Abstract
Objective(s):
This study aimed to investigate the role and the possible mechanisms involved in the immunoregulation of experimental periodontitis by Th17/Treg.
Materials and Methods:
Experimental periodontitis was established by silk thread ligation with Porphyromonas gingivalis daubing in the bilateral maxillary second molar of Male Sprague-Dawley (SD) rats. Alveolar bones were scanned by Micro-CT. Histological examination was stained with H&E. The proportions of Th17 and Treg cells in peripheral blood were detected by flow cytometry. RT-PCR was used to measure the expression of RORγt, Foxp3 mRNA in the gingival tissues. The concentrations of IL-17, IL-10, and TGF-β in peripheral blood and gingival crevicular fluid were measured by ELISA.
Results:
Experimental rats showed profound bone resorption and inflammatory cell infiltration. The percentages of Th17 significantly increased in the peripheral blood, which was consistent with gingival tissues study that Th17 cells related transcription factor RORγt mRNA and IL-17 increased in the course of periodontitis. The percentages of CD25+Foxp3+ Treg significantly increased in the peripheral blood, which was consistent with gingival tissues study that Treg cells related transcription factor Foxp3 mRNA and cytokines IL-10 and TGF-β increased in the course of periodontitis. The ratio of Th17/Treg cells was significantly increased in the peripheral circulation, however, the Th17/Treg balance is in wave motion in inflamed gingival tissues in the different stages of periodontitis.
Conclusion:
Th17/Treg balance may be associated with the progression of periodontitis and pathological tissue destruction. Moreover, local inflammation would result in the up-regulation ratio of Th17/Treg in peripheral blood, which may influence some periodontally involved systemic diseases.
Keywords: Bone resorption, Cytokine, Experimental periodontitis, Th17, Treg
Introduction
Periodontitis is a chronic inflammatory disease characterized by the inflammatory bone resorption of the teeth-supporting structures, being the most prevalent form of bone pathology (1, 2). It is widely accepted that periodontal disease can be caused by bacteria, which not only causes periodontal attachment and alveolar bone loss but can also have an impact on systemic diseases, such as diabetes, and cardiovascular disease (3-5). Although periodontal bacteria such as Porphyromonas gingivalis (P. gingivalis) are necessary for the initiation of periodontitis, the host immune response to periodontal bacteria, particularly the CD4+T cell-mediated cellular response, plays an important role in subsequent disease progression and periodontal tissue destruction (6-8). After priming and activation, naive CD4+T cells differentiate into different subsets with either promoter or suppressor functions during the progression of periodontitis (9).
Regulatory T cells (Tregs) and Th17 cells are two closely related CD4+ Th-cell subsets, and they have reciprocal effects on the development of autoimmune diseases. Th17 cells express the specific transcription factor retinoid-related orphan receptor γτ (RORγτ), a major pathogenic cell that orchestrates the complex network of the sustained inflammation and disease progression (10). Th17 produces IL-17, which is abundantly expressed in the inflammation sites, such as arthritis joints (11). IL-17 not only induces proinflammatory cytokines but also directly enhances osteoclastogenesis by upregulating receptor activator nuclear kappa ligand (RANKL) on osteoblasts, contributing to bone resorption in periodontitis (12, 13).
Treg cells, whose functions are different from other T cell subpopulations, retain tolerance to self-antigens and inhibit autoimmunity (14). CD4+CD25+ Treg cells express the transcription factor forkhead box P3 and they play an important role in the maintenance of immune self-tolerance and homeostasis. Foxp3+ Treg cells can migrate into inflammation sites and inhibit various effector lymphocytes, including Th1, Th2, and Th17 (15). There is accumulating evidence indicating that Tregs’ suppressive function can be mediated by TGF-β and IL-10 (16). Tregs have been identified in human periodontal lesions by the expression of the phenotypic markers FOXp3, IL-10, and TGF-β. A previous study demonstrated an increased frequency of Tregs in healthy tissues, suggesting a role for Tregs in the maintenance of periodontal health (17).
Treg/Th17 balance plays an important role in maintaining immune homeostasis. The proportion of Th17/Treg cells has been studied in some inflammatory diseases, such as inflammatory bowel disease and chronic airway inflammation (18, 19). Maintaining an appropriate balance between Th17 and Treg cells can ensure effective immunity while avoiding inflammatory and tumor immunosurveillance. Accumulated evidence has demonstrated quantitative or functional imbalance between Th17 and Tregs and these subsets’ expression correlation with prognosis in inflammatory and tumor (20).
However, the roles of Tregs and Th17 in the course of periodontitis are unknown. To clarify these questions, we investigated the percentage of Th17/Treg cells and related transcription factor and cytokines in peripheral blood, gingival tissues and gingival crevicular fluid of SD rats with experimental periodontitis in different stages of progression. Based upon this research, we evaluated the role and the possible mechanisms involved in the immunoregulation of experimental periodontitis by Th17/Treg.
Materials and Methods
Reagents
Fluorescence-conjugated mAbs to CD4, CD25, Foxp3, and IL-17 were from eBiosciences Co (USA); Reagents for qPCR were purchased from Takara Bio (China); TGF-β, IL-10, IL-17 ELISA kits from Raybio Co., Ltd. (USA).
Animals
Male Sprague-Dawley (SD) rats weighing 220±20 g were purchased from the Experimental Animal Center of Sun Yat-sen University, Guangzhou, China. The animals were housed in a room with a controlled ambient temperature (22±2 °C) and humidity (50%±10%), with food and water ad libitum. All experiments were approved by the Ethics Review Committee for Animal Experimentation of Sun Yat-sen University.
Bacteria
Porphyromonas gingivalis from Xiangf bio co. (China) (ATCC: 33277). P. gingivalis was grown on brain-heart infusion (BHI) blood agar plates supplemented with vitamin K (10 μg/ml), hemin (0.25%), and sterilized sheep blood (5%) under anaerobic conditions at 37 °C. The bacteria were collected during the logarithmic growth phase of oral infection.
Induction and groups of periodontitis rats
SD rats were anesthetized and silk thread ligation was operated in the bilateral maxillary second molar. Periodontal infection was performed as described previously (17). Periodontal infection was established through oral inoculation using 1010 colony-forming units of P. gingivalis suspended in 100 μl 4% carboxymethyl cellulose (CMC) every other day within a week. The rats were euthanized 2 weeks, 4 weeks, 8 weeks, and 12 weeks after the first oral inoculation. An equivalent number of untreated rats were employed as controls.
40 rats were randomly divided into 5 groups, controls, 2 weeks, 4 weeks, 8 weeks, and 12 weeks groups. Each group contained 8 rats.
Alveolar bone loss measurement
Bone loss measurements were made along the axis of each root surface of all the molar teeth (14 sites) to accurately determine the distance between the alveolar bone crest (ABC) and the cement-enamel junction (CEJ). The buccal and palatal root surfaces of the molar teeth were photographed at 32× with a dissecting microscope. To calculate alveolar bone resorption, the average distance between the ABC and the CEJ at each of the 14 predetermined sites was subtracted from the average distance between the ABC and the CEJ. All the bone level measurements were performed by two blinded evaluators.
Histological examination
Alveolar bones were fixed in formalin for 2 days and scanned by Micro-CT. For histological examination, after being fixed overnight in 4% paraformaldehyde, the maxilla were decalcified in 10% ethylenediamine -tetraacetic acid disodium salt (EDTA) for 4 weeks. The decalcified specimens were then dehydrated and embedded in paraffin. The tissue blocks were cut into a half series of slices five-micrometers-thick in the mesiodistal direction. The sections were fixed on slides, the paraffin removed, and then the samples were stained with hematoxylin and eosin (H&E). The sections were examined with a stereomicroscope (Olympus).
Detection of CD4+IL-17+ T cells and CD4+CD25+Foxp3+ Treg
Fresh heparinized peripheral blood samples from each group were collected. PBMCs were isolated from blood by standard density gradient separation using Ficoll density gradient (21). Isolated cells were washed three times with PBS and used for flow cytometry. A total of 1 × 107 PBMCs prepared above were acquired for each sample and surface stained with CD4-FITC or CD4-FITC and CD25-APC at 4 °C for 30 min, and subsequently intracellularly stained with a PE anti-rat Foxp3 or IL-17 staining kit according to the manufacturer’s instructions. Cells were detected by flow cytometry using a Beckman Coulter Epics Elite and data were analyzed by FCS Express 4. The results were expressed by the percentage of CD25+Foxp3+ and IL-17+ in gated CD4+ cell.
Real-time quantitative PCR
Gingival tissues from each group were homogenized for RNA extraction. Total RNA was extracted with TRI reagent. Total RNA (1 μg) was then reverse-transcribed in a reaction mixture containing 1U RNase inhibitor, 500 ng random primers, 3 mM MgCl2, 0.5 mM dNTP, and 10 U reverse transcriptase in RT buffer. The sequences of the synthetic oligonucleotides were as Table 1.
Table 1.
The sequnces of the synthetic oligonucleotices
| Molecule | Sequence (5’–3’) |
|---|---|
| Foxp3 | Forward:5’-CCCAGGAAAGACAGCAACC-3’ |
| Reverse:5’-TTCTCACAACCAGGCCACTTG-3’ | |
| ROR γt | Forward:5’- AGTGTAATGTGGCCTACTCCT-3’ |
| Reverse:5’- GCTGCTGTTGCAGTTGTTTCT -3’ | |
| GAPDH | Forward:5’-ACTCCACGACGTACTCAGCG-3’ |
| Reverse:5’-GGTCGGAGTCAACGGATTTG-3’ |
Q-PCR analysis of GAPDH, Foxp3, and ROR γt was performed using the Roche universal probe library detection system. Relative quantification of gene expression was performed using the comparative threshold (CT) method (22). Changes in mRNA expression level were calculated following normalization to GAPDH. All experiments were performed in triplicate independently.
Detection of cytokines IL-10, TGF-β, and IL-17 by ELISA
The serum and gingival crevicular fluid of experimental and control rats were taken at 2, 4, 8, and 12 weeks after ligation. IL-10, TGF-β, and IL-17 level in the serum and gingival crevicular fluid were measured using enzyme-linked immunosorbent assay (ELISA) kits according to the protocols provided by the manufacturer.
Statistical analysis
Data are presented as the mean±standard deviation (SD). Differences between groups were analyzed using Student’s t-test for continuous variables. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS, version 17.0; SPSS, Inc.), and P<0.05 was considered statistically significant.
Results
Experimental periodontitis was established successfully
We first observed periodontal bone by Micro-CT and stereomicroscope to investigate periodontal bone loss induced by P. gingivalis. From the figure we found bone resorption and furcation were involved in periodontitis groups (Figure 1A). We measured the distance from the CEJ to the ABC to quantitate periodontal bone loss. The average distance between the CEJ and the ABC increased gradually from 2 weeks to 12 weeks after ligation compared to the control group (Figure 1B).
Figure 1.
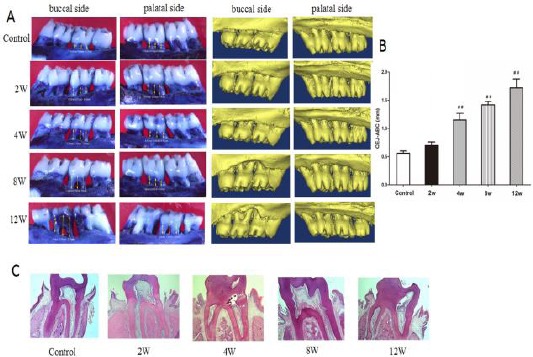
Experimental periodontitis was induced P. gingivalis. Alveolar bone resorption was analyzed by measuring the distance from the cement-enamel junction (CEJ) to the alveolar bone crest (ABC). A: Periodontal bone is observed by stereomicroscope and Micro-CT. B: The distance between the CEJ and ABC. C: HE staining of periodontium. ##P<0.01, compared with the control group
It is an important pathological change that inflammatory cells infiltrate periodontal tissues in the pathogenesis of periodontitis. H&E staining revealed that inflammatory cells were observed in both the connective tissue and epithelium, collagenous fibers and apparent attachment were mainly destroyed in the experimental periodontitis groups (2, 4, 8, and 12 weeks after ligation) (Figure 1C).
The proportion and ratio of Th17/Treg were disordered in peripheral blood of experimental periodontitis rats
The percentages of Th17 and Treg cells in peripheral blood were determined by flow cytometry in different stages of experimental periodontitis. The percentage of IL-17+ T cells in gated CD4+ cell significantly increased in the experimental periodontitis groups (2, 4, 8, and 12 weeks groups) compared to the control group. Among them, the percentages of Th17 in 8 weeks group was the highest (Figure 2A).
Figure 2.
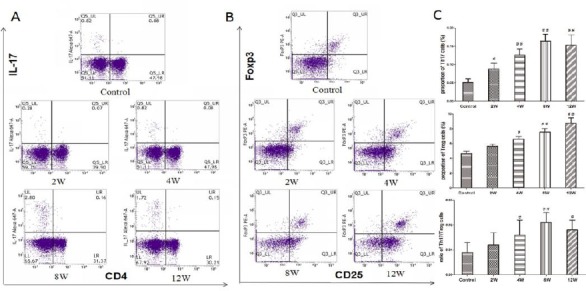
Proportion of Th17 cells and Treg cells and the ratio of Th17/Treg in experimental periodontitis rats’ peripheral blood. Lymphocytes were isolated and purified from peripheral blood. These cells were harvested, then stained with antibodies to CD4, CD25, IL-17, and Foxp3, and analyzed by flow cytometry. A: Dot plot in the upper right quadrant represents CD25+Foxp3+ in gated CD4+cell. B: Dot plot in the upper right quadrant represents CD4+IL-17+Th17. C: Histogram expression of CD25+Foxp3+Treg percentages in gated CD4+cell CD4+IL-17+ Th17 percentages and the ratio of Th17/Treg. Data are shown as the means±SD from 8 animals. #P<0.05, ##P<0.01 versus control
Similar results were obtained in detecting Treg cells proportion. The percentage of CD25+Foxp3+ cells in gated CD4+ T cells significantly increased in the experimental periodontitis groups (2, 4, 8, and 12 weeks groups) compared to the control group. Among them, the percentages of Treg in 12 weeks groups were highest (Figure 2B).
The ratio of IL-17+T/CD25+Foxp3+Treg cells increased gradually from 0 weeks to 8 weeks, it especially increased significantly in the experimental periodontitis groups (8, 12 weeks groups) compared to control group (Figure 2C).
Expression of RORγt mRNA and Foxp3 mRNA in gingival tissues
We detected the specific transcription factor RORγτ of Th17 to further reflect the Th17 related gene level. The expression of RORγt mRNA increased gradually from 0 weeks to 8 weeks, and the expression significantly increased in the experimental periodontitis groups (2, 4, 8, and 12 weeks groups) compared to the control group. Among these groups, the expression of RORγt mRNA in the 8 weeks group was the highest (Figure 3A).
Figure 3.
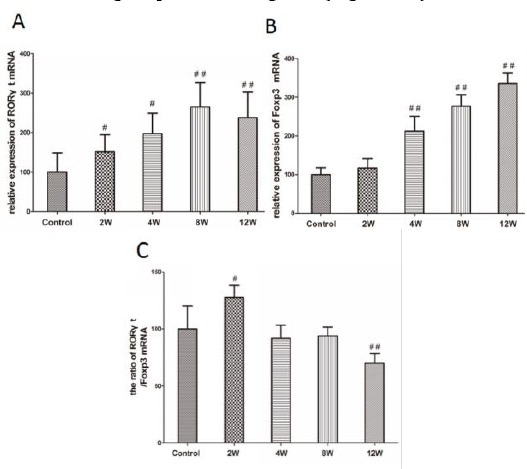
Expression of RORγt and Foxp3 mRNA and the ratio of RORγt/Foxp3 in experimental periodontitis rats’ gingival tissues. A: Analysis of expression of RORγt mRNA in rats’ gingival tissues. B: Analysis of expression of Foxp3 mRNA in rats’ gingival tissues. C: Analysis of the ratio of RORγt/Foxp3 in experimental periodontitis rats’ gingival tissues. Data are shown as the means±SD from 8 animals. #P<0.05, ##P<0.01 versus control
We detected the specific transcription factor Foxp3 mRNA of Treg. Similar results were obtained in the expression of Foxp3 mRNA. The expression of Foxp3 mRNA increased gradually from 2 weeks to 12 weeks, and the expression significantly increased in the experimental periodontitis groups (4, 8, and 12 weeks groups) compared to the control group. Among these periodontitis groups, the expression of Foxp3 mRNA in the 12 weeks group was the highest (Figure 3B).
The ratio of RORγt/Foxp3 mRNA significantly increased in 2 weeks groups compared to the control group and 4, 8, and 12 weeks groups, that is to say, the ratio was highest in 2 weeks groups. In contrast, the ratio significantly decreased in 12 weeks groups compared to the control group and 2, 4, and 8 weeks groups (Figure 3C).
The expression of IL-10, TGF-β, and IL-17 in the serum and gingival crevicular fluid
We detected IL-10, TGF-beta, and IL-17 by the ELISA method to further explore Th17/Treg related cytokines. There are no significant differences in IL-17 level between the control group and experimental groups in the serum. The level of IL-10 increased gradually from 2 weeks to 12 weeks, and the level significantly increased in the experimental periodontitis groups (4, 8, and 12 weeks groups) compared to the control group. Among these periodontitis groups, the level of IL-10 in 12 weeks groups was highest. The level of TGF-β increased in experimental groups, but there was no significant difference between the control group and the experimental groups (Figure 4A).
Figure 4.
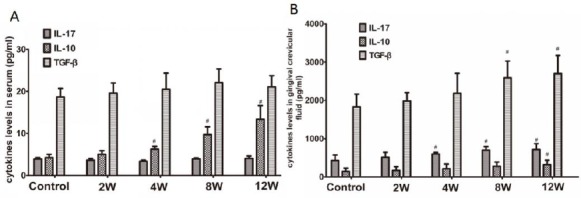
Expression of IL-17, IL-10, and TGF-β in experimental periodontitis rats’ serum and gingival crevicular fluid. A: Analysis of expression of IL-17, IL-10, and TGF-β in experimental periodontitis rats’ serum. B: Analysis of expression of IL-17, IL-10, and TGF-β in experimental periodontitis rats’ gingival crevicular fluid. Data are shown as the means±SD from 8 animals. #P<0.05 versus control
The level of IL-17 increased gradually from 2 weeks to12 weeks in the gingival crevicular fluid, and the level significantly increased in the experimental periodontitis groups (4, 8, and 12 weeks groups) compared to the control group. Cytokines IL-10 and TGF-β increased gradually from 2 weeks to 12 weeks. Compared to the control group, IL-10 significantly increased in 12 weeks, while TGF-β significantly increased in 8 and 12 weeks groups (Figure 4B).
Discussion
As previously reported, mice inoculated with P. gingivalis had a significantly increased number of alveolar bone osteoclasts, which could contribute to their bone loss (23, 24). In the present study, we specifically demonstrated that bone loss was increased gradually in different stages of P. gingivalis-induced experimental periodontitis. H&E staining revealed that inflammatory cells were observed in both the connective tissue and epithelium, collagenous fibers and apparent attachments were destroyed at various degrees with the progress of the disease. It also shows that experimental periodontitis was established successfully in the present study.
Several studies have shown that Th17 and Treg cells are involved in periodontal inflammation and tissue destruction during periodontitis (25), but the roles of Tregs and Th17 in the course of periodontitis are unknown. To clarify these questions, we investigated the percentage of Th17/Treg cells and related transcription factor and cytokines. Th17 cell activation has been linked to bone resorption (26); accordingly, high levels of IL-17 were found in the crevicular fluid of periodontal pockets from periodontitis patients (27), and Th17 cells have been identified in chronic periodontitis lesions (28). We can detect Th17 and Treg cell populations by flow cytometry in peripheral blood, however, it is difficult to detect Th17 and Treg cell populations in gingival tissues, so we detected RORγτ, Foxp3 mRNA and related cytokines in gingival tissues. The results of the present study found that the percentages of Th17 significantly increased in peripheral blood in the course of periodontitis, which was consistent with gingival tissues study that Th17 cells related transcription factor RORγt mRNA and cytokines IL-17 significantly increased in the course of periodontitis. As a pro-inflammatory cytokine, IL-17 plays a critical role through the recruitment of neutrophils and induction of granulopoiesis to clear periodontal pathogens (29). The current study showed that IL-17 levels increased during the pathogenesis of periodontitis and were strongly associated with periodontal destruction. That is to say, Th17 cells increased in the local inflammation tissue in different stages of P. gingivalis-induced experimental periodontitis, which may affect Th17 cells in the peripheral circulation. However, it is interesting that there were no significant differences in IL-17 levels in serum between the control and experimental groups. The exact mechanism for unchanged IL-17 levels in serum is not clear. We inferred that the sensitivity of the detection is not enough for the low level of IL-17 in peripheral blood, or perhaps it was related to IL-17 gathering from peripheral blood into the local inflammation tissue.
Recent studies suggest that Foxp3+ Treg cells may have an essential role in regulating periodontal inflammation and alveolar bone resorption in inflamed gingival tissues (30). Treg cells have been reported to attenuate periodontitis through the expression of membrane inhibitory molecules, such as glucocorticoid-induced tumor necrosis factor receptor, and anti-inflammatory cytokines, such as IL-10 and TGF-β (31). In the present study, we examined the percentage of CD25+Foxp3+ Treg cells from peripheral blood and Treg cells related transcription factor Foxp3 mRNA and cytokines IL-10 and TGF-β in gingival tissues with significant inflammation. Our results indicated the percentages of CD25+Foxp3+ Treg significantly increased in peripheral blood in the course of periodontitis, which was consistent with a gingival tissues study showing that Treg cells related transcription factor Foxp3 mRNA and cytokines IL-10 and TGF-β significantly increased in the course of periodontitis. That is to say, Treg cells increased in the local inflammation tissue in different stages of P. gingivalis-induced experimental periodontitis, which may affect Treg cells in the peripheral circulation. Our experimental results were not completely consistent with previous reports, for example, some reports suggest that the percentages of Treg cells tended to decrease in periodontitis (32). Why was there such a different result? The feature of this study is observing Treg in the different stages of periodontitis, and the percentages of Treg may be related to its self-protective increase.
Previous studies suggested that Th17/Treg cell imbalances are associated with the development of periodontitis and that increased amounts of the ratio of Th17/Treg contribute to the pathogenesis of periodontitis (25). Consistent with these studies, in the present study, we found that the ratio of Th17/Treg cells was significantly increased in the peripheral circulation in the different stages of periodontitis. In the 12th week, the proportion of Treg continued to rise. The protective rise of Treg inhibited Th17, which probably lead to decreased Th17/ Treg ratio from the 12th week, but the ratio was still higher than the control. However, the Th17/Treg balance is in wave motion in inflamed gingival tissues in different stages, which may be associated with the progression of periodontitis and pathological tissue destruction.
Conclusion
We demonstrated that the percentages of Th17 and Treg cells were up-regulated significantly in inflamed gingival tissues in different stages of periodontitis. However, the ratio of Th17/Treg is in wave motion, which may be associated with the progression of periodontitis and pathological tissue destruction. Moreover, local inflammation would result in the up-regulation ratio of Th17/Treg in peripheral blood, which may influence some periodontally involved systemic diseases. These findings should be the subject of further inquiry through a carefully conducted, larger, prospective study to determine whether Tregs/Th17 ratio can be used as a marker and whether it may serve as a potential therapeutic target. The percentages and the ratio of Th17/Treg have been detected in different stages of periodontitis and their applications for clinical value remain to be investigated in future studies.
Acknowledgment
The experiment was performed in conformity with the laws and regulations for live animals. All experiments were approved by the Ethics Review Committee for Animal Experimentation of Sun Yat-sen University, Guangzhou, China. This project was supported by grants from the National Natural Science of China (No: 30801296, 81300887).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Lavu V, Venkatesan V, Rao SR. The epigenetic paradigm in periodontitis pathogenesis. J Indian Soc Periodontol. 2015;19:142–149. doi: 10.4103/0972-124X.145784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2014;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llambés F, Arias-Herrera S, Caffesse R. Relationship between diabetes and periodontal infection. World J Diabetes. 2015;6:927–935. doi: 10.4239/wjd.v6.i7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kholy KE, Genco RJ, Van Dyke TE. Oral infections and cardiovascular disease. Trends Endocrinol Metab. 2015;26:315–321. doi: 10.1016/j.tem.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Hussain M, Stover CM, Dupont A. P. gingivalis in Periodontal Disease and Atherosclerosis-Scenes of Action for Antimicrobial Peptides and Complement. Front Immunol. 2015;6:45. doi: 10.3389/fimmu.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernández M, Dutzan N, García-Sesnich J, Abusleme L, Dezerega A, Silva N, et al. Host-pathogen interactions in progressive chronic periodontitis. J Dent Res. 2011;90:1164–1170. doi: 10.1177/0022034511401405. [DOI] [PubMed] [Google Scholar]
- 7.Teng YT. The role of acquired immunity and periodontal disease progression. Crit Rev Oral Biol Med. 2003;14:237–252. doi: 10.1177/154411130301400402. [DOI] [PubMed] [Google Scholar]
- 8.Gemmell E, Yamazaki K, Seymour GJ. The role of T cells in periodontal disease: homeostasis and autoimmunity. Periodontol. 2007;43:14–40. doi: 10.1111/j.1600-0757.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 9.Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. 2008;79:1585–1591. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- 10.Peters A, Lee Y, Kuchroo VK. The many faces of Th17 cells. Curr Opin Immunol. 2011;23:702–706. doi: 10.1016/j.coi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isailovic N, Daigo K, Mantovani A, Selmi C. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1–11. doi: 10.1016/j.jaut.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 13.Hienz SA, Paliwal S, Ivanovski S. Mechanisms of Bone Resorption in Periodontitis. J Immunol Res. 2015;2015:615486. doi: 10.1155/2015/615486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Y, Chen X, Huang ZM, Ye XH, Niu Q. Increased frequency of Foxp3+ regulatory T cells in mice with hepatocellular carcinoma. Asian Pac J Cancer Prev. 2013;13:3815–3819. doi: 10.7314/apjcp.2012.13.8.3815. [DOI] [PubMed] [Google Scholar]
- 15.Haque M, Fino K, Lei F, Xiong X, Song J. Utilizing regulatory T cells against rheumatoid arthritis. Front Oncol. 2014;4:209. doi: 10.3389/fonc.2014.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakaguchi S. Naturally arising CD4+ regulatory T-cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 17.Garlet GP, Cardoso CR, Mariano FS, Claudino M, de Assis GF, Campanelli AP, et al. Regulatory T cells attenuate experimental periodontitis progression in mice. J Clin Periodontol. 2010;37:591–600. doi: 10.1111/j.1600-051X.2010.01586.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee SY, Lee SH, Yang EJ, Kim EK, Kim JK, Shin DY, Cho ML. Metformin Ameliorates Inflammatory Bowel Disease by Suppression of the STAT3 Signaling Pathway and Regulation of the between Th17/Treg Balance. PLoS One. 2015;10:e0135858. doi: 10.1371/journal.pone.0135858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei Y, Luo QL, Sun J, Chen MX, Liu F, Dong JC. Bu-Shen-Yi-Qi formulae suppress chronic airway inflammation and regulate Th17/Treg imbalance in the murine ovalbumin asthma model. J Ethnopharmacol. 2015;164:368–377. doi: 10.1016/j.jep.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Duan MC, Zhong XN, Liu GN, Wei JR. The Treg/Th17 paradigm in lung cancer. J Immunol Res. 2014;2014:730380. doi: 10.1155/2014/730380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li XS, Li S, Kellermann G. An integrated liquid chromatography-tandem mass spectrometry approach for the ultra-sensitive determination of catecholamines in human peripheral blood mononuclear cells to assess neural-immune communication. J Chromatogr A. 2016;1449:54–61. doi: 10.1016/j.chroma.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 22.Bleda S, de Haro J, Varela C, Ferruelo A, Acin F. Elevated levels of triglycerides and vldl-cholesterol provoke activation of nlrp1 inflammasome in endothelial cells. Int J Cardiol. 2016;220:52–55. doi: 10.1016/j.ijcard.2016.06.193. [DOI] [PubMed] [Google Scholar]
- 23.Imai K, Victoriano AF, Ochiai K, Okamoto T. Microbial interaction of periodontopathic bacterium Porphyromonas gingivalis and HIV-possible causal link of periodontal diseases to AIDS progression. Curr HIV Res. 2012;10:238–244. doi: 10.2174/157016212800618183. [DOI] [PubMed] [Google Scholar]
- 24.Norowski PA, Jr, Bumgardner JD. Biomaterial and antibiotic strategies for peri-implantitis: a review. J Biomed Mater Res B Appl Biomater. 2009;88:530–543. doi: 10.1002/jbm.b.31152. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Wang J, Jin Y, Gao H, Lin X. Oral administration of all-trans retinoic acid suppresses experimental periodontitis by modulating the Th17/Treg imbalance. J Periodontol. 2014;85:740–750. doi: 10.1902/jop.2013.130132. [DOI] [PubMed] [Google Scholar]
- 26.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vernal R, Dutzan N, Chaparro A, Puente J, Antonieta Valenzuela M, Gamonal J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J Clin Periodontol. 2005;32:383–389. doi: 10.1111/j.1600-051X.2005.00684.x. [DOI] [PubMed] [Google Scholar]
- 28.Cardoso CR, Garlet GP, Crippa GE, Rosa AL, Júnior WM, Rossi MA, et al. Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol Immunol. 2009;24:1–6. doi: 10.1111/j.1399-302X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 29.Yu JJ, Ruddy MJ, Wong GC, Sfintescu C, Baker PJ, Smith JB, et al. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood. 2007;109:3794–3802. doi: 10.1182/blood-2005-09-010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi R, Kono T, Bolerjack BA, Fukuyama Y, Gilbert RS, Fujihashi K, et al. Induction of IL-10-producing CD4+ T-cells in chronic periodontitis. J Dent Res. 2011;90:653–658. doi: 10.1177/0022034510397838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okui T, Aoki Y, Ito H, Honda T, Yamazaki K. The presence of IL-17+/FOXP3+ double-positive cells in periodontitis. J Dent Res. 2012;91:574–579. doi: 10.1177/0022034512446341. [DOI] [PubMed] [Google Scholar]
- 32.Jin Y, Wang LY, Liu DX, Lin XP. Tamibarotene modulates the local immune response in experimental periodontitis. Int Immunopharmacol. 2014;23:537–545. doi: 10.1016/j.intimp.2014.10.003. [DOI] [PubMed] [Google Scholar]


