Abstract
Background Perception and activation of plant immunity require a remarkable level of signalling plasticity and control. In Arabidopsis and other plant species, constitutive defence activation leads to resistance to a broad spectrum of biotrophic pathogens, but also frequently to stunted growth and reduced seed set. Plant hormones are important integrators of the physiological responses that influence the outcome of plant–pathogen interactions.
Scope We review the mechanisms by which the plant hormone cytokinin regulates both plant growth and response to pathogens, and how cytokinins may connect these two processes, ultimately affecting the growth trade-offs observed in plant immunity.
Keywords: Cytokinin, defence, plant immunity, pathogens, plant growth, plant development, growth–defence trade-offs, fitness costs
INTRODUCTION
Upon pathogen perception, plants initiate the concerted activation of a complex suite of structural and physiological defence responses, a process that requires a remarkable degree of signalling plasticity and control. Insufficient or untimely immunity activation may fail to restrain the pathogen, resulting in the host succumbing to disease. Conversely, constitutive or excessive defence activation leads to resistance to a broad spectrum of pathogens, but also often to suppression of plant growth, a phenomenon described as the growth–defence trade-off.
The existence of growth–defence trade-offs associated with defence activation underscores the need of plants to maintain a delicate balance between growing and defending against pathogens. While the underlying mechanisms associated with growth suppression during increased states of immunity are not well understood, energy diversion to the production of defence proteins and metabolites, as well as alteration of developmental programmes that promote growth, have been proposed as possible means of growth suppression. Thus, in order to survive pathogen attack, as well as successfully grow and reproduce, plants must effectively integrate signals initiated upon defence activation with those responsible for growth and developmental programmes.
Several lines of evidence suggest the existence of plant fitness costs associated with mechanisms of defence activation against pathogens (Bergelson and Purrington, 1996). This is especially true in the case of resistance to biotrophic pathogens, which are pathogens that obtain their nutrients from living plant cells. For example, yield penalties associated with resistance controlled by recognition of biotrophic pathogen effectors by resistance (R) proteins [effector-triggered immunity (ETI)] have been reported (Bjornstad and Aastveit, 1990; Ortelli et al., 1996; Brown, 2002). In the model plant Arabidopsis thaliana (hereafter arabidopsis), effective association of R gene-mediated resistance and fitness costs has been demonstrated (Tian et al., 2003). Introgression of RPM1, a nucleotide binding leucine-rich repeat (NLR) R gene conferring resistance to the bacterium Pseudomonas syringae pv. tomato DC3000 (Pst) expressing avrRPM1 or avrB, into an RPM1− ecotype leads to fitness costs characterized by reduction of seed set, decreased number of siliques and lower dry biomass (Tian et al., 2003). Further, recognition by plant cells of certain pathogen-associated molecular patterns (PAMPs), such as the conserved flg22 epitope from the bacterial protein flagellin, leads not only to immunity activation [PAMP-triggered immunity (PTI)] but also compromised plant growth (Gomez-Gomez et al., 1999), indicating that fitness costs of resistance are not restricted to ETI activation.
Similarly to the effect of defence activation by pathogen perception, plants with mutations that lead to constitutive activation of defence responses are also frequently dwarf, often with reduced seed set, demonstrating the fitness costs of constitutive defence activation. For example, arabidopsis constitutive defence mutants snc1 (SUPPRESSOR OF NPR1 CONSTITUTIVE), cpr1 (CONSTITUTIVE PATHOGENESIS-RELATED 1) and cpr5 (CONSTITUTIVE PATHOGENESIS-RELATED 5) are dwarf plants with reduced biomass, altered morphology and decreased seed yield compared with wild-type plants (Bowling et al., 1994, 1997; Li et al., 2001). The snc1 plants contain a gain-of-function mutation in a gene encoding an NLR protein that leads to constitutive expression of PATHOGENESIS-RELATED (PR) genes and activated levels of defence responses, including accumulation of the plant defence hormone salicylic acid (SA). Likewise, cpr1 and cpr5 mutants also have elevated PR gene expression and SA levels, with cpr5 exhibiting early senescence (Bowling et al., 1994, 1997).
Because activation of resistance to biotrophic pathogens is mostly dependent on SA signalling pathways (Glazebrook, 2005), and because SA-accumulating mutants tend to display decreased plant growth, high levels of SA have been suggested as one of the mechanisms by which the growth–defence trade-off may occur in plants. In agreement with this hypothesis, mutants with reduced SA content or signalling have been shown to display increased biomass in comparison with wild-type plants (Abreu and Munne-Bosch, 2009). How exactly SA mediates growth inhibition is still unknown.
The plant hormone cytokinin has long been associated with the regulation of plant growth and stress tolerance (Argueso et al., 2009). In recent years, cytokinins have been determined to play an important role in defence against biotrophic pathogens, which has led to the elucidation of hormonal crosstalk between SA and cytokinins in the orchestration of plant defence. In this review we highlight the cytokinin-regulated physiological and molecular processes associated with plant development and also with plant immunity, and point to a role for this class of plant hormones in the regulation of the growth–defence trade-offs in plants.
IF I DON’T FIGHT THERE WILL BE TROUBLE: CYTOKININS IN PLANT–PATHOGEN INTERACTIONS
Cytokinins are N6-substituted adenine derivatives that were discovered based on their role in regulating cell division in plants. Since then these plant hormones have been shown to regulate several other aspects of plant development and physiology, as well as responses to the environment (Argueso et al., 2009). Cytokinins constitute a group of structurally similar compounds, which can be classified as isoprenoid or aromatic cytokinins depending on whether they have an isoprene-derived or an aromatic side chain at the N6-terminus (Kudo et al., 2010). Isoprenoid cytokinins are considered the predominant type of cytokinin in plants and are synthesized through the transfer of an isopentenyl group to an ATP/ADP moiety, a reaction catalysed by isopentenyl transferase (IPT) enzymes (Kakimoto, 2001). The resulting isopentenyladenine (iP) ribosides can be converted to active free base forms by the lonely guy (LOG) enzymes (Kurakawa et al., 2007). Cytokinin content is also tightly regulated by cytokinin oxidase/dehydrogenases (CKXs), which catalyse degradation of cytokinins into either adenine or adenoside (Houba-Herin et al., 1999).
Cytokinin signal transduction in plant cells utilizes a two-component phosphorelay system, a signalling pathway commonly used by bacteria and fungi to perceive and respond to environmental signals (Hwang et al., 2012). Briefly, cytokinins are perceived by histidine kinases (HKs), which are mostly present on the endoplasmic reticulum membrane and act as cytokinin receptors. Binding of cytokinin to HKs leads to HK autophosphorylation and conformational changes. The cytokinin signal is then transduced from HKs to response regulators (RRs) through histidine-containing phosphotransfer proteins (HPs), which shuttle between the cytoplasm and nucleus (Hutchison et al., 2006; Punwani et al., 2010). Response regulators can be grouped into at least two classes, depending on the plant species. Type-B RRs contain DNA-binding domains and act as transcription factors, activating the transcription of primary cytokinin response genes, including type-A RRs (Argyros et al., 2008). Type-A RRs, on the other hand, lack any DNA-binding domains and function to inhibit cytokinin signalling, forming a negative feedback loop that regulates the cytokinin signalling pathway (To et al., 2004).
A role for cytokinins in plant–pathogen interactions has long been suggested, mostly from studies where exogenous application of molecules with cytokinin activity to plants resulted in altered levels of host resistance or susceptibility to pathogens. For example, plant cell cultures grown under high cytokinin concentrations showed increased expression of defence and stress genes (Schafer et al., 2000). Application of cytokinins to bean plants resulted in decreased susceptibility to white clover mosaic potexvirus, also accompanied by the induction of defence gene expression (Clarke et al., 2000). The results of exogenous application of cytokinins to plants raised the question of whether the reduction in pathogen growth originated from antimicrobial activities of biologically active cytokinins or from host-regulated processes that impeded pathogen growth.
Helping the plant defend: cytokinin-induced immunity
A definitive role for host cytokinins in plant immunity came from studies in arabidopsis. Exogenous application of high concentrations (10–100 μm) of the isoprenoid-derived synthetic cytokinin 6-benzylaminopurine (BA) to arabidopsis plants before pathogen inoculation led to decreased susceptibility to the biotrophic oomycete Hyaloperonospora arabidopsidis (Hpa) (Argueso et al., 2012). Similar results were obtained in arabidopsis treated with 1 μm of trans-zeatin, a natural isoprenoid biologically active cytokinin for which the cytokinin receptors have very high affinity, in response to the bacterial pathogen Pseudomonas syringae pv. tomato (Pst) DC3000 (Choi et al., 2010). The same disease-protective effect of cytokinins could not be observed in ahk2,3 plants, which harbour mutations in two of the three genes encoding cytokinin receptors (ARABIDOPSIS HISTIDINE KINASE 2 and 3), indicating that the action of cytokinins in this context is indeed due to cytokinin-regulated physiological processes (Choi et al., 2010; Argueso et al., 2012). An additional concern in elucidating a role of cytokinins in plant immunity was the fact that many of the experiments were performed with exogenous hormone applications to plants, therefore confounding the contributions of exogenous and endogenous levels of cytokinin to the process. This was conclusively addressed by the use of transgenic arabidopsis plants overexpressing IPT genes, in which the endogenous levels of cytokinin are increased up to 100-fold (Kakimoto, 2001). IPT-overexpressing arabidopsis plants showed a decrease in Pst growth, confirming that highly increased levels of cytokinins help deter pathogen growth, and that this can be achieved by either exogenous or endogenous cytokinins (Choi et al., 2010). The decreased pathogen growth observed in cytokinin-treated plants was accompanied by enhanced upregulation of defence gene expression and callose deposition, to levels far superior to those obtained with pathogen treatment alone (Choi et al., 2010; Argueso et al., 2012). It is important to note that treatment of plants with cytokinin alone, without pathogen challenge or elicitors, does not lead to high levels of defence activation. In this way, the action of cytokinin in plant immunity is similar to the action of chemicals known as priming agents, which act to potentiate defence responses, but are only activated upon pathogen/elicitor perception (Conrath et al., 2015).
While the initial mechanistic studies on cytokinin action in plant immunity were primarily focused on arabidopsis, they were shortly followed by studies in other plant species. In tobacco, inducible expression of an IPT gene responsible for cytokinin biosynthesis, as well as exogenous application of cytokinins, substantially reduced disease progression of the biotrophic bacterial pathogen Pseudomonas syringae pv. tabaci (P.s. tabaci) (Grosskinsky et al., 2011). In rice, treatment of plants with high levels of cytokinins led to increased defence gene expression against the biotrophic rice blast fungus Magnaporthe oryzae (Jiang et al., 2013). Added to other reports from the literature where high levels of cytokinins in plants have been linked to resistance to viruses (Clarke et al., 2000; Pogany et al., 2004) and even nematodes (Shanks et al., 2016), these data point to a role for cytokinins in activating defence responses and contributing to physiological conditions that help contain invading biotrophic pathogens, a process we have now named cytokinin-induced immunity.
Continued work in arabidopsis and other plant species has shown that the decrease in pathogen growth seen in cytokinin-induced immunity is at least partially dependent on content and signalling of the plant hormone SA. Arabidopsis mutants in the gene encoding the SA biosynthetic enzyme isochorismate synthase 1 (ISC1) failed to show the same effect of suppression of biotrophic pathogen growth due to cytokinin treatment (Choi et al., 2010; Argueso et al., 2012; Naseem et al., 2012). A similar lack of cytokinin-induced immunity was also observed using npr1 (NON-EXPRESSOR OF PR-1) plants, which contain a mutation in a known master regulator of SA signalling (Choi et al., 2010). The SA-dependence of cytokinin-induced immunity further supported the observed priming activity of cytokinins, in which cytokinin-treated plants show enhancement of SA-dependent gene expression upon pathogen perception (Choi et al., 2010; Argueso et al., 2012). Similarly, in rice, exogenous co-treatment of plants with the SA analogue benzothiadiazole S-methyl ester (BTH) and the cytokinin kinetin dramatically increased expression of the defence genes OsPR1b and PBZ1, while treatment with either hormone alone did not show a significant increase in defence gene expression, nor did co-treatment with SA plus several other hormones (Jiang et al., 2013). This potentiation of SA-dependent defence gene expression by cytokinin is determined by the major regulators of SA-dependent defence responses in rice (Wu et al., 2012), OsNPR1 and WRKY45 (Shimono et al., 2007; Sugano et al., 2010). Interestingly, the increased immunity state induced by cytokinin and SA treatment in rice does not translate into decreased M.oryzae growth. It is important to note that in arabidopsis and tobacco high levels of SA are produced in response to pathogen invasion, while in rice the basal levels of SA are already high and do not change significantly following pathogen invasion (Silverman et al., 1995). Therefore, the observed differences in disease outcome in cytokinin-induced immunity may reflect the differences in SA content and signalling between rice and dicotyledonous plant species.
Despite the recognizable role for SA in cytokinin-induced immunity described above, some evidence points to a role for cytokinins in the activation of defence responses in a manner that is independent of SA. Transgenic tobacco lines overexpressing the bacterial gene nahG, encoding an SA degradation enzyme, did not alter the protective effect of cytokinin against P.s. tabaci infection seen in wild-type plants (Grosskinsky et al., 2011). The positive effects of cytokinin on defence responses of wild-type tobacco plants were attributed to the production of the key phytoalexins scopoletin and capsidiol, which act to restrict pathogen growth. A time-course analysis showed that the production of these phytoalexins occurs early during infection in response to pretreatment with cytokinin, before SA accumulation (Grosskinsky et al., 2011). Therefore, the fact that cytokinin-induced immunity in this pathosystem seems to be independent of SA may be simply due to the timing of scopoletin and capsidiol accumulation, which occurs before SA-dependent defence responses are activated (Grosskinsky et al., 2011). Nevertheless, co-treatment of wild-type tobacco plants with cytokinin and P.s. tabaci increased SA levels and PR-1a expression significantly more during late infection stages than treatment with cytokinin or P. s. tabaci alone (Grosskinsky et al., 2011). Thus, while SA may not be essential for cytokinin-induced immunity in early defence responses such as scopoletin and capsidiol biosynthesis, it certainly contributes to the overall defence response. In agreement with this, a synergistic action of cytokinins and SA was found to regulate the production of another type of phytoalexin, known as diterpenoid phytoalexins, during defence responses of rice to M. oryzae (Ko et al., 2010; Akagi et al., 2014), and this response is dependent on both SA and the SA signalling regulator WRKY45 (Akagi et al., 2014).
The signalling mechanisms regulating immunity activation by cytokinins are slowly being revealed. Choi et al. (2010) demonstrated that ARR2 (arabidopsis response regulator 2), the a type-B positive regulator of the cytokinin signalling, directly interacts with the SA-responsive transcription factor TGA3 and master regulator of SA signalling NPR1, forming a transcriptional complex that activates expression of the SA-dependent defence marker PR1. On the other hand, type-A ARRs, which function as negative regulators of cytokinin signalling, also act to suppress SA-dependent defence gene expression, in a manner dependent on their phosphorylation status (Argueso et al., 2012). Stimulation of reactive oxygen species (ROS) has also been linked to cytokinin and is a likely mechanism by which defence responses can be modulated by this plant hormone. Transgenic tobacco plants overexpressing IPT genes showed an increase in activity of the antioxidant enzymes ascorbate peroxidase, glutathione S-transferase and catalase, accompanied by lower H2O2 content in infected leaves. This suggests that increases in cytokinin content in leaves provide more efficient ROS scavenging activity, inhibiting symptom development of necrotic lesions upon infection with tobacco necrosis virus (TNV) (Pogany et al., 2004), which is in agreement with the role of cytokinin in oxidative stress (Zavaleta-Mancera et al., 2007; Shi et al., 2014). In another study, overexpression in tobacco of S-adenosylhomocysteine hydrolase (SAHH), a key enzyme in transmethylation reactions, conferred resistance to infection by several host viruses, and transgenic tobacco plants overexpressing SAHH (Masuta et al., 1995). Through physiological analysis it was shown that resistance to viral infection was correlated with elevated levels of cytokinins (Masuta et al., 1995). Interestingly, a similar result was obtained with viral-induced gene co-silencing of the three genes in the tomato genome that encode SAHHs (Li et al., 2015). The SAHH-silenced tomato plants showed increased activation of immunity and resistance to Pst, as well as growth alterations similar to IPT-overexpressing plants. Further, the transgenic plants also showed increased drought tolerance (Li et al., 2015), a phenotype associated with cytokinin accumulation. Together, these results point to a role for cytokinin-regulated ROS homeostasis as a factor in the defence activation observed during cytokinin-induced immunity.
Helping the pathogen grow: cytokinin-induced susceptibility
Adding to the complexity of the roles of cytokinins in plant–pathogen interactions is the fact that in some cases an increase in the levels of biologically active cytokinins in plants is associated with increased pathogen growth, a process that we have named cytokinin-induced susceptibility. Given its beneficial effect in pathogenic organisms, cytokinin-induced susceptibility is likely a pathogen-driven process, by which manipulation of in planta cytokinin signalling and/or content, or direct production of cytokinins by the pathogens themselves, culminates in host physiological responses that help the pathogen thrive.
Cytokinin-induced susceptibility is usually associated with low to moderate levels of cytokinin content in plants. In one of the first demonstrations of cytokinin-induced susceptibility, Argueso et al. (2012) showed that lower concentrations of cytokinins can actually help pathogen success. Exogenous application of low concentrations of the cytokinin BA (<1 μm) to arabidopsis led to increased growth of the oomycete Hpa on wild-type plants, in comparison with mock-treated controls (Argueso et al., 2012). Similarly, a moderate increase in the levels of biologically active cytokinins was associated with increased growth of powdery mildew on wheat leaves, rather than increased resistance (Babosha, 2009). How low to moderate concentrations of cytokinins may help pathogen success is unclear, but it likely involves improved physiological conditions for pathogen growth. For example, the fungal pathogen M. oryzae produces and secretes cytokinins. Magnaportheoryzae-derived cytokinins are biologically active in the host plant, as shown by activation of the cytokinin responsive promoter of the type-A response regulator OsRR6 (Jiang et al., 2013). Production of cytokinins by M. oryzae was shown to alter rice metabolism near sites of infection, leading to an increase in the levels of key sugars and amino acids, which may act to help support fungal growth, increasing plant susceptibility (Chanclud et al., 2016).
Another possible explanation for cytokinin-induced susceptibility is decreased defence activation. Consistent with this hypothesis, examination of the molecular mechanism by which the bacterial effector protein HopQ1 suppresses defence responses revealed that low levels of cytokinin signalling increase susceptibility of arabidopsis to Pst by decreasing defence responses (Hann et al., 2014). HopQ1 is an effector protein produced by Pst and secreted via the type III secretion system. Transgenic arabidopsis plants expressing HopQ1 show suppression of PTI responses when exposed to flg22, including reduced accumulation of ROS and PTI-associated mitogen-activated protein kinase (MAPK) activation. The reduced PTI responses are attributed to the attenuated expression of the FLS2 (FLAGELLIN-SENSITIVE 2) gene, encoding the receptor for flg22. This reduced expression of FLS2 corresponds to increased levels of active cytokinins and increased expression of cytokinin-responsive genes. Interestingly, HopQ1-expressing plants had severe developmental defects, including reduced root growth and branching, anthocyanin build-up and loss of apical dominance, consistent with phenotypes observed in a cytokinin-signalling mutant. Further, exogenous application of a low concentration of trans-zeatin (100 nm) to wild-type arabidopsis plants can recapitulate the reduced levels of FLS2 transcript and protein amounts seen in HopQ1-transgenic plants, along with suppression of both ROS accumulation and PTI-associated MAPK activation (Hann et al., 2014). The authors hypothesize that the bacterial effector HopQ1 acts similarly to the LOG enzymes in arabidopsis, converting inactive cytokinin nucleotides into moderate levels of cytokinin active forms. The moderate increases in active cytokinins attenuate FLS2 expression, diminishing PTI responses and ultimately helping pathogen growth. The action of HopQ1 seems limited by the existing pool of the inactive forms, which fluctuates with the developmental stage and environmental conditions of the plant (Hann et al., 2014). As in other plant–pathogen interactions, HopQ1 is an example of effector-triggered susceptibility and of how pathogens can exploit hormone biosynthesis to facilitate colonization.
IF I DON’T GROW THERE WILL BE DOUBLE: REGULATION OF PLANT GROWTH BY CYTOKININS AND POTENTIAL AVENUES FOR REGULATION OF GROWTH–DEFENCE TRADE-OFFS
The main implication of a role of cytokinins in the growth–defence trade-off comes from the fact that this plant hormone regulates not only plant immunity, as described in the sections above, but also plant growth (Kieber and Schaller, 2014). The decreased cytokinin content observed in ipt mutant plants, as well as in transgenic plants overexpressing CKX genes, positively correlates with decreased shoot size and decreased shoot meristem activity (Werner et al., 2003; Miyawaki et al., 2006). Conversely, overexpression of IPT genes causes increases in cytokinin content, leading to larger embryos and often to increased shoot growth (Smigocki and Owens, 1988; Ma et al., 2002).
In the process of analysing the hormonal crosstalk between cytokinin and SA, SA was determined to also have an inhibitory effect on cytokinin signalling (Argueso et al., 2012). Plants lacking SA biosynthesis (eds16 mutants) are more sensitive to root growth inhibition by cytokinin and express higher levels of cytokinin-regulated genes, indicating a negative regulatory effect of SA on cytokinin signalling in wild-type plants (Argueso et al., 2012). Therefore, it is possible to envision a scenario where plants that accumulate high levels of SA, due to mutations or immunity activation by pathogens, also have reduced cytokinin content and/or signalling, which can then be translated into reduced plant growth and the fitness costs seen in the growth–defence trade-off. In the next sections we discuss some of the cytokinin-regulated processes that may affect the observed growth–defence trade-offs during immunity activation.
Cytokinins and the control of cell division
Plant growth depends on cell division and expansion. Cytokinins were first discovered as molecules with the ability to promote cell division in plant cells. Initial studies identified a substance present in autoclaved herring sperm DNA that could promote division of plant cells in culture as a purine derivative, today known as the cytokinin kinetin (Miller et al., 1955, 1956). Since then, several other cytokinin species have been identified, and while these molecules have now been found to play roles in many developmental processes and biotic/abiotic responses, promotion of cell proliferation continues to be the hallmark role of this class of plant hormones (Schaller et al., 2014).
The control of cell growth and proliferation by cytokinins is directly tied to cell cycle regulation. The levels of cytokinins are known to change during cell cycle progression in cultured plant cells (Redig et al., 1996; Hartig and Beck, 2005). In addition, cytokinin treatment induces the expression of CYCLIN D3 genes in arabidopsis (Riou-Khamlichi et al., 1999), which are conserved regulators of the gap transitions during cell cycle progression in plants and animals. Cyclin proteins activate cyclin-dependent kinases (CDKs), whose ultimate function is the activation of the EF2 protein complex, which regulates G1/S and G2/M gap transitions. Further connecting cytokinins and cell cycle control, the overexpression of CYCLIN D3 genes in plants can bypass the requirement for cytokinin in culture media for shoot regeneration (Riou-Khamlichi et al., 1999). Moreover, treatment of tobacco BY-2 cells with a cytokinin biosynthesis inhibitor demonstrated that cytokinin biosynthesis is indispensable for the G2/M transition (Laureys et al., 1998), and a delay in the G2/M transition is observed in root cells of cytokinin receptor (ahk) multiple mutants. Interestingly, perturbations of the cell cycle can lead to activation of plant immunity (Bao et al., 2013). Recently, two cell cycle CDK proteins, SIAMESE (SIM) and SIM-RELATED 1 (SMR1), were found to be essential for cell cycle control and proper activation of programmed cell death during ETI (Wang et al., 2014; Hamdoun et al., 2016). The activation of SIM and SMR1 is also dependent on the CPR5 gene, encoding a nuclear envelope protein, and whose mutations show constitutive activation of defence and growth defects. It would be interesting to know whether the activation of these CDK proteins is dependent on the transcriptional regulation of CYCLIN D3 by cytokinins. It is noteworthy that cytokinins have also been implicated in the control of HR-like programmed cell death, through mechanisms that may involve cell cycle control (Suda et al., 2009; Novak et al., 2013).
Cytokinins and the control of meristem function
Given their important role in cell cycle control and regulation of cell division, it is not surprising that cytokinins have a direct role in the regulation of meristem function. Cytokinins have long been known to promote, in conjunction with auxin, the induction of organogenesis, with a predominant role for cytokinins in shoot initiation (Kieber and Schaller, 2014). In the shoot apical meristem (SAM), plants with decreased cytokinin content due to disruption of cytokinin biosynthesis or signalling have smaller SAMs (Miyawaki et al., 2006; Kurakawa et al., 2007; Kuroha et al., 2009) and show reduced growth rate (Higuchi et al., 2004; Nishimura et al., 2004; Miyawaki et al., 2006; Kurakawa et al., 2007). On the other hand, disruption of CKX genes or mutations in the type-A RRs, which increased levels of cytokinin content and signalling respectively, led to an enlarged SAM (To et al., 2004; Leibfried et al., 2005; Bartrina et al., 2011). Transgenic arabidopsis plants expressing CKX genes specifically in young shoot tissue have a lower cytokinin content and a dramatic decrease in the size of the leaves and number of leaf epidermal cells (Werner et al., 2003; Holst et al., 2011). The reduction of cytokinin content in the SAM also compromises the ability of the plant to form new leaf primordia and flowers (Holst et al., 2011), which is consistent with the increased number of inflorescences and seed yield seen in ckx mutant plants (Bartrina et al., 2011).
Maintenance and differentiation of SAM cells is under the spatial–temporal transcriptional control of the meristem-defining transcription factor WUSCHEL (WUS), the signalling peptide CLAVATA3 (CLV3) and its cognate receptor CLAVATA1 (CLV1) (Fletcher et al., 1999; Brand et al., 2000). The link between meristem size and cytokinins comes from data showing that cytokinin upregulated WUS expression (Lindsay et al., 2006). In turn, WUS upregulation induces CLV3 expression, which then binds to its receptor, CLV1 (Mayer et al., 1998; Schoof et al., 2000). Binding of CLV3 to CLV1 then represses WUS, forming a negative feedback loop that regulates meristem organization. In addition, upregulation of WUS was shown to downregulate expression of type-A ARRs, including ARR7 and ARR15, relieving inhibition of cytokinin signalling in the SAM (Leibfried et al., 2005) and forming a second feedback loop that amplifies cytokinin signalling in the SAM. Because SA was determined to negatively regulate cytokinin signalling (Argueso et al., 2012), it is tempting to speculate a function for SA in the inhibition of cytokinin signalling in the SAM, leading to altered shoot development and growth trade-offs during defence.
In the root apical meristem (RAM) auxin regulates cell division while cytokinin regulates cell differentiation, and a balance between these two growth hormones is critical for proper RAM maintenance. Exogenous application of cytokinins leads to a reduced cell division zone in the RAM, culminating in reduced root growth and root branching, while cytokinin receptor mutants have the opposite phenotype, indicating a role for cytokinins in root growth inhibition (Bertell and Eliasson, 1992; Riefler et al., 2006). The control by cytokinins of RAM function is achieved by inhibition of auxin signalling and transport. Cytokinin signalling activates type-B ARRs, which promotes transcription of the SHY2/IAA3 (SHORT HYPOCOTYL 2/INDOLE-3-ACETIC ACID INDUCIBLE 3) gene (Dello Ioio et al., 2008). The SHY2 protein, in turn, inhibits auxin signalling by forming heterodimers with auxin response factors (ARFs), which are responsible for the expression of auxin response genes. SHY2/ARF dimerization limits the number of cell divisions in the RAM before differentiation occurs, leading to decreased root growth (Dello Ioio et al., 2008). SHY2 also downregulates expression of PIN genes, responsible for auxin transport, and this relocation of auxin stimulates cell differentiation (Dello Ioio et al., 2008). In contrast to shoots, the control of plant immunity in roots has been largely understudied. Recent work has demonstrated that FLS2 is under different transcriptional control in roots and shoots (Beck et al., 2014). Given the importance of cytokinin for root development and the recent discovery of transcriptional regulation of FLS2 through altered cytokinin levels (Hann et al., 2014), it is possible to suggest that cytokinins may be involved in the control of root immunity, conceivably by regulation of the FLS2 transcript levels in root cells. While most examples of growth–defence trade-offs have been focused on shoots and inflorescences, reduced root growth or altered root architecture has severe consequences for shoot growth, including inflorescence growth and seed set. It is interesting to note that cytokinins are synthesized in the roots and transported to the shoot, where they exert control of different physiological processes (Kudo et al., 2010). Therefore, changes in development that result in reduced root growth may lead to diminished overall cytokinin content in the plants, and likely to the shoot growth defects that are typical of reduced cytokinin levels.
Cytokinins and the control of the source–sink relationships
Since the work of Mothes and Engelbrecht (1961), which showed that application of cytokinins can redirect the localization of plant assimilates in leaves of fava beans, cytokinins have been considered to have a fundamental function in the regulation of source–sink relationships. Today cytokinins are known to regulate the metabolism and transport of amino acids and carbohydrates important for plant growth, as well as several macronutrients, including nitrogen, phosphorus, sulphur and iron (Argueso et al., 2009).
One of the main ways by which cytokinins regulate the establishment of source and sink tissue is through the mediation of carbohydrate availability and transport (Roitsch and Ehness, 2000). Overexpression in tobacco of CKX genes, which encode enzymes that degrade cytokinin, led to a dramatic decrease in soluble sugar content in sink tissues accompanied by reduced shoot growth and seed set; however, no significant changes were observed in source tissues (Werner et al., 2008). These results suggest that cytokinin may be responsible for the availability of soluble sugars in sink tissues, such as young leaves, developing roots, fruits and seeds, positively affecting plant growth and yield.
A potential mechanism by which cytokinins may affect sugar availability in sink tissues involves invertases, which hydrolyse phloem-transported sucrose into hexose sugars. While plant cells possess different types of enzymes with invertase activity that differ in subcellular localization, cell wall invertase activity during phloem unloading constitutes an essential part of the establishment of metabolic sinks (Tauzin and Giardina, 2014). During attack by biotrophic pathogens, infected tissues such as leaves are known to transition from source to sink, and this transition is accompanied by increased cell wall invertase activity and increased expression of genes encoding sucrose transporters (Fotopoulos et al., 2003; Hayes et al., 2010). In some cases, as in the case of gall-forming pathogens, increased cell wall invertase activity helps pathogen growth, possibly by increasing the supply of sugars to pathogens (Siemens et al., 2011). In other instances, increased cell wall invertase activity is linked to increased resistance; silencing of a cell wall invertase in tobacco led to plants that displayed reduced defence responses and that were more susceptible to the oomycete Phytophthora nicotianae, indicating that availability of carbohydrates is essential to support defence reactions to invading pathogens (Essmann et al., 2008). Similarly, downregulation of proteinaceous invertase inhibitors during the defence reaction of arabidopsis to Pst culminates in elevated cell wall invertase activity that is needed for defence (Bonfig et al., 2010). It is interesting to note that exogenous application of cytokinin and overexpression of cytokinin biosynthetic IPT genes results in increased expression of genes encoding cell wall invertases (Gan and Amasino, 1995; Ehness and Roitsch, 1997; Kim et al., 2006), while plants with reduced cytokinin content have decreased cell wall invertase activity and plant growth (Werner et al., 2008). Thus, cytokinins are considered positive regulators of cell wall invertase activity, and are important for plant growth, defence and pathogen success.
Another possible role for cytokinin in source-sink relationships and plant growth is through the regulation of sugar transporters, which transport sucrose between photosynthetically active cells and phloem for further transport to sink tissues. During cytokinin-induced susceptibility, pathogens are able to manipulate cytokinin signalling and/or content to create physiological conditions that help their growth. In this scenario, sucrose transporters could also be targeted by pathogens to increase nutrient availability at sites of infection. A well-known example is the formation of green islands, which occurs in cereals infected with some rust and powdery mildew fungi. Green islands are regions of photosynthetically active leaf tissue at sites of infection surrounded by tissue undergoing senescence (Walters et al., 2008). Cytokinin content was demonstrated to be higher in green islands than in the surrounding senescing tissue (Lopez-Carbonell et al., 1998), where the effect of cytokinins on cell wall invertase activity is thought to prevent senescence and maintain the metabolically active tissue to support biotrophy and pathogen growth (Lara et al., 2004). Similarly, transcriptional activation of the SWEET class of rice sucrose transporters by Xanthomonas oryzae effectors has been implicated in the susceptibility of rice to this pathogen, possibly as a way to increase sucrose transport into the apoplast for pathogen feeding (Chen et al., 2010). Whether sucrose transporter expression and activity are dependent on cytokinins is unknown, but evidence exists supporting this hypothesis (Lee and Huang, 2013). Given the role of cytokinins in carbohydrate allocation during plant growth, and manipulation of these processes by invading pathogens, it could be hypothesized that competition among host and pathogen for cytokinin-regulated metabolic sinks is a potential avenue for the growth–defence trade-off to occur.
SO YOU’VE GOT TO LET ME KNOW: SHOULD I FIGHT OR SHOULD I GROW? REGULATION OF GROWTH–DEFENCE TRADE-OFFS BY CYTOKININS
In immunity against pathogens, modes of action in the cytokinin regulation of defence responses are dependent on the cytokinin concentration and the stage of infection. Moderate levels of cytokinins can help the biotrophic pathogen thrive by creating favourable physiological conditions (Argueso et al., 2012; Hann et al., 2014), while higher concentrations of cytokinin activate plant immunity primarily through SA-dependent processes (Choi et al., 2010; Argueso et al., 2012).
There are multiple areas of growth and development regulated by cytokinin that are likely processes during which the growth–defence trade-off could occur (Fig. 1). Defence gene expression, production of ROS and phytoalexin biosynthesis are processes that are partly mediated by cytokinins and also known to have a major role in the success of pathogen infection and disease progression. As cytokinins appear to both positively and negatively regulate sucrose transport and differentially regulate the expression of various hexose transporters, definition of sink tissues by cytokinins affords plants the ability to prioritize carbohydrate transport and metabolism to young growing tissues during optimal environmental conditions, while allowing for adjustment of sink tissue identity upon pathogen perception, leading to downregulation of growth. While metabolic reprogramming and competing resource allocation may account for growth suppression during immunity activation, several pieces of evidence indicate that such a scenario may not represent a complete picture of growth–defence trade-offs. For example, activation of PTI by the PAMP chitin does not lead to growth suppression (Wan et al., 2008; Petutschnig et al., 2010), which is in stark contrast to the effect of another PAMP, flg22 (Gomez-Gomez et al., 1999). Further, metabolic shifts under nitrogen- and carbon-limiting conditions have not been found to correlate with defence activation (Kleessen et al., 2014). Finally, decreased plant growth does not necessarily translate into increased immunity, as seen by the phenotype of cytokinin receptor mutants, which display reduced shoot growth but not increased defence activation. It is therefore more probable that some combination of energy diversion and altered regulation of developmental control pathways is responsible for the growth fitness costs of defence activation. In this regard, the roles of cytokinins in both energy partitioning and the control of cell division and meristem function indicate that this class of plant hormones is likely to play an important part in the regulation of growth–defence trade-offs. This existence of several physiological mechanisms that could account for growth–defence trade-offs stimulates one to hypothesize that growth suppression can be decoupled from defence activation. Studies that examine the fitness cost of defence have found that ROS produced during pathogen invasion are partly responsible for growth suppression independently of defence activation (Zhu et al., 2013).
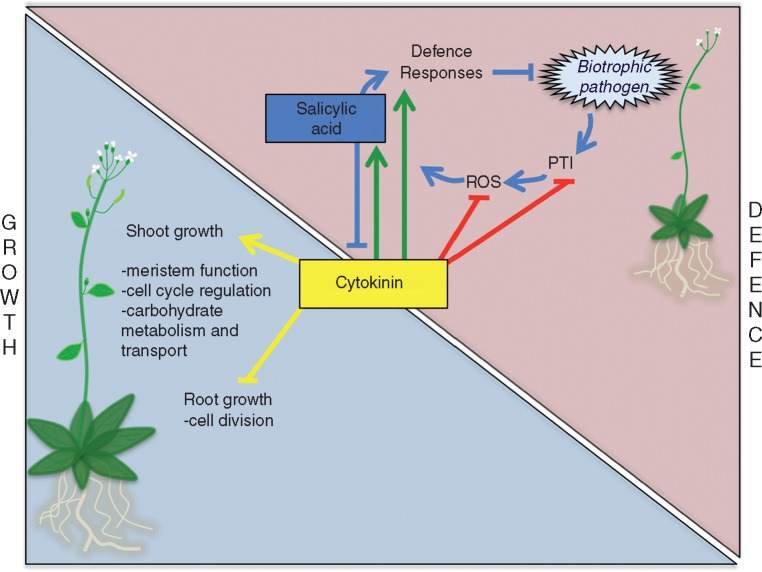
Fig. 1.
Schematic representation of the role of cytokinins in plant growth and defence against biotrophic pathogens, and in the growth–defence trade-off. Under normal growing conditions, cytokinin promotes shoot growth while inhibiting root growth (yellow arrows). Infection by a biotrophic pathogen stimulates pattern-triggered immunity (PTI) activation, oxidative stress (ROS) and salicylic acid biosynthesis, culminating in salicylic acid-dependent defence responses that suppress biotrophic pathogen growth (blue arrows). Cytokinins can enhance defence activation by salicylic acid-dependent and -independent processes (cytokinin-induced immunity; green arrows). Cytokinins can also help pathogen growth, by mechanisms that include suppression of PTI and ROS (cytokinin-induced susceptibility; red arrows). Increased salicylic acid content/signalling inhibits cytokinin-regulated processes, potentially causing inhibition of plant growth, a likely mechanism by which the growth–defence trade-off may occur. Arrows indicate positive interaction; blunt ends indicate negative interaction (inhibition).
Finally, the contribution of other plant hormones in conjunction with cytokinin in the regulation of the growth–defence trade-off also has to be considered (Belkhadir et al., 2014; Huot et al., 2014; Lozano-Duran and Zipfel, 2015). For example, auxin and cytokinin signalling pathways interact in multiple ways to modulate key aspects of growth and development (Schaller et al., 2015). Salicylic acid represses expression of auxin signalling genes, resulting in inhibition of pathogen growth (Wang et al., 2007). Furthermore, Pst DC3000 stimulates auxin production upon infection in arabidopsis, presumably to facilitate colonization (Naseem et al., 2012). Auxins and cytokinins appear to act antagonistically in the mediation of immune responses, with auxin promoting pathogen growth while cytokinin inhibits growth. Co-treatment of plants with both hormones shows decreased pathogen growth relative to treatment with auxin alone (Naseem et al., 2012). This illustrates the well-known fact that it is the interactions between multiple hormone signalling pathways that regulate defence responses, and unravelling the complexities of the growth–defence trade-off requires accounting for hormonal signalling crosstalk.
Understanding of the role of cytokinin in the growth–defence trade-off requires more research that should define cytokinin actions in the responses of plants to pathogens, using different pathosystems, as well as examination during various stages of infection. Considerations that should be accounted for include cytokinin origin (host or pathogen), as well as types and concentrations of cytokinins, as differences in biological activity and effective concentrations can lead to states of increased immunity or susceptibility (Table 1). The matter of tissue specificity of growth–defence trade-offs should also be further explored, and in this regard the opposing regulation of shoot and root growth by cytokinins indicates that this class of plant hormones may play an important role. For example, plants overexpressing genes encoding CKX enzymes involved in cytokinin degradation display decreased shoot growth but increased root growth; however, whether this also translates into mutually decreased susceptibility to shoot pathogens and increased susceptibility to root pathogens is not known.
Table 1.
Summary of research studying the effects of cytokinin on plant–pathogen interactions
| Pathogen | Host plant | Cytokinin alteration | Effect observed | Reference | |
|---|---|---|---|---|---|
| Bacteria | |||||
| Pseudomonas syringae pv. tomato DC3000 | Arabidopsis thaliana | Exogenous trans-zeatin (1 μm) and endogenous increase (IPT/CKX overexpression) | CII | Choi et al. (2010) | |
| Pseudomonas syringae pv. tabaci | Nicotiana tobacum | Exogenous kinetin (1–18 μm) and endogenous increase (upregulated IPT) | CII | Grosskinsky et al. (2011) | |
| Pseudomonas syringae pv. tomato DC3000 | Arabidopsis thaliana | Exogenous kinetin (10 μm) | CII | Naseem et al. (2012) | |
| Pseudomonas syringae pv. tomato DC3000 | Arabidopsis thaliana | Exogenous trans-zeatin (0·001–1 μm) | CIS | Hann et al. (2014) | |
| Rhodococcus fascians | Arabidopsis thaliana | Pathogen-secreted isopentenyladenine, trans-zeatin, cis-zeatin | CIS | Pertry et al. (2009) | |
| Oomycete | |||||
| Hyaloporenospora arabidopsidis | Arabidopsis thaliana | Exogenous benzyladenine (0·01–100 μm) | CIS and CII | Argueso et al. (2012) | |
| Fungi | |||||
| Erysiphe graminis f. sp. tritici | Triticum aestivum | Exogenous trans-zeatin (0·25–9 μm) | CIS and CII | Babosha (2009) | |
| Magnaporthe oryzae | Oryza sativa subsp. japonica | Exogenous kinetin or isopentenyladenine (1–100 μm) plus SA analogue | CII | Akagi et al. (2014), Jiang et al. (2013) | |
| Magnaporthe oryzae | Oryza sativa subsp. japonica | Pathogen-secreted cis-zeatin nucleotide, isopentenyladenine, cis-zeatin riboside, isopentenyladenosine | CIS | Chanclud et al. (2016) | |
| Pyrenopeziza brassicae | Brassica napus | Pathogen-secreted zeatin riboside, isopentenyl adenosine | CIS | Ashby (2000) | |
| Viruses | |||||
| Cucumber mosaic virus, tobacco mosaic virus, potato virus X, potato virus Y | Nicotiana tobacum | Endogenous upregulation (SAHH overexpression) | CII | Masuta et al. (1995) | |
| Tobacco necrosis virus | Nicotiana tobacum | Endogenous increase (IPT overexpression) | CII | Pogany et al. (2004) | |
| White clover mosaic potexvirus | Phaseolus vulgaris | Exogenous dihydrozeatin (0·0025 μm) | CII | Clarke et al. (2000) | |
| Tobacco mosaic virus | Nicotiana tobacum | Endogenous increase (RGPT1 overexpression) | CII | Sano et al. (1994) | |
| Plasmodiophoromycetes | |||||
| Plasmodiophora brassicae | Arabidopsis thaliana | Endogenous decrease (CKX overexpression) | CIS | Siemens et al. (2011) | |
| Nematodes | |||||
| Heterodera schachtii | Arabidopsis thaliana | Cytokinin-hyper/hyposensitive signalling mutants | CII and CIS | Shanks et al. (2016) | |
CII, cytokinin-induced immunity; CIS, cytokinin-induced susceptibility.
In a breeding programme for new varieties of crops, disease resistance is only one of the several factors taken into account when deciding whether a cultivar may be of commercial significance, and any fitness costs associated with traits that increase resistance to pathogens must be weighed against other traits of agronomical importance, especially crop yield (Brown, 2002). Therefore, understanding of the mechanisms that regulate the growth–defence trade-off in plants is an important step for the production of advanced crop varieties that are both high-yielding and resistant to biotrophic pathogens. Possible applications exist for the breeding and engineering of crop species with enhanced, broad-spectrum, durable disease resistance to biotrophic pathogens with reduced yield penalties by uncoupling defence activation from growth reduction through manipulation of cytokinin levels and signalling.
ACKNOWLEDGEMENTS
We thank Dawn Hajdu for her help in the writing of previous versions of this manuscript. We also thank Joe Strummer, Mick Jones, Paul Simmon and Nick Topper Headon for inspiration. We apologize to colleagues whose work could not be cited due to space limitations. This work was supported by a grant from the US Department of Agriculture to C.T.A.
LITERATURE CITED
- Abreu ME, Munne-Bosch S.. 2009. Salicylic acid deficiency in NahG transgenic lines and sid2 mutants increases seed yield in the annual plant Arabidopsis thaliana. Journal of Experimental Botany 60: 1261–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi A, Fukushima S, Okada K, et al. 2014. WRKY45-dependent priming of diterpenoid phytoalexin biosynthesis in rice and the role of cytokinin in triggering the reaction. Plant Molecular Biology 86: 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso CT, Ferreira FJ, Kieber JJ.. 2009. Environmental perception avenues: the interaction of cytokinin and environmental response pathways. Plant Cell & Environment 32: 1147–1160. [DOI] [PubMed] [Google Scholar]
- Argueso CT, Ferreira FJ, Epple P, et al. 2012. Two-component elements mediate interactions between cytokinin and salicylic acid in plant immunity. PLoS Genetics 8 e1002448. doi:10.1371/journal.pgen.1002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyros RD, Mathews DE, Chiang Y, et al. 2008. Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development. The Plant Cell 20: 2102–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby AM. 2000. Biotrophy and the cytokinin conundrum. Physiological and Molecular Plant Pathology 57: 147–158. [Google Scholar]
- Babosha AV. 2009. Regulation of resistance and susceptibility in wheat-powdery mildew pathosystem with exogenous cytokinins. Journal of Plant Physiology 166: 1892–1903. [DOI] [PubMed] [Google Scholar]
- Bao Z, Yang H, Hua J.. 2013. Perturbation of cell cycle regulation triggers plant immune response via activation of disease resistance genes. Proceedings of the National Academy of Sciences of the USA 110: 2407–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartrina I, Otto E, Strnad M, Werner T, Schmuelling T.. 2011. Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. The Plant Cell 23: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M, Wyrsch I, Strutt J, et al. 2014. Expression patterns of FLAGELLIN SENSING 2 map to bacterial entry sites in plant shoots and roots. Journal of Experimental Botany 65: 6487–6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkhadir Y, Yang L, Hetzel J, Dangl JL, Chory J.. 2014. The growth-defense pivot: crisis management in plants mediated by LRR-RK surface receptors. Trends in Biochemical Sciences 39: 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergelson J, Purrington CB.. 1996. Surveying patterns in the cost of resistance in plants. American Naturalist 148: 536–558. [Google Scholar]
- Bertell G, Eliasson L.. 1992. Cytokinin effects on root-growth and possible interactions with ethylene and indole-3-acetic-acid. Physiologia Plantarum 84: 255–261. [Google Scholar]
- Bjornstad A, Aastveit K.. 1990. Pleiotropic effects on the ML-O mildew resistance gene in barley in different genetic backgrounds. Euphytica 46: 217–226. [Google Scholar]
- Bonfig KB, Gabler A, Simon UK, et al. 2010. Post-translational derepression of invertase activity in source leaves via down-regulation of invertase inhibitor expression is part of the plant defense response. Molecular Plant 3: 1037–1048. [DOI] [PubMed] [Google Scholar]
- Bowling SA, Guo A, Cao H, Gordon AS, Klessig DF, Dong XI.. 1994. A mutation in arabidopsis that leads to constitutive expression of systemic acquired-resistance. The Plant Cell 6: 1845–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SA, Clarke JD, Liu YD, Klessig DF, Dong XN.. 1997. The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. The Plant Cell 9: 1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand U, Fletcher JC, Hobe M, Meyerowitz EM, Simon R.. 2000. Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289: 617–619. [DOI] [PubMed] [Google Scholar]
- Brown JKM. 2002. Yield penalties of disease resistance in crops. Current Opinion in Plant Biology 5: 339–344. [DOI] [PubMed] [Google Scholar]
- Chanclud E, Kisiala A, Emery N, et al. 2016. Cytokinin production by the rice blast fungus is a pivotal requirement for virulence. PLoS Pathogens 12: e1005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Hou B-H, Lalonde S, et al. 2010. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468: 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Huh SU, Kojima M, Sakakibara H, Paek KH, Hwang I.. 2010. The cytokinin-activated transcription factor ARR2 promotes plant immunity via TGA3/NPR1-dependent salicylic acid signaling in Arabidopsis. Developmental Cell 19: 284–295. [DOI] [PubMed] [Google Scholar]
- Clarke SF, Burritt DJ, Jameson PE, Guy PL.. 2000. Effects of plant hormones on white clover mosaic potexvirus double-stranded RNA. Plant Pathology 49: 428–434. [Google Scholar]
- Conrath U, Beckers GJM, Langenbach CJG, Jaskiewicz MR.. 2015. Priming for enhanced defense. Annual Review of Phytopathology 53: 97–119. [DOI] [PubMed] [Google Scholar]
- Dello Ioio R, Nakamura K, Moubayidin L, et al. 2008. A genetic framework for the control of cell division and differentiation in the root meristem. Science 322: 1380–1384. [DOI] [PubMed] [Google Scholar]
- Ehness R, Roitsch T.. 1997. Co-ordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. The Plant Journal 11: 539–548. [DOI] [PubMed] [Google Scholar]
- Essmann J, Schmitz-Thom I, Schon H, Sonnewald S, Weis E, Scharte J.. 2008. RNA interference-mediated repression of cell wall invertase impairs defense in source leaves of tobacco. Plant Physiology 147: 1288–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher LC, Brand U, Running MP, Simon R, Meyerowitz EM.. 1999. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science, 283: 1911–1914. [DOI] [PubMed] [Google Scholar]
- Fotopoulos V, Gilbert MJ, Pittman JK, et al. 2003. The monosaccharide transporter gene, AtSTP4, and the cell-wall invertase, At beta fruct1, are induced in Arabidopsis during infection with the fungal biotroph Erysiphe cichoracearum. Plant Physiology 132: 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan SS, Amasino RM.. 1995. Inhibition of leaf senescence by autoregulated production of cytokinin. Science 270: 1986–1988. [DOI] [PubMed] [Google Scholar]
- Glazebrook J, Chen WJ, Estes B, et al. 2003. Topology of the network integrating salicylate and jasmonate signal transduction derived from global expression phenotyping. The Plant Journal 34: 217–228. [DOI] [PubMed] [Google Scholar]
- Gomez-Gomez L, Felix G, Boller T.. 1999. A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. The Plant Journal 18: 277–284. [DOI] [PubMed] [Google Scholar]
- Grosskinsky DK, Naseem M, Abdelmohsen UR, et al. 2011. Cytokinins mediate resistance against Pseudomonas syringae in tobacco through increased antimicrobial phytoalexin synthesis independent of salicylic acid signaling. Plant Physiology 157: 815–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdoun S, Zhang C, Gill M, et al. 2016. Differential roles of two homologous cyclin-dependent kinase inhibitor genes in regulating cell cycle and innate immunity in Arabidopsis. Plant Physiology 170: 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann DR, Dominguez-Ferreras A, Motyka V, et al. 2014. The Pseudomonas type III effector HopQ1 activates cytokinin signaling and interferes with plant innate immunity. New Phytologist 201: 585–598. [DOI] [PubMed] [Google Scholar]
- Hartig K, Beck E.. 2005. Endogenous cytokinin oscillations control cell cycle progression of tobacco BY-2 cells. Plant Biology 7: 33–40. [DOI] [PubMed] [Google Scholar]
- Hayes MA, Feechan A, Dry IB.. 2010. Involvement of abscisic acid in the coordinated regulation of a stress-inducible hexose transporter (VvHT5) and a cell wall invertase in grapevine in response to biotrophic fungal infection. Plant Physiology 153: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Pischke MS, Mahonen AP, et al. 2004. In planta functions of the Arabidopsis cytokinin receptor family. Proceedings of the National Academy of Sciences of the USA 101: 8821–8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst K, Schmulling T, Werner T.. 2011. Enhanced cytokinin degradation in leaf primordia of transgenic Arabidopsis plants reduces leaf size and shoot organ primordia formation. Journal of Plant Physiology 168: 1328–1334. [DOI] [PubMed] [Google Scholar]
- Houba-Herin N, Pethe C, d'Alayer J, Laloue M.. 1999. Cytokinin oxidase from Zea mays: purification, cDNA cloning and expression in moss protoplasts. Plant Journal 17: 615–626. [DOI] [PubMed] [Google Scholar]
- Huot B, Yao J, Montgomery BL, He SY.. 2014. Growth-defense tradeoffs in plants: a balancing act to optimize fitness. Molecular Plant 7: 1267–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison CE, Li J, Argueso C, et al. 2006. The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. The Plant Cell 18: 3073–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Sheen J, Muller B.. 2012. Cytokinin signaling networks. Annual Review of Plant Biology 63: 353–380. [DOI] [PubMed] [Google Scholar]
- Jiang CJ, Shimono M, Sugano S, et al. 2013. Cytokinins act synergistically with salicylic acid to activate defense gene expression in rice. Molecular Plant-Microbe Interactions 26: 287–296. [DOI] [PubMed] [Google Scholar]
- Kakimoto T. 2001. Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate: ATP/ADP isopentenyltransferases. Plant Cell Physiology 42: 677–685. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Schaller GE.. 2014. Cytokinins. The Arabidopsis Book 12: e0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Ryu H, Hong SH, et al. 2006. Cytokinin-mediated control of leaf longevity by AHK3 through phosphorylation of ARR2 in Arabidopsis. Proceedings of the National Academy of Sciences of the USA 103: 814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleessen S, Laitinen R, Fusari CM, et al. 2014. Metabolic efficiency underpins performance trade-offs in growth of Arabidopsis thaliana. Nature Communications 5: 3537. doi:10.1038/ncomms4537. [DOI] [PubMed] [Google Scholar]
- Ko KW, Okada K, Koga J, Jikumaru Y, Nojiri H, Yamane H.. 2010. Effects of cytokinin on production of diterpenoid phytoalexins in rice. Journal of Pesticide Science 35: 412–418. [Google Scholar]
- Kudo T, Kiba T, Sakakibara H.. 2010. Metabolism and long-distance translocation of cytokinins. Journal of Integrative Plant Biology 52: 53–60. [DOI] [PubMed] [Google Scholar]
- Kurakawa T, Ueda N, Maekawa M, et al. 2007. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445: 652–655. [DOI] [PubMed] [Google Scholar]
- Kuroha T, Tokunaga H, Kojima M, et al. 2009. Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis. The Plant Cell 21: 3152–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara MEB, Garcia MCG, Fatima T, et al. 2004. Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. The Plant Cell 16: 1276–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys F, Dewitte W, Witters E, Van Montagu M, Inze D, Van Onckelen H.. 1998. Zeatin is indispensable for the G(2)-M transition in tobacco BY-2 cells. FEBS Letters 426: 29–32. [DOI] [PubMed] [Google Scholar]
- Lee ST, Huang WL.. 2013. Cytokinin, auxin, and abscisic acid affects sucrose metabolism conduce to de novo shoot organogenesis in rice (Oryza sativa L.) callus. Botanical Studies 54: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibfried A, To JPC, Busch W, et al. 2005. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438: 1172–1175. [DOI] [PubMed] [Google Scholar]
- Li X, Clarke JD, Zhang YL, Dong XN.. 2001. Activation of an EDS1-mediated R-gene pathway in the snc1 mutant leads to constitutive, NPR1-independent pathogen resistance. Molecular Plant-Microbe Interactions 14: 1131–1139. [DOI] [PubMed] [Google Scholar]
- Li X, Huang L, Hong Y, et al. 2015. Co-silencing of tomato S-adenosylhomocysteine hydrolase genes confers increased immunity against Pseudomonas syringae pv. tomato DC3000 and enhanced tolerance to drought stress. Frontiers in Plant Science 6. doi:10.3389/fpls.2015.00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay DL, Sawhney VK, Bonham-Smith PC.. 2006. Cytokinin-induced changes in CLAVATA1 and WUSCHEL expression temporally coincide with altered floral development in Arabidopsis. Plant Science 170: 1111–1117. [Google Scholar]
- Lopez-Carbonell M, Moret A, Nadal M.. 1998. Changes in cell ultrastructure and zeatin riboside concentrations in Hedera helix, Pelargonium zonale, Prunus avium, and Rubus ulmifolius leaves infected by fungi. Plant Disease 82: 914–918. [DOI] [PubMed] [Google Scholar]
- Lozano-Duran R, Zipfel C.. 2015. Trade-off between growth and immunity: role of brassinosteroids. Trends in Plant Science 20: 12–19. [DOI] [PubMed] [Google Scholar]
- Ma QH, Lin ZB, Fu DZ.. 2002. Increased seed cytokinin levels in transgenic tobacco influence embryo and seedling development. Functional Plant Biology 29: 1107–1113. [DOI] [PubMed] [Google Scholar]
- Masuta C, Tanaka H, Uehara K, Kuwata S, Koiwai A, Noma I.. 1995. Broad resistance to plant-viruses in transgenic plants conferred by antisense inhibition of a host gene essential in S-adenosylmethionine-dependent transmethylation reactions. Proceedings of the National Academy of Sciences of the USA 92: 6117–6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer KFX, Schoof H, Haecker A, Lenhard M, Jurgens G, Laux T.. 1998. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95: 805–815. [DOI] [PubMed] [Google Scholar]
- Miller CO, Skoog F, Vonsaltza MH, Strong FM.. 1955. Kinetin, a cell division factor from deoxyribonucleic acid. Journal of the American Chemical Society 77: 1392–1392. [Google Scholar]
- Miller CO, Skoog F, Okumura FS, Vonsaltza MH, Strong FM.. 1956. Isolation, structure and synthesis of kinetin, a substance promoting cell division. Journal of the American Chemical Society 78: 1375–1380. [Google Scholar]
- Miyawaki K, Tarkowski P, Matsumoto-Kitano M, et al. 2006. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proceedings of the National Academy of Sciences of the USA 103: 16598–16603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothes K, Engelbrecht L.. 1961. Kinetin-induced directed transport of substances in excised leaves in the dark. Phytochemistry 1: 58–62. [Google Scholar]
- Naseem M, Philippi N, Hussain A, Wangorsch G, Ahmed N, Dandekar T.. 2012. Integrated systems view on networking by hormones in Arabidopsis immunity reveals multiple crosstalk for cytokinin. The Plant Cell 24: 1793–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura C, Ohashi Y, Sato S, Kato T, Tabata S, Ueguchi C.. 2004. Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. The Plant Cell 16: 1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak J, Pavlu J, Novak O, et al. 2013. High cytokinin levels induce a hypersensitive-like response in tobacco. Annals of Botany 112: 41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortelli S, Winzeler H, Winzeler M, Fried PM, Nosberger J.. 1996. Leaf rust resistance gene Lr9 and winter wheat yield reduction. Yield and yield components. Crop Science 36: 1590–1595. [Google Scholar]
- Pertry I, Vaclavikova K, Depuydt S, et al. 2009. Identification of Rhodococcus fascians cytokinins and their modus operandi to reshape the plant. Proceedings of the National Academy of Sciences of the USA 106: 929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petutschnig EK, Jones AME, Serazetdinova L, Lipka U, Lipka V.. 2010. The lysin motif receptor-like kinase (LysM-RLK) CERK1 is a major chitin-binding protein in Arabidopsis thaliana and subject to chitin-induced phosphorylation. Journal of Biological Chemistry 285: 28902–28911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogany M, Koehl J, Heiser I, Elstner EF, Barna B.. 2004. Juvenility of tobacco induced by cytokinin gene introduction decreases susceptibility to Tobacco necrosis virus and confers tolerance to oxidative stress. Physiological and Molecular Plant Pathology 65: 39–47. [Google Scholar]
- Punwani JA, Hutchison CE, Schaller GE, Kieber JJ.. 2010. The subcellular distribution of the Arabidopsis histidine phosphotransfer proteins is independent of cytokinin signaling. The Plant Journal 62: 473–482. [DOI] [PubMed] [Google Scholar]
- Redig P, Shaul O, Inze D, VanMontagu M, VanOnckelen H.. 1996. Levels of endogenous cytokinins, indole-3-acetic acid and abscisic acid during the cell cycle of synchronized tobacco BY-2 cells. FEBS Letters 391: 175–180. [DOI] [PubMed] [Google Scholar]
- Riefler M, Novak O, Strnad M, Schmulling T.. 2006. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. The Plant Cell 18: 40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JAH.. 1999. Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283: 1541–1544. [DOI] [PubMed] [Google Scholar]
- Roitsch T, Ehness R.. 2000. Regulation of source/sink relations by cytokinins. Plant Growth Regulation 32: 359–367. [Google Scholar]
- Sano H, Seo S, Orudgev E, Youssefian S, Ishizuka K, Ohashi Y.. 1994. Expression of the gene for a small GTP-binding protein in transgenic tobacco elevates endogenous cytokinin levels, abnormally induces salicylic-acid in response to wounding, and increases resistance to tobacco mosaic virus infection. Proceedings of the National Academy of Sciences of the USA 91: 10556–10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer S, Krolzik S, Romanov GA, Schmulling T.. 2000. Cytokinin-regulated transcripts in tobacco cell culture. Plant Growth Regulation 32: 307–313. [Google Scholar]
- Schaller GE, Street IH, Kieber JJ.. 2014. Cytokinin and the cell cycle. Current Opinion in Plant Biology 21: 7–15. [DOI] [PubMed] [Google Scholar]
- Schaller GE, Bishopp A, Kieber JJ.. 2015. The yin-yang of hormones: cytokinin and auxin interactions in plant development. The Plant Cell 27: 44–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof H, Lenhard M, Haecker A, Mayer KFX, Jurgens G, Laux T.. 2000. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100: 635–644. [DOI] [PubMed] [Google Scholar]
- Shanks CM, Rice JH, Yan ZB, Schaller GE, Hewezi T, Kieber JJ.. 2016. The role of cytokinin during infection of Arabidopsis thaliana by the cyst nematode Heterodera schachtii. Molecular Plant-Microbe Interactions 29: 57–68. [DOI] [PubMed] [Google Scholar]
- Shi XL, Gupta S, Rashotte AM.. 2014. Characterization of two tomato AP2/ERF genes, SlCRF1 and SlCRF2 in hormone and stress responses. Plant Cell Reports 33: 35–45. [DOI] [PubMed] [Google Scholar]
- Shimono M, Sugano S, Nakayama A, et al. 2007. Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. The Plant Cell 19: 2064–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemens J, Gonzalez MC, Wolf S, et al. 2011. Extracellular invertase is involved in the regulation of clubroot disease in Arabidopsis thaliana. Molecular Plant Pathology 12: 247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman P, Seskar M, Kanter D, Schweizer P, Metraux JP, Raskin I.. 1995. Salicylic acid in rice (biosynthesis, conjugation, and possible role). Plant Physiology 108: 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smigocki AC, Owens LD.. 1988. Cytokinin gene fused with a strong promoter enhances shoot organogenesis and zeatin levels in transformed plant cells. Proceedings of the National Academy of Sciences of the USA 85: 5131–5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda N, Iwai H, Satoh S, Sakai S.. 2009. Benzyladenine arrests cell cycle progression in G1 phase in tobacco BY-2 cells preceding induction of cell death. Plant Biotechnology 26: 225–235. [Google Scholar]
- Sugano S, Jiang CJ, Miyazawa S, et al. 2010. Role of OsNPR1 in rice defense program as revealed by genome-wide expression analysis. Plant Molecular Biology 74: 549–562. [DOI] [PubMed] [Google Scholar]
- Tauzin AS, Giardina T.. 2014. Sucrose and invertases, a part of the plant defense response to the biotic stresses. Frontiers in Plant Science 5: 293. doi:org/10.3389/fpls.2014.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D, Traw MB, Chen JQ, Kreitman M, Bergelson J.. 2003. Fitness costs of R-gene-mediated resistance in Arabidopsis thaliana. Nature 423: 74–77. [DOI] [PubMed] [Google Scholar]
- To JPC, Haberer G, Ferreira FJ, et al. 2004. Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. The Plant Cell 16: 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters DR, McRoberts N, Fitt BDL.. 2008. Are green islands red herrings? Significance of green islands in plant interactions with pathogens and pests. Biological Reviews 83: 79–102. [DOI] [PubMed] [Google Scholar]
- Wan J, Zhang X, Neece D, et al. 2008. A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. The Plant Cell 20: 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Pajerowska-Mukhtar K, Culler AH, Dong X.. 2007. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Current Biology 17: 1784–1790. [DOI] [PubMed] [Google Scholar]
- Wang S, Gu Y, Zebell SG, et al. 2014. A noncanonical role for the CKI-RB-E2F cell-cycle signaling pathway in plant effector-triggered immunity. Cell Host & Microbe 16: 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmulling T.. 2003. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. The Plant Cell 15: 2532–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Holst K, Pors Y, et al. 2008. Cytokinin deficiency causes distinct changes of sink and source parameters in tobacco shoots and roots. Journal of Experimental Botany 59: 2659–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhang D, Chu JY, et al. 2012. The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Reports 1: 639–647. [DOI] [PubMed] [Google Scholar]
- Zavaleta-Mancera HA, Lopez-Delgado H, Loza-Tavera H, et al. 2007. Cytokinin promotes catalase and ascorbate peroxidase activities and preserves the chloroplast integrity during dark-senescence. Journal of Plant Physiology 164: 1572–1582. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Du BJ, Qian J, Zou BH, Hua J.. 2013. Disease resistance gene-induced growth inhibition is enhanced by rcd1 independent of defense activation in Arabidopsis. Plant Physiology 161: 2005–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]