Abstract
Objective(s):
Ischemia/reperfusion (I/R) injury of spinal cord is leading to the paraplegia observed. In this study, we investigated the protective effect of the saffron extract on spinal cord I/R injury.
Materials and Methods:
Thirty five male Sprague-Dawley rats were divided into 5 groups: intact, sham surgery, normal saline (NS), low dose saffron aqua extract, high dose saffron aqua extract.
Results:
The mean motor deficit index (MDI) scores were significantly lower in the saffron extract groups than in the NS group at 48 hr after spinal cord ischemia (P<0.001). Saffron extract groups significantly decreased plasma level of malondialdehyde than in the NS Group (P<0.05). The number of motor normal neurons was significantly greater in the high saffron extract group than in the NS and low saffron group (P<0.05).
Conclusion:
These data suggest that a saffron extract may protect spinal cord neurons from I/R injury.
Keywords: Ischemia, Reperfusion, Spinal cord, Saffron extract
Introduction
Ischemia/reperfusion (I/R) injury may occur in a variety of clinical settings, including organ transplan-tation, aortic cross-clamping or cardiopulmonary bypass (1). Spinal cord I/R injury is a serious compli-cation of thoracoabdominal aortic surgery (2), and so severe postoperative complications may arise, such as paraplegia (3). It is known that the major proportion of the damage occurs during reperfusion when free oxygen radicals induce lipid peroxidation (4). In the literature some antioxidative and anti-inflammatory agents are used to prevent paraplegia due to aortic ischemia in animal models (5). Saffron consists of the dried stigmas of Crocus sativus L., is used in folk medicine for various purposes such as an antinociceptive (6), anti-inflammatory (7). Previous studies have demonstrated that saffron extract and its active constituents have a protective effect against hippocampal (8), skeletal muscle (9), and kidney I/R injury (10).
However, there are no studies assessing the neuroprotective actions of saffron extract on spinal cord I/R. For this reason, we planned this study to determine neurological, biochemical and histotologic evaluation of saffron extract on I/R spinal cord injury in a rat model.
Materials and Methods
Experimental design
Thirty five male Sprague-Dawley rats (weighing 250 to 300 g) were divided randomly into 5 equal groups: intact, sham surgery, normal saline or NS, low dose saffron extract (20 mg/kg saffron extract), high dose saffron extract (80 mg/kg saffron extract). This study was approved by the ethical committee of Urmia University of Medical Sciences.
Preparation of saffron extract
Saffron was purchased from Novin Saffron (Iran) in 2015. The powdered stigma was macerated in water (80%, v/v) for 3 days at 4 °C, in the absence of light and with continuous stirring. The extract was filtered (0.2 µm), and then it was concentrated by freeze dryer.
Surgery procedure
Animals were anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg) via intraperitoneal (IP). Under sterile conditions, making a midline laparatomy incision of approximately 5 cm in length, the abdominal aorta was exposed. The operation was terminated at this point in the sham surgery group.
The abdominal aorta was clamped for 45 min with mini aneurysm clamps between just below the left renal artery and just proximal to the aortic bifurcation (11). After ischemia, the cross-clamps were removed and distal perfusion was observed visually. Then, abdomen was closed. 30 min before an operation, a single dose of 20 mg/kg and 80 mg/kg saffron extract was administrated intraperitoneally to rats of saffron experimental groups (12), while 1 ml normal saline was administrated intraperitonea-lly to rats of the control group. The Crede maneuver was used to empty the urinary bladders of the paraplegic animals at least twice daily.
Neurologic evaluation
At 48 hr after spinal cord ischemia, an indepen-dent observer, who was blinded to the protocol and group assignments, assessed the motor deficit index (MDI) score (13). MDI was scored using the assessment of ambulation using the hind limbs and by the placing/stepping reflex. Animals with MDI < 3 were considered a nonparaplegic, and animals with MDI ≥ 3 were considered as paraplegics.
Blood sampling
After neurologic evaluation, blood samples were collected by a direct cardiac puncture and transferred on ice to be centrifuged at 1500 g for 15 min at 4 °C to obtain plasma. The plasma samples were stored at –80 °C until the time of assay for plasma levels of MDA and TAC
Biochemical measurements
Plasma level of malondialdehyde (MDA) is formed as an end product of lipid peroxidation, which reacts with the TBA reagent under acidic conditions to generate a pink-colored product whose absorbance was measured spectrophotometrically (Jasco, UV-975, Tokyo, Japan) at 532 nm. Plasma level of total antioxidative capacity (TAC) was assessed using a kit (LDN Labor Diagnostika Nord GmbH and Co KG, Germany). The determination of the TAC is based on the reaction of peroxides with peroxidase followed by a color reaction of the chromogenic substrate tetramethyl benzidine. Its blue color turns to yellow after addition of the stop solution and can be measured spectrophotometri-cally at 450 nm.
Histologic evaluation
After the blood samples have been taken, the rats were then transcardially perfused by buffered formalin. The spinal cords were removed and postfixed in the same fixative for 1-2 days. The fourth lumbar spinal segment was dissected, embedded in paraffin, cut transversely at 5 µm, and stained with H-E. The number of normal motor neurons in the ventral part of the gray matter at ×400 magnification. Cells that contained Nissle substance in the cytoplasm, loose chromatin, and prominent nucleoli were considered to be normal neurons. The number of normal motor neurons was counted in 3 sections for each animal and averaged by an observer unaware of group assignment (14).
Statistical analysis
Statistical analysis and calculations were performed by using SPSS 16.0 for Windows (Chicago, IL, USA). All values were presented as means±standard deviation. Statistical analysis was done by one-way ANOVA followed by Tukey´s post hoc test for multiple comparison. Kruskal- Wallis analysis of variance was used to detect differences of MDI among groups and statistical comparison was made using the Mann-Whitney U test. A P<0.05 was considered statistically significant.
Results
Neurological assessment based on MDI is shown in Figure 1. The mean MDI scores were significantly lower in the saffron extract groups than in the NS group at 48 hr after spinal cord ischemia (P<0.001) (Figure 1).
Figure 1.

The mean neurological scores assessed at 48 hr after spinal cord ischemia. *demonstrated a significant difference normal saline group in comparison to other groups (P<0.05); **demonstrated a significant difference saffron 20 mg/kg group in comparison to intact, sham surgery, and normal saline groups (P<0.05)
Findings from the NS group show significantly decreased plasma level of TAC when compared with high dose saffron extract group (P<0.001). Saffron extract groups significantly decreased plasma level of MDA than in the NS Group (P<0.05). The plasma levels of MDA and TAC were almost similar in the sham and low saffron extract group, whereas at high saffron extract group significantly increased plasma level of TAC (P<0.001). The significant lowering effect of plasma level of MDA was observed in saffron groups than NS group (P<0.001). The plasma level of TAC was significantly increased in the high saffron group compared with sham surgical group (P<0.05), but there was no significant difference between the NS and the low saffron extract groups (P<0.05).
The number of motor normal neurons was significantly greater in the high saffron extract group than in the NS and low saffron group (P<0.05). Although approximately 64% of motor neurons in the ventral gray matter were lost in NS group, only approximately 17% and 35% were lost in animals given high and low saffron extract, respectively (Figure 2).
Figure 2.
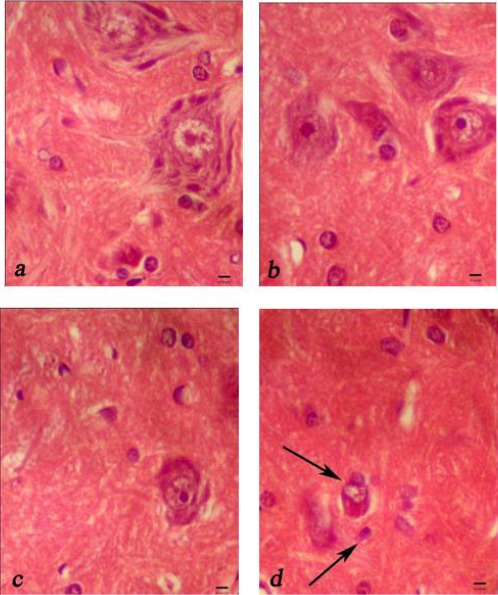
Representative light microphotographs of the anterior horn of spinal cord at 48 hr after ischemia in the sham surgery group (a), saffron extract (80 mg/kg) group (b), saffron extract (20 mg/kg) group (c), and normal saline group (d). Shrunken neurons contained dark hyperchromatic nuclei and Nissl granules had disappeard, were shown (arrow). Sacle bar = 10 µm
Discussion
Our results showed that the animal in saffron extract groups had a better hind limb motor function and less gray matter injury 48 hr after spinal cord ischemia. The present study is the first report describing the protective effect of saffron extract on I/R of spinal cord in rats.
This study assessed the effect of the saffron extract, because the saffron extract was more potent than its constituents such as crocin, crocetin, and safranal which quench free radicals and have antioxidant effects and may have a role in the protective effect of saffron on the spinal cord (14). In addition saffron extract may be assigned to a synergistic action of many constituents, and its constituents may be impure (9).
Saffron extract prevented lipid peroxidation and showed antioxidant activity in this study. A potential cause of delayed neuronal damage is an over inflammatory response that continues after reperfusion (15). Hosseinzadeh et al (2002) concluded that aqueous extracts of saffron have an anti-inflammatory activity (16). Because saffron extract can inhibit MDA production, the preservation of hind limb motor function observed in the present study may have been associated with a protective effect of saffron extract against nervous injury.
The present study has shown that the protective effect against I/R injury of the saffron extract is dependent on dose. Similar to another study (12), histologic evaluation revealed that pretreatment with aqueous saffron extract (80 mg/kg) as compared to control group yielded strong protection of motor neurons.
The present study has shown that a saffron extract could have a protective effect against I/R injury through an increase in the TAC and a reduction in MDA. Nam et al (2010) shown that a saffron extract has potent neuroprotective effects, so that blocked the effect of lipopolysaccaride on hippocampal cell (17). It seems that at least one part of the neuroproecive effect of saffron extract in spinal cord I/R is due to antioxidant activity and superoxide production. In our study, plasma levels of MDA were found to be significantly higher, and TAC levels, significantly lower, in NS group, compared with other groups. Reactive oxygen intermediates cause direct cellular injury, which leads to the destruction of the cell membrane by oxidative injury to cellular proteins and nucleic acids and by inducing lipid peroxidation (18).
Conclusion
These data suggest that a saffron extract may protect spinal cord neurons from I/R injury and it significantly decreased the levels of free radicals and act as an antioxidant. The protective effect of saffron extract is probably multifactorial, and further extensive study is needed to identify the mechanisms of action of saffron extract and its constituents on I/R of spinal cord in different conditions.
Acknowledgment
This paper has been extracted from an MSc thesis in the Urmia University of Medical Sciences. Funding for this research project was supported by a grant (contract No. 1551) from Urmia University of Medical Sciences in Urmia, Iran.
References
- 1.Eltzschig HK, Collard CD. Vascular ischemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 2.Li XQ, Lv HW, Tan WF, Fang B, Wang H, Ma H. Role of the TLR4 pathway in blood-spinal cord barrier dysfunction during the biomodal stage after ischemia/reperfusion injury in rats. J Neuroinflamm. 2014;11:62. doi: 10.1186/1742-2094-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wan IY, Angelini GD, Bryan AJ, Ryder I, Underwood MJ. Prevention of spinal cord ischemia during descending thoracic and thoracoabdominal aortic surgery. Eur J Cardiothorac Surg. 2001;19:203–213. doi: 10.1016/s1010-7940(00)00646-1. [DOI] [PubMed] [Google Scholar]
- 4.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 5.Saito T, Tsuchida M, Umehara S, Kohno T, Yamamoto H, Hayashi J. Reduction of spinal cord ischemia/reperfusion injury with simvastatin in rats. Anesth Analg. 2011;113:565–567. doi: 10.1213/ANE.0b013e318224ac35. [DOI] [PubMed] [Google Scholar]
- 6.Tamaddonfard E, Farshid AA, Eghdami K, Samadi F, Erfanparast E. Comparison of the effects of crocin, safranal and diclofenac on local inflammation and inflammatory pain responces induced by carrageenan in rats. Pharmacol Rep. 2013;65:1272–1280. doi: 10.1016/s1734-1140(13)71485-3. [DOI] [PubMed] [Google Scholar]
- 7.Boskababy MH, Tabatabaee A, Byrami G. The effect of the extract of Crocus sativus and its constituent safranal, on lung inflammation of ovalbumin sensitized guinea-pigs. Phytomedicine. 2012;19:904–911. doi: 10.1016/j.phymed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Hossenzadeh H, Sadeghnia HR. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J Pharm Pharm Sci. 2005;8:394–399. [PubMed] [Google Scholar]
- 9.Hossenzadeh H, Modaghegh MH, Saffari Z. Crocus sativus L. (saffron) extract and its active constituents (crocin and safranal) on ischemia-reperfusion in rat skeletal muscle. Evid Based Complement Alternat Med. 2009;6:343–350. doi: 10.1093/ecam/nem125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makhlouf H, Saksouk M, Habib J, Chahine R. Determination of antioxidant activity of saffron taken from the flower of Crocus sativus grown in Lebanon. Afr J Biotechnol. 2011;10:8093–8100. [Google Scholar]
- 11.Wang B, Zhu Q, Man X, Guo L, Hao L. Ginsenoside Rd inhibits apoptosis following spinal cord ischemia/reperfusion injury. Neural Regen Res. 2014;9:1678–1687. doi: 10.4103/1673-5374.141802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehdizadeh R, Parizadeh MR, Khooei AR, Mehri S, Hosseinzadeh H. Cardioprotective effect of saffron extract and safranal in isoproterenol-induced myocardial infarction in Wister rats. Iran J Basic Med Sci. 2013;16:56–63. [PMC free article] [PubMed] [Google Scholar]
- 13.Taira Y, Marsala M. Effect of proximal arterial perfusion pressure on function, spinal cord blood flow, and histopathologic changes after increasing intervals of aortic occlusion in the rat. Stroke. 1996;27:1850–1858. doi: 10.1161/01.str.27.10.1850. [DOI] [PubMed] [Google Scholar]
- 14.Asdag SM, Inamdar MN. Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Appl Biochem Biotechnol. 2010;162:358–372. doi: 10.1007/s12010-009-8740-7. [DOI] [PubMed] [Google Scholar]
- 15.Akuzawa S, Kazui T, Shi E, Yamashita K, Bashar AH, Terada H. Interleukin-1 receptor antagonist attenuates the severity of spinal cord ischemia injury in rabbits. J Vasc Surg. 2008;48:694–700. doi: 10.1016/j.jvs.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Hossenzadeh H, Khosravan V. Anticonvulsant effects of aqueous and ethanolic extracts of Crocus sativus L. stigmas in mice. Arch Iran Med. 2002;5:44–47. [Google Scholar]
- 17.Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur Pharmacol. 2010;648:110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Bulger EM, Maier RV. Antioxidants in critical illness. Arch Surg. 2001;136:1201–1207. doi: 10.1001/archsurg.136.10.1201. [DOI] [PubMed] [Google Scholar]


