Abstract
Polymorphisms in NKX2-3 gene have been inconsistently associated with Crohn's disease (CD) and ulcerative colitis (UC). To generate large-scale evidence on whether NKX2-3 polymorphisms are associated with CD or UC susceptibility we have conducted a meta-analysis of 17 studies involving 17329 patients and 18029 controls. A significantly increased CD or UC risk was observed in persons carrying a G allele at rs10883365 polymorphism (A/G) compared with those with a A allele. (OR = 1.226, 95%CI: 1.177–1.277 and OR = 1.274, 95%CI: 1.175–1.382 respectively). In the subgroup analysis, a significantly increased CD risk was found in both Europeans and Asians. For rs11190140 polymorphism (C/T) and CD risk, the risk estimate for the allele contrast was OR = 1.201 (1.136–1.269). This meta-analysis provided a robust result that persons with a G or T allele may have a moderately increased risk of CD, and suggested that rs10883365 polymorphism was also a candidate gene polymorphism for UC susceptibility.
Inflammatory bowel diseases (IBDs) are chronic inflammatory disorders characterized by chronic relapsing inflammation of the gastrointestinal tract that affect 0.1% of Western populations, comprising two major forms, Crohn's disease (CD) and ulcerative colitis (UC)1. In Crohn's disease the inflammation is often transmural, whereas in ulcerative colitis the inflammation is typically confined to the mucosa. Additionally, Crohn's disease can be associated with intestinal granulomas, strictures, and fistulas, but these are not typical findings in ulcerative colitis. Although our understanding of disease pathogenesis remains incomplete, accumulating evidence suggests that that IBD is a complex, multifactorial disease partly determined by a genetic predisposition2. Strong familial aggregation, twin studies, and established genetic associations3,4,5. indicate that there is a genetic component to the disease susceptibility in IBD. Recently, sequence variations associated with IBD have been reported for several genes, including NOD2, IL23R, IRGM, ATG16L1, PTPN2, and NK2 transcription factor related and locus 3 (NKX2-3)6,7,8,9,10,11.
NKX2-3, located on 10q24, is a member of a family of genes that encodes transcription factors containing homeodomains and, therefore, is implicated in basic developmental functions. During development, NKX2-3 is expressed in midgut and hindgut mesoderm and spleen, as well as in pharyngeal endoderm12,13. The association between the NKX2-3 polymorphism and susceptibility of IBD was first reported in Caucasian patients14. After the first report of the association, several studies confirmed the association of tag-SNPs (rs10883365 and rs1190140) in the NKX2-3 gene with CD15,16 as well as with UC in Caucasian or Asian populations17,18,19. However, several studies could not replicate the genetic association between IBD and NKX2-3 polymorphsims15,20,21.
Thus, a quantitative synthesis may help to provide clearer evidence on the association of such genetic polymorphisms with IBD. In the present study, we conducted a meta-analysis of all eligible studies to quantitatively assess the associations between three common polymorphisms (rs10883365 and rs11190140) in the NKX2-3 gene and IBD susceptibility.
Results
Characteristics of the included studies
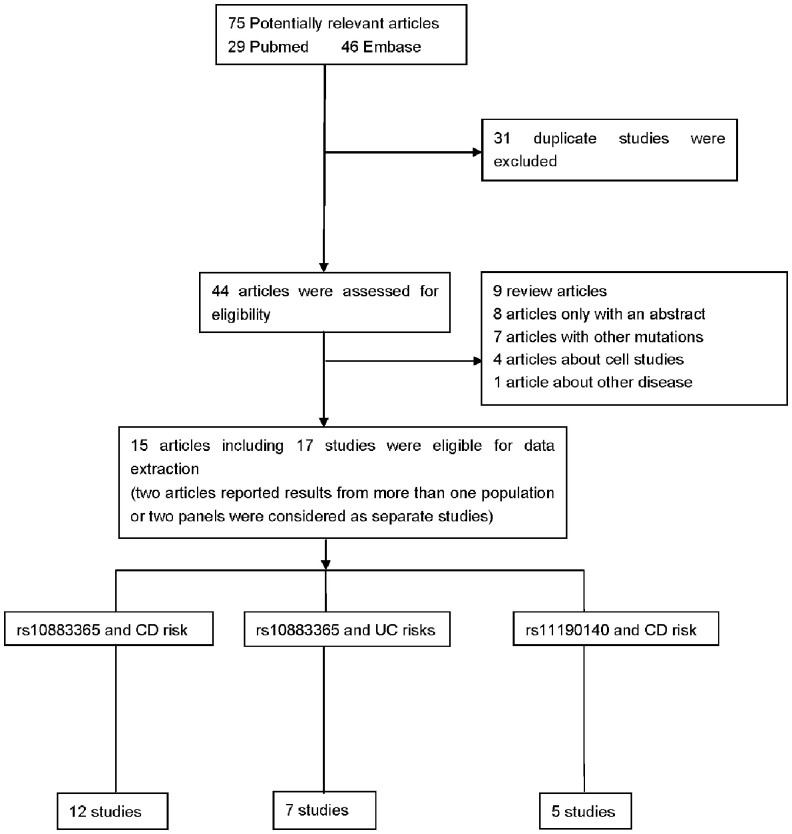
The combined search yielded 75 references, of which 31 were duplicate studies, 9 were reviews, 4 were about cell studies, 8 were only with abstracts, 7 reported other mutations, 1 reported other disease. Finally, a total of 15 articles were finally included. Among them, one publication15 contained data on two different subpopulations, one16 included Wellcome Trust Case Control Consortium (WTCCC) samples and replication Crohn's disease (RCD) samples, and we treated them independently. In total, 17 studies comprising 17329 cases and 18029 controls were included in the present meta-analysis11,15,16,17,18,19,20,21,22,23,24,25,26,27,28. The 17 separate studies consisted of 13 European and 4 Asian. The distribution of genotypes in the control groups of all studies was in agreement with HWE except for 1 study19. Summaries of all included studies were summarized in Table 1, and the flow chart of study selection process was shown in Figure 1.
Table 1. Main Characteristics of Studies Involved in NKX2-3 polymorphism and Crohn's disease or ulcerative colitis Risk.
| Cases | Controls | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year of publishcation | Ethnicity | NKX2-3 variant | Phenotype Studied | Number | Males (%) | Age or Age at diagnosis | Number | Males (%) | Age | Matching |
| Tanaka, 2009 | Asians | rs10883365 | CD and UC | CD: 174 | CD: 65.5 | CD: 16–61 | 394 | 48.0 | 19–76 | nr |
| separately | UC: 296 | UC: 48.0 | UC: 15–79 | |||||||
| Meggyesi, 2010 | Europeans | rs10883365 | CD and UC separately | CD: 810 | CD: 53.6 | CD: 37.1 ± 12.6 and 26.5 ± 10.6 at diagnosis | 469 | 53.5 | 40.5 ± 11.5 | Age and sex |
| Meggyesi, 2010 | Europeans | rs10883365 | CD and UC separately | UC: 428 | UC: 47.2 | UC: 43.7 ± 15.0 and 31.3 ± 13.4 at diagnosis | ||||
| Fisher, 2008 | Europeans | rs10883365 | UC | UC: 1841 | nr | nr | 1470 | nr | nr | nr |
| Franke, 2008 | Europeans | rs10883365 rs11190140 | CD and UC separately | CD: 1850UC: 1103 | CD: 32.0 | CD: mean 38 and 21at diagnosis | 1817 | nr | nr | Age and sex |
| Parkes, 2007 | Europeans | rs10883365 | CD | CD: 1182 | CD: 40.3 | CD: mean 43.9 and 25.5 at diagnosis | 2024 | nr | nr | nr |
| Parkes, 2007 | Europeans | rs10883365 | CD | CD: 1748 | CD: 39.2 | CD: mean 45.7 and 26.1 at diagnosis | 5740 | nr | nr | nr |
| Yu, 2009 | Europeans | rs10883365 | CD | CD: 75 | nr | nr | 255 | nr | nr | nr |
| Yamazaki, 2009 | Asians | rs10883365 | CD | CD: 484 | CD: 72.8 | CD: 22.4 (7–55) at diagnosis | 470 | 50.2 | 38.7(21–77) | |
| Pang, 2010 | Asians | rs10883365 | CD | CD: 66 | CD: 48.5 | CD: 36.26 ± 11.82 | 66 | 50.0 | 35.42 ± 13.14 | Age and sex |
| Arai, 2011 | Asians | rs10883365 | CD and UC separately | CD: 344UC: 253 | nrnr | nrnr | 243 | nr | nr | nr |
| Weersma, 2009 | Europeans | rs10883365 | CD and UC separately | CD: 1656UC: 1075 | nr | nr | 1086 | nr | nr | nr |
| van der Heide, 2010 | Europeans | rs10883365 | CD | CD: 310 | 34.5 | CD:26.6 (7.5–73.9) at diagnosis | 976 | nr | nr | nr |
| Latiano, 2011 | Europeans | rs11190140 | CD | CD: 1070 | CD: 56% | nr | 783 | nr | nr | nr |
| Laukens, 2010 | Europeans | rs11190140 | CD | CD: 1051 | nr | nr | 676 | nr | nr | nr |
| Peter, 2011 | Europeans | rs11190140 | CD | CD: 369 | nr | nr | 503 | nr | nr | nr |
| Waterman, 2011 | Europeans | rs11190140 | CD | CD: 1144 | CD: 53% | CD: 16(2–62) at diagnosis | 1057 | 36% | nr | nr |
NKX2-3: NK2 transcription factor related and locus 3; CD: Crohn's disease, UC: ulcerative colitis, nr: not report.
Figure 1. Study selection procedures for a meta-analysis of NKX2-3 polymorphisms and risk of CD or UC.
NKX2-3: NK2 transcription factor related and locus 3; CD: Crohn's disease; UC: ulcerative colitis.
Quantitative synthesis
Crohn's disease
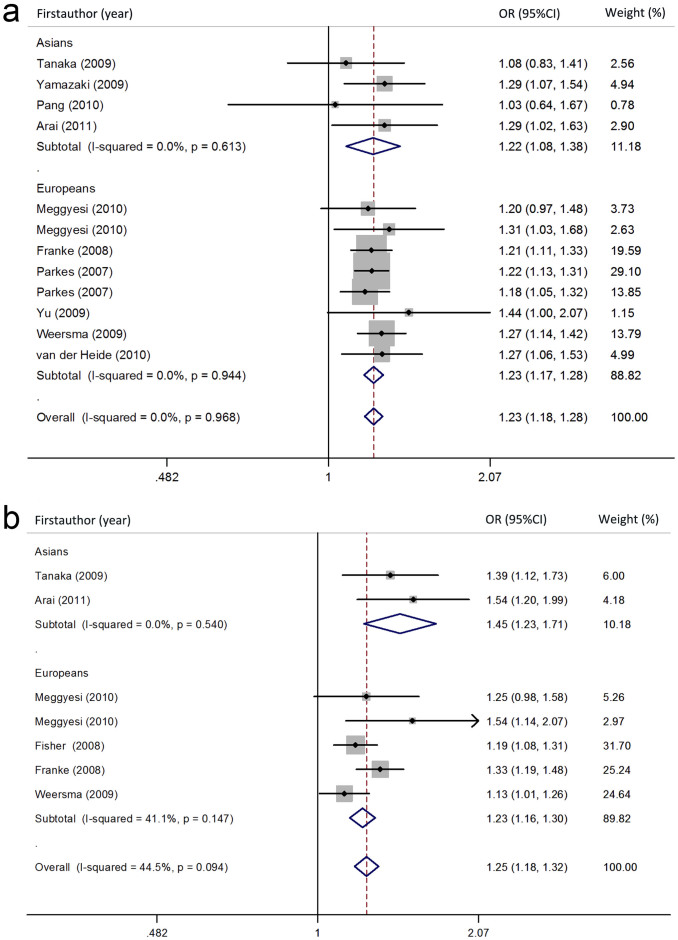
The summary of meta-analysis for the NKX2-3 polymorphisms with CD is shown in Table 2, Figure 2A and Supplementary Figure S1. Regarding rs10883365 polymorphism, the results of combined analyses comprising 8699 cases and 13540 controls revealed a significantly increased risk of CD in all genetic models. In addition, the OR was 1.481 (1.351–1.623) in carriers of two risk G alleles compared with non-risk allele carriers (GG vs AA), which was higher than the risk of one G allele carriers (GA vs AA, OR = 1.141 (1.055–1.234), suggesting a dose–response with increasing number of the variant allele. In the subgroup analysis, significantly increased risks were found both among European and Asian population. No between-study significant heterogeneity was observed in all genetic models.
Table 2. Pooled analysis for the associations between the polymorphism of NKX2-3 and the risk of Crohn's disease.
| Variant | Comparison | Variables | No. of | Sample Size | Test of association | Model | Test of heterogeneity | |||
|---|---|---|---|---|---|---|---|---|---|---|
| studies | Case | Control | OR (95% CI) | P-value | I2(%) | P-value | ||||
| rs10883365 | G vs A | Overall | 12 | 8699 | 13540 | 1.226 (1.177–1.277) | <0.001 | F | 0.0 | 0.968 |
| All in HWE | 9 | 4883 | 9661 | 1.215 (1.154–1.280) | <0.001 | F | 0.0 | 0.913 | ||
| European | 8 | 7631 | 12367 | 1.226 (1.174–1.280) | <0.001 | F | 0.0 | 0.944 | ||
| Asian | 4 | 1068 | 1173 | 1.223 (1.082–1.382) | 0.001 | F | 0.0 | 0.613 | ||
| GG vs AA | overall | 10 | 6733 | 11478 | 1.481 (1.351–1.623) | <0.001 | F | 0.0 | 0.936 | |
| All in HWE | 9 | 4883 | 9661 | 1.476 (1.328–1.639) | <0.001 | F | 0.0 | 0.893 | ||
| European | 6 | 5665 | 10305 | 1.481 (1.342–1.635) | <0.001 | F | 0.0 | 0.905 | ||
| Asian | 4 | 1068 | 1173 | 1.477 (1.148–1.901) | 0.002 | F | 0.0 | 0.566 | ||
| GA vs AA | Overall | 10 | 6733 | 11478 | 1.141 (1.055–1.234) | 0.001 | F | 0.0 | 0.836 | |
| All in HWE | 9 | 4883 | 9661 | 1.159 (1.059–1.268) | 0.001 | F | 0.0 | 0.807 | ||
| European | 6 | 5665 | 10305 | 1.116 (1.024–1.215) | 0.012 | F | 0.0 | 0.796 | ||
| Asian | 4 | 1068 | 1173 | 1.280 (1.055–1.553) | 0.012 | F | 0.0 | 0.807 | ||
| GG + GA vs AA | Overall | 10 | 6733 | 11478 | 1.241 (1.153–1.336) | <0.001 | F | 0.0 | 0.887 | |
| All in HWE | 9 | 4883 | 9661 | 1.254 (1.152–1.365) | <0.001 | F | 0.0 | 0.846 | ||
| European | 6 | 5665 | 10305 | 1.225 (1.130–1.328) | <0.001 | F | 0.0 | 0.814 | ||
| Asian | 4 | 1068 | 1173 | 1.328 (1.106–1.595) | 0.002 | F | 0.0 | 0.693 | ||
| GG vs GA + GA | Overall | 10 | 6733 | 11478 | 1.362 (1.263–1.468) | <0.001 | F | 0.0 | 0.960 | |
| All in HWE | 9 | 4883 | 9661 | 1.345 (1.235–1.465) | <0.001 | F | 0.0 | 0.948 | ||
| European | 6 | 5665 | 10305 | 1.373 (1.268–1.486) | <0.001 | F | 0.0 | 0.927 | ||
| Asian | 4 | 1068 | 1173 | 1.297 (1.024–1.598) | 0.030 | F | 0.0 | 0.710 | ||
| rs11190140 | T vs C | Over(Europeans) | 5 | 5484 | 4836 | 1.201 (1.136–1.269) | <0.001 | F | 0.0 | 0.773 |
| All in HWE | 2 | 2121 | 1426 | 1.190 (1.080–1.311) | <0.001 | F | 0.0 | 0.544 | ||
| TT vs CC | Over(Europeans) | 3 | 3971 | 3276 | 1.485 (1.297–1.700) | <0.001 | F | 0.0 | 0.631 | |
| All in HWE | 2 | 2121 | 1426 | 1.412 (1.162–1.716) | 0.001 | F | 0.0 | 0.516 | ||
| TC vs CC | Over(Europeans) | 3 | 3971 | 3276 | 1.155 (1.029–1.298) | 0.015 | F | 0.0 | 0.478 | |
| All in HWE | 2 | 2121 | 1426 | 1.227 (1.033–1.458) | 0.020 | F | 0.0 | 0.430 | ||
| TT + TC vs CC | Over(Europeans) | 3 | 3971 | 3276 | 1.253 (1.122–1.398) | <0.001 | F | 0.0 | 0.867 | |
| All in HWE | 2 | 2121 | 1426 | 1.289 (1.095–1.516) | 0.002 | F | 0.0 | 0.785 | ||
| TT vs TC + CC | Over(Europeans) | 3 | 3971 | 3276 | 1.344 (1.204–1.501) | <0.001 | F | 50.1 | 0.135 | |
| All in HWE | 2 | 2121 | 1426 | 1.239 (1.061–1.446) | 0.007 | F | 45.4 | 0.176 | ||
NKX2-3: NK2 transcription factor related and locus 3; R: Random-effects model, F: fixed-effects model, HWE: Hardy-Weinberg equilibrium; OR: odds ratio; CI: confidence interval.
Figure 2. OR estimates with the corresponding 95% CI for the association between rs10883365 polymorphism in NKX2-3 gene and CD or UC risk.
(a): rs10883365 polymorphism and CD risk (G vs. A), (b): rs10883365 polymorphism and UC risk (G vs. A). The sizes of the squares reflect the weighting of included studies. OR: odds ratio; CI: confidence interval.
A total of 5484 patients and 4863 controls were investigated for rs11190140 variant, a significant association was found in all genetic models. (see Supplementary Fig. S1) Similar to rs10883365, the OR (OR = 1.485, P < 0.001) in carriers of 2 risk alleles was higher than that (OR = 1.155, P < 0.001) in those of 1 risk allele. No between-study heterogeneity was detected in any genetic models of rs11190140 variant and CD risk.
Ulcerative colitis
Seven studies with 4996 UC patients and 5479 controls for rs10883365 polymorphism were investigated. Meta-analysis findings of associations between rs10883365 in NKX2-3 gene and the risk of UC were shown in Table 3 and Figure 2B. Significantly increased UC risk was observed in all comparisons (G vs A: OR = 1.274 (1.175–1.382), GG vs AA: OR = 1.672 (1.474–1.896), GA vs AA = 1.207 (1.084–1.343), dominant model: OR = 1.342 (1.213–1.485), and recessive model: OR = 1.470 (1.325–1.630)). (Fig. 2B) When stratified by ethnicity, significant association was found both in European and Asian subgroups except for one genetic model in Asian (GA vs AA: OR = 1.260 (0.971–1.634)). No heterogeneity was detected in major genetic models.
Table 3. Pooled analysis for the associations between the polymorphism of NKX2-3 and the risk of ulcerative colitis.
| Variant | Comparison | Variables | No. of | Sample Size | Test of association | Model | Test of heterogeneity | |||
|---|---|---|---|---|---|---|---|---|---|---|
| studies | Case | Control | OR (95% CI) | P-value | I2(%) | P-value | ||||
| rs10883365 | G vs A | Overall | 7 | 4996 | 5479 | 1.274 (1.175–1.382) | <0.001 | R | 44.5 | 0.094 |
| All in HWE | 5 | 2818 | 2576 | 1.268 (1.174–1.369) | <0.001 | F | 36.5 | 0.178 | ||
| Europeans | 5 | 4447 | 4842 | 1.225 (1.156–1.298) | <0.001 | F | 41.1 | 0.147 | ||
| Asians | 2 | 549 | 637 | 1.452 (1.232–1.712) | <0.001 | F | 0.0 | 0.176 | ||
| GG vs AA | overall | 6 | 3921 | 4393 | 1.672 (1.474–1.896) | <0.001 | F | 20.1 | 0.282 | |
| All in HWE | 5 | 2818 | 2576 | 1.619 (1.387–1.889) | <0.001 | F | 30.5 | 0.218 | ||
| Europeans | 4 | 3372 | 3756 | 1.609 (1.404–1.844) | <0.001 | F | 26.0 | 0.255 | ||
| Asians | 2 | 549 | 637 | 2.078 (1.500–2.878) | <0.001 | F | 0.0 | 0.654 | ||
| GA vs AA | Overall | 6 | 3921 | 4393 | 1.207 (1.084–1.343) | 0.001 | F | 0.0 | 0.901 | |
| All in HWE | 5 | 2818 | 2576 | 1.242 (1.090–1.416) | 0.001 | F | 0.0 | 0.905 | ||
| Europeans | 4 | 3372 | 3756 | 1.196 (1.063–1.345) | 0.003 | F | 0.0 | 0.814 | ||
| Asians | 2 | 549 | 637 | 1.260 (0.971–1.634) | 0.082 | F | 0.0 | 0.466 | ||
| GG + GA vs AA | Overall | 6 | 3921 | 4393 | 1.342 (1.213–1.485) | <0.001 | F | 0.0 | 0.801 | |
| All in HWE | 5 | 2818 | 2576 | 1.356 (1.199–1.533) | <0.001 | F | 0.0 | 0.688 | ||
| Europeans | 4 | 3372 | 3756 | 1.317 (1.179–1.472) | <0.001 | F | 0.0 | 0.767 | ||
| Asians | 2 | 549 | 637 | 1.467 (1.151–1.869) | 0.002 | F | 0.0 | 0.454 | ||
| GG vs GA + GA | Overall | 6 | 3921 | 4393 | 1.470 (1.325–1.630) | <0.001 | F | 43.9 | 0.112 | |
| All in HWE | 5 | 2818 | 2576 | 1.391 (1.223–1.581) | <0.001 | F | 41.2 | 0.146 | ||
| Europeans | 4 | 3372 | 3756 | 1.455 (1.202–1.761) | <0.001 | R | 53.9 | 0.089 | ||
| Asians | 2 | 549 | 637 | 1.534 (1.160–2.028) | <0.001 | F | 0.0 | 0.894 | ||
NKX2-3: NK2 transcription factor related and locus 3; R: Random-effects model, F: fixed-effects model, HWE: Hardy-Weinberg equilibrium; OR: odds ratio; CI: confidence interval.
Sensitivity analyses and cumulative meta-analysis
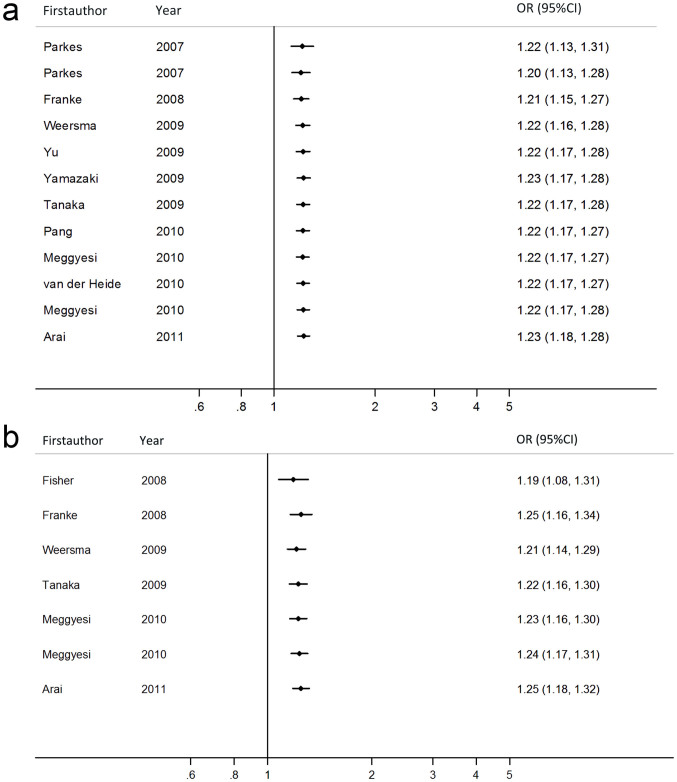
Sensitivity analysis showed no single study qualitatively changed the pooled ORs. (see Supplementary Fig. S2 and S3) Moreover, there was a study which deviated from HWE, when excluded, the estimated pooled OR still did not change at all, indicating that the results of this meta-analysis were high stable. (Table 2 and 3) In the cumulative meta-analysis, the pooled ORs tended to be stable and the associations tended toward significant associations with accumulation of more data over time between rs10883365 or rs11190140 variant and CD risk, as well as between rs10883365 and UC risk (see Fig. 3 and Supplementary Fig. S4).
Figure 3. Cumulative meta-analysis on the association between rs10883365 polymorphism and CD or UC risk.
(a): rs10883365 variant and CD risk (G vs. A); (b): rs10883365 variant and UC risk (G vs. A). Pooled OR estimates with the 95% CI as information accumulates at the end of each year (left column). CD: Crohn's disease; UC: ulcerative colitis; OR: odds ratio; CI: confidence interval.
Publication bias
Funnel plots and Egger's test were performed to assess publication bias. No publication bias was detected for rs10883365 polymorphism. (G vs A: t = −0.04, p = 0.966 and t = 1.56, p = 0.181 in CD and UC, respectively). Similarly, no publication bias was detected for T vs C contrast of rs11190140 polymorphism in CD. (see Supplementary Fig. S6) As shown in Supplementary Figure S5, the shapes of the funnel plots did not indicate any evidence of obvious asymmetry for rs10883365 variant and CD or UC risk.
Discussion
Presently the mechanisms of the etiology and progression of IBD are far from clear. Several genes have been identified to be associated with IBD risk, including NOD2, NKX2-3 and IL-23. Recently, genome-wide association studies (GWAS) have identified SNPs implicating hundreds of replicated loci for common traits and becomes a powerful tool to detect the susceptibility genes in the IBD diseases16,29,30. Several GWAS and GWAS meta-analysis have provided strong evidences for the association between NKX2-3 single nucleotide polymorphisms (SNPs) (rs6584283 and rs4409764) and risk of IBDs31,32,33. However, two common variants (rs10883365 and rs11190140) in NKX2-3 gene were not included in the GWAS meta-analyses.
A meta-analysis can combine results from individual studies to overcome the limitation of small sample sizes and inadequate statistical power, and produce a single estimate of the major effect. Recently, accumulated meta-analysis has been performed to investigate the association of genetic variants with susceptibility to CD or UC. Polymorphisms in several genes, including ATG16L1 T300A34, TGF-α G308A35, MIF G173C36, OCTN1 C1672T37, CD14 C260T38 and MDR1 C3435T39, were identified as risk factors of CD or UC. Patients with mutant allele of NOD1 rs695857140 and 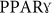 Pro12Ala41 might have a decreased susceptibility to IBD. Additionally, some genetic variants were not association with CD or UC risk, such as MDR1 C1236T39, IL-10 G1082A42, and IL-18 A607C43. Therefore, we saw the need to perform pooled analyses with larger sample size by summarizing previous case–control or cohort studies in order to better understand the association between the NKX2-3 variants and IBD risk.
Pro12Ala41 might have a decreased susceptibility to IBD. Additionally, some genetic variants were not association with CD or UC risk, such as MDR1 C1236T39, IL-10 G1082A42, and IL-18 A607C43. Therefore, we saw the need to perform pooled analyses with larger sample size by summarizing previous case–control or cohort studies in order to better understand the association between the NKX2-3 variants and IBD risk.
NKX2-3, located on chromosome 10q24, is predominantly expressed in mesoderm of midgut and hindgut during embryonic mouse development12. Postnatally, Nkx2-3 expression continues in gut mesenchyme and in spleen. In addition, mice lacking Nkx2-3 exhibit severe defects in gut development; primarily in the epithelium of the small intestine44. The perturbations of the gut tissue architecture lead to early postnatal death presumably due to digestive malfunctions. Moreover, analysis of Nkx2-3-deficient mice has revealed a critical role for NKX2-3 in spleen development and in establishing the correct environment for normal B cell development and T cell dependent immune response45,46. Recently, associations between the two common polymorphisms (rs10883365 and rs11190140) in NKX2-3 gene and susceptibility of CD or UC have been reported in several studies.
To the best of our knowledge, the present study involving 37039 subjects represents the first comprehensive meta-analysis investigating the association between NKX2-3 polymorphisms (rs10883365 and rs11190140) and IBD susceptibility. For the analysis of rs10883365 polymorphism, a significantly increased CD risk was observed in all genetic models. In the ethnicity-stratified analyses, significant association was found both in Asian and European populations. Similar results were found between the rs11190140 variant and risk of CD where a significant association was found European population (no Asian population reported). No between-study heterogeneity was observed in most genetic models. Sensitivity analysis indicated that when excluding studies departed from HWE, the pooled OR still did not change, demonstrating the results of this meta-analysis were stable. Since these two SNPs are close to each other, we use 1000 Genomes Pilot sequence data to identify whether these SNPs are in linkage disequilibrium (LD) (r2 > 0.8). The results indicated that rs10883365 and rs11190140 are in perfect LD (r2 = 1.0).
CD and UC, as two major subtypes of IBD, are believed to share overlapping but distinct clinical and pathological features, and have great differences in genetic backgrounds11. Some genes, such as NOD2 and ATG16L1, were associated with CD, but not with UC47,48. However, recent GWAS meta-analysis identified 163 IBD loci that meet genome-wide significance thresholds, 50 of these have an indistinguishable effect size in UC and CD, including IL23R (rs11209026), IL10 (rs3024505) and MST1 (rs3197999). In the present meta-analysis, significant association between rs10883365 and risk of UC was found in all genetic models. When stratified by ethnicity, similar correlation was observed both in Asians and Europeans. No between-study heterogeneity existed in major genetic models. Sensitivity analysis showed no single study qualitatively changed the pooled ORs. Moreover, excluding studies departed from HWE, the pooled OR still did not change, demonstrating the results of this meta-analysis were stable. These results indicated that rs10883365 polymorphism in NKX2-3 gene may be significantly associated with both CD and UC.
By combining the data of individual studies, we increased the statistical power to detect subtle associations, however,several limitations should be considered in our meta-analysis. Only studies published in the English and Chinese language were included in this meta-analysis; therefore, publication bias may have occurred. In addition, this meta-analysis was designed to analyze single polymorphism, a haplotype analysis may have been more powerful for finding significant associations with UC and CD. Finally, gene–environment interactions were not analyzed because of insufficient data.
Despite these limitations, our results still yield statistical results. Taken together, we expand previously individual studies on IBD by suggesting that NKX2-3 gene rs10883365 polymorphisms might contribute to the occurrence of both CD and UC, and suggested that persons with a T allele of rs1190140 variant might have a significantly increased risk of CD. Further studies or large case-control studies, especially studies emphasizing genotype–phenotype interaction should be performed to clarify possible roles of NKX2-3in IBD. Moreover, studies involved in NKX2-3 polymorphisms in different populations with larger sample size might need to be performed.
Methods
Search strategy
We searched PubMed and Embase to identify genetic association studies of the rs10883365 or rs11190140 polymorphism and IBD risks. Electronic searches were performed by using the following search terms: ‘Inflammatory Bowel Disease' or ‘IBD', ‘Crohn's disease' or ‘CD', ‘ulcerative colitis' or ‘UC', ‘NKX2-3', ‘rs10883365', or ‘rs11190140', (the last search update was 1 September 2013). In addition, the reference lists of reviews and retrieved articles were checked by hand-search for additional potential studies. A study reported results from more than one population was considered as separate studies.
Inclusion and exclusion criteria
Studies were considered eligible if they had to meet the following criteria: (1) association between NKX2-3 polymorphisms and risk of IBD (CD or UC), (2) case-control or cohort studies. Studies were excluded for the following reasons: (1) articles only with an abstract and review articles (2) no control population, (3) studies considered overlapped with other studies.
Data extraction
Two authors extracted the following data independently from each of the eligible articles: first author, publication year, ethnicity of the participants involved (categorized as Europeans or Asians), number of cases and controls, information (age, mean age at diagnosis and sex) of cases and controls, and number of genotypes or allele frequency in cases and controls. Study authors were contacted for detailed data when there was insufficient information to determine the relationship between genetic polymorphism and IBD risk. Disagreements were resolved by discussion between the two authors.
Statistical analysis
Pooled odds ratios (ORs) and 95% confidence intervals (CIs) used to assess the strength of the association between IBD risk (CD or UC) and NKX2-3 polymorphisms (rs10883365 or rs11190140). The significance of the pooled OR was determined by the Z-test; a P-value of <0.05 was considered significant. The Hardy-Weinberg equilibrium (HWE) in the control group was assessed, and a P < 0.05 was considered as significant disequilibrium. For rs10883365 polymorphism, the pooled ORs were estimated for G versus A, GG versus AA, GA versus AA, dominant model (GG + GA versus AA), and recessive model (GG versus GA + AA). Because of only three studies19,49,50 available for the association between rs11190140 variant and UC risk, we have performed meta-analysis of correlation between rs11190140 polymorphism and CD risk. Subgroup analysis was performed according to ethnicity.
Between-study heterogeneity was evaluated by using the Chi-square based Q test and I2 test51. Heterogeneity was considered significant for P < 0.10, and a random-effects model was used, otherwise, fixed-effects model was used. In addition, if heterogeneity was detected, Galbraith plots were used to visualize the impact of individual studies on the overall homogeneity, which spot the outliers as the possible major sources of heterogeneity52,53. Moreover, a meta-regression was used to delineate the major sources of between-study heterogeneity54.
Sensitivity analysis was carried out to evaluate the stability of the results after sequential removal of each study or by excluding those studies deviated from HWE. In addition, cumulative meta-analyses were carried out for each polymorphism through assortment of studies with publication time. Graphical evaluation of funnel plots and Egger's linear regression test were performed to assess publication bias55. If significant publication bias was detected, ORs and 95% CI would be adjusted by trim and fill methods56. All statistical analyses were performed by STATA software, version 12 (StataCorp LP, College Station, Texas).
Supplementary Material
supplementary figures
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 81171147), “Xingwei Project” Key Personal Medical Research Foundation of Health Department of Jiangsu Province (No. RC201156), “Six Categories of Key Person” Research Foundation of Jiangsu Province (No. 069), Program Sponsored for Scientific Innovation Research of College Graduate in Jiangsu Province (No. CXZZ12_0583), and Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (No. JX10231801).
Footnotes
The authors declare no competing financial interests.
Author Contributions Conceived and designed the experiments: L.X.C., L.L.X. Performed the experiments: L.X.C., T.L.J., L.K. Analyzed the data: L.X.C., Z.J.Y., Z.P.L. Contributed reagents/materials/analysis tools: L.X.C., T.L.J., T.Y. Wrote the paper: L.X.C., L.L.X.
References
- Vermeire S., Van Assche G. & Rutgeerts P. Classification of inflammatory bowel disease: the old and the new. Curr Opin Gastroenterol 28, 321–26 (2012). [DOI] [PubMed] [Google Scholar]
- Abraham C. & Cho J. H. Inflammatory bowel disease. N Engl J Med 361, 2066–2078 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern D. P. et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet 42, 332–337 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tysk C., Lindberg E., Jarnerot G. & Floderus-Myrhed B. Ulcerative colitis and Crohn's disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking. Gut 29, 990–996 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombel J. F. et al. Clinical characteristics of Crohn's disease in 72 families. Gastroenterology 111, 604–607 (1996). [DOI] [PubMed] [Google Scholar]
- Glas J. et al. IRGM variants and susceptibility to inflammatory bowel disease in the German population. PLoS One 8, e54338 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar T. et al. NOD1 gene E266K polymorphism is associated with disease susceptibility but not with disease phenotype or NOD2/CARD15 in Hungarian patients with Crohn's disease. Dig Liver Dis 39, 1064–1070 (2007). [DOI] [PubMed] [Google Scholar]
- Lakatos P. L. et al. ATG16L1 and IL23 receptor (IL23R) genes are associated with disease susceptibility in Hungarian CD patients. Dig Liver Dis 40, 867–873 (2008). [DOI] [PubMed] [Google Scholar]
- Kabi A., Nickerson K. P., Homer C. R. & McDonald C. Digesting the genetics of inflammatory bowel disease: insights from studies of autophagy risk genes. Inflamm Bowel Dis 18, 782–792 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharl M. et al. Crohn's disease-associated polymorphism within the PTPN2 gene affects muramyl-dipeptide-induced cytokine secretion and autophagy. Inflamm Bowel Dis 18, 900–912 (2012). [DOI] [PubMed] [Google Scholar]
- Waterman M. et al. Distinct and overlapping genetic loci in Crohn's disease and ulcerative colitis: correlations with pathogenesis. Inflamm Bowel Dis 17, 1936–1942 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst O., Schneider A., Brand T. & Arnold H. H. The mouse Nkx2-3 homeodomain gene is expressed in gut mesenchyme during pre- and postnatal mouse development. Dev Dyn 209, 29–35 (1997). [DOI] [PubMed] [Google Scholar]
- Fu Y., Yan W., Mohun T. J. & Evans S. M. Vertebrate tinman homologues XNkx2-3 and XNkx2-5 are required for heart formation in a functionally redundant manner. Development 125, 4439–4449 (1998). [DOI] [PubMed] [Google Scholar]
- Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447, 661–678 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meggyesi N. et al. NKX2-3 and IRGM variants are associated with disease susceptibility to IBD in Eastern European patients. World J Gastroenterol 16, 5233–5240 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes M. et al. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn's disease susceptibility. Nat Genet 39, 830–832 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai T. et al. Increased expression of NKX2.3 mRNA transcribed from the risk haplotype for ulcerative colitis in the involved colonic mucosa. Hum Immunol 72, 587–591 (2011). [DOI] [PubMed] [Google Scholar]
- Fisher S. A. et al. Genetic determinants of ulcerative colitis include the ECM1 locus and five loci implicated in Crohn's disease. Nat Genet 40, 710–712 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A. et al. Replication of signals from recent studies of Crohn's disease identifies previously unknown disease loci for ulcerative colitis. Nat Genet 40, 713–715 (2008). [DOI] [PubMed] [Google Scholar]
- Tanaka M. et al. Genetic variants in surfactant, pulmonary-associated protein D (SFTPD) and Japanese susceptibility to ulcerative colitis. Inflamm Bowel Dis 15, 918–925 (2009). [DOI] [PubMed] [Google Scholar]
- Pang Z., Cao K. & Wei W. X. Correlation of rs10883365 Polymorphism in Upstream Region of NKX2-3 Gene with Crohn' Disease in Chinese Han Population. Chin J Gastroenterol 15, 532–535 (2010). (Chinese). [Google Scholar]
- Latiano A. et al. Investigation of multiple susceptibility loci for inflammatory bowel disease in an Italian cohort of patients. PLoS One 6, e22688 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W. et al. Association of a Nkx2-3 polymorphism with Crohn's disease and expression of Nkx2-3 is up-regulated in B cell lines and intestinal tissues with Crohn's disease. J Crohns Colitis 3, 189–195 (2009). [DOI] [PubMed] [Google Scholar]
- Weersma R. K. et al. Confirmation of multiple Crohn's disease susceptibility loci in a large Dutch-Belgian cohort. Am J Gastroenterol 104, 630–638 (2009). [DOI] [PubMed] [Google Scholar]
- Yamazaki K. et al. Positive association of genetic variants in the upstream region of NKX2-3 with Crohn's disease in Japanese patients. Gut 58, 228–232 (2009). [DOI] [PubMed] [Google Scholar]
- Laukens D. et al. Evidence for significant overlap between common risk variants for Crohn's disease and ankylosing spondylitis. PLoS One 5, e13795 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heide F. et al. Differences in genetic background between active smokers, passive smokers, and non-smokers with Crohn's disease. Am J Gastroenterol 105, 1165–1172 (2010). [DOI] [PubMed] [Google Scholar]
- Peter I. et al. Evaluation of 22 genetic variants with Crohn's disease risk in the Ashkenazi Jewish population: a case-control study. BMC Med Genet 12, 63 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr R. H. et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imielinski M. et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat Genet 41, 1335–1340 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A. et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet 42, 1118–1125 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. A. et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet 43, 246–252 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jostins L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J. F., Ning Y. J., Zhang W., Lu Z. H. & Lin L. T300A polymorphism of ATG16L1 and susceptibility to inflammatory bowel diseases: a meta-analysis. World J Gastroenterol 16, 1258–1266 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W. et al. Relationship between the polymorphism of tumor necrosis factor-alpha-308 G > A and susceptibility to inflammatory bowel diseases and colorectal cancer: a meta-analysis. Eur J Hum Genet 19, 432–437 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y. et al. The −173 G/C Polymorphism of the MIF Gene and Inflammatory Bowel Disease Risk: A Meta-Analysis. Int J Mol Sci 14, 11392–11401 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan C. et al. Association between OCTN1/2 gene polymorphisms (1672C-T, 207G-C) and susceptibility of Crohn's disease: a meta-analysis. Int J Colorectal Dis 27, 11–19 (2012). [DOI] [PubMed] [Google Scholar]
- Wang Z., Hu J., Fan R., Zhou J. & Zhong J. Association between CD14 gene C-260T polymorphism and inflammatory bowel disease: a meta-analysis. PLoS One 7, e45144 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zintzaras E. Is there evidence to claim or deny association between variants of the multidrug resistance gene (MDR1 or ABCB1) and inflammatory bowel disease? Inflamm Bowel Dis 18, 562–572 (2012). [DOI] [PubMed] [Google Scholar]
- Lu W. G. et al. Association of NOD1 (CARD4) insertion/deletion polymorphism with susceptibility to IBD: a meta-analysis. World J Gastroenterol 16, 4348–4356 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. X. et al. Associations between PTPN2 polymorphisms and susceptibility to ulcerative colitis and Crohn's disease: a meta-analysis. Inflamm Res 63, 71–79 (2014). [DOI] [PubMed] [Google Scholar]
- Zou L. et al. The association between three promoter polymorphisms of IL-10 and inflammatory bowel diseases (IBD): A meta-analysis. Autoimmunity 47, 27–39 (2014). [DOI] [PubMed] [Google Scholar]
- Pan H. F., Leng R. X. & Ye D. Q. Lack of association of interleukin-18 gene promoter −607 A/C polymorphism with susceptibility to autoimmune diseases: a meta-analysis. Lupus 20, 945–951 (2011). [DOI] [PubMed] [Google Scholar]
- Pabst O., Zweigerdt R. & Arnold H. H. Targeted disruption of the homeobox transcription factor Nkx2-3 in mice results in postnatal lethality and abnormal development of small intestine and spleen. Development 126, 2215–2225 (1999). [DOI] [PubMed] [Google Scholar]
- Pabst O., Forster R., Lipp M., Engel H. & Arnold H. H. NKX2.3 is required for MAdCAM-1 expression and homing of lymphocytes in spleen and mucosa-associated lymphoid tissue. EMBO J 19, 2015–2023 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlinton D., Light A., Metcalf D., Harvey R. P. & Robb L. Architectural defects in the spleens of Nkx2-3-deficient mice are intrinsic and associated with defects in both B cell maturation and T cell-dependent immune responses. J Immunol 170, 4002–4010 (2003). [DOI] [PubMed] [Google Scholar]
- Yazdanyar S., Weischer M. & Nordestgaard B. G. Genotyping for NOD2 genetic variants and crohn disease: a metaanalysis. Clin Chem 55, 1950–1957 (2009). [DOI] [PubMed] [Google Scholar]
- Marquez A. et al. Role of ATG16L1 Thr300Ala polymorphism in inflammatory bowel disease: a Study in the Spanish population and a meta-analysis. Inflamm Bowel Dis 15, 1697–1704 (2009). [DOI] [PubMed] [Google Scholar]
- John G. et al. NKX2-3 variant rs11190140 is associated with IBD and alters binding of NFAT. Mol Genet Metab 104, 174–179 (2011). [DOI] [PubMed] [Google Scholar]
- Skieceviciene J. et al. Replication Study of Ulcerative Colitis Risk Loci in a Lithuanian-Latvian Case-Control Sample. Inflamm Bowel Dis 19, 2349–2355 (2013). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558 (2002). [DOI] [PubMed] [Google Scholar]
- Galbraith R. F. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med 7, 889–894 (1988). [DOI] [PubMed] [Google Scholar]
- Huy N. T. et al. Cerebrospinal fluid lactate concentration to distinguish bacterial from aseptic meningitis: a systemic review and meta-analysis. Crit Care 14, R240 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriston L., Harms A. & Berner M. M. A meta-regression analysis of treatment effect modifiers in trials with flexible-dose oral sildenafil for erectile dysfunction in broad-spectrum populations. Int J Impot Res 18, 559–565 (2006). [DOI] [PubMed] [Google Scholar]
- Tang J. L. & Liu J. L. Misleading funnel plot for detection of bias in meta-analysis. J Clin Epidemiol 53, 477–484 (2000). [DOI] [PubMed] [Google Scholar]
- Fan W. et al. Relationship between the polymorphism of tumor necrosis factor-alpha-308 G > A and susceptibility to inflammatory bowel diseases and colorectal cancer: a meta-analysis. Eur J Hum Genet 19, 432–437 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary figures