Abstract
The objectives of this study were to (1) determine the distribution and synthesis of pericellular matrix (PCM) molecules (collagen VI, collagen IV and laminin) in rat temporomandibular joint (TMJ) and (2) investigate the effects of PCM molecules on chondrocytes against inflammation in osteoarthritis. Four zones (fibrous, proliferating, mature and hypertrophic) of condylar cartilage and three bands (anterior, intermediate and posterior) of disc were analysed by immunohistochemistry for the presence of PCM molecules in rat TMJs. Isolated chondrocytes were pre-treated with PCM molecules before being subjected to interleukin (IL)-1β treatment to stimulate inflammation. The responses of the chondrocytes were analysed using gene expression, nitric oxide release and matrix metalloproteinase (MMP)-13 production measures. Histomorphometric analyses revealed that the highest areal deposition of collagen VI (67.4%), collagen IV (45.7%) and laminin (52.4%) was in the proliferating zone of TMJ condylar cartilage. No significant difference in the distribution of PCM molecules was noted among the three bands of the TMJ disc. All three PCM molecules were expressed intracellularly by chondrocytes cultured in the monolayer. Among the PCM molecules, pre-treatment with collagen VI enhanced cellular proliferation, ameliorated IL-1β-induced MMP-3, MMP-9, MMP-13 and inducible nitric oxide synthase gene expression, and attenuated the downregulation of cartilage matrix genes, including collagen I, aggrecan and cartilage oligomeric matrix protein (COMP). Concurrently, collagen VI pretreatment inhibited nitric oxide and MMP-13 production. Our study demonstrates for the first time the distribution and role of PCM molecules, particularly collagen VI, in the protection of chondrocytes against inflammation.
Keywords: cartilage, chondrocytes, collagen IV, collagen VI, inflammation, laminin, pericellular matrix, temporomandibular joint
Introduction
The pericellular matrix (PCM) was first described as a distinct feature of cartilage several decades ago.1 However, there is a recent emerging paradigm in the field to decipher the components of the PCM and their functional roles in chondrocyte biology and chondrogenesis.2, 3 PCM is a specialized, thin layer of the extracellular matrix (ECM) that immediately surrounds the chondrocytes and serves as a transducer for both biochemical and biomechanical signals to the chondrocyte.4 The chondrocyte and PCM constitute the chondron. Therefore, the PCM plays an important role in maintaining the phenotype and integrity of the chondrocytes3, 5 and is involved in (1) biochemical interactions with regulatory proteins (for example, growth factors); (2) the regulation of the mechanical environment of the chondrocyte; and (3) the maintenance of the microenvironment of the chondrocyte and control (prevention) of the chondrocyte interaction with the extracellular matrix molecules of the territorial matrix.
Biglycan, decorin, matrilins, collagen VI and XVIII/endostatin are key components of the PCM.6, 7, 8 Proteins that are normally associated with the basement membrane, including collagen IV, laminins, perlecan and nidogens, have been recently found to be prominently in the PCM surrounding the chondrocytes.9 Notably, perlecan, type IV collagen and laminins have been reported to be present in murine and human articular cartilage during development.10, 11 Our prior study also demonstrated that the deposition of collagen IV and laminin in mesenchymal stem cell (MSC) pellet cultures followed an orderly spatiotemporal shift in pattern during chondrogenesis from a diffuse territorial and interterritorial distribution to a distinct pericellular localization, as observed in normal articular cartilage.12
Although previous studies have demonstrated the presence of PCM molecules in various cartilage tissues, including knee joint articular cartilage, intervertebral disc and meniscus,1, 13, 14 no prior study has investigated the role and distribution of PCM molecules in temporomandibular joint (TMJ) condylar cartilage and discs. The TMJ is unique compared with other synovial joints (articular cartilages) because it is composed primarily of fibrocartilage in both the condylar cartilage and discs. Therefore, the aim of our study was to investigate the presence and distribution of three PCM molecules, namely collagen VI, collagen IV and laminin, in the rat TMJ condylar cartilage and disc. We also examined the role of PCM molecules in the anabolic and catabolic responses of TMJ condylar chondrocytes and disc cells via the pretreatment of cells with PCM molecules before a pro-inflammatory interleukin (IL)-1β treatment to stimulate the inflammation that is present in osteoarthritis (OA). We hypothesized that collagen VI, collagen IV and laminin are synthesized by chondrocytes and disc cells, and these molecules are differentially distributed in TMJ condylar cartilage and disc. These proteins and distribution may protect condylar and disc chondrocytes against inflammation during OA.
Materials and methods
Animals
This study was conducted in compliance with the Institutional Animal Care and Use Committee at the National University of Singapore under protocol number R14-913. Twelve 8-week-old female Sprague–Dawley rats with a mean weight of (212.5±17.1)g (range 180–230 g) were used in this study.
Histology, immunohistochemistry and histomorphometry
Three rats were euthanized, and the heads were resected. After the removal of overlying skin and connective tissue, the left and right TMJs of each animal (n=6) were separated and fixed in 10% neutral-buffered formalin (Sigma, St. Louis, MO, USA) for 1 week and decalcified in 30% buffered formic acid. After the decalcification, the samples were dehydrated and embedded in paraffin. Ten serial sagittal sections were cut at 5 μm for each TMJ sample and randomly assigned to haematoxylin and eosin, and immunohistochemical staining. Immunohistochemistry to detect collagen VI (ab6588; 0.1 μg·mL−1; Abcam, Cambridge, MA, USA), collagen IV (ab6586; 0.5 mg·mL−1; Abcam, Cambridge, MA, USA) and laminin (ab11575; 0.5 μg·mL−1; Abcam, Cambridge, MA, USA) were performed using a biotin-streptavidin Lab Vision UltraVision detection system (Thermo Fisher Scientific, Waltham, MA, USA), as previously described.12 An antibody isotype control (X0903; Dako, Glostrup, Denmark) was included to ensure the specificity of the immunohistochemical staining.
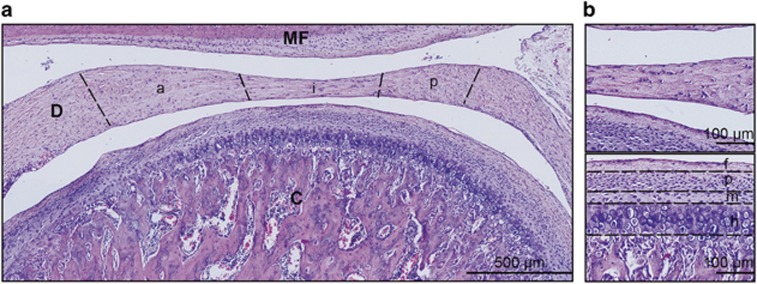
For the histomorphometric analysis, 40–60 images were taken for each sample at a magnification of × 20 under an inverted microscope (Olympus IX70; Olympus, Tokyo, Japan). All the images taken for each sample were assembled to form an image of the entire TMJ, and all the samples were included in the analysis. The anterior, intermediate and posterior bands of the disc, excluding the vascularized ends, were identified for the analysis.15 The fibrous, proliferative, mature and hypertrophy zones of the condylar cartilage were demarcated for the analysis (Figure 1).16, 17 The percentage of the positively stained areas was analysed using ImageJ software (National Institutes of Health, Bethesda, MD, USA), as previously described.12
Figure 1.
Histological sagittal section of a rat TMJ. (a) Morphological evaluation of rat TMJ using H&E staining showing the condyle (C), disc (D), mandibular fossa (MF), and disc bands: anterior (a), intermediate (i) and posterior (p) bands. Scale bar, 500 μm. (b) Top panel shows a high magnification view of the disc. Bottom panel shows the zones of condylar cartilage: fibrous (f), proliferating (p), mature (m) and hypertrophic (h) layers. Scale bar, 100 μm. Representative images of six TMJs (n=6) harvested from three rats. TMJ, temporomandibular joint; H&E, haematoxylin and eosin.
Cell culture
Condylar chondrocytes and disc cells were harvested from nine rats. The tissues were washed 3 times with phosphate-buffered saline (PBS) and digested with 0.2% collagenase I and II (Worthington, Lakewood, NJ, USA) overnight at 37 °C. The cells were passed through a 40-μm cell strainer (Corning, Corning, NY, USA) to obtain single cells before seeding at a density of 20 000 cells per cm2. The culture was maintained in Dulbecco's modified Eagle's Medium-Ham's F12 (DMEM-F12, Biowest, Nuaillé, France) supplemented with 10% foetal bovine serum (FBS, Biowest, Nuaillé, France), 25 μg·mL−1 ascorbic acid 2-phosphate (AA2P, Sigma, St Louis, MO, USA), and 1% penicillin-streptomycin (PS; Life Technologies, Carlsbad, CA, USA) under a humidified atmosphere of 5% CO2 at 37 °C. The medium was changed every alternate day. Chondrocytes were dissociated at confluence using TrypLe (Life Technologies, Carlsbad, CA, USA) and further sub-cultured to passage 2 (P2) to yield a sufficient number of cells for further experiments.
Immunocytochemistry of cell culture
Condylar and disc chondrocytes were cultured in 24-well plates at a density of 20 000 cells per cm2 overnight. Immunofluorescence staining of monolayer culture samples was performed as previously described.18 In brief, the monolayer culture samples were fixed in 4% paraformaldehyde (Sigma, St. Louis, MO, USA), permeabilized with 0.3% Triton X-100 (Sigma, St. Louis, MO, USA) and incubated overnight at 4 °C with primary antibodies for collagen VI, collagen IV and laminin. After the incubation, the cells were washed twice with 0.3% Triton X-100 in PBS and incubated with an Alexa Fluor 594 goat anti-rabbit secondary antibody (A11012; Life Technologies, Carlsbad, CA, USA). Nuclear staining was performed using 4′,6-diamidino-2-phenylindole (DAPI; Life Technologies, Carlsbad, CA, USA) before imaging under an inverted fluorescence microscope (Olympus, Tokyo, Japan).
Cellular proliferation
Chondrocytes were plated at 5 000 cells per well in 96-well plates (n=4 per culture condition). Cell proliferation was assessed at 24 and 48 h using the CellTiter 96 AQueous One Solution [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] (MTS) cell proliferation assay kit (Promega, Madison, WI, USA) following the manufacturer's instructions. In brief, 20 μL of MTS reagent were added to each well. The cells were incubated at 37 °C in CO2 for 2 h, and the absorbance readings were obtained at 490 nm and 650 nm using an Infinite 2000 plate reader (Tecan, Austria). Untreated cultures served as the control. The percentage cell proliferation was calculated using the normalization of the absorbance readings against that of the untreated control (set as 100%).19
Interleukin-1β treatment
Chondrocytes were seeded at 30 000 cells per well in 24-well plates for 48 h to reach ~90% confluency. Cells were then made quiescent by incubation for 24 h in reduced serum media (DMEM-F12 supplemented with 0.5% FBS and 1% PS). To study the effect of PCM, chondrocytes were incubated with or without 2 μg·mL−1 PCM molecules (collagen VI, collagen IV or laminin (Corning, Corning, NY, USA)) for 18 h followed by stimulation with 1 ng·mL−1 IL-1β (R&D Systems, Minneapolis, MN, USA) for 6 or 24 h (n=3). This exposure duration and concentration of IL-1β has been reported to induce a catabolic response in TMJ fibrochondrocytes.20 By the end of the treatment, the cell samples and culture supernatants were harvested for analysis.
Real-time reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was isolated from the samples (n=3) using a PureLink RNA Mini kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions. Reverse-transcription of total RNA to complementary DNA (cDNA) was performed using the iScript reverse transcription supermix (Bio-Rad Laboratories, Hercules, CA, USA). RT-PCR was performed using iTaq Universal SYBR Green Supermix (Bio-Rad) with CFX Connect real-time PCR system (Bio-Rad) for the metalloproteinase (MMP)-3, -9 and -13 (MMP-3, MMP-9, MMP-13), inducible nitric oxide synthase (iNOS), aggrecan (ACAN), collagen I (COL-1) and cartilage oligomeric matrix protein (COMP) genes. The primer sequences are listed in Supplementary Table 1. The PCR cycling consisted of 40 cycles of amplification of the template cDNA with primer annealing at 60 °C. The relative gene expression data were calculated using the 2-delta delta Ct method by comparing the cycle threshold (Ct) values of the targeted genes and normalizing to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (ΔCt) in the treated and non-treated conditions (ΔΔCt).
Nitric oxide production
Nitric oxide (NO) was measured in the cell culture supernatants collected 24 h after the addition of IL-1β using the Griess reaction and nitrite standards from the Griess reagent kit (Life Technologies, Carlsbad, CA, USA) following the manufacturer's protocol. Duplicate assays were performed on each sample (n=3), and absorbance readings were obtained at 548 nm using a plate reader (Infinite 2000, Tecan, Männedorf, Switzerland).
MMP-13 enzyme-linked immunosorbent assay
Cells were treated with IL-1β for 24 h, and the culture media were collected. The cells were lysed using CelLytic M cell lysis reagent (Sigma, St. Louis, MO, USA). Protein concentrations were determined using a Pierce BCA assay kit (Thermo Fisher Scientific, Waltham, MA, USA). A rat MMP-13 ELISA kit (MBS702112; Mybiosource, San Diego, CA, USA) was used to quantify the MMP-13 levels according to the manufacturer's protocol. The level of MMP-13 production was normalized to the total protein concentration.
Statistical analysis
The data are presented as the mean±standard error of the mean (s.e.m.) Statistical analyses were performed using SPSS 21.0 (SPSS, Chicago, IL, USA) at a significance level of 0.05. Statistical analyses between multiple groups were performed using one-way analysis of variance (ANOVA) and Scheffe's post hoc test.
Results
Distribution of PCM molecules in the TMJ
TMJ sections from all three animals (six TMJs) displayed immunoreactivity for PCM molecules, including collagen VI, collagen IV and laminin. Positive staining of PCM molecules was observed in the subchondral bone underlying the condylar cartilage and the lining of blood vessels at the vascularized ends of the TMJ disc. However, these regions were excluded from the analysis since this study intended to investigate the pericellular localization of these molecules in the cartilaginous tissues of TMJ, namely the condylar cartilage and disc. All six TMJs examined from the three animals showed positive staining for the PCM molecules (collagen VI, collagen IV and laminin) in both the condylar cartilage and disc. However, no distinguishable difference in the staining was noted between the left and right TMJs.
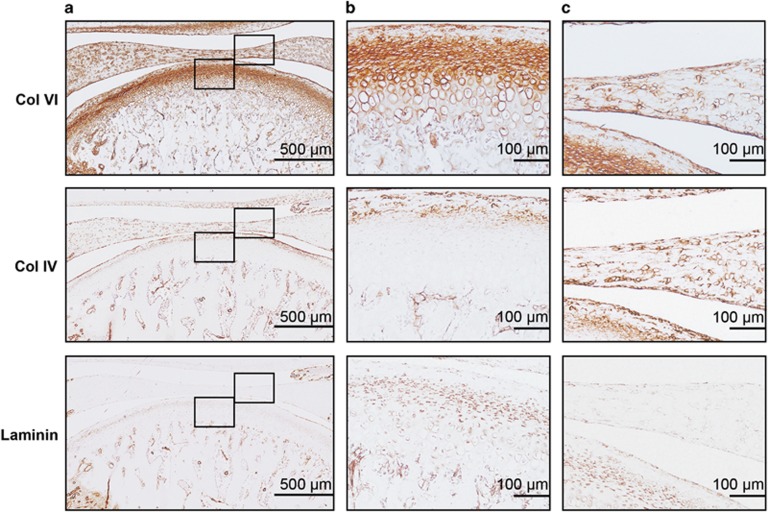
The TMJ condylar cartilage displayed pericellular localization of the PCM molecules surrounding the chondrocytes, albeit at varying amounts in the different zones. The total area of positive staining varied among the three PCM molecules, with collagen VI showing the highest areal deposition (42.68%±2.88%) compared with collagen IV (18.32%±1.64%) and laminin (23.93%±2.45% P<0.001). Histomorphometric analyses in different zones of the condylar cartilage revealed the highest areal deposition of collagen VI (67.39%±1.97%), collagen IV (45.69%±5.61%) and laminin (52.38%±7.81%) in the proliferating zone of TMJ condylar cartilage (Figure 2a and 2b, Table 1). In contrast, the hypertrophic zone of TMJ condylar cartilage showed the lowest level of collagen VI (22.8%±2.67%), collagen IV (1.82%±0.25%) and laminin (10.5%±1.26%). No chromogen was detected in any of the isotype control TMJ sections (data not shown).
Figure 2.
Expression of PCM molecules in rat TMJ. (a) Histological sections of rat TMJ stained for collagen VI, collagen IV and laminin. Scale bars, 500 μm. (b) High magnification view of condylar cartilage showing the pericellular staining of collagen VI, collagen IV and laminin. Scale bars, 100 μm. (c) High magnification view of disc showing the pericellular staining of collagen VI, collagen IV and laminin. Scale bars, 100 μm. Representative images; n=6. PCM, pericellular matrix; TMJ, temporomandibular joint.
Table 1. Distribution of PCM molecules in rat TMJ condylar cartilage and disc.
| Tissue | Total area/mm2 | Collagen VI stained area/% | Collagen IV stained area/% | Laminin stained area/% |
|---|---|---|---|---|
| Condylar Cartilage | 0.26±0.03 | 42.68±2.88* | 18.32±1.64 | 23.93±2.45 |
| Fibrous | 0.05±0.00 | 35.01±0.76 | 34.42±2.4 | 21.06±3.23 |
| Proliferative | 0.06±0.01 | 67.39±1.97† | 45.69±5.61‡ | 52.38±7.81† |
| Mature | 0.05±0.00 | 48.15±3.67 | 9.29±1.74 | 20.30±2.20 |
| Hypertrophic | 0.09±0.01 | 22.81±2.67 | 1.82±0.25 | 10.50±1.26 |
| Disc | 0.15±0.01 | 27.77±1.03§ | 24.91±1.58 | 12.95±2.21 |
| Anterior | 0.06±0.01 | 27.67±3.01 | 23.62±1.52 | 15.52±4.84 |
| Intermediate | 0.03±0.00 | 30.24±2.98 | 29.33±3.71 | 13.29±2.38 |
| Posterior | 0.06±0.01 | 27.39±1.22 | 25.47±2.01 | 11.52±1.78 |
PCM, pericellular matrix; TMJ, temporomandibular joint.
P<0.001, compared with % collagen IV and % laminin stained areas for condylar cartilage.
P<0.001, compared with fibrous, mature and hypertrophic zones of condylar cartilage.
P<0.001, compared with mature and hypertrophic zones of condylar cartilage.
P<0.001, compared with % laminin stained area for disc.
Data are presented as mean±s.e.m.; n=5–6.
Pericellular localization of the PCM molecules surrounding individual chondrocytes was found throughout the length of the disc in the TMJ disc samples. The total area of positive staining varied among the three PCM molecules. The highest areal deposition of collagen VI (27.77%±1.03%) compared with collagen IV (24.91%±1.58%) and laminin (12.95%±2.21%) was also found in the TMJ disc. However, there was no significant difference in the distribution of PCM molecules (collagen VI, collagen IV and laminin) among the three bands of the TMJ disc (Figure 2a and 2c, Table 1).
Presence of PCM molecules in monolayer cultures of TMJ condylar and disc chondrocytes
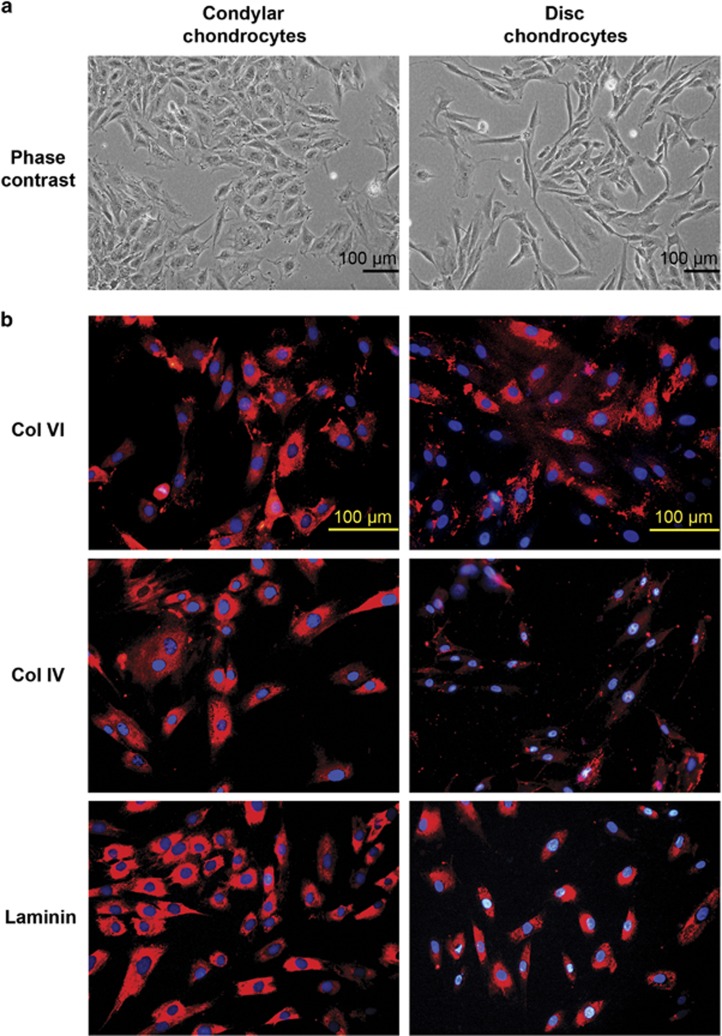
Condylar chondrocytes appeared flattened and fibroblastic in the monolayer cultures, and the disc cells appeared spindle-shaped and slightly elongated (Figure 3a). All three PCM molecules, collagen VI, collagen IV and laminin, were observed in the monolayer cultures of cells derived independently from three rats (Figure 3b). A similar pattern of intracellular staining was observed for all three PCM molecules in both condylar and disc cells (Figure 3b). Some deposition of these molecules was also observed in the culture environment surrounding the cells.
Figure 3.
Monolayer cultures of condylar and disc chondrocytes. (a) Phase-contrast micrographs of condylar and disc chondrocytes. (b) Immunofluorescent (red) staining for collagen VI, collagen IV and laminin produced by condylar and disc chondrocytes. Nuclei were stained using DAPI (blue). Scale bars, 100 μm. Representative images; n=4 per cell type. DAPI, 4′,6-diamidino-2-phenylindole.
Effects of PCM molecules on the proliferation of TMJ condylar and disc chondrocytes
The TMJ condylar and disc chondrocytes were individually pooled from six animals for culture to P2 for the cellular experiments. We investigated the proliferative effect of the PCM molecules on condylar and disc chondrocytes over 48 h. Notably, soluble collagen VI significantly increased cell proliferation in a dose-dependent manner in both condylar and disc chondrocytes (Figure 4a and 4b). A significant enhancement in cell proliferation by collagen VI was observed as early as 24 h. By the end of the 48 h treatment, a significant enhancement (>20%) in proliferation was observed in cells treated with 2 μg·mL−1 collagen VI compared with the untreated controls (P<0.001). A moderate increase (~10%) in the cell proliferation of condylar and disc chondrocytes was observed with the collagen IV treatment. Minimal changes in cell proliferation were observed with the laminin treatment (Figure 4a and 4b).
Figure 4.
Effect of PCM molecules on condylar and disc chondrocyte proliferation. (a) Condylar chondrocytes and (b) disc chondrocytes treated with various concentrations of collagen VI, collagen IV or laminin were assessed for proliferation using the MTS assay at 24 and 48 h. (c) Condylar chondrocytes (left panel) and disc chondrocytes (right panel) pre-treated with collagen VI, collagen IV and laminin were subjected to IL-1β-induced inflammation for 24 h. Cell viability was assessed using the MTS assay. Mean±s.e.m.; n=4. #P<0.05; ##P<0.01; ###P<0.001, compared with untreated control. ***P<0.001, compared with IL-1β-treated group. h, hours; IL-1β, interleukin-1β PCM, pericellular matrix.
To test the proliferative effects of PCM molecules against inflammation, both condylar and disc chondrocytes were pre-treated with PCM molecules before the treatment with IL-1β to stimulate inflammation. Among the three PCM molecules, pre-treatment with collagen VI consistently enhanced the cellular viability and proliferation of condylar chondrocytes (~10%) and disc chondrocytes (~20%) compared with untreated and IL-β-treated cells (P<0.001; Figure 4c). In contrast, pretreatment with collagen IV and laminin did not produce notable proliferative effects in the presence of inflammation.
Pre-treatment effects of PCM molecules on IL-1β-induced gene expression
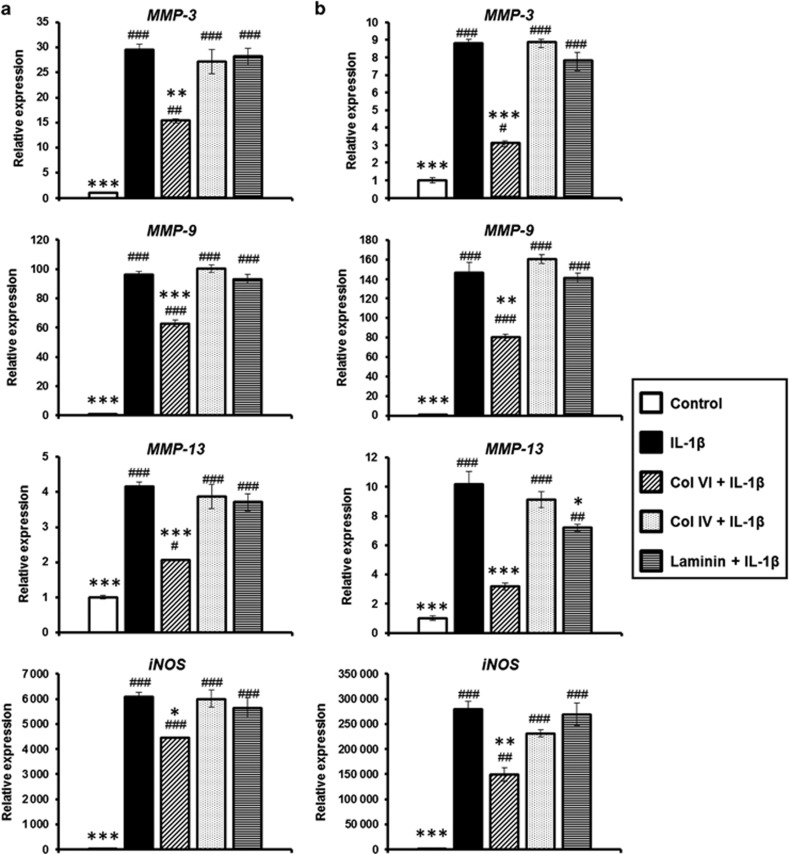
To test the protective effects of PCM molecules against inflammation, both condylar and disc chondrocytes were pre-treated with the PCM molecules before the treatment with IL-1β to stimulate inflammation. Both condylar and disc chondrocytes showed similar patterns in gene expression in response to the catabolic effects of the IL-1β treatment (Figure 5a and 5b). Notably, the IL-1β treatment significantly upregulated the expression of genes associated with matrix degradation, including MMP-3, MMP-9, MMP-13 and iNOS, for both the condylar and disc chondrocytes compared with their untreated counterparts (P<0.001; Figure 5a and 5b). Among the three PCM molecules, pre-treatment with collagen VI consistently reduced the IL-1β-induced upregulation of MMP-3, MMP-9, MMP-13 and iNOS in both the condylar and disc chondrocytes compared with IL-1β-treated cells (P<0.05; Figure 5a and 5b). In contrast, pretreatment with collagen IV and laminin did not reduce the expression of MMP-3, MMP-9 and iNOS in either cell type treated with IL-1β. However, laminin pretreatment significantly reduced MMP-13 expression in disc chondrocytes (P<0.05) but not in condylar chondrocytes (P=0.622; Figure 5a and 5b).
Figure 5.
Effect of PCM molecules on matrix degradative gene expression. (a) Condylar chondrocytes and (b) disc chondrocytes pre-treated with collagen VI, collagen IV or laminin were subjected to IL-1β-induced inflammation for 6 h. Gene expression of MMP-3, MMP-9, MMP-13 and iNOS were measured using RT-PCR. Mean±s.e.m.; n=3. #P<0.05; ##P<0.01; ###P<0.001, compared with untreated control. *P<0.05, **P<0.01, ***P<0.001, compared with IL-1β-treated group. iNOS, inducible nitric oxide synthase; IL-1β, interleukin-1β MMP, matrix metalloproteinase; PCM, pericellular matrix; RT-PCR, real-time reverse transcriptase polymerase chain reaction; s.e.m.: standard error of the mean.
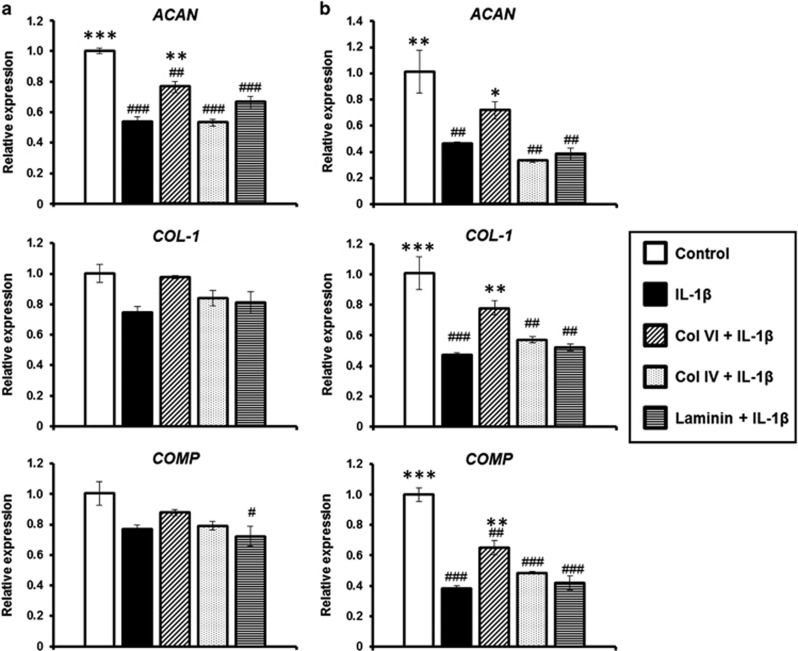
The IL-1β treatment consistently downregulated the expression of cartilage matrix genes, including ACAN, COL-1 and COMP, in condylar and disc chondrocytes (Figure 6a and 6b). In condylar chondrocytes, IL-1β significantly downregulated ACAN, but no significant differences in the levels of COL-1 and COMP were observed compared with the untreated controls. In the disc cells, there were at least twofold reductions in the expression of ACAN (P<0.01), COL-1 (P<0.001) and COMP (P<0.001) with the IL-1β treatment. Among the PCM molecules, pre-treatment with collagen VI generally reduced the adverse effect of the IL-1β-induced downregulation of ACAN, COL-1 and COMP in both condylar and disc chondrocytes. In the condylar chondrocytes, pre-treatment with collagen VI significantly reduced the downregulation of ACAN (P<0.01), but not COL-1 (P=0.106) or COMP (P=0.658), compared with IL-1β-treated cells (Figure 6a and 6b). In the disc chondrocytes, pre-treatment with collagen VI significantly ameliorated the downregulation of ACAN, COL-1 and COMP compared with their IL-1β-treated counterparts (P<0.05; Figure 6a and 6b). In contrast, collagen IV and laminin pretreatment did not reduce the IL-1β-induced downregulation of ACAN, COL-1 and COMP expression in either cell type (Figure 6a and 6b).
Figure 6.
Effects of PCM molecules on matrix synthetic gene expression. (a) Condylar chondrocytes and (b) disc chondrocytes pre-treated with collagen VI, collagen IV or laminin were subjected to IL-1β-induced inflammation for 6 h. Gene expression of COL-1, ACAN and COMP was measured using RT-PCR. Representative results of the mean±s.e.m.; n=3. #P<0.05, ##P<0.01, ###P<0.001, compared with untreated control. *P<0.05, **P<0.01, ***P<0.001, compared with IL-1β-treated group. ACAN, aggrecan; COMP, cartilage oligomeric matrix protein; COL-1, collagen I; IL-1β, interleukin-1β PCM, pericellular matrix; RT-PCR, real-time reverse transcriptase polymerase chain reaction; s.e.m.: standard error of the mean.
Pre-treatment effects of PCM molecules on IL-1β-induced NO and MMP-13 production
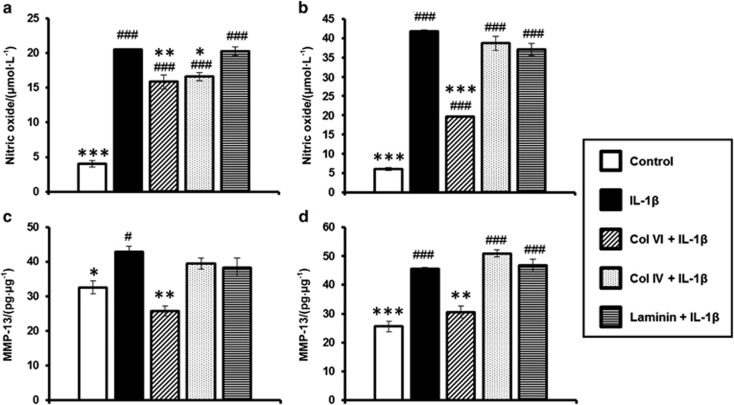
We further investigated the effect of PCM molecules on NO and MMP-13 production by primary condylar and disc chondrocytes. The IL-1β treatment significantly increased NO production in both condylar and disc chondrocytes compared with the untreated controls (P<0.001; Figure 7a and 7b). Among the three PCM molecules, pre-treatment with collagen VI consistently reduced the IL-1β-induced upregulation of NO production in both condylar and disc chondrocytes compared with IL-1β-treated cells (P<0.01; Figure 7a and 7b). In contrast, collagen IV pretreatment significantly reduced NO production in condylar chondrocytes (P<0.05), but not disc chondrocytes (P=0.482), compared with their IL-1β-treated counterparts. However, there was no effect of laminin on IL-1β-induced NO production in either cell type.
Figure 7.
Effects of PCM molecules on NO and MMP-13 production. Condylar chondrocytes (a, c) and disc chondrocytes (b, d) pre-treated with collagen VI, collagen IV or laminin were subjected to IL-1β-induced inflammation for 24 h. NO concentration in culture supernatant was measured using the Griess reaction (a, b). MMP-13 level was measured using ELISA and normalized to the total protein concentration (c,d). Mean±s.e.m.; n=3. #P<0.05, ###P<0.001, compared with untreated control. *P<0.05, **P<0.01, ***P<0.001, compared with IL-1β-treated group. ELISA, enzyme-linked immunosorbent assay; IL-1β, interleukin-1β MMP, matrix metalloproteinase; NO, nitric oxide; PCM, pericellular matrix; s.e.m.: standard error of the mean.
Similarly, IL-1β treatment significantly increased MMP-13 production in both condylar and disc chondrocytes compared with the untreated controls (P<0.05; Figure 7c and 7d). Among the three PCM molecules, pre-treatment with collagen VI consistently reduced the IL-1β-induced upregulation of MMP-13 production in both condylar and disc chondrocytes compared with IL-1β-treated cells (P<0.01; Figure 7c and 7d). However, collagen IV and laminin demonstrated no effect on IL-1β-induced MMP-13 production in either cell type.
Discussion
This study describes the spatial distribution of PCM molecules, including collagen VI, collagen IV and laminin, in normal TMJ condylar cartilage and disc tissues and demonstrates for first time the differential protective roles of these molecules against the IL-1β-induced inflammation that is present in OA. Several molecules have been identified to be present in the PCM surrounding individual chondrocytic cells in different cartilage tissues, including knee joint cartilage, meniscus and intervertebral discs.1, 13, 14 Among these PCM molecules, collagen VI and, more recently, the basement membrane molecules (collagen IV and laminin) have been widely postulated to have a role in the maintenance of cartilage homeostasis and mechanical integrity.3, 14, 21
Our results demonstrated the presence and distribution of collagen VI, collagen IV and laminin in healthy TMJ condylar and disc tissues in rats. These molecules are uniquely localized in the PCM and surround individual chondrocytes. The question of the origin of these PCM molecules in the TMJ is central to these findings. Of interest, chondrocytes are the major source of PCM molecules.
In the TMJ condylar cartilage, it has been well-established that collagen I is predominantly detected in the fibrous zone while collagen II is primarily localized in mature and hypertrophic zones.22 The proliferative zone is primarily a cellular region with relatively few collagen fibres.23 Notably, all three PCM molecules were most abundant in the proliferative zone of the condylar cartilage, which consists of mesenchymal precursor cells involved in matrix synthesis and organization in response to the external environment.24 In contrast, all three PCM molecules were evenly distributed across the three bands of the TMJ disc. The highest zonal deposition of all three PCM molecules in the proliferative zone of TMJ condylar cartilage may be related to the role of these molecules in the cellular proliferation of TMJ chondrocytes. Notably, collagen VI exerted the most potent proliferative effect on both condylar and disc chondrocytes, even in the presence of IL-1β-induced inflammation. This result may be related to the relatively higher amounts of collagen VI than collagen IV and laminin in the condylar cartilage and disc tissues. This finding is consistent with a recent study that demonstrated enhanced proliferation of normal and OA human chondrocytes after treatment with soluble collagen VI.25
Previous studies reported a perturbation and/or changes in PCM as a result of degenerative changes during OA.7, 14, 26 It is well-established that IL-1β levels in the synovial fluid of TMJ correlate with OA changes.27 IL-1β is a major pro-inflammatory cytokine in OA that exhibits destructive effects that encompass increased cartilage degradation and suppression of cartilage matrix synthesis.28 This study demonstrated that collagen VI pretreatment ameliorated IL-1β-induced matrix degradation and reduced the catabolic loss in the chondrocytic phenotype of condylar and disc chondrocytes. This finding is somewhat consistent with a recent study that collagen VI pretreatment effectively prevented knee articular chondrocytes from monoiodoacetate-induced cell apoptosis,29 which suggests protective effects of collagen VI in overall chondrocyte survival and inflammatory response. This role of collagen VI is further supported by transgenic knockout mouse studies, which demonstrated that collagen VI knockout mice showed accelerated osteoarthritic changes in the hip accompanied with decreased bone mass density because of a collagen VI deficiency.21
Although our results demonstrated little to no effect of collagen IV and laminin on both condylar and disc chondrocytes against IL-1β-induced inflammation, these basement membrane molecules (collagen IV and laminin) are likely involved in other major cellular processes, such as chondrogenic differentiation. Schminke et al.3 recently reported the role of laminin and nidogen-2 in the chondrogenic differentiation of chondrogenic progenitor cells and chondrocytes in three-dimensional (3-D) alginate bead cultures.
Future studies should examine the changes in expression of these PCM molecules in relevant TMJ-OA animal models to better establish the role of these molecules, particularly collagen VI, in cartilage metabolism during inflammation that is present in TMJ-OA. It would also be of interest to investigate the effects of endogenous and soluble exogenous PCM molecules on the survival, proliferation and differentiation of chondrocytes and mesenchymal progenitor cells.
Collectively, this study shows the presence and distribution of PCM molecules, including collagen VI, collagen IV and laminin, in TMJ condylar cartilage and discs. Among the PCM molecules, collagen VI demonstrates proliferative and protective effects on TMJ chondrocytes against IL-1β-induced inflammation. These findings of PCM molecules, particularly collagen VI, may aid in the discovery of early biomarkers for TMJ-OA and the elucidation of new measures and modalities focusing on the PCM for the effective prevention and/or treatment of TMJ-OA.30, 31
Acknowledgments
This work was supported by grants from the National University Healthcare System (R221000077733) and the National University of Singapore (R221000090112).
Footnotes
Supplementary Information for this article can be found on the International Journal of Oral Science website (http://www.nature.com/ijos).
Supplementary Material
References
- Poole CA, Flint MH, Beaumont BW. Chondrons in cartilage: ultrastructural analysis of the pericellular microenvironment in adult human articular cartilages. J Orthop Res 1987; 5 (4): 509–522. [DOI] [PubMed] [Google Scholar]
- Wilusz RE, Sanchez-Adams J, Guilak F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol 2014; 39: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schminke B, Frese J, Bode C et al. Laminins and nidogens in the pericellular matrix of chondrocytes: their role in osteoarthritis and chondrogenic differentiation. Am J Pathol 2016; 186 (2): 410–418. [DOI] [PubMed] [Google Scholar]
- Guilak F, Alexopoulos LG, Upton ML et al. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Ann N Y Acad Sci 2006; 1068: 498–512. [DOI] [PubMed] [Google Scholar]
- Hing WA, Sherwin AF, Poole CA. The influence of the pericellular microenvironment on the chondrocyte response to osmotic challenge. Osteoarthr Cartil 2002; 10 (4): 297–307. [DOI] [PubMed] [Google Scholar]
- Poole CA, Ayad S, Schofield JR. Chondrons from articular cartilage: I. Immunolocalization of type VI collagen in the pericellular capsule of isolated canine tibial chondrons. J Cell Sci 1988; 90 (4): 635–643. [DOI] [PubMed] [Google Scholar]
- Poole AR, Rosenberg LC, Reiner A et al. Contents and distributions of the proteoglycans decorin and biglycan in normal and osteoarthritic human articular cartilage. J Orthop Res 1996; 14 (5): 681–689. [DOI] [PubMed] [Google Scholar]
- Pufe T, Petersen WJ, Miosge N et al. Endostatin/collagen XVIII—an inhibitor of angiogenesis—is expressed in cartilage and fibrocartilage. Matrix Biol 2004; 23 (5): 267–276. [DOI] [PubMed] [Google Scholar]
- Kvist AJ, Nyström A, Hultenby K et al. The major basement membrane components localize to the chondrocyte pericellular matrix—a cartilage basement membrane equivalent? Matrix Biol 2008; 27 (1): 22–33. [DOI] [PubMed] [Google Scholar]
- Kruegel J, Miosge N. Basement membrane components are key players in specialized extracellular matrices. Cell Mol Life Sci 2010; 67 (17): 2879–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Shu C, Melrose J. Comparative immunolocalisation of perlecan with collagen II and aggrecan in human foetal, newborn and adult ovine joint tissues demonstrates perlecan as an early developmental chondrogenic marker. Histochem Cell Biol 2010; 134 (3): 251–263. [DOI] [PubMed] [Google Scholar]
- Toh WS, Foldager CB, Olsen BR et al. Basement membrane molecule expression attendant to chondrogenesis by nucleus pulposus cells and mesenchymal stem cells. J Orthop Res 2013; 31 (7): 1136–1143. [DOI] [PubMed] [Google Scholar]
- Cao L, Guilak F, Setton LA. Three-dimensional morphology of the pericellular matrix of intervertebral disc cells in the rat. J Anat 2007; 211 (4): 444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldager CB, Toh WS, Gomoll AH et al. Distribution of basement membrane molecules, laminin and collagen type IV, in normal and degenerated cartilage tissues. Cartilage 2014; 5 (2): 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto C, Galanti C, Almeida LE et al. Expression and localization of aquaporin-1 in temporomandibular joint disc with internal derangement. J Oral Pathol Med 2012; 41 (8): 642–647. [DOI] [PubMed] [Google Scholar]
- Fang W, Friis TE, Long X et al. Expression of chondromodulin-1 in the temporomandibular joint condylar cartilage and disc. J Oral Pathol Med 2010; 39 (4): 356–360. [DOI] [PubMed] [Google Scholar]
- Figueroba SR, Desjardins MP, Ferreira LE et al. The influence of altered occlusion on pro-inflammatory cytokine levels in the TMJ synovial tissues of rats. Arch Oral Biol 2014; 59 (11): 1164–1171. [DOI] [PubMed] [Google Scholar]
- Toh WS, Guo XM, Choo AB et al. Differentiation and enrichment of expandable chondrogenic cells from human embryonic stem cells in vitro. J Cell Mol Med 2009; 13 (9b): 3570–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh WS, Yap AU, Lim SY. In vitro biocompatibility of contemporary bulk-fill composites. Oper Dent 2015; 40 (6): 644–652. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Long P, Gassner R et al. Cyclic tensile strain suppresses catabolic effects of interleukin-1beta in fibrochondrocytes from the temporomandibular joint. Arthritis Rheum 2001; 44 (3): 608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos LG, Youn I, bonaldo P et al. Developmental and osteoarthritic changes in Col6a1-knockout mice: biomechanics of type VI collagen in the cartilage pericellular matrix. Arthritis Rheum 2009; 60 (3): 771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi I, Takahashi I, Nakamura M et al. An immunohistochemical study of regional differences in the distribution of type I and type II collagens in rat mandibular condylar cartilage. Arch Oral Biol 1996; 41 (8/9): 863–869. [DOI] [PubMed] [Google Scholar]
- de Bont LG, Boering G, Havinga P et al. Spatial arrangement of collagen fibrils in the articular cartilage of the mandibular condyle: a light microscopic and scanning electron microscopic study. J Oral Maxillofac Surg 1984; 42 (5): 306–313. [DOI] [PubMed] [Google Scholar]
- Wadhwa S, Kapila S. TMJ disorders: future innovations in diagnostics and therapeutics. J Dent Educ 2008; 72 (8): 930–947. [PMC free article] [PubMed] [Google Scholar]
- Smeriglio P, Dhulipala L, Lai JH et al. Collagen VI enhances cartilage tissue generation by stimulating chondrocyte proliferation. Tissue Eng Part A 2015; 21 (3/4): 840–849. [DOI] [PubMed] [Google Scholar]
- Pullig O, Weseloh G, Swoboda B. Expression of type VI collagen in normal and osteoarthritic human cartilage. Osteoarthr Cartil 1999; 7 (2): 191–202. [DOI] [PubMed] [Google Scholar]
- Kubota E, Kubota T, Matsumoto J et al. Synovial fluid cytokines and proteinases as markers of temporomandibular joint disease. J Oral Maxillofac Surg 1998; 56 (2): 192–198. [DOI] [PubMed] [Google Scholar]
- Daheshia M, Yao JQ. The interleukin 1β pathway in the pathogenesis of osteoarthritis. J Rheumatol 2008; 35 (12): 2306–2312. [DOI] [PubMed] [Google Scholar]
- Peters HC, Otto TJ, Enders TJ et al. The protective role of the pericellular matrix in chondrocyte apoptosis. Tissue Eng Part A 2011; 17 (15/16): 2017–2024. [DOI] [PubMed] [Google Scholar]
- Zhang S, Yap, Toh WS. Stem cells for temporomandibular joint repair and regeneration. Stem Cell Rev 2015; 11(5): 728–742. [DOI] [PubMed] [Google Scholar]
- Toh WS, Foldager CB, Hui JH et al. Exploiting stem cell-extracellular matrix interactions for cartilage regeneration: a focus on basement membrane molecules. Curr Stem Cell Res Ther 2016; 11 (8): 618–625. [DOI] [PubMed] [Google Scholar]
- Toh WS, Foldager CB, Pei M et al. Advances in mesenchymal stem cell-based strategies for cartilage repair and regeneration. Stem Cell Rev 2014; 10 (5): 686–696. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.