The crystal structure of human Ca2+-bound S100A1 at 2.25 Å resolution is described.
Keywords: S100, S100A1, crystal structure, calcium-binding proteins, S100B, Alzheimer’s disease, cardiomyopathy
Abstract
S100A1 is a member of the S100 family of Ca2+-binding proteins and regulates several cellular processes, including those involved in Ca2+ signaling and cardiac and skeletal muscle function. In Alzheimer’s disease, brain S100A1 is overexpressed and gives rise to disease pathologies, making it a potential therapeutic target. The 2.25 Å resolution crystal structure of Ca2+-S100A1 is solved here and is compared with the structures of other S100 proteins, most notably S100B, which is a highly homologous S100-family member that is implicated in the progression of malignant melanoma. The observed structural differences in S100A1 versus S100B provide insights regarding target protein-binding specificity and for targeting these two S100 proteins in human diseases using structure-based drug-design approaches.
1. Introduction
S100 proteins are a family of Ca2+-binding proteins with cell-specific and tissue-specific expression and a variety of intracellular and extracellular regulatory functions (Baudier et al., 1986 ▸; Donato et al., 2013 ▸; Donato, 2001 ▸; Zimmer et al., 1995 ▸). Despite having no enzymatic activity, S100 proteins are involved in a range of biological processes, including Ca2+ homeostasis, cell growth, metabolism and phosphorylation (Donato et al., 2013 ▸; Kligman & Hilt, 1988 ▸; Schäfer & Heizmann, 1996 ▸; Strynadka & James, 1989 ▸; Zimmer et al., 1995 ▸). The influence of S100 proteins is typically mediated through Ca2+-dependent protein–protein interactions (PPIs). With calbindin as the exception, S100 proteins assemble as homodimers or heterodimers, with each subunit having two EF-hand domains termed EF1 and EF2. The N-terminal EF-hand, EF1, is a pseudo EF-hand having 14 amino-acid residues (versus 12) and binds calcium with a lower affinity than EF2. The canonical EF-hand, EF2, resides in the C-terminal region of an S100 subunit and Ca2+-binding to EF2 activates the rotation of helix 3 necessary for target protein binding (Wright, Inman et al., 2008 ▸; Wright et al., 2009 ▸). The altered expression of several S100 proteins is associated with a variety of disease states, including cancer (Bresnick et al., 2015 ▸; Harpio & Einarsson, 2004 ▸; Rustandi et al., 1998 ▸; Salama et al., 2008 ▸), neurological disorders (Bresnick et al., 2015 ▸; Most et al., 2007 ▸; Salama et al., 2008 ▸) and cardiomyopathies (Bresnick et al., 2015 ▸; Most et al., 2007 ▸; Salama et al., 2008 ▸), with several S100 proteins (i.e. S100A1, S100A4, S100A7, S100B and others) being considered as potential therapeutic targets for drug development (Bresnick et al., 2015 ▸; Harpio & Einarsson, 2004 ▸; Malashkevich et al., 2008 ▸; Salama et al., 2008 ▸).
S100A1 is highly expressed in cardiac and skeletal muscle and interacts with a number of proteins, including the ryanodine receptor (RyR), an interaction that regulates Ca2+ transients in heart and skeletal muscle (Garbuglia et al., 1999 ▸; Landar et al., 1998 ▸; Most, Remppis, Pleger et al., 2003 ▸; Most, Remppis, Weber et al., 2003 ▸; Most et al., 2004 ▸; Osterloh et al., 1998 ▸; Treves et al., 1997 ▸; Wang et al., 2005 ▸; Wright et al., 2009 ▸). In a heart-failure model, decreased S100A1 expression followed by adenoviral reintroduction of the S100A1 gene restored diminished Ca2+ transients and reversed contractile dysfunction (Most et al., 2004 ▸). However, in the brain overexpressed S100A1 contributes to pathologies related to neurological diseases (Wright et al., 2009 ▸). Specifically, high levels of extracellular S100A1 are cytotoxic and its intracellular processes augment Alzheimer’s disease pathologies, including altered amyloid precursor protein expression, destabilization of calcium homeostasis, increased cell growth, decreased dendritic arborization and lower tubulin polymerization/stability (Wright et al., 2009 ▸; Zimmer et al., 1998 ▸). Thus, incentives to develop S100A1 antagonists are in place to regulate both its intracellular and extracellular functions when elevated in neurological diseases. On the other hand, blocking of S100A1 needs to be avoided when S100A1 levels are in the normal range so its critical roles in Ca2+-signaling and muscle contraction remain functional. The approach of avoiding S100A1 blockade remains an important consideration for developing other S100 inhibitors, including S100B inhibitors for treating melanoma (Bresnick et al., 2015 ▸). The crystal structure of Ca2+-S100A1 is reported here to provide details for better understanding of S100-specific targets and for the development of S100A1-specific and/or S100B-specific inhibitors. Thus, the crystal structure of Ca2+-S100A1 was determined and compared with previously published structures of S100A1 (NMR) and S100B (NMR, X-ray) to facilitate these goals as well as to better understand the structure–function relationships within the S100 protein family.
2. Methods
2.1. Materials
Chromatography columns and resins were obtained from GE Healthcare Life Sciences. All buffers were passed through Chelex-100 (Bio-Rad) to remove trace metals and divalent ions. All recombinant proteins were dialyzed using Chelex-100 resin for the same purpose. All other chemical reagents used were ACS grade or higher and were purchased from Sigma–Aldrich unless otherwise stated.
2.2. Expression and purification of S100A1
Recombinant human S100A1 was expressed in Escherichia coli strain BL21(DE3) cells transformed with an expression plasmid (pET-11b; Novagen) containing the gene for codon-optimized human S100A1. E. coli cultures were allowed to grow at 37°C to an OD600 of 0.8–1.0 and were induced with 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) for 4 h or overnight (8 h) at 25°C. The cultures were then pelleted and stored at −80°C before purification. Lysis was performed at room temperature with buffer A (50 mM Tris–HCl pH 7.50, 0.5 mM DTT), 0.5 mM AEBSF, 1 mg lysozyme per gram of pellet, 0.5 mg DNAse I and 10 mM MgCl2. Following this, sonication was performed on ice to ensure total cell lysis. The lysate was then pelleted and the supernatant was kept for precipitation with ammonium sulfate and streptomycin sulfate. Bacterial lysates were precipitated by the slow addition of ammonium sulfate to 65% saturation and streptomycin sulfate to 1%(w/v). The lysate was then pelleted again and the supernatant was dialyzed against buffer A to remove ammonium sulfate. The sample was loaded onto a DE52 diethylaminoethyl Sepharose (DEAE) column (Whatman). The column was equilibrated and unbound proteins were eluted with buffer A. S100A1 bound to the column was next eluted via stepwise additions of buffer with increasing NaCl concentrations. Fractions were analysed by SDS–PAGE, and S100A1 protein-containing fractions were pooled. Pure S100A1 (>99%) was concentrated (to >5 mM) and buffer-exchanged using gel filtration on a Sephadex G-25 column equilibrated in buffer B (0.25 mM Tris–HCl pH 7.50, 50 mM NaCl, 0.25 mM DTT). S100A1 from this column was concentrated (>5 mM) and stored frozen as separate aliquots to avoid repeated freeze–thaw cycles.
2.3. Crystallography
Diffraction-quality crystals of human S100A1 were obtained via sitting-drop vapor diffusion in 24-well trays (Hampton Research) using 250 µl reservoir solution and a 0.75:0.75 µl drop size. The mother liquor for crystal formation consisted of 100 mM cacodylate pH 7.0–7.5, 10 mM CaCl2, 25–28% PEG 8000. The initial crystals were obtained under identical conditions using PEG 3350 in place of PEG 8000. The protein-sample conditions for crystal formation consisted of 20 mg ml−1 S100A1, 10 mM cacodylate pH 7.20, 7.5 mM CaCl2; no cryoprotectants were used. Crystals were flash-cooled in liquid nitrogen for storage before data collection.
Remote data collection was performed on beamline 7-1 at the Stanford Synchrotron Radiation Lightsource (SSRL), SLAC National Accelerator Laboratory, Menlo Park, California, USA (Cohen et al., 2002 ▸; Soltis et al., 2008 ▸). Data were collected at 100 K on an ADSC Q315 (315 × 315 mm) detector using data-collection strategies generated by Web-Ice (González et al., 2008 ▸). The diffraction statistics are summarized in Table 1 ▸. Processing of reflections was accomplished using the AUTOXDS script (González & Tsai, 2010 ▸) developed at SSRL to automate the existing X-ray Detector Software (XDS; Kabsch, 2010 ▸). The structure was initially solved in space group C2. Following this, however, we determined that the correct space group was in fact P3221, as confirmed by Zanuda (Lebedev & Isupov, 2014 ▸), and the structure was solved again in this space group. The BALBES server (Long et al., 2008 ▸) was utilized to identify an appropriate search model and to perform molecular replacement with MOLREP (Vagin, 1989 ▸; Vagin & Teplyakov, 1997 ▸, 2010 ▸). S100Z (PDB entry 2y5i) was identified as the best search model (Moroz et al., 2011 ▸). Restrained refinement was performed using phenix.refine (Adams et al., 2010 ▸; Afonine et al., 2012 ▸) and BUSTER-TNT (Bricogne et al., 2016 ▸), and manual building was performed in Coot (Emsley et al., 2010 ▸). The structure-refinement statistics are summarized in Table 1 ▸, and final figures and alignments were made using PyMOL (http://www.pymol.org) unless otherwise stated.
Table 1. Crystallographic statistics.
Values in parentheses are for the highest resolution shell.
| Diffraction source | Beamline 7-1, SSRL |
| Wavelength (Å) | 1.1271 |
| Resolution range (Å) | 33.47–2.25 (2.33–2.25) |
| Space group | P3221 |
| a, b, c (Å) | 53.5, 53.5, 193.4 |
| α, β, γ (°) | 90, 90, 120 |
| Total No. of reflections | 81192 (5079) |
| No. of unique reflections | 15829 (765) |
| Mosaicity (°) | 1.7 |
| Multiplicity | 5.1 (3.8) |
| Completeness (%) | 92 (85) |
| 〈I/σ(I)〉 | 14.44 (0.39) |
| Overall B factor from Wilson plot (Å2) | 50.78 |
| R merge | 0.185 (3.61) |
| R meas | 0.205 (4.17) |
| CC1/2 | 0.99 (0.16) |
| CC* | 1.00 (0.53) |
| No. of reflections, working set | 16102 (764) |
| No. of reflections, test set | 757 (38) |
| R work | 0.275 (0.491) |
| R free | 0.290 (0.528) |
| Cruickshank DPI | 0.245 |
| No. of non-H atoms | |
| Total | 2093 |
| Protein | 2065 |
| Ligand | 14 |
| Solvent | 14 |
| R.m.s. deviations | |
| Bonds (Å) | 0.009 |
| Angles (°) | 0.96 |
| Average B factors (Å2) | |
| Overall | 82.60 |
| Protein | 82.83 |
| Ligand | 73.56 |
| Ramachandran plot | |
| Most favored (%) | 96.0 |
| Allowed (%) | 4.0 |
| PDB code | 5k89 |
3. Results and discussion
S100A1 is an enhancer of cardiac and skeletal muscle contractility and exhibits potential as a gene-therapeutic agent for the treatment of cardiomyopathies. S100A1 competes with calmodulin for binding RyR1 and acts as a sensitizer and activator of Ca2+ release (Prosser et al., 2008 ▸, 2011 ▸; Schneider et al., 2009 ▸; Wright, Prosser et al., 2008 ▸; Yamaguchi et al., 2011 ▸). On the other hand, when overexpressed in the brain, S100A1 contributes to pathologies related to neurological diseases (Wright et al., 2009 ▸). Therefore, it is important to understand the specifics involved in the S100A1-dependent regulation of biological targets at atomic resolution. For this, the X-ray crystal structure of human Ca2+-S100A1 is presented here, and comparisons of S100A1 and S100B are discussed in order to better understand the target-binding specificities required to generate unique biological responses.
3.1. X-ray crystal structure of calcium-bound S100A1
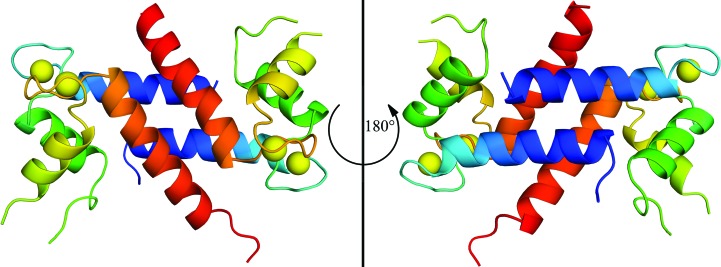
X-ray diffraction data were collected and the crystal structure of Ca2+-bound S100A1 was solved at 2.25 Å resolution with R work and R free values of 0.275 and 0.290, respectively (Table 1 ▸; Fig. 1 ▸). The electron density was limited in places owing to anisotropy, particularly in loop regions such as the hinge region (loop 2) and the C-terminal loop. The asymmetric unit contains three S100A1 molecules: one full dimer and another monomer that forms the biological dimer by crystal symmetry. Like other members of the S100 protein family, S100A1 is a homodimer with an X-type four-helical bundle comprising its dimer interface (Fig. 1 ▸). Ca2+ coordination in the helix–loop–helix EF-hand domains of S100A1 could be modeled but not without some ambiguity owing to weaker electron density in these loops. For example, the density for a water ligand at position 7 of each EF-hand was lacking, so it was not modeled for EF1 or EF2, as is typically found. Nonetheless, as in other S100 proteins, Ca2+ coordination in EF1 of S100A1 is via the O atoms of the backbone carbonyl moieties of Ser20, Glu23, Asp25, Lys28 and the side-chain carboxylate of Glu33, which coordinates in a bidentate manner. In EF2, Ca2+ coordination is via the side-chain carboxylate O atoms of Asp63, Asn65, Asp67, Glu74 and the O atom of the backbone carbonyl moiety of Glu69, as is typical for canonical EF-hand motifs such as those in other S100 proteins, calmodulin, troponin C and other EF-hand-containing proteins (Strynadka & James, 1989 ▸). The one difference is that the O atom of Asn65 coordinates calcium in the EF2 domain of Ca2+-S100A1 and this analogous coordination is via an O atom of an aspartate residue in S100B (Matsumura et al., 1998 ▸).
Figure 1.
Ribbon diagram of the Ca2+-S100A1 dimer crystal structure viewed from the front (left) and rear (right). Helices are colored blue, green, yellow and red for helices 1, 2, 3 and 4, respectively; Ca2+ ions are yellow.
The four helices in each subunit of Ca2+-S100A1 and their alignment at the dimer interface of the crystal structure are very similar to that found previously via NMR (Table 2 ▸). This includes the perpendicular arrangement of helices 3 and 4 of Ca2+-S100A1 to give an open conformation as is necessary to bind its protein targets, including a peptide derived from RyR1. In the absence of the RyR1 target peptide, the crystal and NMR structures of Ca2+-S100A1 show a reasonable level of similarity when interhelical angles and metal-ion coordination distances are compared (Table 2 ▸). The crystal structure of Ca2+-S100A1 aligns similarly with the NMR structures of Ca2+-S100A1 in the absence (PDB entries 1zfs and 2lp3; Nowakowski et al., 2013 ▸; Wright et al., 2005 ▸) or presence (PDB entries 2k2f and 2kbm; Wright, Prosser et al., 2008 ▸) of peptide targets (r.m.s.d. 2.3–2.6 Å) as well as other X-type helical bundle S100 proteins (Table 2 ▸). Differences in these and other S100 proteins are typically localized to the most dynamic regions of the proteins, including the hinge region (loop 2) and the C-terminus, which are involved in target binding.
Table 2. Intrahelical angles and distances for Ca2+-S100A1 and other Ca2+-S100 structures.
Intrahelical angles and distances were calculated using UCSF Chimera and previously established methods (Drohat et al., 1998 ▸).
| Intrahelical angles (°) | Intrahelical distances (Å) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R.m.s.d. (Å) | I to II | I to III | I to IV | II to III | II to IV | III to IV | I to II | I to III | I to IV | II to III | II to IV | III to IV | |
| S100A1 (X-ray)† | 0.5 | 140.0 | −116.5 | 127.4 | 103.4 | −28.8 | 110.2 | 8.1 | 16.8 | 11.3 | 9.9 | 13.9 | 9.1 |
| S100A1 (NMR)‡ | 2.6 | 133.3 | −106.5 | 129.4 | 117.5 | −33.0 | 119.7 | 9.8 | 19.5 | 13.4 | 10.2 | 12.4 | 8.5 |
| S100A1–RyRP (NMR)§ | 2.5 | 137.8 | −94.4 | 123.5 | 121.5 | −40.6 | 137.2 | 9.7 | 19.3 | 12.4 | 10.4 | 12.6 | 9.9 |
| S100A4 (X-ray)¶ | 1.5 | 140.2 | −112.6 | 131.3 | 106.2 | −34.9 | 108.5 | 8.1 | 17.2 | 11.1 | 10.0 | 13.6 | 8.8 |
| S100B (X-ray)†† | 1.0 | 138.7 | −126.6 | 126.7 | 93.8 | −36.6 | 100.1 | 8.7 | 17.3 | 11.0 | 9.5 | 13.9 | 9.0 |
| S100B (NMR)‡‡ | 2.9 | 131.9 | −124.0 | 124.3 | 103.4 | −38.7 | 103.1 | 10.4 | 20.1 | 12.1 | 10.2 | 13.2 | 9.9 |
| S100Z (X-ray)§§ | 0.7 | 136.2 | −107.6 | 131.8 | 116.2 | −25.6 | 112.7 | 7.6 | 17.0 | 11.2 | 9.7 | 12.7 | 8.9 |
The r.m.s.d. for the S100A1 crystal structure corresponds to the average alignment of each monomer in the asymmetric unit.
3.2. Detailed comparison of S100A1 and S100B structures
When comparisons are made with S100B in particular, the global fold of Ca2+-S100A1 determined here was also similar to those of several crystal structures available for S100B (Cavalier, Melville et al., 2016 ▸; Drohat et al., 1998 ▸; Gógl et al., 2016 ▸; Jensen et al., 2015 ▸). In such comparisons, the r.m.s.d. ranged between 0.7 and 1.2 Å, with the exception of S100B bound to a peptide derived from the receptor for advanced glycation endproducts (RAGE; PDB entry 5d7f, J. L. Jensen & C. L. Colbert, unpublished work; 1.6 Å r.m.s.d.), which diverges primarily in the dynamic C-terminus. When Ca2+-coordination distances were compared for the S100A1 and S100B structures, they were also found to be essentially within the margin of error (all-atom alignment r.m.s.d. of 1.0 Å; Cruickshank DPI of the model of approximately 0.24 Å; PyMOL), so like other determined S100 protein structures, the crystal structure of Ca2+-S100A1 determined here is similar in its overall global fold to other available S100 protein structures. In addition, similarities exist in the binding pockets of Ca2+-S100A1 and Ca2+-S100B. This includes the presence of a cysteine residue, Cys86 (Cys84 in S100B), bridging what are termed sites 1 and 2 of the target-binding pocket (Cavalier et al., 2014 ▸; Charpentier et al., 2009 ▸). While the target-binding pockets in Ca2+-S100A1 and Ca2+-S100B are composed largely of hydrophobic residues, it is clear that these pockets are not identical.
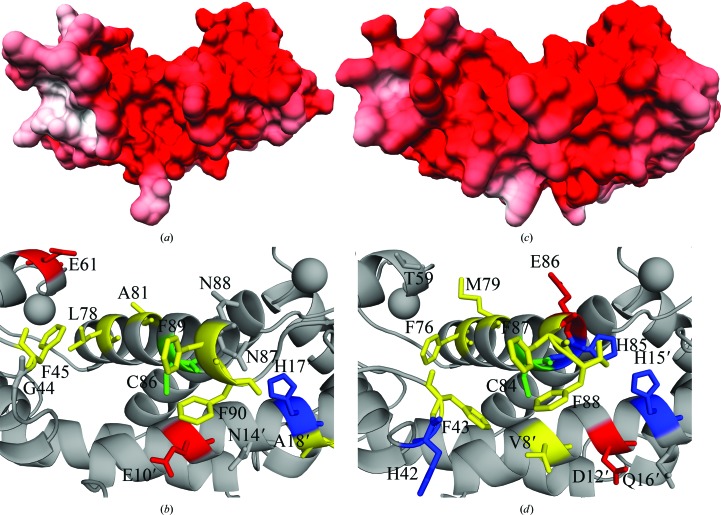
Upon closer comparison of Ca2+-S100A1 and Ca2+-S100B, there are structural differences that provide a means for specificity in target and inhibitor binding. Specifically, the binding pocket in Ca2+-S100B has an additional aromatic residue (Phe76) available for π-stacking compared with an aliphatic residue (i.e. Leu78) in the analogous location in Ca2+-S100A1 (Figs. 2 ▸ and 3 ▸). There is a negatively charged side chain on helix 3 (Glu61) in Ca2+-S100A1 that is occupied by a polar residue (i.e. Thr59) in the analogous position in Ca2+-S100B. In helix 4, a polar residue Asn87 in Ca2+-S100A1 occupies a position where a positively charged His85 resides in Ca2+-S100B. This histidine residue is also part of the Zn2+-binding site in S100B (Wilder et al., 2003 ▸), and S100A1 does not have this Zn2+-binding site. In the hinge region (loop 2), the positively charged His42 in Ca2+-S100B differs from the Gly44 in Ca2+-S100A1. In the hinge region, the positions of Glu45, Glu46 and Ile47 in Ca2+-S100B are occupied by Asp47, Ala48 and Gln49 in Ca2+-S100A1 (Fig. 3 ▸). Another difference can be observed for Glu10, Asn14 and Ala18 in the target-binding pocket of Ca2+-S100A1 versus Val8, Asp12 and Gln16 in the analogous positions of Ca2+-S100B, which again would alter the mode of target and inhibitor binding. Thus, there are important differences in charge and polarity when the two binding pockets of these S100s are compared. These would additionally contribute to the specificity upon target binding and functional folding events necessary for Ca2+-binding affinities to increase, as have been observed previously for S100B (Charpentier et al., 2010 ▸; Liriano et al., 2012 ▸; Rustandi et al., 1998 ▸; Zimmer & Weber, 2010 ▸). With these observed differences, there are numerous structural and dynamic properties within Ca2+-S100A1 and Ca2+-S100B that can be used to explain mechanistically how these two proteins bind specific protein targets in a cell-specific manner to generate unique biological responses, as has been observed in many biological studies (Bresnick et al., 2015 ▸; Donato et al., 2013 ▸; Hernández-Ochoa et al., 2009 ▸; Lin et al., 2010 ▸; Wright, Prosser et al., 2008 ▸; Wright et al., 2009 ▸; Zimmer et al., 1995 ▸; Zimmer & Weber, 2010 ▸).
Figure 2.
Left: electrostatic surface model (a) and ribbon diagram (b) of the Ca2+-S100A1 crystal structure, highlighting the binding pocket. Right: electrostatic surface model (c) and ribbon diagram (d) of the Ca2+-S100B crystal structure (PDB entry 1mho; Matsumura et al., 1998 ▸). In the ribbon diagrams, hydrophobic residues are in yellow, positively charged residues are in blue and negatively charged residues are in red. Key residues are labeled; see text for additional details. Electrostatic surface models were generated using PDB2PQR (Dolinsky et al., 2004 ▸, 2007 ▸), APBS (Czodrowski et al., 2006 ▸) and UCSF Chimera (Pettersen et al., 2004 ▸).
Figure 3.
Sequence alignments.. Sequence alignments were generated using T-Coffee (Di Tommaso et al., 2011 ▸; Notredame et al., 2000 ▸) and BoxShade. S100A sequences correspond to the human sequence, S100B corresponds to the bovine sequence and S100Z corresponds to the zebrafish sequence.
4. Conclusions
The crystal structure of Ca2+-S100A1 is presented here at 2.25 Å resolution. As a complement to the previously published NMR data, crystallography provides a detailed examination of the atomic structure of S100A1 and its binding pocket to reveal small but significant differences between S100A1 and other S100 proteins (i.e. S100B) that are necessary to understand its specific biological functions (Cavalier, Ansari et al., 2016 ▸; Donato et al., 2013 ▸; Lin et al., 2010 ▸; Wright, Prosser et al., 2008 ▸; Zimmer et al., 1995 ▸). These data can be used to aid in the development of more specific, efficacious inhibitors that target one S100 protein versus another. They also provide support for the modeling of the Ca2+ ion coordination sphere by previous NMR studies, which is necessary to understand the role that S100A1 plays as a calcium-signaling protein in mammals. While numerous similarities are found among S100-family members, including their global fold and overall Ca2+-dependent conformational change, detailed differences need to be catalogued in order to understand their specific S100–target protein interactions and perhaps for the development of therapeutics targeting such family members in various disease states. Some of these differences include one of the Ca2+-coordinating residues (Asn65 in S100A1 and Asp65 in S100B), numerous residues in the binding pocket of the two proteins (Figs. 2 ▸ and 3 ▸), and other residues throughout the protein that are likely to affect the binding and functional folding events associated with increasing Ca2+ binding upon binding its specific protein targets. The crystal structure of Ca2+-S100A1 will also have an impact on our understanding of protein–protein interactions involving S100A1 with full-length targets such as the ryanodine receptors in skeletal (RyR1) and cardiac (RyR2) muscle as well as how it responds to Ca2+ concentration fluctuations within mammalian cells (Hernández-Ochoa et al., 2009 ▸; Prosser et al., 2008 ▸, 2011 ▸; Wright, Prosser et al., 2008 ▸).
Supplementary Material
PDB reference: S100A1, 5k89
Supplementary Figure S1,. DOI: 10.1107/S2053230X17003983/rr5138sup1.pdf
Acknowledgments
Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory is supported by the US DOE, Office of Science and Office of Basic Energy. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research and by the National Institutes of Health, National Institute of General Medical Sciences. This work was also supported by NIH grants (CA107331 and GM58888 to DJW). The training of ZM was also supported by an NIH grant (NIH T32 AR007592). We also thank Drs Michael C. Cavalier and Sean D. Stowe for advice with solving this crystal structure.
References
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
- Afonine, P. V., Grosse-Kunstleve, R. W., Echols, N., Headd, J. J., Moriarty, N. W., Mustyakimov, M., Terwilliger, T. C., Urzhumtsev, A., Zwart, P. H. & Adams, P. D. (2012). Acta Cryst. D68, 352–367. [DOI] [PMC free article] [PubMed]
- Baudier, J., Glasser, N. & Gerard, D. (1986). J. Biol. Chem. 261, 8192–8203. [PubMed]
- Bresnick, A. R., Weber, D. J. & Zimmer, D. B. (2015). Nature Rev. Cancer, 15, 96–109. [DOI] [PMC free article] [PubMed]
- Bricogne, G., Blanc, E., Brandl, M., Flensburg, C., Keller, P., Paciorek, W., Roversi, P., Sharff, A., Smart, O. S., Vonrhein, C. & Womack, T. O. (2016). BUSTER. Cambridge: Global Phasing Ltd.
- Cavalier, M. C., Ansari, M. I., Pierce, A. D., Wilder, P. T., McKnight, L. E., Raman, E. P., Neau, D. B., Bezawada, P., Alasady, M. J., Charpentier, T. H., Varney, K. M., Toth, E. A., MacKerell, A. D., Coop, A. & Weber, D. J. (2016). J. Med. Chem. 59, 592–608. [DOI] [PMC free article] [PubMed]
- Cavalier, M. C., Melville, Z., Aligholizadeh, E., Raman, E. P., Yu, W., Fang, L., Alasady, M., Pierce, A. D., Wilder, P. T., MacKerell, A. D. & Weber, D. J. (2016). Acta Cryst. D72, 753–760. [DOI] [PMC free article] [PubMed]
- Cavalier, M. C., Pierce, A. D., Wilder, P. T., Alasady, M. J., Hartman, K. G., Neau, D. B., Foley, T. L., Jadhav, A., Maloney, D. J., Simeonov, A., Toth, E. A. & Weber, D. J. (2014). Biochemistry, 53, 6628–6640. [DOI] [PMC free article] [PubMed]
- Charpentier, T. H., Thompson, L. E., Liriano, M. A., Varney, K. M., Wilder, P. T., Pozharski, E., Toth, E. A. & Weber, D. J. (2010). J. Mol. Biol. 396, 1227–1243. [DOI] [PMC free article] [PubMed]
- Charpentier, T. H., Wilder, P. T., Liriano, M. A., Varney, K. M., Zhong, S., Coop, A., Pozharski, E., MacKerell, A. D., Toth, E. A. & Weber, D. J. (2009). Biochemistry, 48, 6202–6212. [DOI] [PMC free article] [PubMed]
- Cohen, A. E., Ellis, P. J., Miller, M. D., Deacon, A. M. & Phizackerley, R. P. (2002). J. Appl. Cryst. 35, 720–726. [DOI] [PMC free article] [PubMed]
- Czodrowski, P., Dramburg, I., Sotriffer, C. A. & Klebe, G. (2006). Proteins, 65, 424–437. [DOI] [PubMed]
- Di Tommaso, P., Moretti, S., Xenarios, I., Orobitg, M., Montanyola, A., Chang, J.-M., Taly, J.-F. & Notredame, C. (2011). Nucleic Acids Res. 39, W13–W17. [DOI] [PMC free article] [PubMed]
- Dolinsky, T. J., Czodrowski, P., Li, H., Nielsen, J. E., Jensen, J. H., Klebe, G. & Baker, N. A. (2007). Nucleic Acids Res. 35, W522–W525. [DOI] [PMC free article] [PubMed]
- Dolinsky, T. J., Nielsen, J. E., McCammon, J. A. & Baker, N. A. (2004). Nucleic Acids Res. 32, W665–W667. [DOI] [PMC free article] [PubMed]
- Donato, R. (2001). Int. J. Biochem. Cell Biol. 33, 637–668. [DOI] [PubMed]
- Donato, R., Cannon, B. R., Sorci, G., Riuzzi, F., Hsu, K., Weber, D. J. & Geczy, C. L. (2013). Curr. Mol. Med. 13, 24–57. [PMC free article] [PubMed]
- Drohat, A. C., Baldisseri, D. M., Rustandi, R. R. & Weber, D. J. (1998). Biochemistry, 37, 2729–2740. [DOI] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Garbuglia, M., Verzini, M., Rustandi, R. R., Osterloh, D., Weber, D. J., Gerke, V. & Donato, R. (1999). Biochem. Biophys. Res. Commun. 254, 36–41. [DOI] [PubMed]
- Gógl, G., Alexa, A., Kiss, B., Katona, G., Kovács, M., Bodor, A., Reményi, A. & Nyitray, L. (2016). J. Biol. Chem. 291, 11–27. [DOI] [PMC free article] [PubMed]
- González, A., Moorhead, P., McPhillips, S. E., Song, J., Sharp, K., Taylor, J. R., Adams, P. D., Sauter, N. K. & Soltis, S. M. (2008). J. Appl. Cryst. 41, 176–184.
- González, A. & Tsai, Y. A. (2010). A Quick XDS Tutorial for SSRL. http://smb.slac.stanford.edu/facilities/software/xds/#autoxds_script.
- Harpio, R. & Einarsson, R. (2004). Clin. Biochem. 37, 512–518. [DOI] [PubMed]
- Hernández-Ochoa, E. O., Prosser, B. L., Wright, N. T., Contreras, M., Weber, D. J. & Schneider, M. F. (2009). Am. J. Physiol. Cell Physiol. 297, C955–C970. [DOI] [PMC free article] [PubMed]
- Jensen, J. L., Indurthi, V. S. K., Neau, D. B., Vetter, S. W. & Colbert, C. L. (2015). Acta Cryst. D71, 1176–1183. [DOI] [PMC free article] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Kligman, D. & Hilt, D. C. (1988). Trends Biochem. Sci. 13, 437–443. [DOI] [PubMed]
- Landar, A., Rustandi, R. R., Weber, D. J. & Zimmer, D. B. (1998). Biochemistry, 37, 17429–17438. [DOI] [PubMed]
- Lebedev, A. A. & Isupov, M. N. (2014). Acta Cryst. D70, 2430–2443. [DOI] [PubMed]
- Lin, J., Yang, Q., Wilder, P. T., Carrier, F. & Weber, D. J. (2010). J. Biol. Chem. 285, 27487–27498. [DOI] [PMC free article] [PubMed]
- Liriano, M. A., Varney, K. M., Wright, N. T., Hoffman, C. L., Toth, E. A., Ishima, R. & Weber, D. J. (2012). J. Mol. Biol. 423, 365–385. [DOI] [PMC free article] [PubMed]
- Long, F., Vagin, A. A., Young, P. & Murshudov, G. N. (2008). Acta Cryst. D64, 125–132. [DOI] [PMC free article] [PubMed]
- Malashkevich, V. N., Varney, K. M., Garrett, S. C., Wilder, P. T., Knight, D., Charpentier, T. H., Ramagopal, U. A., Almo, S. C., Weber, D. J. & Bresnick, A. R. (2008). Biochemistry, 47, 5111–5126. [DOI] [PMC free article] [PubMed]
- Matsumura, H., Shiba, T., Inoue, T., Harada, S. & Kai, Y. (1998). Structure, 6, 233–241. [DOI] [PubMed]
- Moroz, O. V., Bronstein, I. B. & Wilson, K. S. (2011). J. Mol. Biol. 411, 1072–1082. [DOI] [PubMed]
- Most, P., Pleger, S. T., Völkers, M., Heidt, B., Boerries, M., Weichenhan, D., Löffler, E., Janssen, P. M. L., Eckhart, A. D., Martini, J., Williams, M. L., Katus, H. A., Remppis, A. & Koch, W. J. (2004). J. Clin. Invest. 114, 1550–1563. [DOI] [PMC free article] [PubMed]
- Most, P., Remppis, A., Pleger, S. T., Katus, H. A. & Koch, W. J. (2007). Am. J. Physiol. Regul. Integr. Comput. Physiol. 293, R568–R577. [DOI] [PubMed]
- Most, P., Remppis, A., Pleger, S. T. et al. (2003). J. Biol. Chem. 278, 33809–33817. [DOI] [PubMed]
- Most, P., Remppis, A., Weber, C., Bernotat, J., Ehlermann, P., Pleger, S. T., Kirsch, W., Weber, M., Uttenweiler, D., Smith, G. L., Katus, H. A. & Fink, R. H. A. (2003). J. Biol. Chem. 278, 26356–26364. [DOI] [PubMed]
- Notredame, C., Higgins, D. G. & Heringa, J. (2000). J. Mol. Biol. 302, 205–217. [DOI] [PubMed]
- Nowakowski, M., Ruszczyńska-Bartnik, K., Budzińska, M., Jaremko, Ł., Jaremko, M., Zdanowski, K., Bierzyński, A. & Ejchart, A. (2013). Biochemistry, 52, 1149–1159. [DOI] [PubMed]
- Osterloh, D., Ivanenkov, V. V. & Gerke, V. (1998). Cell Calcium, 24, 137–151. [DOI] [PubMed]
- Pettersen, E. F., Goddard, T. D., Huang, C. C., Couch, G. S., Greenblatt, D. M., Meng, E. C. & Ferrin, T. E. (2004). J. Comput. Chem. 25, 1605–1612. [DOI] [PubMed]
- Prosser, B. L., Hernández-Ochoa, E. O. & Schneider, M. F. (2011). Cell Calcium, 50, 323–331. [DOI] [PMC free article] [PubMed]
- Prosser, B. L., Wright, N. T., Hernãndez-Ochoa, E. O., Varney, K. M., Liu, Y., Olojo, R. O., Zimmer, D. B., Weber, D. J. & Schneider, M. F. (2008). J. Biol. Chem. 283, 5046–5057. [DOI] [PMC free article] [PubMed]
- Rustandi, R. R., Drohat, A. C., Baldisseri, D. M., Wilder, P. T. & Weber, D. J. (1998). Biochemistry, 37, 1951–1960. [DOI] [PubMed]
- Salama, I., Malone, P. S., Mihaimeed, F. & Jones, J. L. (2008). Eur. J. Surg. Oncol. 34, 357–364. [DOI] [PubMed]
- Schäfer, B. W. & Heizmann, C. W. (1996). Trends Biochem. Sci. 21, 134–140. [DOI] [PubMed]
- Schneider, M. C., Prosser, B. E., Caesar, J. J. E., Kugelberg, E., Li, S., Zhang, Q., Quoraishi, S., Lovett, J. E., Deane, J. E., Sim, R. B., Roversi, P., Johnson, S., Tang, C. M. & Lea, S. M. (2009). Nature (London), 458, 890–893. [DOI] [PMC free article] [PubMed]
- Soltis, S. M. et al. (2008). Acta Cryst. D64, 1210–1221. [DOI] [PMC free article] [PubMed]
- Strynadka, N. C. J. & James, M. N. G. (1989). Annu. Rev. Biochem. 58, 951–999. [DOI] [PubMed]
- Treves, S., Scutari, E., Robert, M., Groh, S., Ottolia, M., Prestipino, G., Ronjat, M. & Zorzato, F. (1997). Biochemistry, 36, 11496–11503. [DOI] [PubMed]
- Vagin, A. A. (1989). Jnt CCP4–ESF–EACBM Newsl. Protein Crystallogr. 24, 117–121.
- Vagin, A. & Teplyakov, A. (1997). J. Appl. Cryst. 30, 1022–1025.
- Vagin, A. & Teplyakov, A. (2010). Acta Cryst. D66, 22–25. [DOI] [PubMed]
- Wang, G., Zhang, S., Fernig, D. G., Martin-Fernandez, M., Rudland, P. S. & Barraclough, R. (2005). Oncogene, 24, 1445–1454. [DOI] [PubMed]
- Wilder, P. T., Baldisseri, D. M., Udan, R., Vallely, K. M. & Weber, D. J. (2003). Biochemistry, 42, 13410–13421. [DOI] [PubMed]
- Wright, N. T., Cannon, B. R., Wilder, P. T., Morgan, M. T., Varney, K. M., Zimmer, D. B. & Weber, D. J. (2009). J. Mol. Biol. 386, 1265–1277. [DOI] [PMC free article] [PubMed]
- Wright, N. T., Inman, K. G., Levine, J. A., Cannon, B. R., Varney, K. M. & Weber, D. J. (2008). J. Biomol. NMR, 42, 279–286. [DOI] [PMC free article] [PubMed]
- Wright, N. T., Prosser, B. L., Varney, K. M., Zimmer, D. B., Schneider, M. F. & Weber, D. J. (2008). J. Biol. Chem. 283, 26676–26683. [DOI] [PMC free article] [PubMed]
- Wright, N. T., Varney, K. M., Ellis, K. C., Markowitz, J., Gitti, R. K., Zimmer, D. B. & Weber, D. J. (2005). J. Mol. Biol. 353, 410–426. [DOI] [PubMed]
- Yamaguchi, N., Prosser, B. L., Ghassemi, F., Xu, L., Pasek, D. A., Eu, J. P., Hernández-Ochoa, E. O., Cannon, B. R., Wilder, P. T., Lovering, R. M., Weber, D., Melzer, W., Schneider, M. F. & Meissner, G. (2011). Am. J. Physiol. Cell Physiol. 300, C998–C1012. [DOI] [PMC free article] [PubMed]
- Zimmer, D. B., Cornwall, E. H., Landar, A. & Song, W. (1995). Brain Res. Bull. 37, 417–429. [DOI] [PubMed]
- Zimmer, D. B., Cornwall, E. H., Reynolds, P. D. & Donald, C. M. (1998). J. Biol. Chem. 273, 4705–4711. [DOI] [PubMed]
- Zimmer, D. B. & Weber, D. J. (2010). Cardiovasc. Psychiatr. Neurol., 2010, 728052. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: S100A1, 5k89
Supplementary Figure S1,. DOI: 10.1107/S2053230X17003983/rr5138sup1.pdf