Abstract
In this study, we investigated the association between the FasL -844T/C polymorphism and the risk of developing esophageal squamous cell carcinoma (ESCC) in South China. For the investigation, we randomly selected 248 patients suffering from ESCC from Southern China along with 297 healthy individuals as the control group. The relationship between the FasL gene -844T/C SNP and ESCC was studied using PCR-RFLP and immunohistochemistry. The Fas -1377G/A SNP was also selected for investigation to detect whether it interferes with the functional effect of the FasL -844C/T polymorphism in ESCC development. A significant difference in the FasL -844T/C genotypes between the patients and the control group was observed (P<0.05), with those expressing the C allele having a significantly reduced risk of developing ESCC, however younger patients (<60 years) exhibited a more malignant pathological T grade if they were homozygous for the C allele. FasL -844 CC combined with the Fas -1377 G allele is a protective factor against ESCC. Having said this, even though the C allele has a protective effect prior to development of ESCC, once the host does develop the condition the tumour will develop faster and have a higher degree of malignancy than T carriers.
Apoptosis (programmed cell death) exerts critical roles in development, homeostasis and normal functioning of adult multi-cellular organisms1. It had been reported that various types of malignant diseases acquired ability to resist cell apoptosis, and that the regulatory defects of this cell-death pathway contribute to tumorigenesis, tumor cell invasion and metastasis2. Genetic variations of crucial genes in apoptosis pathway may thus influence the susceptibility to cancer.
The Fas–Fas ligand (FasL) system is the major pathway inducing cell and tissue apoptosis3. Ligation of Fas by either agonistic antibody or its natural ligand transmits a “death signal” to the target cells, potentially triggering apoptosis. Fas and FasL molecules play an important role in immune escape4. Decreased expression levels of Fas and FasL are associated with different malignancies. Increased expression of FasL may favor malignant transformation and progression5.
Single nucleotide polymorphisms (SNPs) have been proposed to play an important role in the genetic susceptibility to cancer. Many studies have reported that functional SNPs can impact the risk of cancer. The functional mutations in the Fas and FasL genes that impair apoptotic signal transduction have been shown to be associated with an increased risk of many types of cancers, e.g esophageal squamous-cell carcinoma and lung cancer6,7. The Fas-FasL system plays important role in cancer initiation, development and progression. SNPs of Fas and/or FasL gene have been proposed to be significant in the genetic susceptibility to cancer through altering the expression of gene.
The aim of the present study was to analyze whether -844T/C (rs763110) polymorphism in FasL gene (GenBank accession no. Z96050) promoter confers host's susceptibility to cancers of esophageal squamous cell in south China. Meanwhile, the expression of FasL was analyzed to conform that the variation of -844T/C in FasL gene is able to significantly modify the levels of expression of FasL in patients. The polymorphism of Fas gene -1377 was analyzed to study whether susceptibility to esophageal cancer is influenced by FasL -844 SNP under the intervention of Fas -1377 SNP.
Results
FasL polymorphism and the association with ESCC
This population-based case-control study included 248 patients with esophageal squamous cell carcinoma (ESCC) and 238 control subjects. All study subjects were south Chinese. Baseline clinical characteristics of cases and controls are summarized in Table 1. Cases were older than controls and had a greater proportion of males.
Table 1. Baseline clinical characteristics of cases and controls.
*P < 0.05.
The genotype and allele distributions of FasL polymorphism in the cases and controls are shown in Table 2. The observed genotype frequencies was in agreement with Hardy–Weinberg equilibrium in the controls (χ2 = 2.663, P = 0.103). The frequencies of the TT, TC and CC genotypes were 27.8%, 44.4% and 27.8%, respectively, among the cases and 20.2%, 33.6% and 46.2%, respectively, among the controls. The frequencies of C alleles in ESCC patients and control samples were 0.50 and 0.63, respectively.
Table 2. Genotypic and allelic frequencies of FasL gene -844 among case patients and control subjects and the association of this SNP with ESCC risk.
| Cases (%) n = 248 | Controls (%) n = 297 | χ2 | P | Crude OR (95%CI) | P | Adjusted OR# (95% CI) | P | |
|---|---|---|---|---|---|---|---|---|
| Genotypes | ||||||||
| TT | 69 (27.8) | 52(17.5) | 1 | 1 | ||||
| TC | 110 (44.4) | 128(43.1) | 11.827 | 0.003** | 0.957(0.599–1.527) | 0.852 | 1.081(0.640–1.826) | 0.771 |
| CC | 69 (27.8) | 117 (39.4) | 0.436(0.271–0.702) | 0.001** | 0.425(0.255–0.708) | 0.001** | ||
| Alleles | ||||||||
| T allele | 248 (50.0) | 232 (39.1) | 13.13 | 0.000** | 1 | 1 | ||
| C allele | 248 (50.0) | 362 (60.9) | 0.641(0.504–0.816) | 0.000** | 0.597(0.460–0.776) | 0.000** |
**P < 0.01.
#. Adjusting for age and gender.
Effect modification of the associations of the SNP with ESCC
Logistic regression analysis was used to estimate associations between the genotypes and risk of ESCC (Table 2). When stratified by age and gender, the FasL gene -844 CC genotype was associated with a decreased risk for the development of ESCC (Adjusted OR = 0.436, 95% CI = 0.271–0.702, P = 0.001) compared with TT genotype. The C haplotype remained a significant protective role for ESCC (Adjusted OR = 0.555, 95% CI = 0.419–0.735, P = 0.000), which was comparable to that of T.
Prognostic significance of FasL polymorphism
The relationship of ESCC clinical pathological parameters and FasL -844 SNP was compared (Table 3). There were no significant differences between the gene polymorphism and age, gender, tumor grade, tumor site, lymph node metastasis, cardiac involvement, vascular invasion. While FasL -844 C/T showed significant modification of genotype and risk by the tumor length, T stage and TNM stage. FasL CC versus TT was dangerous, adjusted OR = 3.300 (1.572–6.929), P = 0.002; adjusted OR = 2.494 (1.242–5.007), P = 0.010; adjusted OR = 2.179 (1.063–4.468), P = 0.033, respectively (Table 4).
Table 3. Distribution of selected characteristics of the patient cohort.
| Cases n = 248 | ||||||
|---|---|---|---|---|---|---|
| n(%) | TT | TC | CC | χ2 | P | |
| Age(year) | <60 | 26(10.5) | 37(14.9) | 28(11.3) | 0.920 | 0.631 |
| ≥60 | 43(17.3) | 73(29.4) | 41(16.5) | |||
| sex | male | 58(23.3) | 94(37.9) | 59(23.8) | 0.079 | 0.961 |
| female | 11(4.4) | 16(6.5) | 10(4.0) | |||
| Grading | well- moderately differentiation | 53(21.4) | 85(34.3) | 51(20.6) | 0.283 | 0.868 |
| poorly differentiation | 16(6.5) | 25(10.1) | 18(7.3) | |||
| T Grade | T1–T2 | 37(5.6) | 49(12.5) | 20(3.6) | 8.820 | 0.012* |
| T3–T4 | 32(12.9) | 61(24.6) | 49(19.8) | |||
| TNM stage | I–II–III | 54(21.8) | 75(30.2) | 40(16.1) | 6.543 | 0.038* |
| IV | 15(6.0) | 35(14.1) | 29(11.7) | |||
| Location of the tumor | Epimere- midportion | 41(16.5) | 58(23.4) | 40(16.1) | 1.065 | 0.587 |
| hypomere | 28(11.3) | 53(21.4) | 29(11.7) | |||
| Length | <5 | 52(21.0) | 70(28.2) | 35(14.1) | 9.025 | 0.011* |
| ≥5 | 17(6.9) | 40(16.1) | 34(13.7) | |||
| Lymphnode metastasis | Yes | 34(13.7) | 65(26.2) | 35(14.1) | 2.066 | 0.356 |
| No | 35(14.1) | 45(18.1) | 34(13.7) | |||
| Nerve involvement | yes | 48(19.4) | 77(31.0) | 48(19.4) | 0.005 | 0.997 |
| no | 21(8.5) | 33(13.3) | 21(8.5) | |||
| Cardiac involvement | yes | 49(19.8) | 88(35.5) | 53(21.4) | 1.913 | 0.384 |
| no | 20(8.1) | 22(8.9) | 16(6.5) | |||
| Vascular tumor thrombus | yes | 58(23.4) | 85(34.3) | 56(22.6) | 1.282 | 0.527 |
| no | 11(4.4) | 25(10.1) | 23(9.3) | |||
*P < 0.05.
Table 4. Risk of ESCC tumor length, T grade and TNM stage according to FasL genotype.
| Length | T grade | TNM stage | |||||||
|---|---|---|---|---|---|---|---|---|---|
| genotypes | OR | CI | P | OR | CI | p | OR | CI | P |
| TT | 1 | 1 | 1 | ||||||
| TC | 1.748 | 0.893–3.421 | 0.103 | 1.439 | 0.787–2.634 | 0.238 | 1.680 | (0.835–3.379) | 0.146 |
| CC | 2.971 | 1.442–6.122 | 0.003** | 2.833 | 1.402–5.722 | 0.004** | 2.610 | (1.238–5.500) | 0.012* |
*P < 0.05.
**P < 0.01.
Association of -844T/C polymorphism and ESCC in selected population
Patients with ESCC and healthy group were stratified by age to explore whether FasL -844T/C polymorphism was associated with ESCC in this selected population of patients (Table 5). Genotype frequencies in two control subgroups (<60, ≥60 years old) agreed with frequencies expected under the Hardy-Weinberg equilibrium (χ2 = 1.852, P = 0.174 and χ2 = 0.792, P = 0.373, respectively). With age <60 years, the frequencies of the TT, TC and CC genotypes were 27.6%, 41.4% and 31.0%, respectively, among the cases and 20.5%, 33.5% and 54.0%, respectively, among the controls. There was no difference between the two groups. While, the frequencies of C alleles in ESCC patients and control samples were 60.2% and 51.1%, respectively, which had statistical difference.
Table 5. Genotypic and allelic frequencies of FasL gene -844 in subgroups according to age.
| n(%) | Genotype(Frequency) | Allele(Frequency) | |||||
|---|---|---|---|---|---|---|---|
| TT | TC | CC | T | C | |||
| <60 | Cases | 91 | 26(28.6) | 37(40.7) | 28(30.8) | 89(48.9) | 93(51.1) |
| Controls | 192 | 35(18.23) | 83(43.23) | 74(38.54) | 153(39.8) | 231(60.2) | |
| χ2 | 4.195 | 4.139 | |||||
| p | 0.123 | 0.042* | |||||
| ≥60 | Cases | 157 | 43(27.4) | 73(46.5) | 41(26.1) | 159(50.6) | 155(49.4) |
| Controls | 105 | 17(16.2) | 45(42.9) | 43(40.9) | 79(37.6) | 131(62.4) | |
| χ2 | 7.951 | 8.602 | |||||
| p | 0.019* | 0.003** | |||||
*P < 0.05.
**P < 0.01.
For those over age 60, there were significant differences between the two groups about genotypes or alleles. The frequencies of the three genotypes were 27.4% (TT), 46.5% (TC) and 26.1% (CC), respectively, among the cases and 16.2% (TT), 42.9% (TC) and 40.9% (CC), respectively, among the controls. The frequencies of C alleles in ESCC patients and control samples were 75.2% and 51.1%, respectively.
We conducted further analyses to explore whether FasL -844T/C polymorphism was associated with clinical parameters in a selected population of patients (Table 6).
Table 6. Distribution of selected characteristics of patients subgroups cohort.
| <60 | ≥60 | |||
|---|---|---|---|---|
| χ2 | P | χ2 | P | |
| sex | 11.052 | 0.004** | 7.385 | 0.025* |
| Grading | 13.305 | 0.010* | 3.554 | 0.470 |
| T Grade | 17.976 | 0.001** | 8.494 | 0.075 |
| TNM stage | 22.716 | 0.001** | 15.906 | 0.014* |
| Location of the tumor | 2.962 | 0.564 | 3.063 | 0.547 |
| Length | 1.826 | 0.401 | 8.383 | 0.015* |
| Lymph node metastasis | 0.878 | 0.645 | 5.067 | 0.079 |
| Nerve involvement | 0.499 | 0.779 | 0.298 | 0.862 |
| Cardiac involvement | 15.721 | 0.000** | 10.085 | 0.006** |
| Vascular tumor thrombus | 3.817 | 0.148 | 0.034 | 0.983 |
*P < 0.05.
**P < 0.01.
In univariate analysis for age <60 years (Table 7), FasL -844 TC and CC genotype showed significant associations with T grade (OR = 3.333, 1.134–9.801, P = 0.029; OR = 5.833, 1.710–19.902, P = 0.005, respectively). With TT as reference, CC was significant with an OR of 0.308 (0.192–0.493), P = 0.000 but TC was not significant in Cardiac involvement.
Table 7. Genotype frequencies of FasL -844 T/C polymorphism in relation to pathological indices of ESCC severity in patients according to the age (<60 vs ≥60).
| <60 | ≥60 | ||||||
|---|---|---|---|---|---|---|---|
| TT | TC | CC | TT | TC | CC | ||
| sex | OR | 1 | 0.333 | 0.357 | 1 | 0.591 | 0.423 |
| CI | 0.100–1.112 | 0.238–0.536 | 0.506–0.690 | 0.326–0.548 | |||
| P | 0.074 | 0.000** | 0.026* | 0.001** | |||
| Grading | OR | 1 | 1.000 | 0.857 | 1 | 1.193 | 1.091 |
| CI | 0.303–3.296 | 0.235–3.129 | 0.481–2.962 | 0.386–3.079 | |||
| P | 1.000 | 0.816 | 0.704 | 0.869 | |||
| T grade | OR | 1 | 3.333 | 5.833 | 1 | 0.785 | 1.575 |
| CI | 1.134–9.801 | 1.710–19.902 | 0.374–1.650 | 0.666–3.726 | |||
| P | 0.029* | 0.005** | 0.523 | 0.301 | |||
| TNM stage | OR | 1 | 2.143 | 2.400 | 1 | 1.019 | 2.062 |
| CI | 0.687–6.680 | 0.726–7.935 | 0.441–2.351 | 0.839–5.073 | |||
| P | 0.189 | 0.151 | 0.966 | 0.115 | |||
| Length | OR | 1 | 1.500 | 2.400 | 1 | 2.298 | 4.000 |
| CI | 0.473–4.761 | 0.726–7.935 | 0.962–5.487 | 1.550–10.326 | |||
| P | 0.491 | 0.151 | 0.061 | 0.004** | |||
| Cardiac involvement | OR | 1 | 0.500 | 0.308 | 1 | 0.900 | 3.611 |
| CI | 0.173–1.441 | 0.192–0.493 | 0.298–2.722 | 1.243–10.489 | |||
| P | 0.199 | 0.000** | 0.852 | 0.018* | |||
*P < 0.05.
**P < 0.05.
For those over age 60, FasL -844 TC and CC genotype showed significant associations with sex (OR = 0.591, 0.506–0.690, P = 0.026; OR = 0.423, 0.326–0.548, P = 0.001, respectively). With TT as reference, CC was significant with an OR of 0.308 (0.192–0.493), P = 0.000 but TC was not significant in Cardiac involvement. The same situation was appeared in tumor length (OR = 4.000, 1.550–10.326, P = 0.004).
Association of -844T/C polymorphism and FasL expression
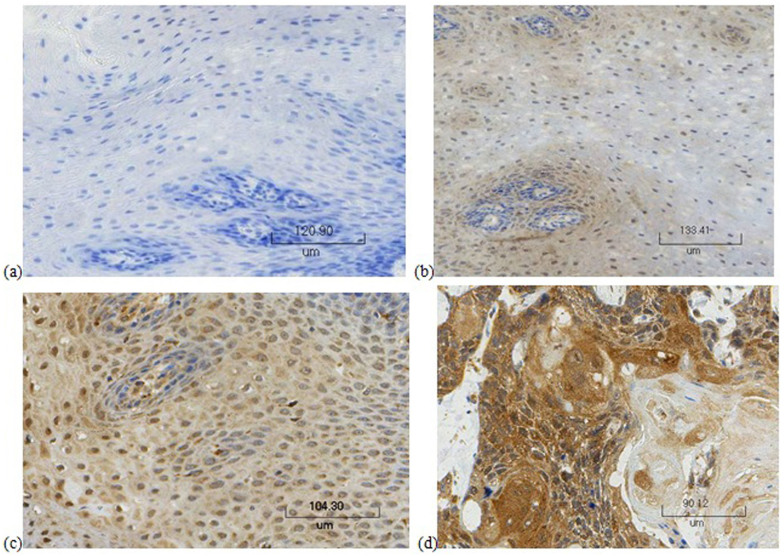
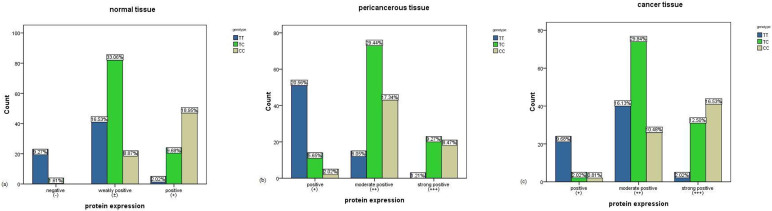
Immunohistochemistry was carried out to research whether FasL -844 variation is able to modify the levels of expression of FasL (Fig. 1). This SNP was correlated with FasL expression in ESCC patients' normal esophagus region, cancer tissue and pericancerous tissue region (Fig. 2). The protein expression in normal esophageal region was weak with only a higher rate of positive expression in CC genotype. No matter what the genotype is, there were FasL expression in cancer tissue and peficancerous tissue and compared with TT and TC genotypes, CC genotype carriers had higher FasL protein expression.
Figure 1. Immunohistochemical stainings of FasL.
(a) normal tissue with negative staining; (b) normal tissue with weakly positive staining; (c) pericancerous tissue; (d) carcinoma tissue.
Figure 2. FasL expression with FasL -844 SNP in normal tissue, pericancerous tissue and carcinoma tissue of patients group (n = 248).
(a) normal tissue, χ2 = 1.047 × 102 P = 0.000; (b) pericancerous tissue, χ2 = 75.451 P = 0.000; (c) carcinoma tissue, χ2 = 1.016 × 102 P = 0.000.
Association of -844T/C polymorphism and Fas -1377G/A polymorphism
The genotype and allele distributions of FasL polymorphism in the cases and controls are shown in Table 8. When stratified by age and gender, the Fas gene -1377 AA genotype was associated with a decreased risk for the development of ESCC (Adjusted OR = 0.511, 95% CI = 0.283–0.921, P = 0.026) compared with GG genotype. The A haplotype remained a significant protective role for ESCC (Adjusted OR = 0.834, 95% CI = 0.727–0.956, P = 0.000), which was comparable to that of G. However, FasL -844 CC combining with Fas -1377 G allele is a protective factor for ESCC (Adjusted OR = 0.488, 95% CI = 0.320–0.743, P = 0.001) (Table 9).
Table 8. Genotypic and allelic frequencies of Fas gene -1377 among case patients and control subjects and the association of this SNP with ESCC risk.
| Cases (%) n = 248 | Controls (%) n = 297 | χ2 | P | Crude OR (95%CI) | P | Adjusted OR# (95% CI) | P | |
|---|---|---|---|---|---|---|---|---|
| Genotypes | ||||||||
| GG | 126(50.8) | 120(40.4) | 1 | 1 | ||||
| GA | 97(39.1) | 135(45.5) | 6.330 | 0.042* | 0.684(0.477–0.982) | 0.040* | 0.694(0.470–1.026) | 0.067 |
| AA | 25(10.1) | 42(14.1) | 0.567(0.326–0.987) | 0.045* | 0.511(0.283–0.921) | 0.026* | ||
| Alleles | ||||||||
| G allele | 349(70.4) | 375(63.1) | 6.338 | 0.012* | 1 | 1 | ||
| A allele | 147(29.6) | 219(36.9) | 0.849(0.748–0.965) | 0.012* | 0.834(0.727–0.956) | 0.000** |
*P < 0.05.
**P < 0.01
#. Adjusting for age and gender.
Table 9. Risk of ESCC associated with FasL -844genotypes by Fas -1377genotypes.
| Genotypes | |||||
|---|---|---|---|---|---|
| FasL -844 | Fas -1377 | Cases (%) n = 248 | Controls (%) N = 297 | Adjusted OR# (95% CI) | P |
| TT + TC | GG + GA | 163 (65.7) | 154 (51.9) | 1 | |
| TT + TC | AA | 16 (6.5) | 26 (8.8) | 0.513(0.251–1.047) | 0.067 |
| CC | GG + GA | 60 (24.2) | 101 (34.0) | 0.488(0.320–0.743) | 0.001** |
| CC | AA | 9 (3.6) | 16 (5.4) | 0.422(0.174–1.021) | 0.056 |
*P < 0.05.
**P < 0.01.
#. Adjusting for age and gender.
Discussion
The FasL gene maps on chromosome 1q23 and its product is a 40-kDa type II transmembrane protein. FasL which exists in cytotoxic T lymphocyte (CTL) and NK cells belongs to TNF family and works as an apoptotic agent. It can bounds Fas to activate target cell apoptosis. The FasL -844T/C polymorphism locates in the gene promoter. Higher basal expression of FasL is significantly associated with the FasL -844 C allele compared with the FasL-844 T allele. The C allele and its flanking sequence constitute CAAT box that is the binding site for CAAT Enhancer Binding Protein Beta (C/EBPβ), resulting in a significantly higher basal FasL expression in peripheral blood fibrocytes with luciferase reporter assay8. The FasL -844 T/C polymorphism may influence FasL expression level and FasL-mediated signaling pathway, and ultimately, the susceptibility to cancer. Many types of tumors express FasL, and aberrant gene expression has been reported to do with cervical carcinogenesis and tumor progression9,10,11. Heightened FasL expression would improve tumor cells to counter attack the immune system by killing Fas-sensitive lymphocytes, thereby contributing to cancer development. High FasL expression in tumor tissues has been linked to poor prognosis in various human carcinomas12,13,14,15,16,17. An animal model study indicated that after neutralized by human FasL antibody, tumor load and colony formation of tumor cells significantly reduced18.
There were two mechanisms for Fas-FasL system participating in immune escape: (I) tumor cells express FasL to counterattack tumor-infiltrating T cells, which results in immune escape19,20,21,22; (II) In tumor microenvironment, Fas-FasL mediated activation-induced cell death (AICD) in tumour-infiltration lymphocyte (TIL) apoptosis. AICD is an important mechanism responsible for the increased apoptosis rate among the tumor-infiltrating lymphocytes, leading to the transformed cells escaping the elimination by anticancer immunosurveillance and contributes to cancer development. The FasL –844C allele, which had higher expression of FasL on T cells, was associated with an enhanced rate of AICD of T cells, which may result in less powerful immune surveillance and increase the susceptibility to cancer23.
It had been reported that FasL-844T/C gene polymorphism is a risk factor for cervical cancer23, lung cancer24, bladder cancer25, breast cancer26, pancreatic cancer27. One meta-analysis suggested that the FasL -844 T/C polymorphism was associated with cancer risk28. The C allele is a low-penetrant cancer susceptibility marker for cancer development while the T allele has a possible protective effect on cancer risk. This association between the SNP and the cancer risk was obvious among Asians. However, Ter-Minassian et al. reported that subjects under the age 60 years with the FasL -844 TC or TT genotype had higher risk for non-small cell lung cancer compared to the CC genotype29. Lei et al found that compared with -844 CC carriers, CT or TT carriers had a sigificant increased risk of the prevalence of second primary cancer after head and neck squamous cell carcinoma30. Sun et al. showed that individuals with both Fas -1377AA and FasL -844 CC genotypes had a higher risk for developing esophageal squamous-cell carcinoma compared with those with both Fas -1377GG and FasL -844 TT genotypes6. We investigated the association between the FasL−844T/C polymorphism on the risk of ESCC in a south China population. In this study, the polymorphism exhibited apparent relationship with the risk of ESCC. Compared with T allele, C allele is protective factors for ESCC susceptibility.
The large case-control study of the association FasL -844 T/C polymorphism with non-small cell lung cancer (NSCLC) found that the association of the FasL -844 SNP in North American Caucasians that appears to be significant in individuals, 60 years old and shows statistically significant effect modification with different measures of age29. Therefore, we stratified the subjects by age (less than 60 years old and greater than or equal to 60 years old. Compared with the control group, there was significant difference in allele frequencies, but no difference in genotypes of patients less than 60 years old. While, there were significant differences in allele and genotypes of patients greater than 60 years old. That means the presence of the CC genotype may be a protective factor for ESCC in these subgroup patients. The C allele has been shown to be associated with higher FasL expression compared with the T allele and it was reported that FasL also expressed in esophageal mucosal. We speculate that patients greater than 60 years old with the CC genotype may be have higher expression level of FasL in esophageal mucosa and immune cells. This may play a killing effect to atypical cells of the esophagus to prevent the formation of esophageal cancer. It is relevant to suggest that esophageal cancer screening should be carried out regularly among TT carriers over the age of 60.
Secondly, there were relationship between -844T/C SNP and ESCC clinicopathological parameters. We found that FasL gene polymorphism of esophageal cancer patients is related to T stage, TNM stage, and tumor length. CC genotype is a risk factor. The tumor with CC genotype is longer and has deeper invasion ability, and reached an advanced stage more easily than other genotypes. That is to say FasL -844CC genotype is shown to be a malignant factor for ESCC. Immune cells of healthy people with CC genotype express more FasL protein, which will kill the mutant cells to prevent the occurrence of cancer. After tumorigenesis, cancer cells increase FasL expression to kill more immune cells and promote tumor development. People with CC genotype are not likely to suffer from ESCC. However, once fall ill, tumor will develop faster and get higher degree of malignancy than other genotypes.
To explore the relationship of FasL -844T/C SNP and ESCC clinicopathological parameters in different ages, we stratified patients by age. CC phenotype is a protective factor in female patients regardless of greater than or equal to 60 years old or less than 60 years old, because male patients had a greater proportion of smokers. Over half of Chinese males over 15 years old are smokers, compared to only 2.4% of women. It had been reported that smoking and alcohol drinking were associated with esophageal cancer risk among Chinese men, but not among Chinese women31. Therefore, CC phenotype as a protective factor for women may have a certain relationship with smoking factors. Smoking may modify the association of the FasL -844 SNP with ESCC. Besides sex, FasL -844 CC showed significant modification of genotype and risk by cardiac involvement in less than 60 years old patients. We speculate that CC carriers less than 60 years old may have strong mucosal immune function of gastrointestinal tract that is easy to prevent tumor to invade cardia. While, CC genotype is a risk factor in T grade (T1 + T2 vs T3 + T4). Among older patients, CC is a risk factor in tumor length and Cardiac involvement. For patients with CC genotype less than 60 years old, tumor cells express more FasL to kill immune cells. Young adults have stronger immune response and immunosuppression is relatively stronger than elder patients leading to more aggressive and much later stage. For patients older than 60 years, the immune function especially the gastrointestinal mucosal immune function, is weakened resulting in more length of cancer. In brief, their tumors will progresse quickly and malignantly in case elder patients with CC genotype get sick.
The expression of FasL in patients was analyzed to conform that the variation of -844T/C in FasL gene is able to significantly modify the levels of expression of FasL. The polymorphism of FasL -844 affects the expression of FasL protein in patients' normal esophageal epithelium, esophageal carcinoma and pericancerous tissue cells. FasL -844CC genotype were accompanied by high expression of FasL. Cells with high level of FasL would kill atypical precancerous cells more rapidly. That is why FasL-844 C allele is a protection factor for ESCC.
According to the previous study32, other polymorphisms of death pathway genes (in particular Fas) might interfere with the functional effect by -844T/C polymorphisms FasL. So, the polymorphism of Fas gene -1377 was analyzed to study whether susceptibility to esophageal cancer is influenced by FasL -844 SNP under the intervention of Fas -1377 SNP. We found that Fas -1377 AA genotype is a protection factor for ESCC. It was reported that Fas –1377A allele disrupt Sp1 transcription factor binding sites, and thus diminish promoter activity and decrease Fas gene expression33.
FasL -844 polymorphism effects together with the contribution from the Fas -1377 polymorphism esophageal cancer susceptibility were considered. FasL -844 CC combining with Fas -1377 GG + GA is a protective factor for ESCC. Cells with Fas -1377 G allele express Fas protein normally. If these cells had FasL -844 CC genotype which results in high expression FasL protein, compared with FasL -844 T carriers, it will kill atypical precancerous cells more rapidly to avoid tumorigenesis. On the contrary, cells, including cancer cells with Fas -1377 G allele express low level Fas that will cause immune escape. In this case, even FasL -844 CC genotype induces high expression of FasL, nor will it plays a protective role in susceptibility to ESCC. Among the same FasL -844 SNP carriers, Fas -1377 polymorphism's effect is small to susceptibility to ESCC.
In this study, we investigated the association between the FasL -844T/C polymorphism on the risk of ESCC in south China. We found that C allele was associated with a significantly decreased risk of ESCC. FasL -844 CC combining with Fas -1377 G allele is a protective factor for ESCC. However, younger patients would suffer more malignant pathologic T grade if they were FasL -844 CC carriers. This polymorphism affects the expression of FasL protein in patients. Further research will be carried out to study the mechanism of above conclusions.
Methods
Subjects
Patients with histopathologically confirmed esophageal squamous cell carcinoma (n = 248) were recruited from Zhejiang Cancer Hospital, affiliated hospital of Zhejiang Medical College, China between January 2008 and October 2011. Control subjects (n = 298) were cancer-free individuals who were randomly selected from medical examination center. All subjects were unrelated ethnic Han Chinese and residents in south China. There was no sex and age restriction. Before recruitment, a standard questionnaire was administered through face-to-face interviews by trained interviewers to obtain information on demographic data and related factors. At recruitment, written informed consent was obtained from each subject to consent to participate in the study and to allow their biological samples to be genetically analyzed. This research was approved by Medical Ethics Committee, Zhejiang Cancer Hospital. The experimental protocol was carried out strictly according to the guidelines.
All patients underwent esophagectomy and had detailed metastatic data. The tumor staging was determined according to the 1997 American Joint Committee on Cancer (AJCC). TNM stage system and the tumor grade were according to WHO guidelines. The presence or absence of lymph node metastasis was evaluated according to the tumor node metastasis classification on the basis of postoperative histopathologic examination of esophageal tumor specimens. Other clinical data (age at diagnosis, sex, tumor length and location), tumor biological data (Nerve involvement, Cardiac involvement, Vascular tumor thrombus) were also documented.
Genotyping
After signing informed consent forms, each subject donated 3 ml of blood to be used for genomic DNA extraction.
Genomic DNA was extracted from peripheral blood using Blood Genome DNA Extraction Kit (Takara Biotechnology Co. Ltd., Dalian). The -844T/C polymorphism of FasL was detected using PCR-RFLP. DNA fragments were amplified in a total volume of 100 ul PCRs containing PCR Premix 50 uL (Bioteke corporation, Beijing, China), forward primer (5′- CAGCTACTCGG AGGCCAAG - 3′) 1 uL, reverse primer (5′- GCTCTGAGGGGAGAGACCAT –3′) 1 ul, DDW 46 uL and DNA 2 uL. Amplification was carried out under the following condition: 1 cycle of 2 min at 95°C, 35 cycles of 30 sec at 94°C, 30 sec at 62°C, and 45 sec at 72°C, followed by 7 min at 72°C. Amplified products were digested with BsrDI (Fermentas, Thermo Fisher Scientific Inc.) at 55°C for 4 h. All products were loaded onto 3% MS-6 Agarose (Takara Biotechnology Co. Ltd., Dalian), and electrophoresed. Bands were visualized and typed after GelRed (Biotium Company, U.S) staining.
The PCR product amplified for the polymorphism was 410 bp. The BsrDI restriction enzymes were used to distinguish -844T/C, resulting in 122 bp and 104 bp fragments in the presence of the -844T allele. The polymorphism analysis was performed by two persons independently in a blind fashion. More than 10% of the samples were randomly selected for confirmation, and the results were 100% concordant.
Fas -1377G/A polymorphism in promoter region was also detected with PCR-RFLP. The primers for amplification were 5′- TGTGTGCACAAGGCTGGCGC–3′ for forward primer and 5′- TGCATCTGTCACTGCACTTACCACCA–3′ for reverse primer, which produce 122 bp fragment. To introduce a restriction endonuclease site, 3′end of forward primer was changed from CAC to CGC, which created a BstUI site. The procedure of amplification was the same as above. After PCR, BstUI (New England Biolabs, Beverly, MA) digestion generated the following fragments: Fas –1377G allele, fragments of 104 bp and 18 bp.
Immunohistochemistry
Paraffin-embedded sections of esophageal carcinoma tissue, pericancerous tissue and normal tissue of 248 ESCC patients which stored in pathology department of our hospital were collected to do immunohistochemical stains. Antibody for human FasL (Santa Cruz Biochemistry Inc., Santa Cruz, CA) was used to detect FasL protein on above tissue sections. Immunohistochemical procedures were performed as described previously34. The intensity of staining was graded as negative (−), weakly positive (±), positive (+), moderately positive (++), or strongly positive (+++).
Statistical analyses
Values were expressed as mean ± SD or percent. To check for genotyping error, we examined departure from Hardy–Weinberg equilibrium (HWE) in controls, using χ2 test. χ2 test analysis was used to detect this SNP in patients with esophageal cancer and health individuals. T test and χ2 test were used to detect age and sex in the two groups, respectively.
Association between FasL -844 T/C or Fas -1377 G/A polymorphism and ESCC was assessed using χ2 test. ESCC risk was estimated by odds ratios (OR) and 95% confidence intervals (CI) using a conditional logistic regression model controlling for age, and gender. This codominant model is defined as heterozygotes (1 variant genotype) versus wild-type (0 variant genotype) or homozygotes (2 variant genotype) versus wild-type.
The relationships of the SNP with the clinicopathologic parameters of patients were analyzed by χ2 test. We used binary logistic regression (Ascendant Wald method) with each parameter as dependant variable and genotypes of FasL -844 T/C polymorphism with other clinical parameters as independent variables. Clinical pathological parameters were dichotomised as follows: grading (well and moderately differentiation versus poorly differentiation), T grade (T1–T2 versus T3–T4), Location of the tumor (Epimere- midportion versus hypomere), tumor length (less than 5 cm versus equal and greater than 5 cm), lymphnode metastasis, nerve involvement, cardiac involvement and vascular tumor thrombus (positive versus negative).
For stratified analyses, we created indicator variable of age greater or less than 60 years old. Then the above statistical analyses were carried out at different age groups.
All statistical testing was done at the two-sided 0.05 level with SPSS 16.0 software.
Acknowledgments
This work was financially supported by Natural Science Foundation of Zhejiang (LQ12H16001) and General Research Project of Zhejiang Medical College (2013XZB02). We thank Dr Jie Zhou in Zhejiang University and Dr Peng Du in Zhejiang Medical College for technical assistance.
The authors declare no competing financial interests.
Author Contributions H.G. Zhao collected samples and all pathological parameters and wrote the main manuscript text. X.R. Li and L.F. Wang prepared table 1–9. L.F. Zheng prepared figure 1–2.
06/27/2014
A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
- Fan X. Q. & Guo Y. J. Apoptosis in oncology. Cell Res. 11, 1–7 (2001). [DOI] [PubMed] [Google Scholar]
- Evan G. I. & Vousden K. H. Proliferation, cell cycle and apoptosis in cancer. Nature. 411, 342–348 (2001). [DOI] [PubMed] [Google Scholar]
- Nagata S. & Golstein P. The Fas death factor. Science (Wash DC). 267, 1449–1456 (1996). [DOI] [PubMed] [Google Scholar]
- Griffith T. S. & Ferguson T. A. The role of FasL-induced apoptosis in immune privilege. Immunol Today. 18, 240–244 (1997). [DOI] [PubMed] [Google Scholar]
- Houston A. & O'Connell J. The Fas signalling pathway and its role in the pathogenesis of cancer. Curr Opin Pharmacol. 4, 321–326 (2004). [DOI] [PubMed] [Google Scholar]
- Sun T. et al. Polymorphisms of death pathway genes FAS and FASL in esophageal squamous-cell carcinoma. J Natl Cancer Inst. 96, 1030–1036 (2004). [DOI] [PubMed] [Google Scholar]
- Zhang X. et al. Functional polymorphisms in cell death pathway genes FAS and FASL contribute to risk of lung cancer. J Med Genet. 42, 479–484 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. et al. A novel polymorphic CAAT/enhancer-binding protein beta element in the FasL gene promoter alters Fas ligand expression: a candidate background gene in African American systemic lupus erythematosus patients. J Immunol. 170, 132–138 (2003). [DOI] [PubMed] [Google Scholar]
- Contreras D. N. et al. Cervical cancer cells induce apoptosis of cytotoxic T lymphocytes. J Immunother. 23, 67–74 (2000). [DOI] [PubMed] [Google Scholar]
- Kase H., Aoki Y. & Tanaka K. Fas ligand expression in cervical adenocarcinoma relevance to lymph node metastasis and tumor progression. Gynecol Oncol. 90, 70–74 (2003). [DOI] [PubMed] [Google Scholar]
- Reesink-Peters N. et al. Death receptors and ligands in cervical carcinogenesis: an immunohistochemical study. Gynecol Oncol. 96, 705–713 (2005). [DOI] [PubMed] [Google Scholar]
- Korkolopoulou P. et al. c-FLIP expression in colorectal carcinomas: association with Fas/FasL expression and prognostic implications. Histopathology. 51, 150–156 (2007). [DOI] [PubMed] [Google Scholar]
- Lee W. C., Yu M. C. & Chen M. F. Prognostic impact of Fas ligand on hepatocellular carcinoma after hepatectomy. World J Surg. 28, 792–796 (2004). [DOI] [PubMed] [Google Scholar]
- Viard-Leveugle I., Veyrenc S., French L. E., Brambilla C. & Brambilla E. Frequent loss of Fas expression and function in human lung tumours with overexpression of FasL in small cell lung carcinoma. J Pathol. 201, 268–277 (2003). [DOI] [PubMed] [Google Scholar]
- Nagashima H. et al. Expression of Fas ligand in gastric carcinoma relates to lymph node metastasis. Int J Onco. 18, 1157–1162 (2001). [DOI] [PubMed] [Google Scholar]
- Lerma E. et al. Prognostic significance of the Fas-receptor/Fas-ligand system in cervical squamous cell carcinoma. Virchows Arch. 452, 65–74 (2008). [DOI] [PubMed] [Google Scholar]
- Okada K. et al. Frequency of apoptosis of tumor-infiltrating lymphocytes induced by Fas counterattack in human colorectal carcinoma and its correlation with prognosis. Clin Cancer Res. 6, 3560–3564 (2000). [PubMed] [Google Scholar]
- Chen L. et al. CD95 promotes tumour growth. Nature. 465, 492–496 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. W. et al. Expression of Fas ligand by human gastric adenocarcinomas: a potential mechanism of immune escape in stomach cancer. Gut. 44, 156–162 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber V. et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 128, 1796–1804 (2005). [DOI] [PubMed] [Google Scholar]
- Minas V. et al. Intratumoral CRH modulates immuno-escape of ovarian cancer cells through FasL regulation. Br J Cancer. 97, 637–645 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell J., O'Sullivan G. C., Collins J. K. & Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 184, 1075–1082 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. et al. FASL –844C polymorphism is associated with increased activation-induced T cell death and risk of cervical cancer. J Exp Med. 202, 967–974 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. et al. Functional polymorphisms in cell death pathway genes Fas and FasL contribute to risk of lung cancer. J Med Genet. 42, 479–484 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. et al. Functional polymorphisms in the promoter regions of the FAS and FAS ligand genes and risk of bladder cancer in south China. Pharmacogenet Genomics. 16, 245–251 (2006). [DOI] [PubMed] [Google Scholar]
- Wang W. et al. Polymorphisms of the FAS and FASL genes and risk of breast cancer. Oncol Lett. 3, 625–628 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. et al. Functional variants in cell death pathway genes and risk of pancreatic cancer. Clin Cancer Res. 14, 3230–3236 (2008). [DOI] [PubMed] [Google Scholar]
- Zhang Z. et al. The FAS ligand promoter polymorphism, rs763110 (-844C > T), contributes to cancer susceptibility: evidence from 19 case-control studies. Eur J Hum Genet. 17, 1294–1303 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter-Minassian M. et al. Apoptosis gene polymorphisms, age, smoking and the risk of non-small cell lung cancer. Carcinogenesis. 29, 2147–2152 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei D. et al. FAS and FASLG genetic variants and risk for second primary malignancy in patients with squamous cell carcinoma of the head and neck. Cancer Epidemiol Biomarkers Prev. 19, 1484–1491 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. et al. Smoking and alcohol drinking increased the risk of esophageal cancer among Chinese men but not women in a high-risk population. Cancer Causes Control. 22, 649–657 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. et al. Polymorphisms of death pathway genes FAS and FASL in esophageal squamous-cell carcinoma. J Natl Cancer Inst. 96, 1030–1036 (2004). [DOI] [PubMed] [Google Scholar]
- Sibley K. et al. Functional FAS promoter polymorphisms are associated with increased risk of acute myeloid leukemia. Cancer Res. 63, 4327–4330 (2003). [PubMed] [Google Scholar]
- Bennett M. W. et al. Fas ligand and Fas receptor are coexpressed in normal human esophageal epithelium: a potential mechanism of apoptotic epithelial turnover. Dis Esophagus. 12, 90–98 (1999). [DOI] [PubMed] [Google Scholar]