Abstract
The neuroprotective actions of Ginsenoside-Rg1 (G-Rg1) have been documented for experimental stroke therapy. We used a systematic review and meta-analysis to assess the efficacy of G-Rg1 in experimental ischemic stroke. We identified studies describing the efficacy of G-Rg1 in animal models of focal cerebral ischemia. Primary outcomes were infarct volume and neurological function score (NFS). In all, eleven studies reported significant effects of G-Rg1 for improving the NFS when compared with the control group (P<0.00001), and four studies reported significant effects of G-Rg1 for reducing infarct volume compared with middle cerebral artery occlusion group (P<0.00001). Meanwhile, studies reported G-Rg1 was more efficacious than positive control drug nimodipine (0.7 or 1 mg/kg, intraperitoneal) according to NFS (P = 0.009) and infarct volume (p = 0.0002). The results demonstrate a marked efficacy of G-Rg1 in experimental acute ischemic stroke, but raise concerns that our value of effect size might be overestimate due to factors such as study quality and possible publication bias. Even so, the findings suggest G-Rg1 as a candidate neuroprotective drug for human ischemic stroke.
Acute ischemic stroke (AIS) is defined as a clinical syndrome of sudden break out of focal or global disturbance of central nervous system function result from an interruption of the cerebral blood flow1. The estimated annual incidence of AIS is 0.25% and has become a leading cause of morbidity and mortality2. In addition, AIS is a costly condition incurring treatment, care and indirect costs. Over the past decades, over 1000 drugs have been tested in experiment studies, and exceed 400 have demonstrated efficacy in animal models of stroke3. However, it is disappointing that most of these treatments have been confirmed to be no effective in the acute phase of stroke4,5, only recombinant-tissue plasminogen activator (rt-PA), aspirin and stroke unit care have convincingly displayed efficacy in clinical trials of AIS6. Therefore, it is necessary to examine other potential neuroprotectants for ischemic stroke. Moreover, various failures of candidate drugs for cerebral ischemia have generated a series of suggestions that aim to improve the likelihood of successful translation. Among them, systematic review and meta-analysis of preclinical studies have been advocated to further inform candidate drug development and supply pool information for jumping into stroke clinical trials7.
For thousands of years, Ginseng has been used in Traditional Chinese Medicine as a tonic to improve stamina and vitality8. It also has supposed adaptogenic properties, making it useful for many health conditions. The main pharmacologically active ingredients of ginseng are ginsenosides,of which Ginsenoside Rg1 (G-Rg1) is regarded as one of the most important bioactive components responsible for pharmaceutical actions for Ginseng (a traditional Chinese tonic drug) with little toxicity and has been shown to have possibly neuroprotective effects9. In recent years, accumulating evidence indicated that G-Rg1 had pivotal role in protecting the brain from ischemic damage10. Zheng et al reported G-Rg1 could improve neurological function outcome and prevent ischemic neuronal death, as well as reduced infarct volume in histology11. Moreover, G-Rg1 increased neurogenesis after transient global ischemia in the dentate gyrus of adult gerbils and attenuated the blood brain barrier disruption by cerebral ischemic stroke12. Meanwhile, numerous studies on specific mechanism of G-Rg1 is now in progress, including potentiating nerve growth factors (NGF), antioxidant, anti-inflammatory, anti-apoptotic, and immune-stimulatory activities, inhibiting excite toxicity and Ca2+ over-influx into neurons, maintaining cellular ATP levels, and preserving structural integrity of neurons et al13.
However, to date, no systematic review has explored the adherence of G-Rg1 experimental research on ischemic stroke models. Meanwhile, the results of the previous preclinical studies often root in relatively small sample size and are quite nuances. Systematically reviewing and meta-analysis of all these papers in an objective and quantitative manner might offer us with credible and solid new evidence on whether or not G-Rg1 treatment exist neuroprotective effect in experimental ischemic stroke, to select the optimal requirements for drug administration for clinical trials. Therefore, in this study, we conducted a systematic review and meta-analysis to identify any evidence of G-Rg1 as a neuroprotectant on ischemic stroke animal models.
Results
Results of the search
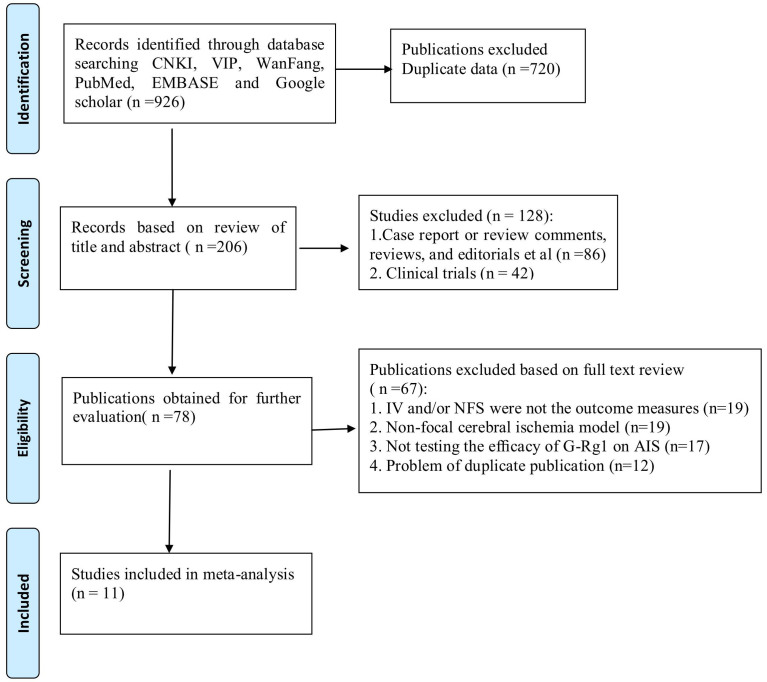
From our searches of the electronic databases and after removing duplicates we identified a total of 206 unique references. After going through the titles and abstracts, we excluded 128 papers with at least one of following reasons: (1) case report or review comments, reviews, and editorials; (2) clinical trials. By reading the full text of the remaining 78 articles which reported the efficacy of G-Rg1 in animal models of focal cerebral ischemia, we obtained the full papers of 11 articles and assessed these for eligibility11,14,15,16,17,18,19,20,21,22,23 (Figure 1). Agreement between the review authors on exclusion was 100%.
Figure 1. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Flow Diagram.
Study characteristics
The studies involved 178 animals (G-Rg1 89, control 89) from two species: C57BL/6 mice (n = 12)23, Sprague-Dawley rats (n = 166). The studies varied in size involving between 12 and 50 animals. The weight of rats varied between 200–300 g. However, only mean data or ranges for each study were available for this analysis instead of individual data. All of the studies were temporary MCAO models and the time of duration ischemia varied from 2 to 4 hours. 10% Chloral hydrate were used in 8 studies (72.7%), Pentobarbital in 1 study (9.1%)23, while no report of anesthetics in the remaining 2 studies (18.2%)17,21. Nine studies performed the dose gradient of G-Rg1 in the included studies, among them three studies adopt 25, 50, 100 mg/kg, respectively14,15,16; the remaining six studies utilized 10, 20, 40 mg/kg, separately17,18,19,21,22,23. Moreover, G-Rg1 compared with positive drugs (Nimodipine 0.7 or 1 mg/kg, intraperitoneal) in 6 studies. Ten studies injected G-Rg1 before ischemia except one study after ischemia20. Meanwhile, infarct volume was used as the outcome measure in 5 studies14,15,16,22,23, and NFS was used in 11 studies. In these 5 studies, we found infarct volume was all appraised by TTC staining for 10 minutes at 37°C, followed by overnight immersion in 4% paraformaldehyd and then unstained areas were referred to as infarct volume. All the studies adopted Zea long criterion24 to assess the muscle force. The scale rates the presence or absence of neurological signs in rats, and the details are as follows: 0 = no neurological deficit; 1 = retracts left forepaw when lifted by the tail; 2 = circles to the left; 3 = falls while walking; 4 = does not walk spontaneously; 5 = dead after surgery (Table 1).
Table 1. Basic characteristics of the included studies.
| Study (years) | Species (sex, n) | Weight | Model (method) | Anesthetic | Interventions | Outcome (time #) | Index ID |
|---|---|---|---|---|---|---|---|
| Hu 2006 [14] | SD Rats (male, 6/6) | 250–300 g | MCAO/2 h (ZL) | 10%Chloralhydrate (350 mg/kg) | G-Rg1 (25,50,100 mg/kg,ip) for 7 d before ischemia; | 1.NFS (Zl,24 h) | 1.p<0.05 |
| 2. IV, BBB | 2.p<0.05 | ||||||
| Nimodipine (0.7 mg/kg,ip) at 1 h before ischemia | 3.MPO, ICAM-1 | 3.p<0.05 | |||||
| HuMX 2006 [15] | SD Rats (male, 6/6) | 250–300 g | MCAO/2 h (ZL) | 10%Chloralhydrate (350 mg/kg) | G-Rg1 (25,50,100 mg/kg,ip) for 7 d before ischemia; | 1.NFS (Zl,24 h) | 1.p<0.01 |
| 2. IV, apoptosis | 2.p<0.01 | ||||||
| Nimodipine (1 mg/kg,ip) at 1 h before ischemia | 3. caspase-3,Bcl-2 | 3.p<0.05 | |||||
| Hu 2007 [16] | SD Rats (male, 10/10) | 250–300 g | MCAO/2 h (ZL) | 10%Chloralhydrate (350 mg/kg) | G-Rg1 (25,50,100 mg/kg,ip) for 7 d before ischemia; | 1.NFS (Zl,24 h) | 1.p<0.05 |
| 2. IV, BWC | 2.p<0.01 | ||||||
| Nimodipine (1 mg/kg,ip) at 1 h before ischemia | 3.AMRE,.SOM | 3.p<0.01 | |||||
| Liu 2010 [17] | SD Rats (male, 10/10) | 250–300 g | MCAO/2 h (ZL) | NR | G-Rg1 (10,20,40 mg/kg,ip) for 7 d before ischemia; | 1.NFS (ZL,24 h) | 1.p<0.05 |
| Nimodipine (1 mg/kg,ip) for 7 d before ischemia | 2. caspase-3 | 2.p<0.05 | |||||
| Wang 2011 [18] | SD Rats (both, 8/8) | 270 ± 30 g | MCAO/2 h (ZL) | 10% Chloralhydrate (400 mg/kg) | G-Rg1 (10,20,40 mg/kg,ip) for 5 d before ischemia | 1.NFS (ZL,24 h) | 1.p<0.01 |
| 2.neuronumber | 2.p<0.01 | ||||||
| 3.NOS,iNOS | 3.p<0.01 | ||||||
| Zhou 2012 [19] | SD Rats (male, 10/10) | 250–300 g | MCAO/2 h (ZL) | 10% Chloralhydrate | G-Rg1 (10,20,40 mg/kg,ip) for 5 d before ischemia; | 1.NFS (ZL,24 h) | 1.p<0.05 |
| 2. iNOS | 2.p<0.05 | ||||||
| Nimodipine (1 mg/kg,ip) for 5 d before ischemia | 3. eNOS | 3.p<0.05 | |||||
| Bao 2012 [20] | SD Rats (male, 5/5) | 250 ± 50 g | MCAO/4 h (ZL) | 10% Chloralhydrate | G-Rg1(20 mg/kg,ip) for 4 w after ischemia; | 1.NFS (ZL,4w) | 1.p<0.05 |
| 2. BrdU,NSE | 2.p<0.05 | ||||||
| Wang 2013 [21] | SD Rats (male, 6/6) | 250–280 g | MCAO/2 h (ZL) | NR | G-Rg1 (10,20,40 mg/kg,ip) for 7 d before ischemia; | 1.NFS (ZL,24 h) | 1.p<0.01 |
| 2. p-JNK,p-ERK1/2 | 2.p<0.05 | ||||||
| Yu 2013 [22] | SD Rats (male, 5/5) | 250 ± 50 g | MCAO/2 h (ZL) | 10%Chloralhydrate (350 mg/kg) | G-Rg1 (10,20,40 mg/kg,ip) for 5 d before ischemia; | 1.NFS (ZL,24 h) | 1.p<0.05 |
| 2.IV | 2.NR | ||||||
| Nimodipine (1 mg/kg,ip) for 5 d before ischemia | 3. PARP-1,caspase-3 | 3.p<0.05 | |||||
| Zeng 2014 [23] | C57BL/6 mice (male, 6/6) | 25–30 g | MCAO/2 h (ZL) | pentobarbital sodium (50 mg/kg) | G-Rg1 (10,20,40 mg/kg,ip) for 7 d before ischemia | 1.NFS (ZL,24 h) | 1.p<0.01 |
| 2.IV; | 2.p<0.05 | ||||||
| 3.caspase-3,MDA,SOD,HSP | 3.p<0.05 | ||||||
| Zheng 2014 [11] | SD Rats (male,12/12) | 230–280 g | MCAO/2 h (ZL) | 10%Chloralhydrate (350 mg/kg) | G-Rg1 (20 mg/kg,ip) for 3 d before ischemia and following 3 w after ischemia | 1.NFS (ZL,3w) | 1.p<0.01 |
| 2. BBB | 2.p<0.05 | ||||||
| 3.AQP4 | 3.p<0.05 |
SD: Sprague-Dawley; MCAO: middle cerebral artery occlusion; ZL: Zea longa; h: hour; NR: NO report; G-Rg1: Ginsenoside-Rg1; IP: Intraperitoneal; D: days; W: Weeks; NFS: neurological function score; IV: infarct volume; BBB: blood brain barrier; MPO: myeloperoxidase; Intercellular adhesion molecule-1; BWC:brain water content; iNOS: inducible nitric oxide synthase; eNOS: endothelial nitric oxide synthase; BrdU: bromodeoxyuridine; NSE: neuronal specific enolase; JNK: c-Jun N—terminal kinases; ERK: extracellular signal—regulated kinase 1/2; PARP-1: poly ADP-ribose polymerase-1; AMRE: activation of mitochondria respiratory enzymes; SOM: structure of mitochondrion; MDA: malondialdehyde; SOD: superoxide dismutase; HSP: shock protein 70; AQP4: aquaporin 4; ID: Intergroup difference
Methodological quality of included studies
The quality score of studies were ranged from 3 to 7 out of a total 10 points. Of whom, three studies got 3 points; four studies got 4; one studies got 5; two studies got 6; and one study got 7 points (Table 2). Six studies described control of temperature, including control of the room or rats anal temperature. Random allocation to treatment group and blinded assessment of outcome were described in 10 and 2 studies, respectively. Nine studies used anesthetic without significant intrinsic neuroprotective activity and there are 2 studies that did not describe the anaesthetic agent used, so we did not have sufficient evidence to give them a point. No animals in this meta-analysis with relevant comorbidities, such as aged, diabetic, or hypertensive. Moreover, no study described the sample size calculation. In terms of compliance with animal welfare regulations, four studies did not give the statement17,19,20,22. Two studies mentioned the statement of potential conflict of interests11,23.
Table 2. Quality assessment of included studies.
| Study (years) | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | Tatal |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hu 2006 [14] | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| HuMX 2006 [15] | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| Hu 2007 [16] | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | |||||
| Liu 2010 [17] | ✓ | ✓ | ✓ | 3 | |||||||
| Wang 2011 [18] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||||
| Zhou 2012 [19] | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| Bao 2012 [20] | ✓ | ✓ | ✓ | 3 | |||||||
| Wang 2013 [21] | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| Yu 2013 [22] | ✓ | ✓ | ✓ | 3 | |||||||
| Zeng 2014 [23] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||||
| Zhou 2014 [11] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 |
Note: Studies fulfilling the criteria of: (1) peer reviewed publication; (2) control of temperature; (3) random allocation to treatment or control; (4) blinded induction of ischemia; (5) blinded assessment of outcome; (6) use of anesthetic without significant intrinsic neuroprotective activity; (7) animal model (aged, diabetic, or hypertensive); (8) sample size calculation; (9) compliance with animal welfare regulations; and (10) statement of potential conflict of interests.
Effectiveness
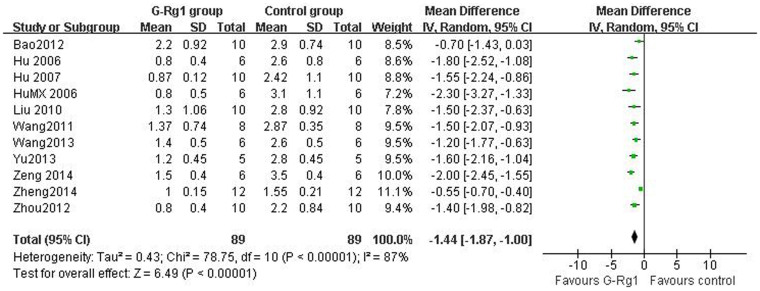
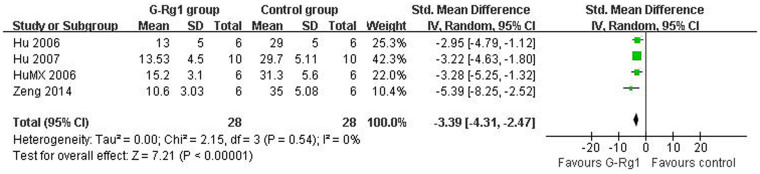
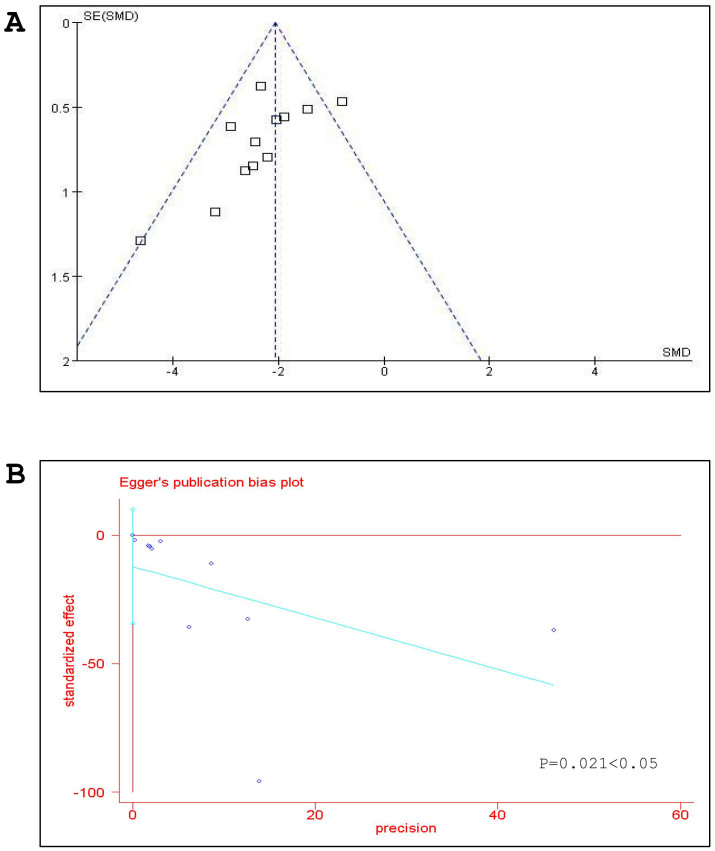
For NFS, there were 178 animals included in the analysis, we pooled the whole data to process and found significant difference when G-Rg1 treatment compared with control group (n = 178, WMD -1.44, 95% CI: -1.87 to -1.00, P<0.00001, Figure 2). Meanwhile, there was obvious heterogeneity for the analysis of NFS between studies (Tau2 = 0.43, Chi2 = 78.75, p<0.00001, I2 = 87%, Figure 2). After sequentially excluding each study, the results of NFS and heterogeneity were inconsistent. Removal of the outlier studies11 led to more homogeneous results (Tau2 = 0.05, Chi2 = 13.05, p = 0.14, I2 = 34%), but increased the effect size by -0.11 yielding a still significant pooled WMD of -1.55 (95% CI -1.80 to -1.30, P <0.00001), which was similar with previous analysis (WMD -1.44 vs WMD -1.55). Five studies showed significant effects of G-Rg1 for reducing the infarct volume based on the TTC staining. However, one study22 excluded for pool analysis due to the data is deficiency, incapable to get the raw mean and standard deviation. Remaining 4 studies reported significant effects of G-Rg1 for reducing infarct volume compared with control group (n = 56, SMD -3.39, 95% CI: -4.31 to -2.47, P<0.00001; heterogeneity:tau2 = 0.00, Chi2 = 2.15, p = 0.54, I2 = 0%, Figure 3). The funnel plot was asymmetric for the effect of G-Rg1 on NFS by visual inspection. Thus, funnel plots suggested a mild publication bias (Figure 4A). Meanwhile, Egger's weighted regression suggested moderate likelihood of publication bias for all analysis (p = 0.021<0.05, Figure 4B).
Figure 2. Pooled estimate of improvement in neurological function score with G-Rg1 according to Zea longa criteria.
Figure 3. Pooled estimate of decrement in infarct volume with G-Rg1.
Figure 4. Bias assessment plot for the effect of G-Rg1 on neurological function score (NFS) by funnel blot (A) and Egger's test (B).
G-Rg1 vs positive control drugs
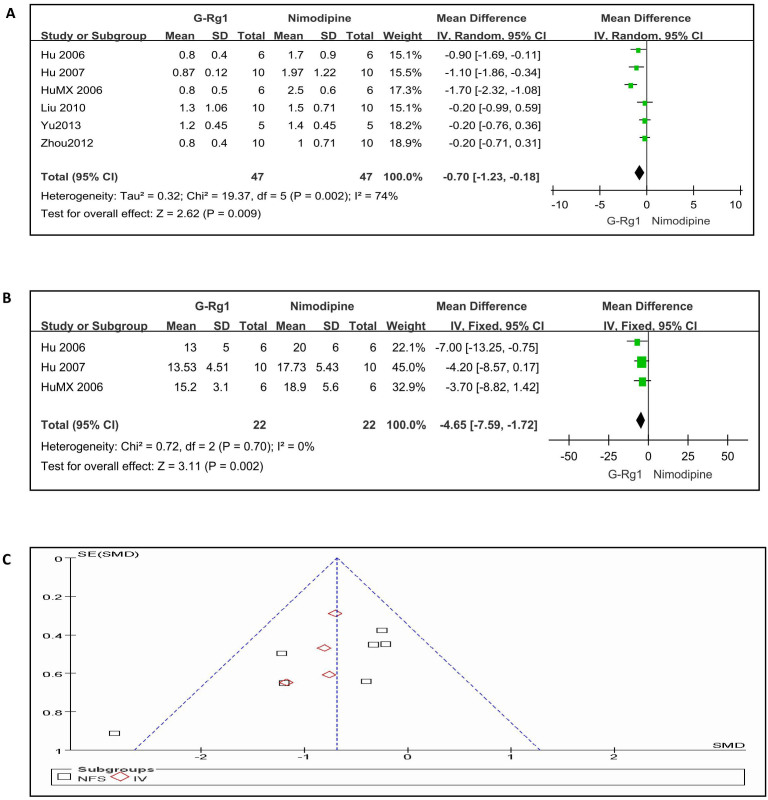
When we compared G-Rg1 with positive control drugs (Nimodipine 0.7 or 1 mg/kg, intraperitoneal), six studies involving 94 animals were included in the analysis according to NFS. The effect size of G-Rg1 was more efficacious than nimodipine administration (n = 94, WMD -0.70, 95% CI: -1.23 to -0.18, P = 0.009; heterogeneity:tau2 = 0.32, Chi2 = 19.37, p = 0.002, I2 = 74%, Figure 5A). In the meta analysis for the outcome measure according to infarct volume, efficacy of G-Rg1 showed a little significant reducing the infarct volume compared with nimodipine group (n = 44, WMD -4.65, 95% CI: -7.59 to -1.72, P = 0.002; heterogeneity:Chi2 = 0.72, p = 0.70, I2 = 0%, Figure 5B). Meanwhile, the funnel plot was roughly symmetric for the effect of G-Rg1 vs positive control drugs on infarct volume and NFS (Figure 5C).
Figure 5. G-Rg1 compared with Nimodipine(0.7 or 1 mg/kg, intraperitoneal)according to neurological function score and infarct volume.
(A) the effect of G-Rg1 compared with Nimodipine in terms of the NFS; (B) the effect of G-Rg1 compared with Nimodipine in terms of the IV; (C) bias assessment plot for the effect of G-Rg1 on NFS.
Pre-specified subgroup analysis
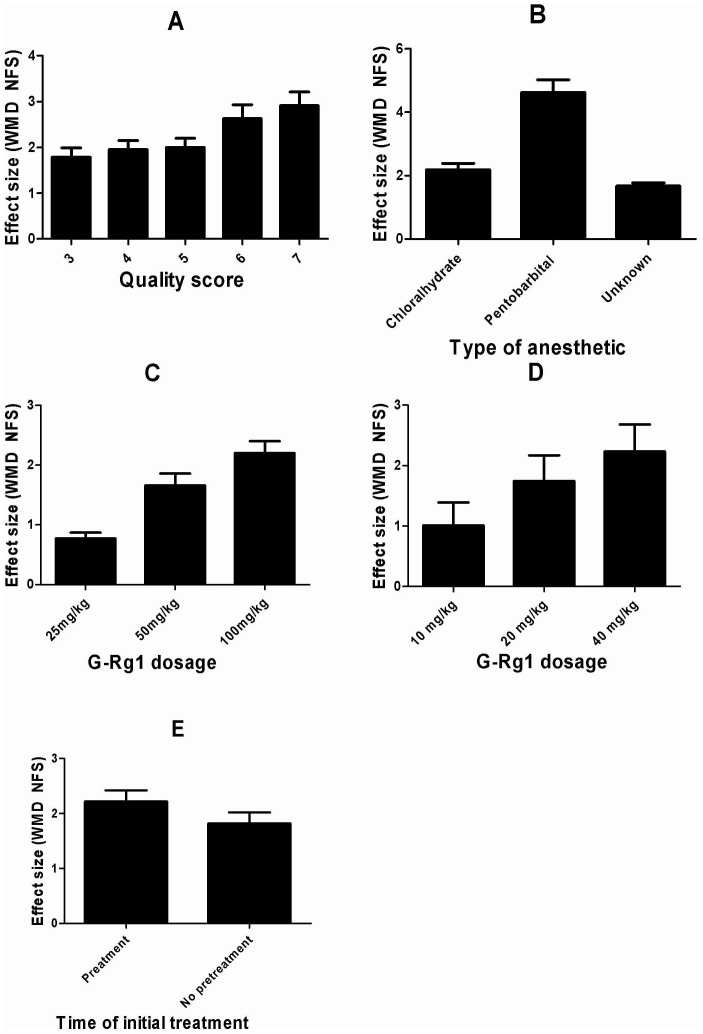
In the subgroup analysis for the outcome measure according to NFS, the effect size of G-Rg1 in high quality studies was larger than in the low quality studies (Figure 6A). Meanwhile, efficacy was observed to be higher with the administration of pentobarbital sodium than Chloralhydrate and unreported anesthetic (Figure 6B). We sought to investigate G-Rg1-dose effect on NFS. High-dose G-Rg1 (100 mg/kg) was more sensitive to improve NFS compared with low-dose (25 mg/kg) and middle-dose G-Rg1 (50 mg/kg) (Figure 6C). Meanwhile, the effect size of G-Rg1 (40 mg/kg) was similarly larger than in the G-Rg1 (20 mg/kg) and G-Rg1 (10 mg/kg) (Figure 6D). Moreover, timing of initiation of treatment ranged from before ischemia 7 days to immediately after the induction of ischemia. Neuroprotection was maximal when G-Rg1 was administered pretreatment before ischemia (Figure 6E).
Figure 6. Subgroup analysis according to neurological function score (NFS).
(A) quality score; (B) type of anesthetic; (C) G-Rg1 dosage (100 mg vs 50 mg vs 25 mg); (D); G-Rg1 dosage (40 mg vs 20 mg vs 10 mg); (E) time of initial treatment. The vertical error bars represent the effect size of G-Rg1 and the error bars represent standard deviations for each group in the subgroup analysis.
Discussion
Summary of main results
To our knowledge, this is the first systematic review and meta-analysis of English and Chinese literatures to determine the efficacy of G-Rg1 for animal models of acute ischemic stroke with infarct volume and NFS as the outcome measures. Our analysis of 11 studies identified a significant improvement in experimental ischemic stroke after G-Rg1 treatment. A separate analysis of the efficacy of G-Rg1 compared with nimodipine revealed that G-Rg1 was more efficicaous than nimodipine (0.7 or 1 mg/kg, intraperitoneal) according to the NFS, as well as reduction in infarct volume. This meta-analysis reinforces the evidence for a neuroprotective role of G-Rg1 in experimental ischemic stroke, but we do not know whether animal models of disease reliably inform human studies. Further evidence is required in this area to assess G-Rg1 in clinical trials.
Limitations
A number of limitations of this study should be considered. Firstly, we confess that our search strategy is likely to include studies in English and Chinese database, nevertheless, takes no account of other languages that may lead to certain degree selective bias25. Meanwhile, there are only two papers that are listed on PubMed database, remaining ten papers seem to be written in Chinese language, which is another weakness that potentially limited the promotion of the findings. Secondly, no studies included in our meta-analysis reported a negative effect on infarct volume reduction or no improvement on neurological behavior, and therefore, there is a chance of overestimation of the results because our analysis only include available data, and hence negative studies that are less likely to be published will be missed. Thirdly, our meta-analysis is observational research rather than experimental, and so we are only able to obtain associations rather than causation. Moreover, no study in this meta-analysis using animals with co-morbidities, which is the typical situation in human stroke. Neither study exploring the efficacy of G-Rg1 in experimental stroke has been conducted in other species such as primates. Fourthly, the funnel plot and Egger test suggested a mild publication bias in this meta-analysis. Who is responsible for publication bias? We know that studies with negative results could remain unpublished because authors fail to write manuscripts and submit them to journals. Selective publishing and reporting are other major causes for bias, which must be considered. Therefore, inclusion of unpublished studies and the use of trial registries become the reasonable means to avoid publication bias26. However, in the present study, all the results from the selected 11 studies were consistently positive without negative results, and found publication bias as a possible explanation27. In terms of clinical trials, the members of the International Committee of Medical Journal Editors published a statement requiring that all clinical trials must be registered in order to be considered for publication28. Finally, as ischemia triggers a multitude of pathophysiological events, combined therapy of G-Rg1 with other neuroprotective should be tested. Due to these limitations, hypothesis arising from this study need proving in reasonably designed adequately powered head-to-head experiments.
Implication for further studies
Significant differences between high and low quality studies were observed, with high quality studies reporting the highest efficacy for NFS outcomes, which is consistent with some previous studies29 suggest that the quality of the research design is an important factor affecting the outcome. It might be that high quality studies have lower variance then the effects will appear larger, or improvement in the quality of reporting studies will also help reduce bias when such trials are included in systematic reviews. However, some studies indicated that statistically significant 30–50% exaggeration of treatment efficacy when results of lower-quality trials were pooled30. Inflated estimates of treatment efficacy were found when the studies with inadequate allocation concealment or randomization31. Therefore, well-designed, high quality studies would be required to test the efficacy of G-Rg1 in AIS. According to the effect size, this study indicated that more effectiveness in NFS improvement in studies using pentobarbital sodium than studies using other two anesthetics. Therefore, future studies for animal research need to select suitable anesthetics. In the present study, no studies investigated G-Rg1 in ischemic stroke models with other conditions such as diabetes, dyslipidemia or aged animals. All animal models of stroke are established on normotensive animals with occlusion of cerebral artery to artificially induce infarction in brain. The relevance of animal models with normal cerebrovascular structure to human conditions remains dubious32. Thus, this lack of information should certainly be addressed in future studies. This meta-analysis suggests efficacy was maximal when G-Rg1 was administered pretreatment before ischemia, but most of the studies in the meta-analysis were pretreatment before induction ischemia. Therefore, the results generated from this subgroup analysis should be interpreted with caution. We have no sufficient evidence to suggest initiating clinical trials based on these data. Consequently, further studies would be demanded to evaluate when the optimum time window and to determine the initial time of administration under which maximum efficacy can be obtained. Moreover, there is a lack of trials exploring the combined effect of G-Rg1 with other neuroprotective drugs that might be investigated in future clinical studies.
The recently published randomized controlled trial (RCT) of ginsenoside-Rd (G-Rd) for acute ischemic stroke has showed G-Rd may be of some benefit in acute ischemic stroke33. The primary end point was NIH Stroke Scale (NIHSS) score at 15 days, Rd-treated patients showed significantly better NIHSS scores at 15 days than the placebo group. Moreover, Liu et al reported G-Rd significantly improved the overall distribution of scores on the modified Rankin score (mRS) compared with the placebo34. To our knowledge, there is still no clinical trial concern on the G-Rg1 for the ischemic stroke. We hope all the results above suggest a potential therapeutic of G-Rg1 on cerebral ischemia in clinic.
Conclusion
In animal models of focal cerebral ischemia, G-Rg1 could improve infarct volume and NFS. Although some factors such as study quality and publication bias may undermine the validity of positive findings, G-Rg1 still probably have potential neuroprotective role in experimental ischemic stroke. Systematic review and meta-analysis here provides a frame work for an evidence-based approach to the development of new treatments for ischemic stroke and for the design of future preclinical and clinical studies.
Methods
The whole process and methods of this meta-analysis were performed according to our previous published paper35.
Search strategy
A computerized literature search and hand searching of abstracts from scientific meetings were performed to find publications studying the effect of G-Rg1 treatment on animal models of acute ischemic stroke from PubMed, EMBASE, Google scholar, Chinese National Knowledge Infrastructure (CNKI), VIP information database, and Wanfang data Information Site. The publication time is from the inception of each database up to March 2014. All searches were limited to studies on animals. Reference lists from the included literature were used to identify further relevant publications. The following search strategy, using the grouped terms, was used for MEDLINE, and was modified to suit other databases.
Medline (Pubmed) search strategy.
Ginseng
Ginsenoside
Ginsenoside-Rg1
G-Rg1
or/1-4
Ischemia
Infarction
Stroke
Middle cerebral artery occlusion
MCAO
or/5-9
5 and 10
Inclusion and exclusion criteria
We included all controlled preclinical studies of the effect of G-Rg1 in animal models of focal cerebral ischemia, where the outcome was measured as infarct volume or/and neurological function score (NFS). NFS is mainly used to assess the effect of new therapeutic methods to indicate the muscle force. To prevent bias, inclusion criteria were pre-specified as follows: (1) the effect of G-Rg1 was tested in animal models of focal cerebral ischemia induced by temporary middle cerebral artery occlusion (MCAO) or permanent MCAO; (2) infarct volume and/or NFS were compared with control animals receiving vehicle or no treatment, as well as compared with positive control drug animals. Pre-specified exclusion criteria were: (1) case reports, abstracts, comments, reviews, and editorials and clinical trials; (2) non-focal cerebral ischemia model (such as global ischemic models, or hypoxic-ischemic models); (3) infarct volume and/or NFS were not the outcome measures; (4) not testing the efficacy of G-Rg1 on AIS.
Data extraction
The following details of the study design were extracted from each study: (1) first author's name and the publication year, method of ischemia induction, and ischemia time; (2) individual data were obtained for each study, including animal number, species, sex, and weight; (3) information on treatment was obtained, including timing and dosage for treatment, method of treatment procedure; (4) outcome measures and timing for outcomes assessment were also included. If outcomes were presented from the preclinical studies of animals at different time points, we extracted data from the last time point to sacrifice. If the data for meta-analysis were missing or only expressed graphically, we tried to contact the authors for further information, and where a response was not received, we measured data from the graphs using digital ruler software or exclude. For each comparison, we extracted data of mean value and standard deviation from every study. The time of lesion was set to zero and the time of drug administration expressed relative to this. All the data were extracted independently by two participants (CLX, WWW).
Quality Assessment
Study quality was assessed based on a ten-item modified scale29: (1) publication in a peer-reviewed journal; (2) statements describing control of temperature; (3) randomization to treatment group; (4) allocation concealment; (5) blinded assessment of outcome; (6) avoidance of anesthetics with known marked intrinsic neuroprotective properties; (7) use of animals with relevant comorbidities; (8) sample size calculation; (9) compliance with animal welfare regulations; (10) declared any potential conflict of interest. For the calculation of an aggregate quality score, each item of the ten-item modified scale was attributed one point. Two authors (WWW, XCL) independently extracted data and assessed study quality.
Statistical analysis
NFS and infarct volume were considered as continuous data. WMD (weighted mean difference) is a standard statistic that measures the absolute difference between the mean values in two groups. It estimates the amount by which the experimental intervention changes the outcome on average compared with the control. It can be used as a summary statistic in meta-analysis when outcome measurements in all studies are made on the same scale. On the contrary, standardised mean difference (SMD) is used as a summary statistic in meta-analysis when the studies all assess the same outcome but measure it in a variety of ways36. We used random effects rather than a fixed effects model because of this takes into account the heterogeneity between multi-studies. Publication bias was assessed by visual inspection of a funnel plot. A very common and simple version of the meta-analysis procedure is commonly referred to as the inverse-variance method. The inverse variance method is so named because the weight given to each study is chosen to be the inverse of the variance of the effect estimate. All analyses were performed with RevMan version 5.1. Probability value P <0.05 was considered significant. Furthermore, to explore the impact of factors modifying on the outcome measures, we performed a pre-stratified subgroup analysis with experiments grouped according to the following: reported quality score; type of anaesthetic used; time of initial treatment and G-Rg1 dosage. To assess the robustness of the results, sensitivity analysis was performed according to quality by removing each individual study in turn from the total and reanalyzing the remainder. The significance of differences between groups was assessed by partitioning heterogeneity and using the x2 distribution with n-1 degrees of freedom, where n equals the number of groups.
Supplementary Material
Text SI
Acknowledgments
The study was supported by the Projects of National Science Foundation of China (No.81171203, 81171204, 81200871, 81400925 and 81471148), and Projects of the Shanghai Committee of Science and Technology, China (12XD1403800).
Footnotes
The authors declare no competing financial interests.
Author Contributions C.L.X. and W.W.W. conceived and participated in its design, searched databases, extracted and assessed studies and helped to draft the manuscript. J.G., S.F.Z. and X.D.X. carried out the statistical analysis and interpretation of data. Z.G.L. participated in the conceptualization and design of the review, performed the selection of studies, data extraction and analysis, and drafted the review. All authors read and approved the final manuscript.
References
- Kidwell C. S., Liebeskind D. S., Starkman S. & Saver J. L. Trends in acute ischemic stroke trials through the 20th century. Stroke 32, 1349–1359 (2001). [DOI] [PubMed] [Google Scholar]
- Simon R. P., Greenberg D. A. & Aminoff M. J. Clinical Neurology. 7th Edition. (McGraw-Hill Publishing House, New York, 2009). [Google Scholar]
- O'Collins V. E. et al. 1,026 experimental treatments in acute stroke. Ann Neurol 59, 467–477 (2006). [DOI] [PubMed] [Google Scholar]
- Muir K. W. & Lees K. R. Excitatory amino acid antagonists for acute stroke. Cochrane Database Syst Rev 3, CD001244 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der, Worp H. B. et al. Can animal models of disease reliably inform human studies? PLoS Med 7, e1000245 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright L. et al. National Stroke Foundation Stroke Guidelines Expert Working Group. Stroke management: updated recommendations for treatment along the care continuum. Intern Med J 42, 562–569 (2012). [DOI] [PubMed] [Google Scholar]
- Sena E., van d. e. r., Worp H. B., Howells D. & Macleod M. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci 30, 433–439 (2007). [DOI] [PubMed] [Google Scholar]
- Ellis J. M. & Reddy P. Effects of Panax ginseng on quality of life. Ann Pharmacother 36, 375–379 (2002). [DOI] [PubMed] [Google Scholar]
- Jiang B. et al. Antidepressant-like effects of ginsenoside Rg1 are due to activation of the BDNF signalling pathway and neurogenesis in the hippocampus. Br J Pharmacol 166, 1872–1887 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L. & Zhang J. NMDA receptor and iNOS are involved in the effects of ginsenoside Rg1 on hippocampal neurogenesis in ischemic gerbils. Neurol Res 29, 270–273 (2007). [DOI] [PubMed] [Google Scholar]
- Zhou Y. et al. Ginsenoside Rg1 provides neuroprotection against blood brain barrier disruption and neurological injury in a rat model of cerebral ischemia/reperfusion through downregulation of aquaporin 4 expression. Phytomedicine 21, 998–1003 (2014). [DOI] [PubMed] [Google Scholar]
- Zheng G. Q. et al. Ginseng total saponins enhance neurogenesis after focal cerebral ischemia. J Ethnopharmacol 133, 724–728 (2011). [DOI] [PubMed] [Google Scholar]
- Rausch W. D., Liu S., Gille G. & Radad K. Neuroprotective effects of ginsenosides. Acta Neurobiol Exp (Wars) 66, 369–375 (2006). [DOI] [PubMed] [Google Scholar]
- Hu X. M., Yan C. K., Xu S. Q., Hu X. M. & Zeng F. D. Effects of ginsenoside Rg1 on endothelial cell adhesion after cerebral ischemia-reperfusion in rats. Chin J Pharmacol Toxicol 20, 19–25 (2006). [Google Scholar]
- Hu M. X., Yan C. K. & Hu X. M. Effects of ginsenoside Rg1 on Cell apoptosis after cerebral ischemia-reperfusion in rats. Chin J Clin Pharmacol Ther 11, 11192–196 (2006). [Google Scholar]
- Hu M. X., Yan C. K., Hu X. M. & Zeng F. D. Protection of cerebral mitochondria function of rats from impairment by cerebral ischemia-reperfhsion with ginsenoside Rg 1. Chin J New Drugs. 15, 514–517 (2006). [Google Scholar]
- Liu X., Bao C. F., Liang J., Wei J. & Qin S. J. Effects of ginsenoside Rg1 on caspase-3 in rats brain tissue after cerebral ischemia reperfhsion. Chin J Histochem Cytochem 19, 88–92 (2010). [Google Scholar]
- Wang Q. Y. & Wu F. J. Effects of ginsenoside Rg1 on activity and protein expression of NOS in rats with cerebral ischemic reperfusion injury. Chin J Pathophysiol 27, 2328–2332 (2011). [Google Scholar]
- Zou X. L., Du J. W., Liang J., Wei J. & Bao C. F. Effects of ginsenoside Rg1 on iNOS and eNOS expression after cerebral ischemia-reperfusion in rats. Shandong Medical Journal 52, 44–45 (2012). [Google Scholar]
- Bao C. F., Bao C. F., Li L. & Liu X. Effects of ginsenoside Rgl joint BMSCs on cerebral ischemia and reperfusion injury in rats. Shandong Medical Journal 52, 9–11 (2012). [Google Scholar]
- Wang Q. Y., Liu F., Wu F. J. & Li J. L. Effects of Ginsenoside Rgl on the expressions of P-ERKl/2 and p-JNK in local cerebraI ischemia/reperfusion injury rats. Chin J integrative medicine 33, 229–234 (2013). [PubMed] [Google Scholar]
- Yu L., Liu X., Bao C. F. & Ji H. C. Effects of ginsenoside Rg1 on cell death model in brain tissue after cerebral ischemia-reperfusion in rats. Chin J Clin Anat 31, 555–559 (2013). [Google Scholar]
- Zeng X. S. et al. Comparative analysis of the neuroprotective effects of ginsenosides Rg1 and Rb1 extracted from Panax notoginseng against cerebral ischemia. Can J Physiol Pharmacol 92, 102–108 (2014). [DOI] [PubMed] [Google Scholar]
- Longa E. Z., Weinstein P. R., Carlson S. & Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20, 84–91 (1989). [DOI] [PubMed] [Google Scholar]
- Guyatt G. H. et al. GRADE guidelines: 5. Rating the quality of evidence--publication bias. J Clin Epidemiol 64, 1277–1282 (2011). [DOI] [PubMed] [Google Scholar]
- Furlan A. D., Pennick V., Bombardier C. & van Tulder. M. Editorial Board, Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 34, 1929–41 (2009). [DOI] [PubMed] [Google Scholar]
- Xie C. L. et al. Efficacy and safety of Suanzaoren decoction for primary insomnia: a systematic review of randomized controlled trials. BMC Complement Altern Med 13, 18 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De, Angelis C. et al. International Committee of Medical Journal Editors. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. Circulation 111, 1337–1338 (2005). [DOI] [PubMed] [Google Scholar]
- Macleod M. R., O'Collins T., Howells D. W. & Donnan G. A. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 35, 1203–1208 (2004). [DOI] [PubMed] [Google Scholar]
- Moher D. et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 352, 609–613 (1998). [DOI] [PubMed] [Google Scholar]
- Bebarta V., Luyten D. & Heard K. Emergency medicine animal research: does use of randomization and blinding affect the results? Acad Emerg Med 10, 684–687 (2003). [DOI] [PubMed] [Google Scholar]
- Wiebers D. O., Adams H. P. Jr & Whisnant J. P. Animal models of stroke: are they relevant to human disease? Stroke 21, 1–3 (1990). [DOI] [PubMed] [Google Scholar]
- Liu X. et al. Efficacy and safety of ginsenoside-Rd for acute ischaemic stroke: a randomized, double-blind, placebo-controlled, phase II multicenter trial. Eur J Neurol 16, 569–575 (2009). [DOI] [PubMed] [Google Scholar]
- Liu X. et al. Ginsenoside-Rd improves outcome of acute ischaemic stroke - a randomized, double-blind, placebo-controlled, multicenter trial. Eur J Neurol 19, 855–863 (2012). [DOI] [PubMed] [Google Scholar]
- Wang W. W., Xie C. L., Lu L. & Zheng G. Q. A systematic review and meta-analysis of Baihui (GV20)-based scalp acupuncture in experimental ischemic stroke. Sci Rep 4, 3981 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterinen H. M. et al. Meta-analysis of data from animal studies: a practical guide. J Neurosci Methods 221, 92–102 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Text SI