D. melanogaster enolase was purified and crystallized. It forms a homodimer with an open conformation.
Keywords: enolase, crystal structure, active site, dimerization, glycolysis, Drosophila melanogaster
Abstract
Enolase is an important enzyme in glycolysis and various biological processes. Its dysfunction is closely associated with diseases. Here, the enolase from Drosophila melanogaster (DmENO) was purified and crystallized. A crystal of DmENO diffracted to 2.0 Å resolution and belonged to space group R32. The structure was solved by molecular replacement. Like most enolases, DmENO forms a homodimer with conserved residues in the dimer interface. DmENO possesses an open conformation in this structure and contains conserved elements for catalytic activity. This work provides a structural basis for further functional and evolutionary studies of enolase.
1. Introduction
Enolase (EC 4.2.1.11), also known as 2-phospho-d-glycerate hydrolase, belongs to the enolase superfamily, which contains the mandelate racemase (MR) and muconate-lactonizing (MLE) families (Gerlt et al., 2005 ▸). Enolase is involved in the glycolytic pathway. It catalyzes the dehydration of 2-phospho-d-glycerate (PGA) to phosphoenolpyruvate (PEP) in catabolic metabolism and the reverse reaction during gluconeogenesis. Besides its role in metabolism, enolase also functions in other physiological processes. In vertebrates, enolase has three isozymes: enolase α (ENO1), β (ENO3) and γ (ENO2). In humans, ENO1 (HsENO1) is ubiquitously expressed, while HsENO2 is specifically expressed in muscle and HsENO3 in neurons (Isgrò et al., 2015 ▸). Although the three isozymes have highly conserved sequences (about 83% identity; Supplementary Fig. S1), they possess specific functions (Pancholi, 2001 ▸; Díaz-Ramos et al., 2012 ▸; Isgrò et al., 2015 ▸). For example, HsENO1 can locate at the cell surface, where it functions as a plasminogen receptor to regulate pericellular fibrinolytic activity, as well as in the nucleus, where it binds to the c-Myc promoter region to regulate gene transcription (López-Alemany et al., 2003 ▸; Feo et al., 2000 ▸; Subramanian & Miller, 2000 ▸). Enolases are also involved in many pathological conditions. The upregulation of HsENO1 is associated with tumour progression, autoimmune diseases and neurological disorders (Díaz-Ramos et al., 2012 ▸). HsENO3 is considered as a biomarker for many tumours, such as those found in the neuroendocrine system and in lung cancer (Isgrò et al., 2015 ▸).
Enolase is an evolutionarily conserved enzyme and is widely present in prokaryotic and eukaryotic cells. There are two enolases, enolase 1 (ScENO1) and 2 (ScENO2), in Saccharomyces cerevisiae and one enolase in Escherichia coli (EcENO), which show 52–63% identity to human enolases (Supplementary Fig. S1). These enolases have similar structural folds and conserved residues for catalysis (Reed et al., 1996 ▸). They have been shown to occur as a homodimer in known structures (Stec & Lebioda, 1990 ▸; Kühnel & Luisi, 2001 ▸; Chai et al., 2004 ▸). However, some enolase proteins from species such as Bacillus subtilis, Staphylococcus aureus, Streptococcus pneumoniae and Streptococcus suis have been found to be octamers (Ehinger et al., 2004 ▸; Lu et al., 2012 ▸; Newman et al., 2012 ▸; Wu et al., 2015 ▸). In these cases, an octameric conformation was found to be required for their catalytic activities.
Enolase possesses a bi-domain architecture with an N-terminal capping domain and a C-terminal β/α-barrel domain (Reed et al., 1996 ▸; Pancholi, 2001 ▸; Díaz-Ramos et al., 2012 ▸). The active site of enolase is located at the interface between the capping domain and the β/α-barrel domain. The catalytic reaction requires a divalent metal ion such as a magnesium ion, which is a natural cofactor of enolase. The two magnesium ions in the active site orchestrate the substrate and the conserved residues for catalysis.
Drosophila melanogaster is a good model organism for genetic and developmental studies. It has one enolase gene that encodes two proteins: the longer isoform 1 and the shorter isoform 2 (UniProt accession code P15007). Compared with isoform 2, D. melanogaster enolase (DmENO) isoform 1 has an extra N-terminal region that is not present in other enolases (Supplementary Fig. S1). This extra N-terminal part was predicted to be folded as a coil with a partially disordered region by the PSIPRED server (http://bioinf.cs.ucl.ac.uk/psipred/; Buchan et al., 2013 ▸). Although DmENO and HsENO are highly conserved (73% sequence identity; Supplementary Fig. S1), it has been shown that these two enolases participate in variable interaction networks by bioinformatics analysis (Paludo et al., 2015 ▸). In this study, we purified and crystallized recombinant DmENO isoform 2. The crystal structure was solved by molecular replacement. In this structure, DmENO forms a homodimer in an open conformation and has a similar overall structure to those of other enolases.
2. Materials and methods
2.1. Cloning and protein expression
The DNA sequence encoding DmENO isoform 2 corresponding to amino acids 68–500 of the longer isoform 1 was amplified from the cDNA of S2 cells and digested with the restriction enzymes EcoRI and XhoI. The forward and reverse primers are 5′-CCGGAATTCATGACCATCAAAGCGATC-3′ and 5′-CCGCTCGAGTTACTGCGGCTTCCTGAAC-3′ (the underlined nucleotides are the recognition sites for the restriction enzymes). The product was ligated into a modified pET-28a vector with an N-terminal 6×His tag and a TEV (Tobacco etch virus) protease site. The plasmid for expression was transformed into E. coli BL21 (DE3) CodonPlus competent cells. The cells were incubated in Terrific Broth medium with 30 µg ml−1 kanamycin and 34 µg ml−1 chloramphenicol at 37°C and protein expression was induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside at 20°C for 16 h.
2.2. Protein purification
Protein purification was performed at 4°C. The E. coli cells were collected at about 6400g for 10 min and suspended in five times the volume of lysis buffer consisting of 20 mM HEPES–Na pH 7.5, 500 mM NaCl, 5% glycerol, 20 mM imidazole, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride and then broken using a high-pressure homogenizer (JNBIO JN-3000 PLUS) at 130 MPa. Cell debris was removed by centrifugation for 40 min at 31 000g. The supernatant was mixed with pre-equilibrated Ni2+ Sepharose (GE Healthcare) for 1 h and the beads were then sequentially washed with lysis buffer containing 20 and 40 mM imidazole. The protein was eluted with lysis buffer plus 300 mM imidazole. The protein was concentrated using a 10 kDa cutoff Amicon Ultra-15 (Millipore) and exchanged into 20 mM HEPES–Na pH 7.5, 250 mM NaCl, 5% glycerol using a HiTrap Desalting column (GE Healthcare). The protein was then treated with His-tagged TEV protease and reapplied onto an Ni2+ Sepharose column to remove the protease and cleaved tag. Finally, the product was concentrated and loaded onto a HiLoad 16/60 Superdex 200 pg column (GE Healthcare) using 20 mM HEPES–Na pH 7.5, 100 mM NaCl, 1 mM DTT as the running buffer. The protein was concentrated to 10 mg ml−1, with the concentration being determined by the Bradford method (Bio-Rad Protein Assay).
2.3. Crystallization and data collection
Crystallization experiments were performed by the sitting-drop vapour-diffusion method at 16°C. Crystallization kits from Hampton Research and Molecular Dimensions were used for crystallization screening. The 2 µl sitting drops consisted of 1 µl protein solution and 1 µl reservoir solution and were equilibrated against 100 µl reservoir solution. Crystals were optimized using the sitting-drop and hanging-drop vapour-diffusion methods.
Crystals were soaked in reservoir solution containing 20% glycerol and flash-cooled in liquid nitrogen. Diffraction data were collected from a single DmENO crystal on beamline BL17U at Shanghai Synchrotron Radiation Facility (SSRF), People’s Republic of China with a Rayonix MX-225 CCD detector using 1° oscillations. The crystal-to-detector distance was 300 mm. A total of 180 diffraction frames were collected. Diffraction data were processed using HKL-2000 (Otwinowski & Minor, 1997 ▸) and iMosflm (Battye et al., 2011 ▸) from the CCP4 software suite (Winn et al., 2011 ▸). The initial space group was suggested to be C2 with an element of twinning, while the correct space group was R32 when the primary data were reprocessed after review.
2.4. Structure determination and refinement
The structure of DmENO was solved by molecular replacement with Phaser (McCoy et al., 2007 ▸) using the HsENO1 structure (PDB entry 3b97; Kang et al., 2008 ▸) as a search model. The model was completed using iterative cycles of manual building in Coot (Emsley et al., 2010 ▸) and refinement with phenix.refine in PHENIX (Afonine et al., 2012 ▸; Adams et al., 2010 ▸) and REFMAC5 in CCP4 (Murshudov et al., 2011 ▸; Winn et al., 2011 ▸). The translation–libration–screw (TLS) method was used during structural refinement. The assignment of metal and chloride ions was based on difference density peak heights and the chemical environment. Structural images were produced in PyMOL (http://www.pymol.org/).
2.5. Structure deposition
The coordinates and structure factors for the crystal structure of DmENO were deposited in the Worldwide Protein Data Bank (wwPDB) under the accession code 5wro.
3. Results and discussion
3.1. Determination of the structure of DmENO
DmENO isoform 2 was purified by affinity chromatography and gel filtration (Supplementary Fig. S2a). There were two major peaks in the gel filtration. Nearly all of the DmENO sample was present in the second peak, which was used for crystal screening. Small crystals appeared in one week using a solution consisting of 10 mM CoCl2, 0.1 M MES pH 6.5, 1.9 M ammonium sulfate. After systematical optimization including additive screening, trigonal crystals grew in 10 mM CoCl2, 0.1 M MES pH 6.5, 1.9 M ammonium sulfate, 0.1 M CdCl2 (Supplementary Fig. S2b). One of these crystals diffracted to 2.02 Å resolution and belonged to space group R32 (Table 1 ▸). The multiplicity and completeness of the data set were 8.9 and 100%, respectively. The structure was determined by molecular replacement using the structure of HsENO1 (PDB entry 3b97; Kang et al., 2008 ▸) as a search model. The model was refined to a final R work of 0.162 and R free of 0.201. Details of the data-collection and refinement statistics are summarized in Table 1 ▸.
Table 1. Data-collection and refinement statistics for DmENO.
Values in parentheses are for the highest resolution shell.
| Data collection | |
| Wavelength (Å) | 0.97915 |
| Space group | R32 |
| Unit-cell parameters (Å, °) | a = b = 118.82, c = 229.79, α = β = 90, γ = 90 |
| Resolution (Å) | 93.9–2.02 (2.07–2.02) |
| Total No. of reflections | 367938 (23567) |
| No. of unique reflections | 41492 (3019) |
| R merge | 0.189 (0.841) |
| 〈I/σ(I)〉 | 7.3 (2.2) |
| Completeness (%) | 100.0 (100.0) |
| Multiplicity | 8.9 (7.8) |
| Wilson B factor (Å2) | 29.0 |
| Refinement | |
| Resolution (Å) | 38.33–2.02 (2.09–2.02) |
| R work | 0.162 |
| R free | 0.201 |
| No. of atoms | |
| Total | 3673 |
| Protein | 3308 |
| Ligand/ion | 32 |
| Water | 333 |
| R.m.s.d.s | |
| Bond lengths (Å) | 0.017 |
| Bond angles (°) | 0.830 |
| Ramachandran plot | |
| Favoured (%) | 97.25 |
| Allowed (%) | 2.29 |
| Outliers (%) | 0.46 |
| Average B factors (Å2) | |
| Overall | 40.99 |
| Protein | 40.16 |
| Ligand/ion | 55.40 |
| Water | 47.82 |
3.2. Crystal structure of DmENO
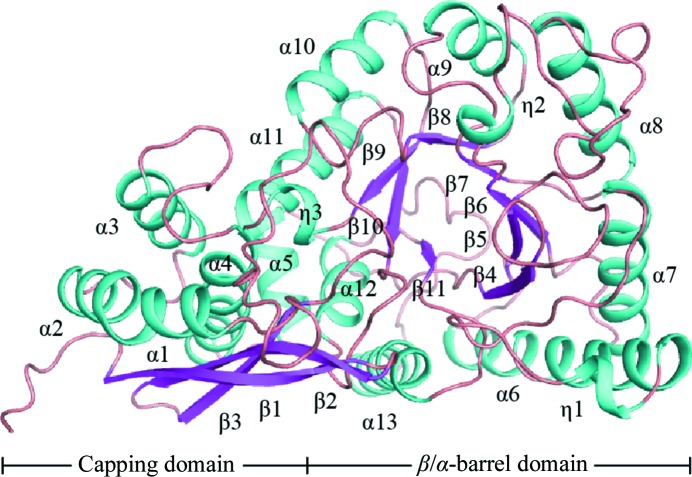
Only one molecule of DmENO is present in the asymmetric unit. DmENO contains 11 β-strands, 13 α-helices and three short 310-helices (notated as η-helices), and can be divided into two domains, like enolases from other species (Fig. 1 ▸). The N-terminal capping domain is composed of the first three β-strands and five α-helices, and the remaining part is the C-terminal β/α-barrel domain.
Figure 1.
The overall structure of DmENO shown as a cartoon representation, with α-helices and η-helices coloured cyan and β-strands coloured pink. Each secondary-structure element is labelled according to its position in the amino-acid sequence.
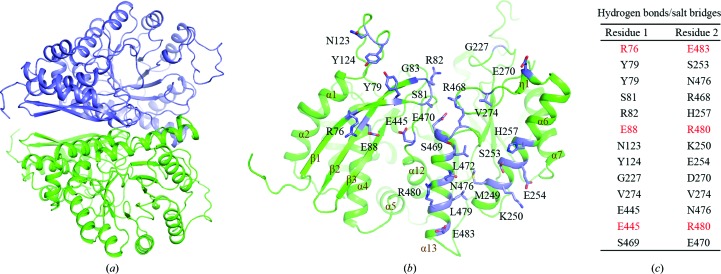
DmENO forms a butterfly-like homodimer with one symmetry-related molecule (Fig. 2 ▸ a). The area of the interface for the DmENO homodimer is 1840 Å2 (PDBePISA server; http://www.ebi.ac.uk/msd-srv/prot_int/pistart.html). Dimerization is mainly mediated by the β1 and β2 strands, the α6, α13 and η1 helices, the β1/β2 turn and the β3/α1, β4/α5, η1/α7 and β10/α12 loops (Fig. 2 ▸ b). Arg76 in the β1 strand, Glu88 in the β2 strand, Glu445 in the β10/α12 loop and Asn123 in the β3/α1 loop of one chain form three ionic bonds and four hydrogen bonds to Lys250 in the α6 helix and Asn476, Arg480 and Glu483 in the α13 helix of the other chain via their side chains (Figs. 2 ▸ b and 2 ▸ c). The side chains of Ser253, Glu254 and His257 in the α6 helix, Asp270 in the η1 helix and Glu470 and Asn476 in the α13 helix from one chain contact the main chains of Tyr79 in the β1 strand, Arg82 and Gly83 in the β1/β2 turn, Tyr124 in the β3/α1 loop, Gly227 in the β4/α5 loop and Ser469 in the α13 helix from the other chain via hydrogen bonds. The main chain of Ser81 in the β1/β2 turn from one molecule forms hydrogen bonds to the main chain of Arg468 in the α13 helix from the other molecule. The main chains of Val274 in the η1/α7 loop from two molecules also contribute two hydrogen bonds. Interestingly, Val274 and Arg468 from two DmENO chains pack with each other through hydrophobic interaction. In addition, Tyr79 in the β1 strand of one chain and Met249 in the α6 helix, and Leu472 and Leu479 in the α13 helix from the other chain form hydrophobic packing. Residues responsible for side-chain-mediated contacts in the interface are highly conserved among different species (Supplementary Fig. S1).
Figure 2.
(a) DmENO forms a homodimer. The two DmENO chains are coloured green and slate. (b) Dimeric interface of DmENO. Residues at the interface are coloured slate and shown as sticks. (c) A list of the residues at the dimeric interface involved in the formation of hydrogen bonds and salt bridges. Residues forming both hydrogen bonds and salt bridges are coloured red.
3.3. The active site of DmENO
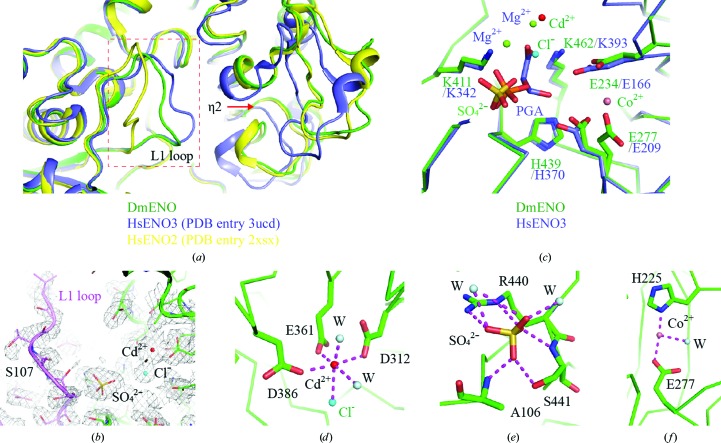
In our structure, DmENO does not contain either a reaction substrate or a product. The β3/α1 loop (also named the L1 loop) of unliganded DmENO, as well as the η2 helix, is distant from the active site (Fig. 3 ▸ a). Poor electron density and high temperature factors (average of 73.2 Å2 for residues 105–112 in the L1 loop versus 41.0 Å2 for the whole subunit) suggest that the L1 loop is structurally flexible (Fig. 3 ▸ b). In addition, Ser107, which is crucial for catalysis, is partially disordered in the DmENO structure. When the DmENO structure was superposed with that of unliganded HsENO3 (PDB entry 3ucd; Qin et al., 2012 ▸) in an open state, the root-mean-square deviation (r.m.s.d.) for Cα atoms only between the two structures was 0.389 Å, whereas the r.m.s.d. was 0.551 Å when it was aligned with liganded HsENO2 (PDB entry 2xsx; M. Vollmar, E. Krysztofinska, A. Chaikuad, T. Krojer, R. Cocking, F. Von Delft, C. Bountra, C. H. Arrowsmith, J. Weigelt, A. Edwards, W. W. Yue & U. Oppermann, unpublished work) in a closed state. The overall conformation of DmENO also resembles that of unliganded HsENO3, indicating that unliganded DmENO is in an open state (Fig. 3 ▸ a).
Figure 3.
(a) Structural comparison of DmENO with HsENO2 and HsENO3. DmENO, HsENO2 and HsENO3 are coloured green, slate and yellow, respectively. The red box indicates the L1 loop region. The red arrow shows that the η2 helix is distant from the active site. (b) The 2mF o − DF c map (grey mesh) for the active site of DmENO contoured at 1.0σ. The L1 loop is coloured purple. (c) Structural comparison of the active sites of DmENO and HsENO3. HsENO3 possesses two magnesium ions and the substrate PGA in its active site, while DmENO has one cadmium ion, one cobalt ion, one chloride ion and one sulfate ion in the active site. Residues involved in substrate binding and catalysis in DmENO and HsENO3 are shown as sticks. (d) The geometry and coordinates of the cadmium ion in the active site of DmENO. (e) The residues and waters interacting with the sulfate ion in the active site of DmENO. (f) The coordination of the cobalt ion in the active site of DmENO. The magenta dashed lines represent salt bridges and hydrogen bonds.
The active site of DmENO is occupied by one cadmium ion, one cobalt ion, one chloride ion and one sulfate ion that are possibly from the crystallization solution (Figs. 3 ▸ b and 3 ▸ c). The cadmium ion replaces the magnesium ion in native enolases such as HsENO3 (PDB entry 3ucd) to coordinate the side chains of Asp312, Glu361 and Asp386 of the enolase (Qin et al., 2012 ▸; Figs. 3 ▸ c and 3 ▸ d). The cadmium ion also coordinates the chloride and two water molecules, forming an octahedral geometry just like the magnesium ion (Rulíšek & Vondrášek, 1998 ▸). The sulfate occupies the position of the phosphate moiety of the enolase substrate and forms salt-bridge and hydrogen-bond interactions with Ala106, Arg440 and Ser441 of DmENO (Figs. 3 ▸ c and 3 ▸ e). In HsENO3, Glu166, Glu209, Lys342, His370 and Lys393 contribute to the substrate-binding and catalytic activities (Qin et al., 2012 ▸; Fig. 3 ▸ c). When comparing the structure of DmENO with that of HsENO3, it was apparent that DmENO possessed the equivalent residues Glu234, Glu277, Lys411, His439 and Lys462. These residues have similar conformations, indicating that DmENO utilizes a similar catalytic mechanism to HsENO and thus has conserved function (Fig. 3 ▸ c and Supplementary Fig. S1). The cobalt ion in the active site interacts with the side chains of His225 and Glu277 in DmENO (Figs. 3 ▸ c and 3 ▸ f). There is no metal ion present in the corresponding position of native enolases such as HsENO2 and HsENO3, suggesting that it is an artifact of crystallization.
3.4. Comparison of DmENO with octameric enolases
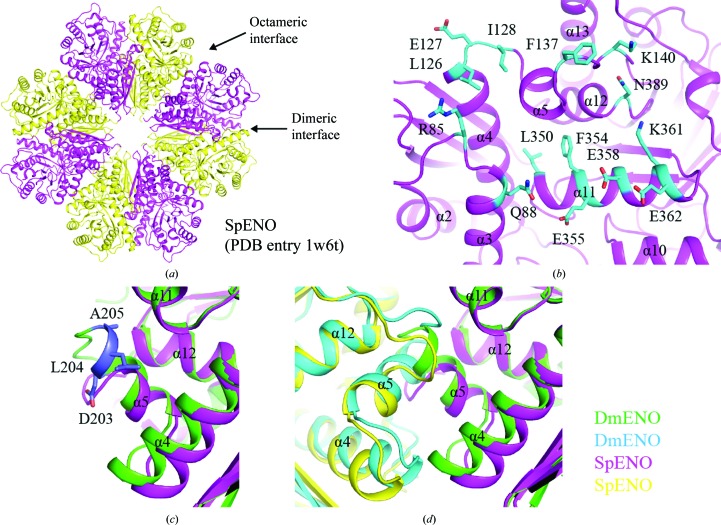
It has been reported that the enolases from some species, such as S. pneumoniae and B. subtilis enolase (SpENO and BsENO), exist as octamers (PDB entries 1w6t and 2pa6; Ehinger et al., 2004 ▸; Newman et al., 2012 ▸; Fig. 4 ▸ a). The formation of the SpENO octameric interface involves many residues, including Arg85, Gln88, Leu126, Glu127, Ile128, Phe137, Lys140, Leu350, Phe354, Glu355, Glu358, Lys361, Glu362 and Asn389, which mediate extensive hydrogen-bonding, electrostatic and hydrophobic interactions (Ehinger et al., 2004 ▸; Fig. 4 ▸ b). When performing the sequence alignment of enolases from different species, we found that some crucial residues in the octameric interface are conserved in SpENO and BsENO, such as Leu126, Phe137, Lys140, Phe354, Glu355 and Asn389 in SpENO, but not in dimeric DmENO, HsENO1/2/3, ScENO1/2 and EcENO (Supplementary Fig. S1). Additionally, the α5 helix present in the octameric interface of SpENO, as well as BsENO, is shorter than that in DmENO (Fig. 4 ▸ c and Supplementary Fig. S1). Three residues, Asp203, Leu204 and Ala205, in DmENO have no corresponding residues in SpENO and BsENO. When superposing the structures of DmENO and SpENO, we found that the longer α5 helix of DmENO clashes with the adjacent molecule, which precludes octamer formation (Fig. 4 ▸ d). This longer α5 helix is also present in the human, yeast and E. coli enolases with a dimeric quaternary structure (Supplementary Fig. S1). Therefore, both specific amino acids and the α5 helix conformation determine that these dimeric enolases including DmENO do not form an octamer.
Figure 4.
(a) Dimeric and octameric interfaces of SpENO. (b) Octameric interface of SpENO. Residues at the interface are coloured cyan and shown as sticks. Each secondary-structure element of SpENO is labelled according to those in DmENO. (c, d) Structural superposition of DmENO and SpENO. The two DmENO chains are coloured green and cyan, while the two SpENO chains are coloured magenta and yellow. The α5 helix of DmENO is longer than that of SpENO. Residues in DmENO that are not present in SpENO are shown as slate sticks.
In this paper, we solved the crystal structure of DmENO. DmENO forms a homodimer just like the human and yeast enolases. In this structure, DmENO possesses an open conformation with a conformationally flexible L1 loop. This work provides a structural basis for further understanding of the functions of enolase in the fruit fly.
4. Related literature
The following references are cited in the Supporting Information for this article: Gouet et al. (1999 ▸) and Sievers et al. (2011 ▸).
Supplementary Material
PDB reference: D. melanogaster enolase, 5wro
Supplementary Figures S1 and S2.. DOI: 10.1107/S2053230X17004022/hv5348sup1.pdf
Acknowledgments
We thank the staff of beamline BL17U at Shanghai Synchrotron Radiation Facility (SSRF) for help with data collection. We appreciate Dr Zhaocai Zhou for helpful support and discussion.
References
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
- Afonine, P. V., Grosse-Kunstleve, R. W., Echols, N., Headd, J. J., Moriarty, N. W., Mustyakimov, M., Terwilliger, T. C., Urzhumtsev, A., Zwart, P. H. & Adams, P. D. (2012). Acta Cryst. D68, 352–367. [DOI] [PMC free article] [PubMed]
- Battye, T. G. G., Kontogiannis, L., Johnson, O., Powell, H. R. & Leslie, A. G. W. (2011). Acta Cryst. D67, 271–281. [DOI] [PMC free article] [PubMed]
- Buchan, D. W., Minneci, F., Nugent, T. C., Bryson, K. & Jones, D. T. (2013). Nucleic Acids Res. 41, W349–W357. [DOI] [PMC free article] [PubMed]
- Chai, G., Brewer, J. M., Lovelace, L. L., Aoki, T., Minor, W. & Lebioda, L. (2004). J. Mol. Biol. 341, 1015–1021. [DOI] [PubMed]
- Díaz-Ramos, À., Roig-Borrellas, A., García-Melero, A. & López-Alemany, R. (2012). J. Biomed. Biotechnol. 2012, 1–12. [DOI] [PMC free article] [PubMed]
- Ehinger, S., Schubert, W. D., Bergmann, S., Hammerschmidt, S. & Heinz, D. W. (2004). J. Mol. Biol. 343, 997–1005. [DOI] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Feo, S., Arcuri, D., Piddini, E., Passantino, R. & Giallongo, A. (2000). FEBS Lett. 473, 47–52. [DOI] [PubMed]
- Gerlt, J. A., Babbitt, P. C. & Rayment, I. (2005). Arch. Biochem. Biophys. 433, 59–70. [DOI] [PubMed]
- Gouet, P., Courcelle, E., Stuart, D. I. & Métoz, F. (1999). Bioinformatics, 15, 305–308. [DOI] [PubMed]
- Isgrò, M. A., Bottoni, P. & Scatena, R. (2015). Adv. Exp. Med. Biol. 867, 125–143. [DOI] [PubMed]
- Kang, H. J., Jung, S.-K., Kim, S. J. & Chung, S. J. (2008). Acta Cryst. D64, 651–657. [DOI] [PubMed]
- Kühnel, K. & Luisi, B. F. (2001). J. Mol. Biol. 313, 583–592. [DOI] [PubMed]
- López-Alemany, R., Longstaff, C., Hawley, S., Mirshahi, M., Fábregas, P., Jardí, M., Merton, E., Miles, L. A. & Félez, J. (2003). Am. J. Hematol. 72, 234–242. [DOI] [PubMed]
- Lu, Q., Lu, H., Qi, J., Lu, G. & Gao, G. F. (2012). Protein Cell, 3, 769–780. [DOI] [PMC free article] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Newman, J. A., Hewitt, L., Rodrigues, C., Solovyova, A. S., Harwood, C. R. & Lewis, R. J. (2012). J. Mol. Biol. 416, 121–136. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Paludo, G. P., Lorenzatto, K. R., Bonatto, D. & Ferreira, H. B. (2015). Comput. Biol. Chem. 58, 1–8. [DOI] [PubMed]
- Pancholi, V. (2001). Cell. Mol. Life Sci. 58, 902–920. [DOI] [PMC free article] [PubMed]
- Qin, J., Chai, G., Brewer, J. M., Lovelace, L. L. & Lebioda, L. (2012). J. Inorg. Biochem. 111, 187–194. [DOI] [PMC free article] [PubMed]
- Reed, G. H., Poyner, R. R., Larsen, T. M., Wedekind, J. E. & Rayment, I. (1996). Curr. Opin. Struct. Biol. 6, 736–743. [DOI] [PubMed]
- Rulíšek, L. & Vondrášek, J. (1998). J. Inorg. Biochem. 71, 115–127. [DOI] [PubMed]
- Sievers, F., Wilm, A., Dineen, D., Gibson, T. J., Karplus, K., Li, W., Lopez, R., McWilliam, H., Remmert, M., Söding, J., Thompson, J. D. & Higgins, D. G. (2011). Mol. Syst. Biol. 7, 539. [DOI] [PMC free article] [PubMed]
- Stec, B. & Lebioda, L. (1990). J. Mol. Biol. 211, 235–248. [DOI] [PubMed]
- Subramanian, A. & Miller, D. M. (2000). J. Biol. Chem. 275, 5958–5965. [DOI] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- Wu, Y., Wang, C., Lin, S., Wu, M., Han, L., Tian, C., Zhang, X. & Zang, J. (2015). Acta Cryst. D71, 2457–2470. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: D. melanogaster enolase, 5wro
Supplementary Figures S1 and S2.. DOI: 10.1107/S2053230X17004022/hv5348sup1.pdf