Abstract
Objective(s):
Tuberculosis (TB) is still one of the problematic infectious diseases in developing countries, especially in Iran. In the present study, we applied ribosome display technique to select single chain variable fragments (scFvs) specific for the 6-kDa early secretory antigenic target (ESAT-6) antigen of Mycobacterium tuberculosis from a mouse scFv library.
Materials and Methods:
The gene encoding ESAT-6 was cloned into pET22b(+) plasmid and expressed in Escherichia coli BL21 (DE3). The purified recombinant ESAT-6 protein was injected into female BALB/c mice for immunization, and then m-RNA was extracted from the spleen of immunized mice. The anti-ESAT-6 VH/k chain library was assembled by joining of VH and k into the VH/k chain with a 72-bp DNA linker by SOE (splicing by overlap extension) PCR. The scFv library was panned against ESAT-6 using a single round of ribosome display via a rabbit reticulocyte lysate system.
Results:
ELISA assay showed that one of the selected scFvs had higher affinity against the recombinant ESAT-6 protein. The affinity of the candidate scFv was ~ 3.74×108 M-1.
Conclusion:
It could be proposed that the isolated scFv in this study may be useful for the diagnosis of TB.
Keywords: Antibody, ESAT-6, Mycobacterium tuberculosis, Ribosome display, scFv
Introduction
Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis. Despite much progression in disease control, the global burden of TB is huge with an estimated incidence of 9 million cases, resulting in 1.5 million deaths in 2013 (1). Existing diagnostic methods such as the purified protein derivatives (PPD) skin test are not very reliable, especially in HIV co-infected or BCG-vaccinated patients (2). The enzyme-linked immunosorbent spot method is more sensitive and dependable than PPD skin test, but this method can be only done by expert staff and needs expensive instruments. Therefore, a simple, fast and cheap diagnostic method is needed for TB control, and use of biomarkers which are peculiar to the pathogen for TB diagnosis has been debatable (3). Genomic studies have revealed 16 regions of differences (RD) in the genome of M. tuberculosis H37Rv which are present in all other M. tuberculosis complex members and absent in the BCG strains. Among these regions, RD1 plays a significant role in the virulence of M. tuberculosis (4). ESAT-6 and CFP-10 (culture filtrate protein 10) are both encoded by RD1 region and only exist in several pathogenic mycobacterial species (5, 6). ESAT-6 is a potent T-cell antigen in the region of difference 1 (7) and suggested as a diagnostic biomarker for TB infection and used in enzyme-linked immune spot assay. Recently, antibody fragments technology has provided several approaches for the manufacture of new diagnostic agents. These fragments are small proteins with the full antigen-binding capacity of the natural antibodies. A single-chain variable fragment (scFv) is a fusion protein (~28 kDa) of the variable (epitope-binding) regions of the heavy (VH) and light chains (VL) of an immunoglobulin, covalently connected with a short flexible polypeptide linker (10-25 amino acids) (8). ScFvs compared with whole antibodies have significant benefits due to their small size and homogeneity. Therefore, genetic manipulation to improve their function can be done easily (9). In recent years, in vitro display techniques such as phage and ribosome displays, offer a robust platform for design, selection, and production of novel scFvs for targeted proteins (10, 11). Ribosome display, a cell-free display method, overcomes the limitations of phage and cell display. For example, ribosome display enables the selection of toxic reagents and increases the capacity and diversity of libraries without transformation step (12, 13). This method developed by Hanes and Pluckthun in 1997, based on earlier work by Mattheakis et al in 1994 (14-16). By this method which is performed completely in vitro, phenotype (antigen binding) link to genotype (mRNA) through the formation of stable antibody-ribosome-mRNA (ARM) complexes (17-19).
In the present study, a single-chain variable fragments (scFvs) antibody library was successfully constructed. Then we applied ribosome display technique to pan scFvs against ESAT-6, aiming to select a specific scFv that bound to this antigen with high affinity.
Materials and Methods
Cloning of ESAT-6 gene into pET22b (+) expression vector
DNA encoding ESAT-6 sequence (288 bp) or EsxA or Rv3875 (GeneBank accession number BX842584) with S-tag sequence was synthesized into pGH plasmid (Bioneer, Korea) and confirmed using specific primers by PCR (94 °C for 40 sec, 57 °C for 40 sec and 72 °C for 1 min, 30 cycles). The PCR product (with S-tag) and pET22b (+) (Novagen, USA) were digested with BamHI and SalI (Fermentas, Lithuania). After gel Extraction (AccuPrep® Gel Purification Kit, Bioneer), ligation was carried out with T4 DNA Ligase (Fermentas, Lithuania) and the ligation reaction was transformed into Escherichia coli Top10 competent cell (20). The recombinant plasmids were confirmed by colony PCR, restriction enzyme analysis, and sequencing procedures.
Expression of the recombinant ESAT-6 protein
BL21(DE3) E. coli which was transformed with pET22b-ESAT, was grown in 100 ml of LB medium (with 100 µg/ml ampicillin) at 37 °C with shaking at 250 RPM to a density of OD600=0.6. Then, the Isopropyl-D-1-thiogalactopyranoside (IPTG) (Merck, Germany) was added to a final concentration of 1 mM. The cells were harvested after 3 hr and treated with lysis buffer (Tris 50 mM, 10% glycerol, 0.1% Triton X-100) (Merck, Germany). The cell lysate was analyzed by sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS-PAGE) (21).
Purification of recombinant protein
S-tagged ESAT-6 protein was purified by affinity chromatography. The cell pellet obtained from 100 ml of culture was washed twice with 5 ml of PBS (1X) and centrifuged at 8000 rpm for 5 min. The cell pellet also was washed twice with 5 ml of Sol I (EDTA 2 mM, Tris-HCl 50 mM, Triton X-100 1%, pH: 7-8) and centrifuged at 8000 rpm for 5 min. After that, the cell pellet was dissolved in 5 ml of Sol II (6 M Urea, 2 mM EDTA, 50 mM Tris-HCl, pH 7-8) and lysed by sonication on ice. The supernatant was loaded on the S-protein agarose (Novagen, USA). The resin was washed 3 times with 10 ml of Sol III (2 M urea, 2 mM EDTA, 50 mM Tris-HCl, pH 7-8), the bounded proteins were eluted with 1 ml of elution buffer (3 M NaSCN, 2 mM EDTA, 50 mM Tris-HCl, pH 7-8) and dialyzed with PBS (1X) at room temperature for 3 hr (22, 23). The purified protein was consequently analyzed by SDS-PAGE and Western blotting using anti-S-tag antibody (Novagen, USA).
Immunization of mice with recombinant ESAT-6 protein
For immunization of mice, three four-week-old female BALB/c mice were purchased from Pasteur Institute (Tehran, Iran) and immunized by intraperi-toneal injection of a 1:1 (v/v) mixture of 25 and 50 (μg/ml) ESAT-6 protein and Freund’s complete adjuvant (Sigma, USA). The first immunization was followed by boosting with the same amount of antigen together with Freund’s incomplete adjuvant after ten days. After 14 days, test bleeds were taken from their eyes for ELISA. Following immunization against ESAT-6, the spleens of the mice with the highest antibody titer were removed and used for RNA extraction.
Construction of the scFv (VH/k) chain library
Total RNA from spleen cells of the immunized mice was isolated using Total RNA Purification kit (Jena Bioscience, Germany) according to the manufacturer’s instructions. The complementary DNA (cDNA) was synthesized by RT-PCR using Oligo(dT) primer. DNA encoding the mouse variable region of the heavy (VH) and k chains were synthesized by PCR. DNA encoding VH was amplified using VH/back (5´(C/G)AGGT(G/C)-CA(G/C)CTCGAG(C/G)AGTCTGG3´) and VH /for (5´TG-AGGAGACGGTGACCGTGGTCCCTTGGCCCC3´) primers; DNA encoding k-chain was amplified using VLF (5´GAC-ATTGAGCTCACCCAGTCTCCA3´) and CKR (5´GCTCT- AGAACACTCATTCCTGTTGGAGCT3´) primers (24). The VH and k chains were assembled by SOE PCR using a 72-bp DNA linker (5’GTCACCGTCTCCTCAGGTGGT-GGTGGTAGCGGCGGCGGCGGCTCTGGTGGTGGTGGATCCGACATTGAGCTCACCC3’) including a flexible DNA linker (Gly4Ser)3. At first VH fragments were amplified by PCR with specific primers mentioned above using Taq polymerase with 25 cycles of PCR (94 °C for 40 sec, 54 °C for 40 sec and 72 °C for 1 min, 30 cycles). DNA Linker was amplified with Link/F (5´GTCACCGTCTCC-TCAGGTGGTGGT3´) and Link/R (5´ATCCGACATTGAGC-TCACCC3´) primers. After gel purification of VH and linker fragments by Gel Purification Kit (Bioneer, Korea) 15 ng of VH DNA and 20 ng of linker DNA were mixed with 25 μl PCR mixture and cycled 25 times (1 cycle 1 min at 94° C, 2 min 60 °C, 2 min 72 °C) to join the linker DNA with VH DNA. The assembled DNA was amplified in a 50 μl PCR mixture for 25 cycles (1 cycle 1 min at 94 °C, 1 min 55 °C, 1 min 72 °C) with T7Ab/back (5´GCAGCTAATACGACTCACTATAGGAAGAACAGACCACCATG(C/G)AGGT(G/C)CA(G/C)CTCGAG(C/G)AGTCTGG3´) and link/R. DNA encoding k-chain was amplified with specific primers by PCR with the same program described for amplification of VH fragments. The PCR products of VH-linker fragments and k-chains were gel purified. The recombinant DNA encoding the single-chain antibody library in VH/k format was constructed by linking of 15 ng VH-linker DNA and 50 ng k-chain DNA with 25 μl PCR mixture without primers. PCR was done by the program used for joining VH to the linker. The assembled DNA was amplified in a 50 μl PCR mixture for 25 cycles (as mentioned above) with T7Ab/back and CKR primers (12, 24). The T7Ab/back primer included a ribosome binding site and a T7 promoter and CKR primer contained nonstop codon. The VH/k PCR library was stored at -20 °C.
In vitro transcription and translation of scFvs
We applied eukaryotic ribosome display for library expression and isolation of scFvs. A 0.5-ml PCR tube was coated with 100 μl of recombinant ESAT-6 antigen (10 μg/ml in PBS) and incubated for 16 hr at room temperature for each DNA sample. After washing with cold PBS, the tubes were blocked with 100 μl BSA-PBS for one hr. For in vitro transcription and translation of antibody-coding genes, we used TNT T7 Quick Master Mix (Promega, USA). The reaction mixture was prepared (in a total volume of 25 μl) by mixing of 20 μl TNT T7 Quick Master Mix, 1 μl of DNA library (0.1-1 μg), 0.5 μl methionine, and 3.5 μl nuclease free water and incubated for 90 min at 30 °C. 6 μl of RNase-free DNaes I (Fermentase, Lithuania) was added and the mixture was incubated for 30 min at 30 °C (25). The combination (containing the antibody-ribosome-mRNA complexes) was added to the prepared ESAT-6 coated tube to bind and select the specific antibody fragments, and incubated on ice for 1 hr. The tube was washed three times with PBS (containing 10 mg/ml BSA and 5 mM MgCl2), the retained ribosomal complexes were dissociated by adding 200 μl PBS with 20 mM EDTA for 10 min on ice (26). The conserved mRNA in separating buffer was isolated by RNA Purification kit (Jena Bioscience, Germany) and recovered by RT-PCR with primers VH/back-NcoI AGGT(G/C)CA(G/C)CTCGAG(C/G)AGTCTGG3´)- and Ck/for-NotI (5´ATATATATATGCGGCCGCTGCA-GCATCAGCCCGTTT3´) which have NcoI and NotI restriction sites (underlined) for cloning into expression vector. The obtained PCR product was cloned into pTG19-T vector (Thermo, USA). After sequencing of derived plasmids, the clones with correct reading frame and without any stop codons were selected for expression of scFvs.
Cloning and expression of selected scFvs into expression vector
After selection, the selected scFv DNA was amplified using specific primers (VH/back-NcoI and Ck/for-NotI). The amplified DNA was digested with NotI and NcoI restriction enzymes and ligated into the expression vector pET28a(+) (Novagen, USA) using the T4 DNA ligase (Fermentase, Lithuania). The recombinant plasmids were transformed into E. coli BL21. Transformed bacteria were grown in LB medium containing 30 µg/ml kanamycin at 37 °C to a density of OD600=0.6. Then, IPTG (final concentration of 0.5 mM) was added to induce expression of recombinant scFvs. After induction, the cells were incubated at 37°C for 3 hr. The cells were pelleted by centrifugation at 8000 rpm for 5 min. Then E. coli lysates of tested clones were run on 12% SDS-PAGE. The Expression of scFvs was confirmed by Western blotting with 1:2000 dilution of anti-His-tag monoclonal antibody (Abcam, UK) conjugated with ALP (alkaline phosphatase). The immunoreactivity was visualized using NBT/BCIP substrate solution (Roche, Germany).
Purification of recombinant His-tagged scFvs
We applied affinity chromatography using Ni-NTA agarose resin (Novagen, USA) for purification of expressed His-tagged scFvs. The cell pellet obtained by centrifugation of 100 ml of the induced culture was resuspended in 5 ml of denaturing buffer (6 M Urea, 20 mM NaH2PO4, 500 mM NaCl, pH 8.0) and lysed by sonication on ice. After centrifugation at 12000 rpm for 25 min, the supernatant (containing the His-tagged scFvs) was loaded on the equilibrated resin. The column was washed 3 times with 10 ml of wash buffer (10 mM, 20 mM and 30 mM gradient of imidazole, 20 mM NaH2PO4, 500 mM NaCl, pH 8.0), the bounded proteins were eluted with 1 ml of elution buffer (500 mM imidazole, 20 mM NaH2PO4, 500 mM NaCl, pH 8.0) (27) and dialyzed in PBS (1X) at room temperature for 3 hr.
Analysis of scFv binding activity by ELISA
ELISA assay was used to examine the binding activity of selected scfv clones to ESAT-6 protein. Microtiter plate was coated with recombinant ESAT-6 (2 µg/ml in PBS) and incubated overnight at room temperature, and PBSB (PBS containing 3% (w/v) BSA) was used for blocking for 2 hr. After washing three times, 100 µl of extracted scFvs antibody diluted with PBST (5 µg/ ml) were added to the well and incubated for 5 hr at 37 °C. To determine the amount of scfv antibody that had bound, horseradish peroxidase (HRP) conjugated anti-His tag antibody (diluted 1/10000 in PBST) (Abcam, UK) was used as a secondary antibody. The immunoreaction was started by addition of 50 µl of TMB (tetramethylbenzidine). After 20 min, the peroxidase reaction was stopped by addition of 50 µl of 2N H2SO4. Absorbance was measured at 450 nm using a microtiter plate reader.
Affinity analysis of selected scFvs
The affinity of selected scfvs against recombinant ESAT-6 was determined by ELISA and affinity constant (Kaff) was calculated using the method described by Beatty et al For ELISA assay, Microplate wells were coated with purified recombinant S-tagged ESAT-6 protein in the concentration of 2, 1, 0.5, 0.25 and 0.125 µg/ml in carbonate–bicarbonate buffer, pH 7.2 at 4 °C overnight. For each concentration of antigen, we used 5, 2.5, 1.25, and 0.625 µg/ml of purified scFv antibody separately and ELISA was performed as described earlier. The following Beatty’s equation was used to calculate the Kaff of isolated scFvs (28):
Kaff = (n-1)/2(n [Ab´]-[Ab])
n= [Ag] / [Ag´]
Results
Cloning, expression, and purification of recombinant ESAT-6
DNA encoding ESAT-6 sequence was cloned into pET22b(+) expression vector and expression of recombinant protein was analyzed by SDS-PAGE and Western blotting that showed in Figure 1.
Figure 1.
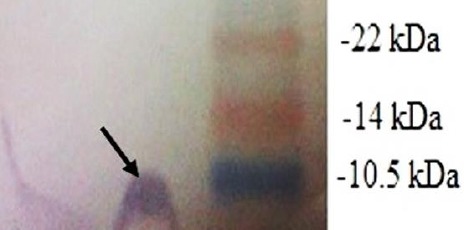
Western blot analysis of the recombinant protein after 3 hr induction
Construction of a VH/k chain library
The spleen of immunized mouse was isolated, and after RNA extraction VH fragments (~ 350 bp) and k-chain DNA (~650 bp) were amplified by PCR with corresponding primers. At first VH fragments and linker sequence (76 bp) were joined by SOE PCR and the resulting product (VH-linker) was joined to k-chain DNA by SOE PCR. The final assembled product (~ 1100 bp) which contained the library of the mouse antibody genes with a T7 promoter and ribosome binding site was used in an in vitro ribosome display (Figure 2).
Figure 2.
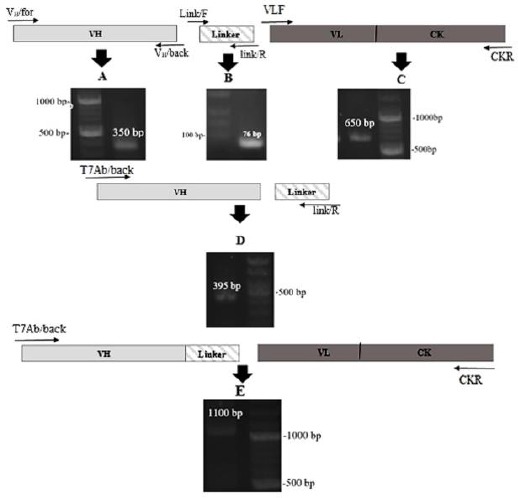
An illustration of the scFv (VH/k) chain library construction steps. Agarose gel electrophoresis of PCR product for each step is shown. (A) VH fragment 350 bp; (B) linker 76 bp; (C) k-chain 650 bp; (D) VH-linker 395 bp; (E) assembled VH/k fragments 1100 bp
In vitro transcription and translation of scFvs
The eukaryotic ribosome display was performed for library expression and selection of scFvs. The final PCR product (720 bp) was cloned into pTG19-T vector, and 15 clones of white colonies were picked randomly and sequenced. After checking the sequences, five clones were chosen for further analysis.
Cloning, expression, and purification of selected scFvs
The scFv sequences of all five clones were amplified separately with primers VH/back-NcoI and Vk/for-NotI. The PCR products were ligated into pET28a(+) vector. The His-tagged scFvs were expressed in E. coli BL21 and purified by affinity chromatography using Ni-NTA agarose resin. The expressed proteins (27 kDa) were analyzed by SDS-PAGE and western blotting (Figure 3).
Figure 3.
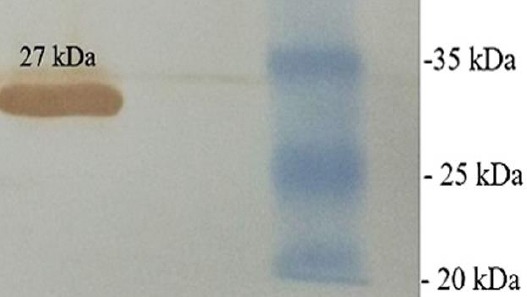
Western blot analysis of scFv (clone 3) expression after 3 hr induction
Characterization of purified scFvs
The binding activity of each purified scFv was tested by ELISA. The results indicated that all five recombinant scFvs had binding activity to ESAT-6 protein but two clones (no.3 and 7) (accession no. LC189555, LC189556) had more binding activity (Figure 4). Affinity analysis of the purified scFvs showed an affinity constant of 3.74×108 M−1 for clone 3 and 1.29×108 M−1 for clone 7. According to these results, Figure 5 explains the result of selected clone no.3.
Figure 4.

Characterization of selected clones by ELISA analysis. The binding activity of the purified scFv from each clone was tested against ESAT-6 protein, E. coli lysate and BSA as a control
Figure 5.

Affinity of clone 3 scFv was assessed by ELISA. The affinity constant of this clone was 3.74×108 M−1
Sequence analysis
DNA sequences of clone three and clone seven were translated into their amino acid sequences and aligned together by PRALINE multiple sequence alignm-ent (http://www.ibi.vu.nl/programs/pralinewww/) (Figure 6). Complementary determining region (CDR) of the VH and VL domains were identified by IMGT/V-Quest database. Sequence analysis showed 13 amino acid substitutions between two clones. Three of these changes were in CDRs (VH-CDR2, VL-CDR1, and VL-CDR2) and the others were in framework regions.
Figure 6.

Alignment and comparison of the amino acid sequences of selected scFv clones. The CDRs and linker are boxed
Discussion
Human TB is a significant infectious disease which is still problematic, especially in developing countries (1). The currently used diagnosis methods for TB are time-consuming or expensive or small sensitive, and some of them can only be done by professional staff. Therefore, the development of a simple, cost-effective, fast, and accurate diagnostic technique is crucial to control this disease and to reduce the public health costs for it (29). Monoclonal antibodies against pathogen-specific antigens could be useful to develop such a technique. Recently, in vitro display techniques such as ribosome display, offer a robust platform for design, selection, and production of novel antibodies for targeted proteins (11). ESAT-6 is a potent T-cell antigen of M. tuberculosis that induces the secretion of INF-γ. This antigen, which is unique to tuberculose mycobacteria has a strong potential for diagnosis both the virulent form and possible form of M. tuberculosis (30). At present study, we selected an anti ESAT-6 scFv by employing ribosome display. Recombinant ESAT-6 was used to immunize BALB/c mice. A DNA library of VH/k antibody fragments was constructed using SOE PCR according to a protocol described by He & Taussig (12). This scFv DNA library was expressed in vitro using an in vitro transcription and translation system. There are two systems for in vitro transcrip-tion and translation: (I) prokaryotic ribosome display (by a coupled E. coli S30 system) and (II) eukaryotic ribosome display (by a coupled rabbit reticulocyte lysate system) (12, 14). Here we used the eukaryotic ribosome display for scFv selection because this scheme has lower RNase activity and so reduces the loss of material during the process and, could recover the translation and folding output of some proteins (14, 31). In most studies the whole cell of a pathogen has been used for immunization of mice and library construction (19, 26), and panning step has been repeated more than once (32, 33). In this work, we used recombinant ESAT-6 (a pathogen-specific antigen) instead of the whole cell and performed only one round of panning. Finally, after evaluating a few clones (5 clones), two clones had high affinity to ESAT-6. Our results and selection of two scFvs with high affinity by only one round of panning may be due to the use of specific antigen in immunization and library construction. Anti-ESAT-6 antibodies which have been produced so far, whether polyclonal or monoclonal, have been generated by traditional methods and all are whole antibodies (34-36). Here we generated anti-ESAT-6 scFv which is a type of antibody fragments by applying a fully cell-free method. ScFv antibodies compared with polyclonal and monoclonal antibodies have significant benefits due to their small size and homogeneity. Therefore, genetic manipulation to improve their function can be done easily. For example to generate bifunctional proteins they can be fused to the marker proteins for use in single-step immunodetection (8). To evaluate scFvs affinity, two scFv molecules can be bound together to form diabodies which are bivalent and bispecific antibody fragments and have higher affinity (9). The affinity of selected scFv (clone 3) was determined 3.74×108 M−1 by using Betty method (28), which was reasonable. Our results show that this anti-ESAT-6 scFv exhibited high affinity to the recombinant ESAT-6. Clinical studies on patient samples are being designed to investigate the potential usefulness of this scFv in the diagnosis of TB.
Conclusion
The anti-ESAT-6 VH/K chain ribosome display library was assembled by joining VH and k into the VH/k chain using a peptide constructed linker via SOE PCR. The scFv library was panned against ESAT-6 using a single round of ribosome display. The selected scFv showed a high affinity in the enzyme-linked immunosorbent assay. The calculated affinity of the selected scFv was 3.74×108 M−1.
Acknowlwgment
This work was carried out in the Cellular & Molecular Biology Research Center of Shahid Beheshti University of Medical Sciences, Tehran, Iran. This article was extracted from Shahrzad Ahangarzadeh’s PhD thesis. This study was funded by deputy of Shahid Beheshti University of Medical Sciences, Tehran, Iran (grant number 5828).
Conflict of interest
The Authors declare that they have no conflict of interest. “All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.”
References
- 1.Zumla A, George A, Sharma V, Herbert RH, Baroness Masham of I, Oxley A, et al. The WHO 2014 global tuberculosis report--further to go. Lancet Glob Health. 2015;3:e10–2. doi: 10.1016/S2214-109X(14)70361-4. [DOI] [PubMed] [Google Scholar]
- 2.Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 3.Mukundan H, Kumar S, Price DN, Ray SM, Lee YJ, Min S, et al. Rapid detection of Mycobacterium tuberculosis biomarkers in a sandwich immunoassay format using a waveguide-based optical biosensor. Tuberculosis (Edinb) 2012;92:407–416. doi: 10.1016/j.tube.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Ingen J, de Zwaan R, Dekhuijzen R, Boeree M, van Soolingen D. Region of difference 1 in nontuberculous Mycobacterium species adds a phylogenetic and taxonomical character. J Bacteriol. 2009;191:5865–5867. doi: 10.1128/JB.00683-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganguly N, Giang PH, Gupta C, Basu SK, Siddiqui I, Salunke DM, et al. Mycobacterium tuberculosis secretory proteins CFP-10, ESAT-6 and the CFP10: ESAT6 complex inhibit lipopolysaccharide-induced NF-kappaB transactivation by downregulation of reactive oxidative species (ROS) production. Immunol Cell Biol. 2008;86:98–106. doi: 10.1038/sj.icb.7100117. [DOI] [PubMed] [Google Scholar]
- 6.van Pinxteren LA, Ravn P, Agger EM, Pollock J, Andersen P. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin Diagn Lab Immunol. 2000;7:155–160. doi: 10.1128/cdli.7.2.155-160.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodin P, de Jonge MI, Majlessi L, Leclerc C, Nilges M, Cole ST, et al. Functional analysis of early secreted antigenic target-6, the dominant T-cell antigen of Mycobacterium tuberculosis reveals key residues involved in secretion, complex formation, virulence, and immunogenicity. J Biol Chem. 2005;280:33953–33959. doi: 10.1074/jbc.M503515200. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad ZA, Yeap SK, Ali AM, Ho WY, Alitheen NB, Hamid M. scFv antibody: principles and clinical application. Clin Dev Immunol. 2012;2012:980250. doi: 10.1155/2012/980250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson AL. Antibody fragments: hope and hype. MAbs. 2010:277–83. doi: 10.4161/mabs.2.1.10786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gan R, Jewett MC. Evolution of translation initiation sequences using in vitro yeast ribosome display. Biotechnol Bioeng. 2016;113:1777–1786. doi: 10.1002/bit.25933. [DOI] [PubMed] [Google Scholar]
- 11.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 12.He M, Taussig MJ. Ribosome display of antibodies: expression, specificity and recovery in a eukaryotic system. J Immunol Methods. 2005;297:73–82. doi: 10.1016/j.jim.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 13.Weichhart T, Horky M, Sollner J, Gangl S, Henics T, Nagy E, et al. Functional selection of vaccine candidate peptides from Staphylococcus aureus whole-genome expression libraries in vitro. Infect Immun. 2003;71:4633–4641. doi: 10.1128/IAI.71.8.4633-4641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanes J, Pluckthun A. In vitro selection and evolution of functional proteins by using ribosome display. Proc Natl Acad Sci U S A. 1997;94:4937–4942. doi: 10.1073/pnas.94.10.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattheakis LC, Bhatt RR, Dower WJ. An in vitro polysome display system for identifying ligands from very large peptide libraries. Proc Natl Acad Sci U S A. 1994;91:9022–9026. doi: 10.1073/pnas.91.19.9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pluckthun A. Ribosome display: a perspective. Methods Mol Biol. 2012;805:3–28. doi: 10.1007/978-1-61779-379-0_1. [DOI] [PubMed] [Google Scholar]
- 17.Binz HK, Amstutz P, Kohl A, Stumpp MT, Briand C, Forrer P, et al. High-affinity binders selected from designed ankyrin repeat protein libraries. Nat Biotechnol. 2004;22:575–582. doi: 10.1038/nbt962. [DOI] [PubMed] [Google Scholar]
- 18.He M, Taussig MJ. Antibody-ribosome-mRNA (ARM) complexes as efficient selection particles for in vitro display and evolution of antibody combining sites. Nucleic Acids Res. 1997;25:5132–5134. doi: 10.1093/nar/25.24.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou L, Mao WP, Fen J, Liu HY, Wei CJ, Li WX, et al. Selection of scFvs specific for the HepG2 cell line using ribosome display. J Biosci. 2009;34:221–226. doi: 10.1007/s12038-009-0026-2. [DOI] [PubMed] [Google Scholar]
- 20.Hosseini ES, Moniri R, Goli YD, Kashani HH. Purification of antibacterial CHAPK protein using a self-cleaving fusion tag and its activity against methicillin-resistant staphylococcus aureus. Probiotics Antimicrob Proteins. 2016;8:202–210. doi: 10.1007/s12602-016-9236-8. [DOI] [PubMed] [Google Scholar]
- 21.Kashani HH, Moniri R. Expression of recombinant pET22b-LysK-cysteine/histidine-dependent amidohydrolase/peptidase bacteriophage thera-peutic protein in escherichia coli BL21 (DE3) Osong Public Health Res Perspect. 2015;6:256–260. doi: 10.1016/j.phrp.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yarian F, Bandehpour M, Seyed N, Kazemi B. Cloning, expression and purification of the factor H binding protein and its interaction with factor H. Iran J Microbiol. 2016;8:29–35. [PMC free article] [PubMed] [Google Scholar]
- 23.Schmelcher M, Powell AM, Becker SC, Camp MJ, Donovan DM. Chimeric phage lysins act synergistically with lysostaphin to kill mastitis-causing Staphylococcus aureus in murine mammary glands. Appl Environ Microbiol. 2012;78:2297–2305. doi: 10.1128/AEM.07050-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee MS, Kwon MH, Kim KH, Shin HJ, Park S, Kim HI. Selection of scFvs specific for HBV DNA polymerase using ribosome display. J Immunol Methods. 2004;284:147–157. doi: 10.1016/j.jim.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Azizi A, Arora A, Markiv A, Lampe DJ, Miller TA, Kang AS. Ribosome display of combinatorial antibody libraries derived from mice immunized with heat-killed Xylella fastidiosa and the selection of MopB-specific single-chain antibodies. Appl Environ Microbiol. 2012;78:2638–26347. doi: 10.1128/AEM.07807-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo Y, Xia Y. Selection of single-chain variable fragment antibodies against fenitrothion by ribosome display. Anal Biochem. 2012;421:130–137. doi: 10.1016/j.ab.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 27.Sankian M, Yousefi M, Pazouki N, Varasteh A. One-step purification of histidine-tagged profilin with high purity and yield by using metalprecipitation. Biotechnol Appl Biochem. 2007;47:185–189. doi: 10.1042/BA20060214. [DOI] [PubMed] [Google Scholar]
- 28.Beatty JD, Beatty BG, Vlahos WG. Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. J Immunol Methods. 1987;100:173–179. doi: 10.1016/0022-1759(87)90187-6. [DOI] [PubMed] [Google Scholar]
- 29.Ireton GC, Greenwald R, Liang H, Esfandiari J, Lyashchenko KP, Reed SG. Identification of Mycobacterium tuberculosis antigens of high serodiagnostic value. Clin Vaccine Immunol. 2010;17:1539–1547. doi: 10.1128/CVI.00198-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sreejit G, Ahmed A, Parveen N, Jha V, Valluri VL, Ghosh S, et al. The ESAT-6 protein of Mycobacterium tuberculosis interacts with beta-2-microglobulin (beta2M) affecting antigen presentation function of macrophage. PLoS Pathog. 2014;10:e1004446. doi: 10.1371/journal.ppat.1004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohashi H, Shimizu Y, Ying BW, Ueda T. Efficient protein selection based on ribosome display system with purified components. Biochem Biophys Res Commun. 2007;352:270–276. doi: 10.1016/j.bbrc.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 32.Qing Yuan YX, Siji Nian, Youping Yin, Yueqing Cao, Wang Z. Selection of single chain fragments against the phytopathogen Xanthomonas axonopodis pv. citri by ribosome display. Enzyme and Microbial Technol. 2007;41:383–389. [Google Scholar]
- 33.Zhao XL, Chen WQ, Yang ZH, Li JM, Zhang SJ, Tian LF. Selection and affinity maturation of human antibodies against rabies virus from a scFv gene library using ribosome display. J Biotechnol. 2009;144:253–258. doi: 10.1016/j.jbiotec.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 34.Feng F, Zhang H, Zhu Z, Li C, Shi Y, Zhang Z. The application of anti-ESAT-6 monoclonal antibody fluorescent probe in ex vivo near-infrared fluorescence imaging in mice with pulmonary tuberculosis. Luminescence. 2014;29:614–620. doi: 10.1002/bio.2593. [DOI] [PubMed] [Google Scholar]
- 35.Feng TT, Shou CM, Shen L, Qian Y, Wu ZG, Fan J, et al. Novel monoclonal antibodies to ESAT-6 and CFP-10 antigens for ELISA-based diagnosis of pleural tuberculosis. Int J Tuberc Lung Dis. 2011;15:804–810. doi: 10.5588/ijtld.10.0393. [DOI] [PubMed] [Google Scholar]
- 36.Leng J, Ding Y, Shou C, Wu Z, Zhuo G, Wang K, et al. Development of a novel anti ESAT-6 monoclonal antibody for screening of Mycobacterium tuberculosis. Int J Clin Exp Med. 2014;7:4238–4243. [PMC free article] [PubMed] [Google Scholar]


