Abstract
Numerous studies have revealed atypical face processing in autism spectrum disorders (ASD) characterized by social interaction and communication difficulties. This study investigated sensitivity to face-likeness in ASD. In Experiment 1, we found a strong positive correlation between the face-likeness ratings of non-face objects in the ASD (11–19 years old) and the typically developing (TD) group (9–21 years old). In Experiment 2 (the scalp-recorded event-related potential experiment), the participants of both groups (ASD, 12–19 years old; TD, 12–18 years old) exhibited an enhanced face-sensitive N170 amplitude to a face-like object. Whereas the TD adolescents showed an enhanced N170 during the face-likeness judgements, adolescents with ASD did not. Thus, both individuals with ASD and TD individuals have a perceptual and neural sensitivity to face-like features in objects. When required to process face-like features, a face-related brain system reacts more strongly in TD individuals but not in individuals with ASD.
Because faces convey rich social information such as facial identity, gaze direction, and facial expression1, the ability to detect faces is crucial for social animals. In humans, even newborns preferentially look at face-like stimuli2,3,4, suggesting the existence of an innate face detection ability. This face detection sensitivity has been observed also in adults5,6. This innate preference for face-like features leads to a specialization of face processing7, which is essential for adequate social interactions. The configuration of the elements is thought to be important for this preferential bias (e.g. two elements in the upper half and one in the bottom half of an object)7. For example, identifying the existence of two eyes and one mouth in objects seems to be crucial for perceiving faces in these objects8. Humans often erroneously perceive faces in non-face objects that have face-like features (e.g. the famous rock on Mars that looks like a face9) and in random visual noise10. Some have argued that this kind of error occurs because humans are hard-wired to detect faces and that we even unconsciously try to see faces11,12,13. From an evolutionary perspective, the tendency to perceive faces in non-face objects might have adaptive consequences, because they might aid in detecting faces in the environment13; this tendency might lead to successful survival in our social system.
Although direct empirical evidence is scarce (Akechi & Hietanen, submitted for publication), social motivation might be related to a tendency to perceive faces in objects14 and it might be diminished in individuals with autism spectrum disorder (ASD)14,15. ASD is a developmental disorder characterized by social interaction and communication difficulties16. Observational studies have shown that children with ASD and those later diagnosed with ASD look at others' faces less frequently17,18,19. Moreover, typically developing (TD) children attended to faces preferentially, but children with ASD did not20. These studies suggest atypical face processing in ASD. However, other observational studies failed to find between-groups differences in response to others' faces21,22,23,24. A recent systematic review showed that facial recognition ability is not qualitatively atypical in individuals with ASD25. In addition, scalp-recorded event-related potential (ERP) studies have revealed that the N170, a face-sensitive negative component at the posterior temporal regions that peaks approximately 170 ms from stimulus onset and is larger for faces than for other objects26, is larger in response to faces also for those with ASD27,28,29. However, the N170 latency in response to inverted faces is longer than that to upright faces for TD individuals, but not for individuals with ASD27,29. Thus, although TD individuals and those with ASD use the same cortical pathway to process facial information, between-groups differences might exist in the manner in which facial information is processed. In brain imaging studies, atypical but not fully impaired face processing has been observed in individuals with ASD30. It has been hypothesized that a diminished interest in others might result in a lack of preferential attention to others. As a result, face processing and social skills might not develop typically7,14,15. As mentioned above, such social interests, which might be diminished in individuals with ASD, might be related to the tendency to perceive faces in objects14 (Akechi & Hietanen, submitted for publication). Additionally, it has been proposed that the sensitivity to face-like stimuli2,3,4 is important for the typical development of face processing7. Therefore, a sensitivity to face-like stimuli might be reduced for those individuals with ASD who show atypical face processing.
Despite its potential importance, it is not well known whether individuals with ASD have the sensitivity to the face-likeness of objects. Brain imaging and electrophysiological studies of TD individuals have shown activation in the fusiform face area (FFA)31,32,33 and the superior temporal sulcus (STS)34 in response to ambiguous stimuli perceived as faces. Importantly, the FFA and STS are candidate sources of the N170 component of the ERP35. Additionally, face-like objects (FLOs)36, schematic faces37, monochrome Mooney faces38, and Arcimboldo paintings39, which are not actually faces but are perceived as faces, elicit larger N170s than non-FLOs. Moreover, the N170 is larger in response to stimuli judged as faces by TD observers40. Therefore, the N170 could be used as a sensitive measure of whether the observer perceives a face in an object. Although individuals with ASD detect faces in degraded monochrome images less frequently41,42, currently, it is unclear whether individuals with ASD have a sensitivity to face-likeness and perceive faces in objects as do TD individuals.
To investigate whether individuals with ASD have an intact sensitivity to face-likeness, we conducted an evaluative rating experiment (Experiment 1) and an ERP experiment (Experiment 2). In Experiment 1, adolescents with ASD and TD adolescents rated how face-like the objects were (i.e. they detected faces in the objects). Because individuals with ASD exhibit differences in neural responses to faces even when the behavioural response is similar to TD individuals43, Experiment 2 was conducted to investigate the neural and behavioural responses to the face-likeness of objects for those with ASD. In Experiment 2, the average N170 amplitudes in response to FLOs and non-FLOs were measured during the time when the participants made face-likeness or roundness judgements of the stimuli. We expected to observe a different pattern in the face-likeness ratings between the participants with ASD and the TD participants. In the ERP experiment, we hypothesized the FLOs would elicit a larger N170 component than the non-FLOs (i.e. we expected to observe a face-likeness effect) in the TD group, as in a previous study36. We hypothesized this face-likeness effect would be absent in the ASD group. Additionally, we predicted that the N170 would be larger during the face-likeness judgments than during the roundness judgment in the TD individuals because FFA activation was enhanced when the participants were instructed to detect a face compared to when they were instructed to detect a house44. In TD individuals, the N170 amplitude is also enhanced for attended over unattended faces45. Because of the reported general atypicality in top-down modulation46 as well as atypical attentional modulation on N17047 in individuals with ASD, we predicted they would not exhibit this difference.
Results
Experiment 1: evaluative rating
On the mean rating scores for face-likeness, roundness, likability, and intensity of smile, t-tests revealed no significant differences between the ASD and TD groups (Table 1; all ts < 1.30, ps > .203). This suggests that adolescents with ASD have a similar sensitivity to the face-likeness of objects as do TD adolescents.
Table 1. The means and standard errors of mean (SEMs) of the rating scores of face-likeness, roundness, likability, and intensity of smile in Experiment 1 and those of the proportion of face-likeness and roundness responses to the FLOs and the non-FLOs in Experiment 2.
| Experiment 1 | ASD (n = 16) | TD (n = 25) |
|---|---|---|
| Face-likeness | 2.58 (0.13) | 2.41 (0.12) |
| Roundness | 2.06 (0.12) | 2.01 (0.11) |
| Likability | 2.02 (0.15) | 2.08 (0.12) |
| Intensity of smile | 2.06 (0.12) | 1.88 (0.11) |
In order to investigate how similar the face-likeness rating was between the two groups, the correlation of the mean face-likeness score for each object between the groups was investigated. The results showed a highly positive correlation (r = .87, p < .001), indicating that the objects that were rated as highly face-like by TD adolescents were also rated so by adolescents with ASD (Figure 1).
Figure 1. The average face-likeness score for each of the objects in the evaluative rating experiment (Experiment 1).
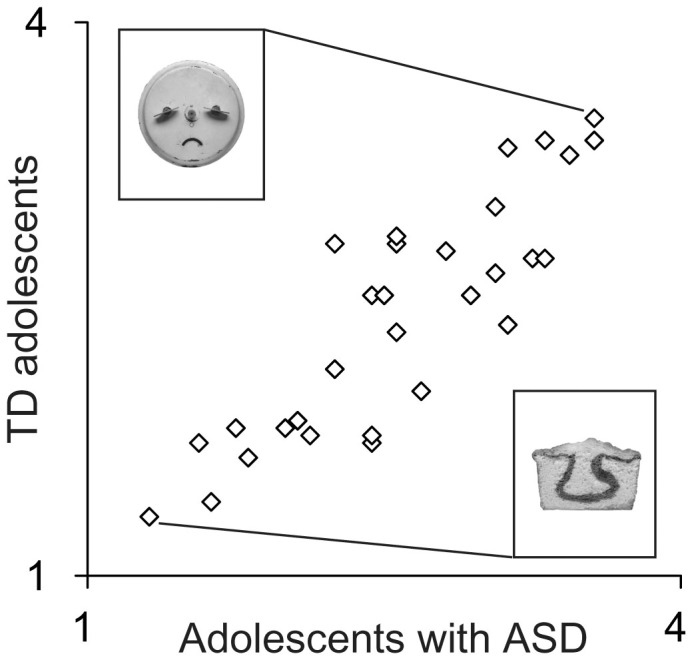
Each dot indicates the mean face-likeness score for each of the objects rated by the adolescents with ASD and the TD adolescents. The object in the top left of the figure was the one given the highest face-likeness rating score. The object in the bottom right of the figure was the one given the lowest score. ASD, autism spectrum disorder; TD, typically developing. Images: from FACES by Francois Robert and Jean Robert. © 2000 by Francois Robert. Used with permission of the authors and Chronicle Books LLC, San Francisco.
Experiment 2: face-likeness/roundness judgement
There was a significant main effect of face-likeness on the face-likeness judgements, (F(1, 27) = 333.19, p < .001, ηp2 = .93); the FLOs (M = .88, SEM = .03) were rated as more face-like than the non-FLOs (Table 1; M = .05, SEM = .02).With regard to the roundness judgements, there was a significant main effect of face-likeness (F(1, 27) = 24.42, p < .001, ηp2 = .47); the non-FLOs (M = .51, SEM = .03) were judged as rounder than the FLOs (M = .34, SEM = .02). For both judgements, the main effect of group and the interaction between group and face-likeness were not significant (all ps > .05).
Experiment 2: event-related potential
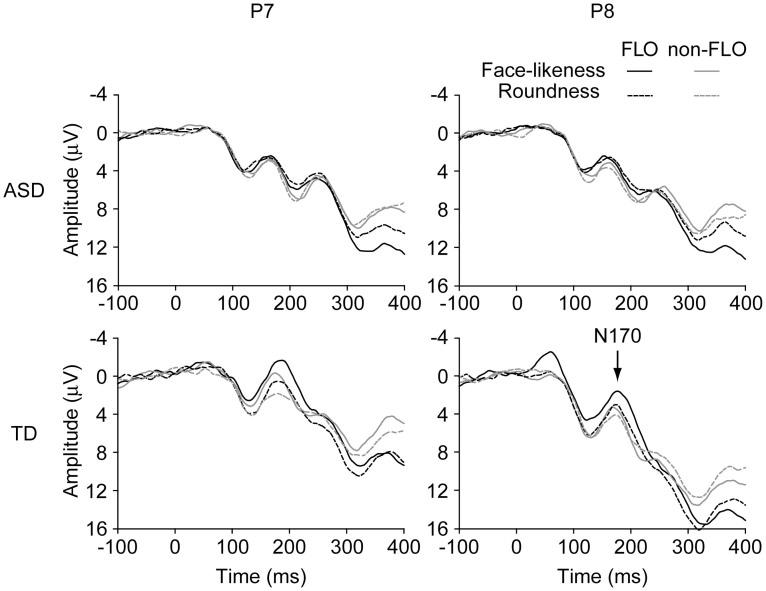
On the N170 amplitude, there was a significant main effect of face-likeness (F(1, 27) = 19.53, p < .001, ηp2 = .42) and electrode site (F(1, 27) = 5.74, p = .024, ηp2 = .18); the N170 amplitude was larger for FLOs (M = 0.57 μV, SEM = 0.63) than for non-FLOs (M = 1.61 μV, SEM = 0.62) and larger at P7 (M = 0.13 μV, SEM = 0.63) than at P8 (Figure 2; M = 2.04 μV, SEM = 0.84). There was also a significant interaction between group and task (F(1, 27) = 4.47, p = .044, ηp2 = .14). A simple effects analysis found a significant simple main effect of task in the TD group (F(1, 13) = 7.83, p = .015, ηp2 = .38), indicating that N170 was larger when judging face-likeness (M = −0.32 μV, SEM = 0.72) than when judging roundness (M = 1.16 μV, SEM = 0.86) of the stimuli, even though this was not true in the ASD group (face-likeness, M = 1.43 μV, SEM = 1.00; roundness, M = 1.40 μV, SEM = 0.76; F(1, 14) < 0.01, p = .947, ηp2 < .01). Other effects were not significant (all ps > .05).
Figure 2. Grand average ERP waveforms.
This figure depicts the grand average ERP waveforms of the adolescents with ASD (top two panels) and the TD adolescents (bottom two panels) in response to the FLOs (black lines) and the non-FLOs (grey lines) at P7 (left panels; left occipitotemporal region) and P8 (right panels; right occipitotemporal region) during the face-likeness (solid lines) and roundness judgement (dashed lines). ERP, event-related potential; ASD, autism spectrum disorder; TD, typically developing; FLO, face-like object.
On the N170 latency, there was a significant main effect of electrode site (F(1, 27) = 5.02, p = .033, ηp2 = .16), with a faster N170 peak at P8 (M = 167.3 ms, SEM = 3.0) than at P7 (M = 176.7 ms, SEM = 3.7). Other effects were not significant (all ps > .05).
The N170 amplitudes were larger at P7 than at P8. The N170 amplitudes had a negative value only in the TD participants. The N170 is usually larger at P8 than at P7, has a negative value in response to face stimuli, and a positive value in response to non-face stimuli; this is true for both TD individuals and individuals with ASD27,29. The opposite laterality and relatively small N170 amplitudes found here might suggest that the effect of FLOs on the activation of face-related brain regions (e.g. the FFA) were smaller than that of an actual face.
Discussion
We investigated the behavioural response and scalp-recorded ERPs to the face-likeness of objects in adolescents with ASD and TD adolescents, using FLOs and non-FLOs as stimuli. We observed a similar pattern of face-likeness ratings and face-sensitive cortical responses (i.e. the N170 amplitude) to the FLOs for the adolescents with ASD and the TD adolescents, and an enhanced N170 amplitude during the face-likeness judgement only for the TD adolescents. In Experiment 1, there was no group difference in the scores for any of the ratings (i.e. face-likeness, roundness, likability, and intensity of smile). This suggests that adolescents with ASD have a sensitivity to the face-likeness of objects, prefer FLOs in the same way as the TD adolescents, and decode the facial expressions of the non-face objects. The lack of group difference in the roundness rating eliminates the possibility that the ASD group simply gave high scores for all of their object ratings. Additionally and importantly, the objects rated as highly face-like by the TD adolescents were also rated so by the adolescents with ASD, suggesting that the two groups have similar criteria for face-likeness; both adolescents with ASD and TD adolescents perceive faces in objects.
In Experiment 2, both adolescents with ASD and TD adolescents showed an enhanced N170 amplitude in response to the FLOs. For the TD adolescents, an enhanced N170 amplitude was observed in response to the objects during the face-likeness judgement. In contrast, for the adolescents with ASD, an N170 amplitude in response to the object was not different during the face-likeness judgement and the roundness judgement. Because an enhanced N170 amplitude occurs when an observer perceives faces in non-face objects40, the results suggest that both the adolescents with ASD and the TD adolescents perceived faces in the FLOs. The absence of task modulation on the N170 amplitudes in the adolescents with ASD suggests atypical top-down modulation. Combined with the results of Experiment 1, we conclude that adolescents with ASD and the TD adolescents both have a sensitivity to face-likeness. Moreover, for the TD adolescents but not the adolescents with ASD, the face-related brain areas become more sensitive and react more strongly to all objects when they are required to process face-likeness information.
First, contrary to our a priori prediction, the adolescents with ASD and the TD adolescents showed highly similar face-likeness ratings (Experiment 1) and judgements (Experiment 2), suggesting that both groups perceive faces in non-face objects. Some argue that the tendency to perceive faces in objects is hard-wired in our brain11,12,13, because finding other's faces in the environment is important for humans and the cost of perceiving faces in non-face objects erroneously might be smaller than that associated with failing to detect other's face13. The present behavioural results suggest that this evolutionary-conserved perceptual tendency is spared in those with ASD. For TD individuals, this tendency is also related to social motivation, especially motivation to interact with other individuals (Akechi & Hietanen, submitted for publication); individuals who give higher face-like ratings to FLOs show a stronger motivation for social contact measured by a questionnaire. A lack of social motivation or a reduced salience of social stimuli in individuals with ASD might decrease the orienting response to other individuals and decrease social learning opportunities, and the result may be the atypical development of face processing and social cognition/skills14,15,48,49. The present results are not consistent with this view. One possible explanation is that social motivation is not reduced in ASD or is reduced only in early age. Because we did not directly measure social motivation here, a study investigating the sensitivity to perceive faces in objects, social motivation, and the link between these two variables in ASD might be beneficial.
Second, again contrary to our a priori hypothesis, in Experiment 2, for both adolescents with ASD and TD adolescents, the N170 amplitudes in response to the FLOs was larger than in response to the non-FLOs (i.e. a face-likeness effect). This face-likeness effect on the N170 amplitudes might be the neural correlate of the sensitivity to perceive faces in objects, because the N170 amplitudes were larger in response to non-face stimuli that are perceived as faces36,37,38,39,40. Note that although familiarity might have been different between the FLOs and non-FLOs in Experiment 2, there is evidence that familiarity of facial stimuli does not affect the N170 amplitudes in individuals with ASD as well as TD individuals50. Therefore, such a possible familiarity difference might not account for the result of the N170 in this study. The previous ERP studies of ASD demonstrated that individuals with ASD show a larger N170 component in response to faces compared to other objects27,28,29, suggesting that they form a visual representation of a face in a same way as the TD individuals51. Additionally, individuals with ASD have a qualitatively typical ability to encode facial identity25. Therefore, the present ERP results extends such a notion by suggesting that individuals with ASD have an intact ability to form the visual representation of faces even from non-face objects having face-like features.
In Experiment 2, the N170 was larger for both FLOs and non-FLOs during the face-likeness judgement than during the roundness judgement for the TD adolescents but not the adolescents with ASD. This suggests that, in TD individuals, face-sensitive brain areas begin to respond more strongly to all objects when they are required to process the face-likeness of the objects. Because the N170 is enhanced when the observer perceives faces40, the present finding suggests that the TD individuals perceived faces more frequently in the FLOs and the non-FLOs when they were required to process the face-likeness of the objects. This modulatory effect might be mediated by the FFA, a candidate source of the N17035, and functional connection between the FFA and prefrontal or parietal cortex. There is greater activation of the FFA in response to noise images when the participant is instructed to detect faces than when instructed to detect houses in pure noise images, even for an individual with face-recognition impairments (i.e. prosopagnosia)44. Functional connections between the medial parietal cortex and the FFA were found only when the observer perceived a face in a degraded image after seeing an undegraded version of the same image33, suggesting that top-down modulation enables us to perceive faces in ambiguous images. A behavioural study showed that enhanced face detection in a degraded image after seeing an undegraded image was absent in individuals with ASD42. Additionally, a functional magnetic resonance imaging study showed an absence of attentional modulation of the FFA activation in response to faces in individuals with ASD52. Moreover, a lack of attentional modulation of N170 amplitudes in individuals with ASD was also reported47. Thus, the absence of the task effect on the N170 amplitudes in adolescents with ASD suggests that top-down modulation is atypical in them, and this might be caused by atypical functional connectivity between the FFA and other brain areas53. Perhaps, such atypical top-down modulation might prevent the N170 increase and the FFA activation, which are related to face perception, when they need to detect faces. Therefore, they might find faces less frequently in an environment, where faces are sometimes less visible and the face-related brain system should be more susceptible to subtle face configurations, and this could result in less frequent looking behaviour toward faces17,18,19 and less eye contact16,54 that are widely reported and diagnostic features of ASD.
In conclusion, in the present study, when the adolescents with ASD and the TD adolescents were compared, similarities and differences in the patterns of processing the face-likeness of objects were found. Here, the adolescents with ASD extracted the face-likeness of the objects in a similar way as the TD adolescents, and they also showed an enhanced face-sensitive cortical response to face-like objects, but they did not show the modulatory effect of task (i.e. a face-likeness judgement) on the face-sensitive cortical response. This suggests that the two groups use the neural pathway involved in face processing to respond to objects having face-like features, and that the readiness to process face-like features enhances the face-sensitive neural responses to all objects in TD individuals but not in individuals with ASD. In future studies, investigating other potential factors, such as social motivation, that might influence the behavioural and neural response to face-likeness would help provide a better understanding of how and why face processing and its development are different between individuals with ASD and TD individuals.
Methods
Participants
Sixteen adolescents with ASD (4 females) and 25 TD adolescents (10 females) participated in Experiment 1 (Table 3; 9–21 years old; IQ, 64–124). Fifteen adolescents with ASD (1 female) and 14 TD adolescents (2 females) participated in Experiment 2 (12–19 years old; IQ, 73–124) of which 7 adolescents with ASD and 4 TD adolescents participated in Experiment 1. Experiment 2 was conducted 1 year after Experiment 1. Participants with ASD had been previously diagnosed by at least one child psychiatrist or pediatrician according to DSM-IV16. The participants' parents all completed the Japanese version of the Social Communication Questionnaire (SCQ)55 to corroborate their clinical presentation. All participants with ASD above the cut-off point (15). The SCQ is a 40-item rating scale and its content is based on that of the ADI-R56 which is a standardized parent interview, and the agreement between SCQ and ADI-R scores is high57. An abbreviated version of the Japanese Wechsler Intelligence Scale for Children-III (WISC-III)58,59 or Wechsler Adults Intelligence Scale-Revised (WAIS-R)60,61 was administered to all of the participants to measure their IQ (the WISC-III for those less than 16.9 years old and the WAIS-R for those greater than 16.9 years old). There were no significant group differences in IQ, chronological age, or sex ratio in Experiments 1 and 2 (all ps > .05). All the participants had normal or corrected-to-normal visual acuity. Written informed consent was obtained from all the participants and their parents. This study was approved by the Research Ethics Committee of the University of Tokyo.
Table 2. Means and standard deviations (SDs) of N170 amplitudes and latencies, and number of trials included in the ERP analysis.
| ASD (n = 15) | TD (n = 14) | ||||
|---|---|---|---|---|---|
| Amplitude (μV) | P7 | P8 | P7 | P8 | |
| Face-likeness | FLOs | 0.73 (3.60) | 1.34 (5.48) | −2.39 (3.24) | 0.53 (5.18) |
| Non-FLOs | 1.51 (4.17) | 2.16 (5.27) | −1.42 (3.57) | 3.07 (4.44) | |
| Roundness | FLOs | 0.78 (3.05) | 1.36 (4.74) | −0.16 (4.70) | 2.20 (4.89) |
| Non-FLOs | 1.23 (2.64) | 2.21 (4.49) | 0.52 (4.61) | 3.56 (4.99) | |
| Latency (ms) | P7 | P8 | P7 | P8 | |
| Face-likeness | FLOs | 179 (28) | 161 (19) | 180 (19) | 169 (21) |
| Non-FLOs | 170 (24) | 168 (24) | 179 (27) | 168 (11) | |
| Roundness | FLOs | 179 (29) | 168 (20) | 180 (20) | 171 (17) |
| Non-FLOs | 168 (23) | 163 (24) | 179 (19) | 171 (14) | |
| Number of accepted trials | |||||
| Face-likeness | FLOs | 53.3 (10.7) | 54.2 (7.4) | ||
| Non-FLOs | 54.1 (7.9) | 54.2 (8.7) | |||
| Roundness | FLOs | 51.9 (10.7) | 52.4 (8.5) | ||
| Non-FLOs | 53.3 (9.2) | 52.0 (8.8) | |||
Table 3. Means, standard deviations (SDs), range of chronological age, IQ, and scores on the Japanese version of the Social Communication Questionnaire (SCQ) of the participants. The P values reflect the levels of significance from the independent samples t-tests.
| Experiment 1 | ASD (n = 16) | TD (n = 25) | |||
|---|---|---|---|---|---|
| M (SD) | range | M (SD) | range | p-value | |
| Age, years | 14.1 (2.5) | 11.3–19.8 | 13.4 (3.6) | 9.4–21.8 | .454 |
| IQ | 97.0 (21.9) | 64–124 | 100.0 (7.8) | 79–113 | .600 |
| SCQ | 24.2 (6.4) | 15–38 | 1.7 (1.9) | 0–7 | <.001 |
Apparatus, stimuli, and procedure
In Experiment 1, stimulus presentation and data collection were done on a laptop PC using E-Prime software (Psychology Software Tools; PST, Inc., Pittsburgh, PA). Before the experiment, 73 candidate objects (from “Faces” book62, which depicts objects with elements arranged in face-like patterns) were rated on an 8-point Likert scale by eight adults (1 = non-face-like, 8 = face-like; M = 4.2, SD = 1.3) and 30 objects were chosen equally from low to high face-likeness (M = 4.0, SD = 1.4, range 1.5–6.5). The participants were seated approximately 60 cm from the monitor. In each block, the greyscale photographs (9.0° high and 6.9° wide) of the 30 objects were presented in random order. There were two practice trials in each block. In each trial, the stimulus was presented until a response was given. The intertrial interval (ITI) was 200 ms. In the first block, participants were asked to rate the “face-likeness” of the objects by using a 4-point Likert scale (1 = non-face-like, 4 = face-like) and press the corresponding key. Next, they rated the “roundness,” “likability,” and “intensity of smile” of each object. The stimuli were presented once in each block, with a total of four blocks. Thus, there were 120 test trials in total.
In Experiment 2, stimulus presentation and data collection were done on a desktop PC with a 17-inch CRT monitor by using E-Prime software (PST, Inc.). The participants were seated approximately 100 cm away from the monitor. The fixation point, consisting of a central cross that subtended at 0.6°, appeared at the centre of the screen; the participants were instructed to fixate on this point before the experiment began. Photographs of 16 face-like objects (FLOs; chosen from 43 objects that were prerated and not used in Experiment 1)62, and comparable 16 non-FLOs (collected from an online catalogue with permission) were used (see Supplementary Information). Luminance and contrast values did not significantly differ between the FLOs and non-FLOs. Before the experiment, 10 adults rated the “face-likeness” of the test stimuli by using a 4-point Likert scale (1 = non-face-like, 4 = face-like). The FLOs (M = 2.89, SD = 0.57) were rated as more face-like than the non-FLOs (M = 1.10, SD = 0.12; p < .001). All the photographs were in greyscale (7.7° high and 6.0° wide).
Each trial started with the presentation of a fixation point. In each trial, the fixation point was replaced after 750 ms with the test stimulus. The participants were asked to judge whether the stimulus was face-like/round or not and press the corresponding mouse button (i.e. two-alternative forced choice). The test stimulus was presented until a response was given. The ITI was randomized between 200 and 400 ms. There were two face-likeness and two roundness judgment blocks. Before each of the two test blocks, there was one practice block. The face-likeness judgment task was conducted in the first half of the experiment for approximately half of the participants (ASD, N = 8; TD, N = 7) and vice versa. The practice block consisted of eight trials (randomly selected from 32 possible test trials; 16 FLOs and 16 non-FLOs trials) and was repeated if the participants had not learned the task yet (once for a participant with ASD and twice for another participant with ASD). There was no group difference on the number of the repeat (p > .05). Each test block consisted of 64 trials. Each of the test stimuli was presented eight times (twice in each test block). The experiment consisted of 256 test trials and the practice trials. The presentation order of each trial was randomized in each block, and the correspondence between the buttons (left/right) and the judgment (face-like/non-face-like or round/not-round) for each task was counterbalanced across the participants.
Electroencephalography recordings
The scalp electroencephalogram (EEG) was recorded using Ag/AgCl electrode caps (Neuroscan, Inc., Charlotte, NC) at 16 scalp electrode sites (F3, Fz, F4, C3, Cz, C4, P3, Pz, P4, O1, Oz, O2, T7, T8, P7, and P8), as well as the left and right earlobes (A1 and A2) and the nose electrode, according to the international 10–20 system. Recordings were referenced to the electrode located between Cz and CPz and then re-referenced offline to the nose electrode. The horizontal electro-oculogram (EOG) was recorded at the outer canthi of both eyes, and the vertical EOG was recorded from electrodes placed below and above the left eye. Electrode impedance was kept below 10 kΩ. The EEG and EOG were recorded and analyzed using the NeuroScan scan system with SynAmps2 (Neuroscan, Inc.) for 400 ms following stimulus onset, with a 100-ms prestimulus baseline and a bandpass of 0.1–30 Hz. The sampling rate was 500 Hz.
Data analysis
In Experiment 2, the practice trials were excluded from the analysis. The mean proportion of face-likeness and roundness responses to the FLOs and the non-FLOs were analyzed using a two-way analysis of variance (ANOVA) with group (ASD, TD) as the between-participants factor and face-likeness (FLO, non-FLO) as the within-participant factor. Trials were rejected from the ERP analysis if any EEG or EOG amplitude exceeded ± 75 μV. The number of the trials included in the analysis did not differ between conditions and groups (Table 2; all ps > .05). The average rejection rate for the ASD group was 16.95% and that for the TD group was 16.85%. The peak amplitudes and latencies of the N170 were measured and manually identified between 140 and 240 ms at P7 and P8. The mean N170 amplitudes and latencies were analyzed using a four-way ANOVA with group (ASD, TD) as the between-participants factor and face-likeness (FLO, non-FLO), task (face-likeness, roundness), and electrode site (P7, P8) as the within-participant factors. Because the N170 peaks were less clear for the ASD group (see Figure 2), Levene's test was used to examine homogeneity of inter-individual variability in N170 peak latency for the ASD and TD groups; there was no group difference in any condition (all ps > .05). To investigate this point further, we also checked whether N170 latency correlated with age, IQ, or SCQ score in the ASD group; there was no significant correlation (all ps > .05). In addition, since both age-range and IQ-range were large in both groups, we examined whether N170 amplitude and latency correlated with age or IQ across groups; there was no significant correlation (all ps > .05).
Supplementary Material
Supplementary Figure
Acknowledgments
We would like to acknowledge all the participants, their family, and the teachers of Musashino Higashi Gakuen. We thank Saori Usui for help in data collection and Motoyuki Sanada for helpful comments on ERP analysis. This study was supported by Japan Society for the Promotion of Science (JSPS): Grant-in-Aid for JSPS Fellows #2310946 (H.A.) and #2310196 (Y.K.), JSPS: Grant-in-Aid for Scientific Research (B) #24330207 (T.H.) and #23330271 (Y.T.), and Center for Evolutionary Cognitive Sciences at University of Tokyo.
Footnotes
The authors declare no competing financial interests.
Author Contributions H.A. designed and conducted the experiments, prepared the materials, developed the computer programs, analyzed the data, and wrote the paper through discussions with Y.K. Y.K. conducted the experiments and prepared the materials. H.O. recruited the participants. Y.T. and T.H. contributed to the materials/apparatuses and supervised the project.
References
- Bruce V. Recognising faces (Lawrence Erlbaum Associates, Hillsdale, 1988). [Google Scholar]
- Farroni T. et al. Newborns' preference for face-relevant stimuli: effects of contrast polarity. Proc. Natl. Acad. Sci. U. S. A. 102, 17245–17250 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondloch C. J. et al. Face perception during early infancy. Psychol. Sci. 10, 419–422 (1999). [Google Scholar]
- Morton J. & Johnson M. H. CONSPEC and CONLERN: a two-process theory of infant face recognition. Psychol. Rev. 98, 164–181 (1991). [DOI] [PubMed] [Google Scholar]
- Stein T., Peelen M. V. & Sterzer P. Adults' awareness of faces follows newborns' looking preferences. PLoS One 6, e29361 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomalski P., Csibra G. & Johnson M. H. Rapid orienting toward face-like stimuli with gaze-relevant contrast information. Perception 38, 569–578 (2009). [DOI] [PubMed] [Google Scholar]
- Johnson M. H. Subcortical face processing. Nat. Rev. Neurosci. 6, 766–774 (2005). [DOI] [PubMed] [Google Scholar]
- Ichikawa H., Kanazawa S. & Yamaguchi M. K. Finding a face in a face-like object. Perception 40, 500–502 (2011). [DOI] [PubMed] [Google Scholar]
- Reichhardt T. NASA critics silenced as Mars loses face. Nature 392, 530–530 (1998). [Google Scholar]
- Zhang H. et al. Detecting faces in pure noise images: a functional MRI study on top-down perception. Neuroreport 19, 229–233 (2008). [DOI] [PubMed] [Google Scholar]
- Guthrie S. Faces in the clouds: a new theory of religion (Oxford University Press, New York, 1993). [Google Scholar]
- Sagan C. The demon-haunted world: science as a candle in the dark (Random House, New York, 1997). [Google Scholar]
- Verpooten J. & Nelissen M. Sensory exploitation and cultural transmission: the late emergence of iconic representations in human evolution. Theory. Biosci. 129, 211–221 (2010). [DOI] [PubMed] [Google Scholar]
- Chevallier C., Kohls G., Troiani V., Brodkin E. S. & Schultz R. T. The social motivation theory of autism. Trends. Cogn. Sci. 16, 231–239 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G., Webb S. J. & McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev. Neuropsychol. 27, 403–424 (2005). [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. (American Psychiatric Association, Washington, D. C., 1994). [Google Scholar]
- Mars A. E., Mauk J. E. & Dowrick P. W. Symptoms of pervasive developmental disorders as observed in prediagnostic home videos of infants and toddlers. J. Pediatr. 132, 500–504 (1998). [DOI] [PubMed] [Google Scholar]
- Osterling J. A., Dawson G. & Munson J. A. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Dev. Psychopathol. 14, 239–251 (2002). [DOI] [PubMed] [Google Scholar]
- Osterling J. & Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J. Autism Dev. Disord. 24, 247–257 (1994). [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Senju A., Tojo Y., Osanai H. & Hasegawa T. Faces do not capture special attention in children with autism spectrum disorder: a change blindness study. Child Dev. 80, 1421–1433 (2009). [DOI] [PubMed] [Google Scholar]
- Dawson G., Hill D., Spencer A., Galpert L. & Watson L. Affective exchanges between young autistic children and their mothers. J. Abnorm. Child Psychol. 18, 335–345 (1990). [DOI] [PubMed] [Google Scholar]
- Joseph R. M. & Tager-Flusberg H. An investigation of attention and affect in children with autism and Down syndrome. J. Autism Dev. Disord. 27, 385–396 (1997). [DOI] [PubMed] [Google Scholar]
- Sigman M., Mundy P., Sherman T. & Ungerer J. Social interactions of autistic, mentally retarded and normal children and their caregivers. J. Child Psychol. Psychiatry 27, 647–655 (1986). [DOI] [PubMed] [Google Scholar]
- Werner E., Dawson G., Osterling J. & Dinno N. Brief report: Recognition of autism spectrum disorder before one year of age: a retrospective study based on home videotapes. J. Autism Dev. Disord. 30, 157–162 (2000). [DOI] [PubMed] [Google Scholar]
- Weigelt S., Koldewyn K. & Kanwisher N. Face identity recognition in autism spectrum disorders: a review of behavioral studies. Neurosci. Biobehav. Rev. 36, 1060–1084 (2011). [DOI] [PubMed] [Google Scholar]
- Bentin S., Allison T., Puce A., Perez E. & McCarthy G. Electrophysiological studies of face perception in human. J. Cogn. Neurosci. 8, 551–565 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland J., Dawson G., Webb S. J., Panagiotides H. & Carver L. J. Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. J. Child Psychol. Psychiatry. 45, 1235–1245 (2004). [DOI] [PubMed] [Google Scholar]
- Webb S. J., Dawson G., Bernier R. & Panagiotides H. ERP evidence of atypical face processing in young children with autism. J. Autism Dev. Disord. 36, 881–890 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S. J. et al. ERP responses differentiate inverted but not upright face processing in adults with ASD. Soc. Cogn. Affect. Neurosci. 7, 578–587 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemel B., Mottron L. & Dawson M. Impaired face processing in autism: fact or artifact? J. Autism Dev. Disord. 36, 91–106 (2006). [DOI] [PubMed] [Google Scholar]
- Andrews T. J. & Schluppeck D. Neural responses to Mooney images reveal a modular representation of faces in human visual cortex. Neuroimage 21, 91–98 (2004). [DOI] [PubMed] [Google Scholar]
- Andrews T. J., Schluppeck D., Homfray D., Matthews P. & Blakemore C. Activity in the fusiform gyrus predicts conscious perception of Rubin's vase-face illusion. Neuroimage 17, 890–901 (2002). [PubMed] [Google Scholar]
- Dolan R. J. et al. How the brain learns to see objects and faces in an impoverished context. Nature 389, 596–599 (1997). [DOI] [PubMed] [Google Scholar]
- Kingstone A., Tipper C., Ristic J. & Ngan E. The eyes have it!: an fMRI investigation. Brain. Cogn. 55, 269–271 (2004). [DOI] [PubMed] [Google Scholar]
- Rossion B. & Jacques C. Does physical interstimulus variance account for early electrophysiological face sensitive responses in the human brain? Ten lessons on the N170. Neuroimage 39, 1959–1979 (2008). [DOI] [PubMed] [Google Scholar]
- Churches O., Baron-Cohen S. & Ring H. Seeing face-like objects: an event-related potential study. Neuroreport 20, 1290–1294 (2009). [DOI] [PubMed] [Google Scholar]
- Sagiv N. & Bentin S. Structural encoding of human and schematic faces: holistic and part-based processes. J. Cogn. Neurosci. 13, 937–951 (2001). [DOI] [PubMed] [Google Scholar]
- Latinus M. & Taylor M. J. Face processing stages: impact of difficulty and the separation of effects. Brain Res. 1123, 179–187 (2006). [DOI] [PubMed] [Google Scholar]
- Caharel S. et al. Early holistic face-like processing of Arcimboldo paintings in the right occipito-temporal cortex: evidence from the N170 ERP component. Int. J. Psychophysiol. 90, 157–164 (2013). [DOI] [PubMed] [Google Scholar]
- George N., Jemel B., Fiori N., Chaby L. & Renault B. Electrophysiological correlates of facial decision: insights from upright and upside-down Mooney-face perception. Cogn. Brain Res. 24, 663–673 (2005). [DOI] [PubMed] [Google Scholar]
- Sun L. et al. Impaired gamma-band activity during perceptual organization in adults with autism spectrum disorders: evidence for dysfunctional network activity in frontal-posterior cortices. J. Neurosci. 32, 9563–9573 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loth E., Gómez J. C. & Happé F. When seeing depends on knowing: adults with autism spectrum conditions show diminished top-down processes in the visual perception of degraded faces but not degraded objects. Neuropsychologia 48, 1227–1236 (2010). [DOI] [PubMed] [Google Scholar]
- Kikuchi Y. et al. Atypical disengagement from faces and its modulation by the control of eye fixation in children with autism spectrum disorder. J. Autism Dev. Disord. 41, 629–645 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righart R., Andersson F., Schwartz S., Mayer E. & Vuilleumier P. Top-down activation of fusiform cortex without seeing faces in prosopagnosia. Cereb. Cortex 20, 1878–1890 (2010). [DOI] [PubMed] [Google Scholar]
- Eimer M. Attentional modulations of event-related brain potentials sensitive to faces. Cogn. Neuropsychol. 17, 103–116 (2000). [DOI] [PubMed] [Google Scholar]
- Cook J., Barbalat G. & Blakemore S.-J. Top-down modulation of the perception of other people in schizophrenia and autism. Front. Hum. Neurosci. 6, 175 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churches O., Wheelwright S., Baron-Cohen S. & Ring H. The N170 is not modulated by attention in autism spectrum conditions. Neuroreport 21, 399–403 (2010). [DOI] [PubMed] [Google Scholar]
- Klin A., Jones W., Schultz R. & Volkmar F. The enactive mind, or from actions to cognition: lessons from autism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358, 345–360 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. T. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int. J. Dev. Neurosci. 23, 125–141 (2005). [DOI] [PubMed] [Google Scholar]
- Webb S. J. et al. Response to familiar faces, newly familiar faces, and novel faces as assessed by ERPs is intact in adults with autism spectrum disorders. Int. J. Psychophysiol. 77, 106–117 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin & Golland Y. Meaningful processing of meaningless stimuli: the influence of perceptual experience on early visual processing of faces. Cognition 86, B1–14 (2002). [DOI] [PubMed] [Google Scholar]
- Bird G., Catmur C., Silani G., Frith C. & Frith U. Attention does not modulate neural responses to social stimuli in autism spectrum disorders. Neuroimage 31, 1614–1624 (2006). [DOI] [PubMed] [Google Scholar]
- Khan S. et al. Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proc. Natl. Acad. Sci. U. S. A. 110, 3107–3112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Rutter M., DiLavore P. C. & Risi S. Autism Diagnostic Observation Schedule-WPS (ADOS-WPS) (Western Psychological Services, Los Angeles, 1999) [Google Scholar]
- Rutter M., Bailey A. & Lord C. Social Communication Questionnaire (Western Psychological Services, Los Angeles, 2003). [Google Scholar]
- Rutter M., Le Couteur A. & Lord C. Autism Diagnostic Interview–Revised Manual (Western Psychological Services, Los Angeles, 2003). [Google Scholar]
- Bishop D. V. & Norbury C. F. Exploring the borderlands of autistic disorder and specific language impairment: a study using standardised diagnostic instruments. J. Child Psychol. Psychiatry 43, 917–929 (2002). [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed. (Psychological Corporation, London, 1992). [Google Scholar]
- Japanese WISC-III Publication Committee. Nihonban WISC-III chinou kensahou [Japanese Wechsler Intelligence Scale for Children 3rd ed.] (Nihon Bunka Kagakusha, Tokyo, 1998). [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Revised (Psychological Corporation, New York, 1981). [Google Scholar]
- Shinagawa F., Kobayashi S., Fujita K. & Maekawa H. Nihonban WAIS-R seijin chinou kensahou [Japanese Wechsler Adult Intelligence Scale – Revised] (Nihon Bunka Kagakusha, Tokyo, 1990). [Google Scholar]
- Robert F. & Robert J. Faces (Chronicle Books, San Francisco, 2000). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure