kg increased the level of fsTnI that was significantly different from the control (P<0.001) (Figure 1). Sub-acute injection of PGB also caused fsTnI to rise. The differences were statistically significant at highest dose in comparison with the control group (P<0.01) (80 mg/kg) (Figure 2). No differences were observed between the first and second treated and control groups.
Figure 1.
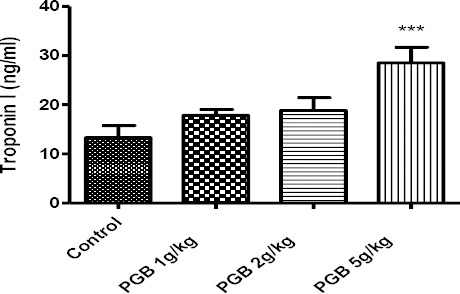
Fast-twitch skeletal muscle troponin I concentration in serum of animals receiving a single IP injection of PGB at doses of 1, 2 or 5 g/kg. Data represent mean±SEM. ***P<0.001 compared to thecontrol group. Tukey–Kramer test, n=6
Figure 2.
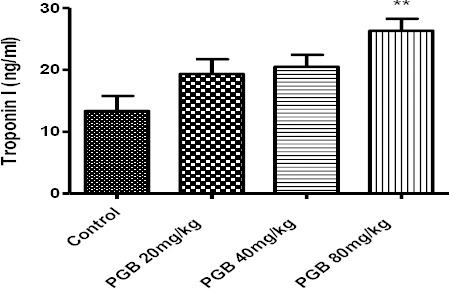
Fast-twitch skeletal muscle troponin I concentration in serum of animals receiving IP injection of PGB at doses of 20, 40 or 80 mg/kg for 21 consecutive days. Data represent mean±SEM **P<0.01 compared to the control group. Tukey–Kramer test, n=6
CK-MM activity
CK-MM activity was also evaluated as an indicator of muscle injury. Single injection of a high dose of PGB significantly increased CK-MM activity compar-ed to the control (P<0.0001) (Figure 3). Also PGB administration in multiple doses for 21 days significantly increased CK-MM activity at 20 and 40 mg/kg, compared to the control group (P<0.05 and P<0.001, respectively) (Figure 4).
Figure 3.
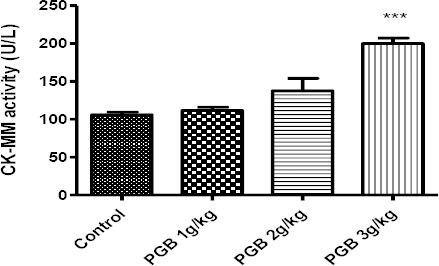
CK-MM activity in serum of animals receiving a single IP injection of PGB at doses 1, 2 or 5 g/kg. Data represent mean±SEM. ***P<0.001 compared to the control group. Tukey–Kramer test, n=6
Figure 4.
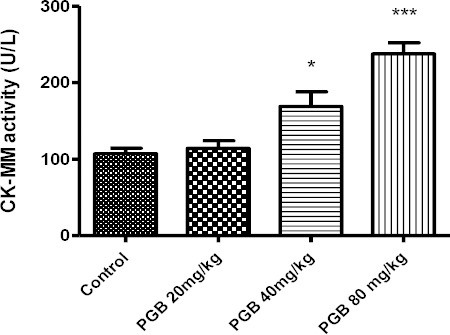
CK-MM activity in serum of animals receiving IP injection of PGB at doses 20, 40 or 80 mg/kg for 21 days. Data represent the mean±SEM. *P<0.05, ***P<0.001 compared to the control group. Tukey–Kramer test, n= 6
Histological observations
Histopathological changes of muscle tissues are presented in Table 1 and Figure 5. Sub-acute exposure to PGB caused damages that include muscle atrophy, infiltration of inflammatory cells and cell degeneration. The cell atrophy was significantly higher in groups that received 40 and 80 mg/kg PGB compared to the control (P<0.05 and P<0.001, respectively). PGB administration induced significant infiltration of inflammatory cells and cell degeneration at highest dose (80 mg/kg)(P<0.001). No pathological findings were observed in groups that were treated with single injections of PGB. There were no changes in cardiac tissues of mice exposed to PGB.
Table 1.
Effect of PGB on histopathological changes in skeletal muscle tissue of mice after 21 days treatment. Results shown as mean±SEM
| Histopathological findings | Control | PGB 20 mg/kg | PGB 40 mg/kg | PGB 80 mg/kg |
|---|---|---|---|---|
| Atrophy | 0.16±0.16 | 0.66±0.21 | 1.16±0.75* | 2±0.25*** |
| Infiltration of inflammatory cells | 0.00±0.00 | 0.66±0.21 | 0.66±0.33 | 1.66±0.42** |
| Cell degeneration | 0.00±0.00 | 0.50±0.22 | 0.66±0.21 | 1.16±0.30** |
| Total lesion | 0.16±0.16 | 1.57±0.42 | 2.14±0.50 | 4.14±0.79*** |
P<0.05,
P<0.01,
P<0.001 compared to control group
Figure 5.
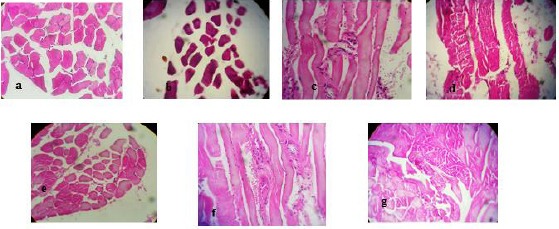
Photomicrographs of hematoxylin and eosin–stained sections of PGB-exposed mice in both acute and sub-acute studies at a magnification of 40X. (a) Normal tissue, (b, c, d) muscle atrophy, inflammatory cells and cell degeneration at groups that received single IP injection at a dose of 5 mg/kg, respectively, (e, f, g) muscle atrophy, inflammatory cells and cell degeneration at groups that received multiple IP injection at a dose of 80 mg/kg, respectively
Discussion
Different types of drugs and substances of abuse cause muscle toxicity including statins, neuromuscular blocking agents, PPAR-α agonists, b-adrenergic receptor agonists, anticonvulsants and morphine (5-7). The Lyrica (PGB) FDA Approved Prescribing Information also does mention that some patients complain of muscle pain, tenderness, or weakness, and creatine kinase elevation (8). There is not enough information available to assess the relationship between PGB use and muscular damage. The result of acute and sub-acute evaluation in this study showed that single and multiple injection of PBG at the highest dose (1 g/kg and 80 mg/kg, respectively) cause elevated troponin-I levels. Following damage to the muscles, troponin-I is released into the blood circulation. There is a strong association between an increase in fsTnI serum level and drug-induced skeletal muscle injury (9, 10). FsTnI is a specific marker for diagnosing muscle toxicity that is unaffected by cardiac muscle injury (5). Another protein in detection of skeletal muscle damage is CK-MM (11). PGB administra-tion leads to elevations in serum CK-MM activity especially at the highest doses in acute and sub-acute toxicity studies. Increases in serum markers of skeletal muscle injury were associated with pathological changes. The total number of lesions increased in a dose-dependent manner. The mechanism behind these injuries is unclear. PGB binds with high affinity to the alpha2-delta-1 (α2/δ1) subunit of the voltage dependent calcium channels (12). The wide distribution of α2/δ1 subunit in the brain and skeletal muscles (13, 14) may be one explanation forthe observed adverse effects on skeletal muscle with PGB treatments. Similar skeletal muscle injury was also detected in other anticonvulsant treatment patients. Valproate has been reported to cause muscle damage with decreased levels of multiple acyl coA dehydrogenase and total and free muscle carnitine (15). Phenytoin- induced muscle injury is reported less commonly with systemic administration (6, 16). The toxicities of antiepileptic drugs in skeletal muscles can occur by different pathways due to the different mechanisms of action. According to medication guides, patients on gabapentin, similar in chemical structure to PGB, may experience unexpected muscle pain. The only case of PGB-induced rhabdomyolysis was reported in combination therapy with simvastatin. The cause of rhabdomyolysis in this patient was attributed to a reduction in clearance of both PGB and simvastatin (17). Combination therapy with drugs that have adverse effects on skeletal muscles may increase the risk of injury and should be avoided.
PGB administration especially in long term care causes muscle atrophy with infiltration of inflammatory cells and cell degeneration. The fsTnI and CK-MM are reliable markers in PGB-related muscle injury. The exact mechanisms behind the muscular damage are unclear and necessitate further investigations.
Acknowledgment
The authors are thankful to the Vice Chancellor of Research, Mashhad University of Medical Sciences for the financial support. We would like to thank Alferd Smith for improving the use of English in the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Etemad L, Moshiri M, Mohammadpour AH, Vahdati Mashhadi N, Moallem SA. Teratogenic effects of pregabalin in mice. Iran J Basic Med Sci. 2013;16:1065–1070. [PMC free article] [PubMed] [Google Scholar]
- 2.Freynhagen R, Grond S, Schupfer G, Hagebeuker A, Schmelz M, Ziegler D, et al. Efficacy and safety of pregabalin in treatment refractory patients with various neuropathic pain entities in clinical routine. Int J Clin Pract. 2007;61:1989–1996. doi: 10.1111/j.1742-1241.2007.01589.x. [DOI] [PubMed] [Google Scholar]
- 3.Sebastian B, Talikoti AT, Nelamangala K, Krishnamurthy D. Effect of oral pregabalin as preemptive analgesic in patients undergoing lower limb orthopedic surgeries under spinal anaesthesia. J Clin Diagn Res. 2016;10:UC01–4. doi: 10.7860/JCDR/2016/18854.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etemad L, Jafarian AH, Moallem SA. Pathogenesis of pregabalin-induced limb defects in mouse embryos. J Pharm Pharm Sci. 2015;18:882–889. doi: 10.18433/j3pp5z. [DOI] [PubMed] [Google Scholar]
- 5.Vassallo JD, Janovitz EB, Wescott DM, Chadwick C, Lowe-Krentz LJ, Lehman-McKeeman LD. Biomarkers of drug-induced skeletal muscle injury in the rat: troponin I and myoglobin. Toxicol Sci. 2009;111:402–412. doi: 10.1093/toxsci/kfp166. [DOI] [PubMed] [Google Scholar]
- 6.Brazeau GA. Drug induced muscle damage. In: Reznic AZ, Packer L, Sen CK, Holloszy JO, Jackson MJ, editors. Oxidative stress in muscle muscle. 1st ed. Basel AG: Springer; 1998. pp. 295–316. [Google Scholar]
- 7.Jones JD, Kirsch HL, Wortmann RL, Pillinger MH. The causes of drug-induced muscle toxicity. Curr Opin Rheumatol. 2014;26:697–703. doi: 10.1097/BOR.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous, Lyrica Prescribing Information. USA: Pfizer Inc; 2004. [Google Scholar]
- 9.Sorichter S, Mair J, Koller A, Gebert W, Rama D, Calzolari C, et al. Skeletal troponin I as a marker of exercise-induced muscle damage. J Appl Physiol. 1997;83:1076–1082. doi: 10.1152/jappl.1997.83.4.1076. [DOI] [PubMed] [Google Scholar]
- 10.Simpson JA, Labugger R, Collier C, Brison RJ, Iscoe S, Van Eyk JE. Fast and slow skeletal troponin I in serum from patients with various skeletal muscle disorders: a pilot study. Clin Chem. 2005;51:966–972. doi: 10.1373/clinchem.2004.042671. [DOI] [PubMed] [Google Scholar]
- 11.Apple FS. Tissue specificity of cardiac troponin I, cardiac troponin T and creatine kinase-MB. Clin Chim Acta. 1999;284:151–159. doi: 10.1016/s0009-8981(99)00077-7. [DOI] [PubMed] [Google Scholar]
- 12.Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol. 2006;6:108–113. doi: 10.1016/j.coph.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Gong HC, Hang J, Kohler W, Li L, Su TZ. Tissue-specific expression and gabapentin-binding properties of calcium channel alpha 2 delta subunit subtypes. J Membr Biol. 2001;184:35–43. doi: 10.1007/s00232-001-0072-7. [DOI] [PubMed] [Google Scholar]
- 14.Patel R, Dickenson AH. Mechanisms of the gabapentinoids and alpha 2 delta-1 calcium channel subunit in neuropathic pain. Pharmacol Res Perspect. 2016;4:e00205. doi: 10.1002/prp2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papadimitriou A, Servidei S. Late onset lipid storage myopathy due to multiple acyl CoA dehydrogenase deficiency triggered by valproate. Neuromuscul Disord. 1991;1:247–252. doi: 10.1016/0960-8966(91)90097-c. [DOI] [PubMed] [Google Scholar]
- 16.Silva MF, Aires CC, Luis PB, Ruiter JP, L IJ, Duran M, et al. Valproic acid metabolism and its effects on mitochondrial fatty acid oxidation: a review. J Inherit Metab Dis. 2008;31:205–216. doi: 10.1007/s10545-008-0841-x. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman MB, Choy M. Pregabalin and simvastatin: first report of a case of rhabdomyolysis. P T. 2012;37:579–595. [PMC free article] [PubMed] [Google Scholar]


