Abstract
Background
Atherogenic dyslipidemia is an important modifiable risk factor for cardiovascular disease among patients of type 2 diabetes mellitus. Timely detection and characterization of this condition help clinicians estimate future risk of cardiovascular disease and take appropriate preventive measures. The aim of this study was to determine the prevalence, pattern and predictors of dyslipidemia in a cohort of Nepalese patients with type 2 diabetes.
Results
We found mixed dyslipidemia as the most prevalent (88.1%) and isolated dyslipidemia (10.1%) as the least prevalent forms of dyslipidemia in our patients. The most prevalent form of single dyslipidemia was high LDL-C (73.8%) and combined dyslipidemia was high TG, high LDL-C and low HDL-C (44.7%). Prevalence of all single and mixed dyslipidemia was higher in patients with poor glycemic control and hypertension. The glycemic status of patients correlated with their fasting serum lipid profile. Dyslipidemia was associated mainly with male gender, poor glycemic control and hypertension.
Conclusions
Atherogenic dyslipidemia is associated mainly with male gender, poor glycemic control and hypertension. It is highly prevalent in Nepalese patients with type 2 diabetes. Urgent lifestyle modification, sustained glycemic control and aggressive lipid lowering treatment plans are necessary to minimize the future risk of cardiovascular disease in this population.
Electronic supplementary material
The online version of this article (doi:10.1186/s13104-017-2465-4) contains supplementary material, which is available to authorized users.
Keywords: Type 2 diabetes, Dyslipidemia, Cardiovascular disease, Prevalence, Pokhara, Nepal
Background
Type 2 diabetes mellitus (T2DM) is the third major non-communicable disease in Nepal, and is approaching pandemic levels due to rapid change in socioeconomic status and life-style of the people [1]. T2DM amplifies the risk of cardiovascular disease (CVD) several fold, making it a significant risk factor of the latter. More than 50% patients with T2DM die due to coronary heart disease (CHD) [2, 3]. Among several modifiable and non-modifiable risk factors for CVD, T2DM is the strongest, as it is strongly associated with atherogenic dyslipidemia [4, 5]. The atherogenic dyslipidemia in diabetic patients results from insulin deficiency or resistance that promotes lipolysis in the visceral adipocytes and increases the flux of free fatty acids in plasma and liver. Moreover, the activity of an endothelial enzyme, lipoprotein lipase, also decreases. These conditions lead to hepatic steatosis, over-secretion of larger triglyceride (TG)-rich very low density lipoprotein 1(VLDL1) particles into the plasma, over-secretion of hepatic apolipoprotein B (ApoB), impaired clearance of chylomicrons and decreased receptor mediated endocytosis in the liver [6, 7]. The most common phenotypic pattern of diabetic dyslipidemia involves lipid triad with raised triglycerides, reduced high density lipoprotein cholesterol (HDL-C) and increased concentration of small, dense low density lipoprotein cholesterol (LDL-C) particles [8, 9]. Additionally, total cholesterol (TC)/HDL-C ratio, non-HDL-C and ApoB have also been shown to be directly involved in the atherogenic process and development of CVD [2, 10]. Diabetic dyslipidemia has therefore emerged as an important biomarker for the increased CVD risk observed in diabetic patients. Significant reduction of CVD related morbidity and mortality by lipid-lowering agents such as statins underscores their importance in the cardiovascular health of diabetic patients [11]. Therefore, early detection and aggressive management of dyslipidemia are very important in saving the lives of diabetic patients from atherogenic cardiovascular diseases.
The Western hilly region of Nepal is mostly populated by ethnic groups like Gurung, Magars and Dalits which differentiates this population genetically from other areas of Nepal. The socioeconomic status, life-style, dietary habit and cultural practices of these ethnic groups make them more vulnerable to high incidence of CVD than any other ethnic groups in this region. Few recent hospital based studies and our own clinical observations have shown that the prevalence of metabolic syndrome and cardiovascular disease is very high in this region particularly among these three ethnic groups [12, 13]. Atherogenic dyslipidemia is a major modifiable risk factor of CVD. However, no systematic study has been carried out so far in this region to map the actual epidemiology of CVD risk factors including the dyslipidemia. Given that the prevalence and pattern of CVD risk factors differ according to geographic location, ethnicity, dietary habits and socio-economic status of the population under study, we hypothesize that epidemiology of dyslipidemia is different for the population of this region compared to others. Our study thus aims to describe the prevalence, pattern and independent predictors of dyslipidemia among type 2 diabetic patients of Western hilly region of Nepal.
Methods
Study design
We conducted a cross-sectional study on patients with type 2 diabetes aged 30–74 years from July 2013 to December 2014.
Study setting
The study was carried out at the Manipal Teaching Hospital (MTH), Pokhara Nepal which is one of the largest multi-specialty tertiary care hospital attached to Manipal College of Medical Sciences (MCOMS). This hospital provides clinical trainings to both Nursing and Medical students in addition to providing comprehensive healthcare services to the general public of hilly districts of Western Development Region of Nepal.
Sample size and selection criteria
We enrolled a total of 497 diabetic patients originating mainly from Gandaki, Dhaulagiri, Lumbini zones of the Western Development Region of Nepal. They were randomly selected from the list of outpatients who were clinically examined in medicine and other outpatient departments and approached the sample collection unit of MTH to have their blood glucose, HbA1c, lipid profile and other parameters measured. All those randomly selected diabetic patients who provided informed consent were enrolled in the study without regard to their treatment for dyslipidemia. The presence of diabetes was confirmed based on their previous medical records, clinical examination and past or current laboratory results. Repeated inclusion of the same patients was avoided by using a filter that consisted of their unique hospital number, full name and age.
Interview, anthropometry and measurement of physiological variables
The demographic data of the patients including their personal and family medical history, smoking and dietary habit were collected by interviewing them with a pre-validated set of questionnaire (see Additional file 1). Anthropometric parameters such as height, weight, body mass index (BMI), waist circumference and blood pressures were measured following standard protocols.
Measurements of biochemical variables
Following the interview and anthropometry, patients were asked to return the next morning for blood collection in overnight (≥8 h) fasting state. Five ml venous blood was drawn from each subject and then divided into fluoride-oxalate, ethylene diamine tetraacetate (EDTA) and plain test tubes. All direct biochemical measurements were made using automated chemistry analyzer and ready-to-use reagent kits according to the standardized protocols provided by the manufacturers (Erbachem XL-300, Germany). All tests were run in duplicate and appropriate standards and quality control sera were used to ensure the accuracy of the measurements. Fasting glucose was measured in fluoridated plasma by glucose oxidase/peroxidase method. TC and TG were estimated by cholesterol oxidase/peroxidase and glycerol phosphate kinase methods, respectively. HDL-C was measured by phosphotungstate precipitation method. The value of LDL-C was calculated using Friedwald formula [14]. Non-HDL-C was calculated by subtracting HDL-C from TC. Total ApoB was estimated using the following equations: ApoB = 0.65 × TC−0.59 × HDL-C + 0.01 × TG when TG < 270 mg/dl and ApoB = 25.6 + 0.58 × TC−0.38 × HDL-C−0.06 × TG when TG > 270 mg/dl [15]. Glycated hemoglobin (HbA1c) was measured on EDTA blood by ion-exchange resin method.
Definitions
Dyslipidemia was defined according to the third report of National Cholesterol Education Program Adult Treatment Panel (NCEP ATP III) criteria [2] with the following cut off values: hypercholesterolemia-serum TC level ≥200 mg/dl; hypertriglyceridemia–serum TG level ≥150 mg/dl; low HDL-C–HDL-C level ≤40 mg/dl for both men and women; high LDL-C–LDL-C level ≥100 mg/dl and high TC/HDL-C ratio ≥5, high non-HDL-C ≥ 130 mg/dl and high ApoB ≥ 90 mg/dl. Mixed or combined dyslipidemia was defined as the combination of two or more single dyslipidemia mentioned before. Isolated dyslipidemia was defined as follows: isolated hypercholesterolemia-serum TC ≥ 200 mg/dl and TG < 150 mg/dl; isolated hypertriglyceridemia–serum TG ≥ 150 mg/dl and TC < 200 mg/dl; isolated low HDL-C–HDL-C < 40 mg/dl (male and female) without hypertriglyceridemia, hypercholesterolemia and high LDL-C; and isolated high LDL-C–LDL-C > 100 mg/dl without hypertriglyceridemia, hypercholesterolemia or low HDL-C.
Patients were designated as to having type 2 diabetes when they had already diagnosed by a physician at the age of 30 or above and on oral hypoglycemic drugs and/or those who had plasma glucose levels above the cut off values recommended by the WHO guidelines i.e. fasting blood glucose ≥126 mg/dl and/or 2 h post meal or random blood glucose value ≥200 mg/dl [16]. Hypertension was deemed to be present when patients were already diagnosed by a physician and were on anti-hypertensive medications and/or those who had systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg as suggested by the Joint National Committee 7 (JNC7) criteria [17]. Generalized and central obesity were defined according to the Asia Pacific guidelines for South Asians [18]. According to this, patients were categorized as overweight if their BMI was between 23 and 25 kg/m2 and obese if their BMI was ≥25 kg/m2. Central obesity was defined as waist circumference ≥90 cm (males), ≥80 cm (females). Poor diabetic control was defined as HBA1c > 7%.
Statistical analyses
The data were analyzed using Microsoft Excel 2007 and SPSS for Windows version 17.0 (SPSS, Inc., Chicago, IL). Continuous variables were reported as mean ± standard deviation (SD) and categorical variables were reported in numbers and/or percentage. The Student’s t test was used to compare two continuous variables, and Chi square test was used to compare categorical variables. One-way analysis of variance (ANOVA) was used to compare three or more subgroups of a continuous variable. Pearson’s bivariate correlation analysis was performed to assess the correlation between dyslipidemia and other independent variables. Multivariate logistic regression was carried out to identify the risk factors that are independently associated with single and mixed dyslipidemia. Statistical significance was set at a p value <0.05.
Results
A total of 497 type 2 diabetic patients, 36.2% females and 63.8% males, were enrolled in the present study, Their mean age was 52.7 ± 10.5 years while mean diabetes duration was 5.1 ± 3.8 years. Among these, 176 (35.4%) patients were not taking any anti-diabetic drugs, 282 (56.7%) were taking only oral hypoglycemic drugs, 26 (5.2%) were taking both oral hypoglycemic drugs and insulin, and remaining 13 (2.6%) were taking only insulin for controlling their blood glucose levels. Their demographic, anthropometric and biochemical characteristics are presented in Table 1. Males were significantly (p < 0.010) older, overweight or obese than females. Majority of the patients were urban residents (74.2%), non-smokers (72.8%) and non-vegetarians (92.8%). Prevalence of smoking habit (36.9%), general obesity (36.3%) and central obesity (51.7%) was significantly higher in males than in females (p < 0.010). There were 24.1% patients with poor glycemic control (HbA1c > 7.0%). The fasting plasma glucose level, duration of diabetes and hypertension, glycemic status and blood pressures did not differ significantly between males and females (p > 0.050).
Table 1.
General and biochemical characteristics of the diabetic patients
| Characteristics | Female (n = 180) | Male (n = 317) | p value | Total (n = 497) |
|---|---|---|---|---|
| Age (year) | 55.6 ± 9.2 | 51.2 ± 10.9 | 0.000 | 52.7 ± 10.5 |
| BMI (kg/m2) | 23.9 ± 2.7 | 24.3 ± 2.2 | 0.069 | 24.2 ± 2.4 |
| Overweight | 68 (37.8) | 126 (39.7) | 0.003 | 194 (39.0) |
| Obese | 45 (25.0) | 115 (36.3) | 160 (32.2) | |
| Waist circumference (cm) | 90.2 ± 8.0 | 94.5 ± 6.7 | 0.000 | 92.9 ± 7.5 |
| Centrally obese | 170 (94.4) | 257 (81.1) | 0.000 | 427 (85.9) |
| Residence | ||||
| Village | 47 (26.1) | 81 (25.6) | 0.886 | 128 (25.8) |
| Urban | 133 (73.9) | 236 (74.4) | 369 (74.2) | |
| Current smokers | 18 (10.0) | 117 (36.9) | 0.000 | 135 (27.2) |
| Diet | ||||
| Vegetarian | 19 (10.6) | 17 (5.4) | 0.032 | 36 (7.2) |
| Non-vegetarian | 161 (89.4) | 300 (94.6) | 461 (92.8) | |
| Fating plasma glucose (mg/dl) | 138.2 ± 40.6 | 134.2 ± 44.0 | 0.314 | 135.7 ± 42.8 |
| DM duration (year) | 5.3 ± 3.8 | 4.9 ± 3.9 | 0.224 | 5.1 ± 3.8 |
| HbA1c (%) | 6.4 ± 0.9 | 6.4 ± 0.9 | 0.932 | 6.4 ± 0.9 |
| Glycemic control | ||||
| Good (HbA1c<7%) | 138 (76.7) | 239 (75.4) | 0.750 | 377 (75.9) |
| Poor (HbA1C>7%) | 42 (23.3) | 78 (24.6) | 120 (24.1) | |
| SBP (mmHg) | 125.1 ± 14.0 | 125.8 ± 11.6 | 0.562 | 125.5 ± 12.5 |
| DBP (mmHg) | 80.9 ± 9.2 | 82.2 ± 7.6 | 0.107 | 81.7 ± 8.2 |
| Hypertension | 75 (41.7) | 130 (41.0) | 0.003 | 205 (41.2) |
| Duration of HTN (year) | 1.9 ± 3.6 | 1.6 ± 3.6 | 0.345 | 1.7 ± 3.6 |
The results are presented as mean ± SD for continuous variables and n (%) for categorical variables
a p < 0.001
b p < 0.05
c p > 0.05 (two tailed); groups were compared using Students t test for quantitative variables and Chi square test for categorical variables
The age- and sex specific values of serum lipid parameters are presented in Fig. 1. Among all the measured lipid parameters, only the serum TG level was higher in males (p < 0.050). The serum TG and HDL-C levels decreased while other lipid parameters increased with age. Gender-wise analysis showed that such age-specific variation of serum lipid parameters was more obvious in males than in females. Serum lipid levels and ratio were either unchanged or decreased with age in female patients. A one-way between-groups analysis of variance was conducted to explore the impact of age on serum lipid parameters. The difference in concentrations and ratio of serum lipid parameters for the three age groups (30–44, 45–59 and 60–74 years) was statistically significant except for TG. Post-hoc comparisons using the Tukey test indicated that the mean serum levels of TC, HDL-C, LDL-C, non-HDL-C and ApoB and TC/HDL-C ratio for age group 30–44 years were significantly different (p < 0.050) from age groups 45–59 and 60–64 years. Serum LDL-C concentration and TC/HDL-C ratio of age group 45–59 years differed from either age groups while serum concentrations of TC and non-HDL-C differed only with age group 30–44 years. No age group specific variation was found in the serum lipid parameters for females. In males, the serum concentrations of TC, LDL-C, non-HDL-C, ApoB and TC/HDL-C ratio of age group 30–44 years differed significantly from age groups 45–59 and 60–74 years. Only LDL-C concentration of age group 45–59 years differed significantly from age groups 30–44 and 60–74 years. Serum TC, non-HDL-C, ApoB concentrations and TC/HDL-C ratio of age group 45–59 years differed significantly only from age group 30–44 years.
Fig. 1.
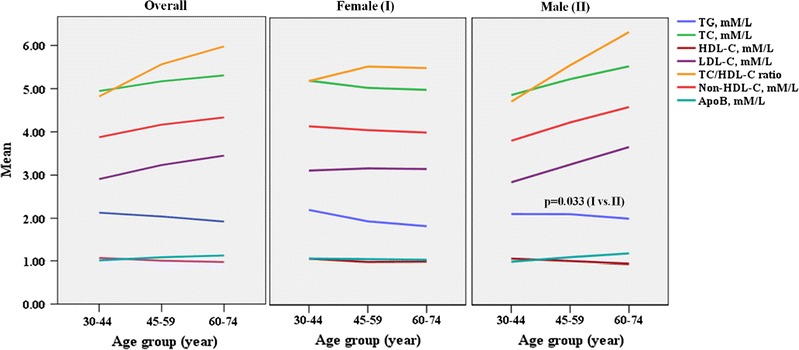
Age- and sex specific mean values of serum lipid parameters and their ratio in diabetic patients. Student's t-test was used to compare the group means of female (I) and male (II) patients. One-way ANOVA was used for comparing serum levels of single lipid parameter within three different age groups (30–44, 45–59 and 60–74). ***p for trend <0.001, **p for trend <0.010 *p for trend <0.05 (two tailed). TG, triglycerides; TC, total cholesterol; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; ApoB, apolipoprotein B. Mean refers to the serum mean concentrations and ratio of lipid parameters
Prevalence of single and mixed dyslipidemia has been presented in Table 2. The most prevalent single lipid disorder was increased non-HDL-C (75.5%) while the least prevalent was hypercholesterolemia (43.7%). Prevalence of mixed dyslipidemia was 88.1%. Prevalence of high LDL-C, non-HDL-C, ApoB and mixed dyslipidemia was significantly higher among males (p < 0.05).
Table 2.
Age-and sex specific prevalence of single and mixed dyslipidemia in diabetic patients
| Overall | Female | Male | p value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 30–44 | 45–59 | 60–74 | Total | 30–44 | 45–59 | 60–74 | Total | 30–44 | 45–59 | 60–74 | ||
| n | 497 | 123 | 214 | 160 | 180 | 24 | 85 | 71 | 317 | 99 | 129 | 89 | |
| Hypertriglyceridemia (%) | 63.8 | 67.5 | 68.2 | 55.0** | 60.0 | 70.8 | 63.5 | 52.1 | 65.9 | 66.7 | 71.3 | 57.3 | 0.186 |
| Hypercholesterolemia (%) | 43.7 | 35 | 44.9 | 48.8 | 41.1 | 54.2 | 37.6 | 40.8 | 45.1 | 30.3 | 49.6 | 55.1*** | 0.388 |
| Low HDL-C (%) | 44.5 | 35 | 48.1 | 46.9 | 45.0 | 41.7 | 48.2 | 42.3 | 44.2 | 33.3 | 48.1 | 40.6 | 0.744 |
| High LDL-C (%) | 73.8 | 69.9 | 75.2 | 75.0 | 68.3 | 70.8 | 69.4 | 66.2 | 77.0 | 69.7 | 79.1 | 82.0 | 0.035 |
| High TC/HDL-C ratio (%) | 45.3 | 35.8 | 48.1 | 48.8 | 43.9 | 41.7 | 44.7 | 43.7 | 46.1 | 34.3 | 50.4 | 52.8* | 0.641 |
| High non-HDL-C (%) | 75.5 | 74.0 | 78.0 | 73.1 | 70.0 | 79.2 | 72.9 | 63.4 | 78.5 | 72.7 | 81.4 | 80.9 | 0.033 |
| High ApoB (%) | 73.6 | 72.4 | 76.6 | 70.6 | 67.8 | 79.2 | 68.2 | 63.4 | 77.0 | 70.7 | 82.2 | 76.4 | 0.025 |
| Mixed dyslipidemia (%) | 88.1 | 87.0 | 91.1 | 85.0 | 88.2 | 83.3 | 88.2 | 78.9 | 93.0 | 87.9 | 93.0 | 89.9 | 0.028 |
p for trend: *** p < 0.001, ** p < 0.010 * p < 0.05 (two tailed) compared to other subgroups within a category using ANOVA; the group means between male and females were compared using Student’s t-test. Dyslipidemia was diagnosed using NCEP ATP III guidelines- hypercholesterolemia: total cholesterol (TC) ≥200 mg/dl; hypertriglyceridemia: triglycerides (TG) ≥150 mg/dl; low HDL cholesterol (HDL-C): HDL-C < 40 mg/dl (both males and females); high LDL cholesterol (LDL-C): LDL-C ≥ 100 mg/dl; high total cholesterol: HDL-C ratio ≥5; High non-HDL-C: non-HDL-C > 130 mg/dl; High apolipoprotein B (ApoB): ApoB > 90 mg/dl; mixed dyslipidemia: when one or more of these lipid parameters were above the cut off values mentioned above
The pattern of dyslipidemia is shown in Fig. 2. Among dyslipidemic patients, there were 36 (8.2%) cases of isolated hypertriglyceridemia, 49 (11.2%) cases of isolated high LDL-C and 13 (3.0%) cases of isolated low HDL-C and no cases of isolated hypercholesterolemia. Likewise, there were 53 (12.1%) cases of combined hypertriglyceridemia and high LDL-C, 21 (4.8%) cases of combined hypertriglyceridemia and low HDL-C, 25 (5.7%) cases of combined high LDL-C and low HDL-C, 24 (5.5%) cases of combined hypertriglyceridemia, low HDL-C and high LDL-C and 119 (27.2%) cases of combined hypertriglyceridemia, hypercholesterolemia, low HDL-C and high LDL-C. Additionally, there were 63 (14.4%) cases of hypercholesterolemia, hypertriglyceridemia and high LDL-C, 16 (3.7%) cases of hypercholesterolemia and high LDL-C, 18 (4.1) case of hypercholesterolemia, low HDL-C and high LDL-C, and 1(0.2%) case of hypercholesterolemia, hypertriglyceridemia and low HDL-C. Prevalence of all single and mixed dyslipidemia was significantly higher (p < 0.001 for all) in patients with poor glycemic control and hypertension. Table 3 presents the prevalence of single and mixed dyslipidemia in diabetic patients based on their characteristics such as duration of diabetes, place of residence, smoking habit, glycemic status and blood pressure. Prevalence of low HDL-C and high TC/HDL-C ratio was significantly higher (p < 0.050) in patients with longer duration of diabetes. Only the prevalence of hypertriglyceridemia was significantly higher (p < 0.050) in non-smoker patients.
Fig. 2.
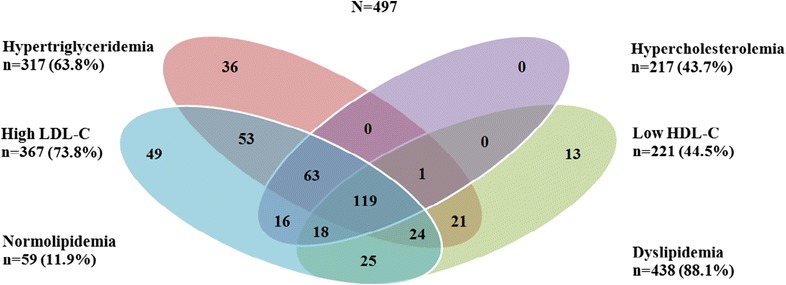
Venn diagram showing the overlapping of the individual components of dyslipidemia (hypertriglyceridemia, hypercholesterolemia, high LDL-C and low HDL-C) in diabetic patients
Table 3.
Prevalence of single and mixed dyslipidemia in diabetic patients based on various characteristics of diabetic patients
| n | High TG (%) | High TC (%) | Low HDL-C (%) | High LDL-C (%) | High TC/HDL-C (%) | High non-HDL-C (%) | High ApoB | Mixed dyslipidemia | |
|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | |||||||||
| <25 | 337 | 58.8 | 40.9 | 43.0 | 70.9 | 42.4 | 73.3 | 71.8 | 83.1 |
| ≥25 | 160 | 74.4 | 49.4 | 47.5 | 80.0 | 51.3 | 80.0 | 77.5 | 92.5 |
| p value | 0.001 | 0.077 | 0.212 | 0.031 | 0.065 | 0.105 | 0.179 | 0.005 | |
| Duration of DM (years) | |||||||||
| ≤7 | 360 | 64.4 | 40.8 | 39.7 | 73.6 | 40.8 | 77.2 | 75.3 | 86.4 |
| 7–13 | 123 | 61.0 | 49.6 | 56.9 | 74.0 | 56.9 | 69.9 | 69.1 | 85.4 |
| ≥14 | 14 | 71.4 | 64.3 | 57.1 | 78.6 | 57.1 | 78.6 | 71.4 | 85.7 |
| p value | 0.656 | 0.069 | 0.016 | 0.917 | 0.006 | 0.257 | 0.399 | 0.960 | |
| Place of residence | |||||||||
| Village | 128 | 65.6 | 46.9 | 42.2 | 77.3 | 43.8 | 79.7 | 78.1 | 87.5 |
| Urban | 369 | 63.1 | 42.5 | 45.3 | 72.6 | 45.8 | 74.0 | 72.1 | 85.6 |
| p value | 0.615 | 0.395 | 0.691 | 0.296 | 0.688 | 0.196 | 0.182 | 0.599 | |
| Current smoker | |||||||||
| No | 135 | 71.9 | 41.5 | 45.2 | 73.3 | 50.4 | 74.8 | 73.3 | 85.9 |
| Yes | 262 | 60.8 | 44.5 | 44.2 | 74 | 43.4 | 75.7 | 73.8 | 86.2 |
| p value | 0.022 | 0.549 | 0.817 | 0.875 | 0.163 | 0.840 | 0.924 | 0.940 | |
| HbA1c (%) | |||||||||
| <7 | 377 | 56.8 | 37.9 | 42.7 | 69.5 | 41.4 | 69.8 | 67.4 | 83.0 |
| >7 | 120 | 85.8 | 61.7 | 50.0 | 87.5 | 57.2 | 93.3 | 93.3 | 95.8 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | |
| Blood pressure | |||||||||
| Normal | 89 | 51 | 32.2 | 37.3 | 67.8 | 35.3 | 67.8 | 65.1 | 80.5 |
| High | 205 | 82.0 | 60 | 54.6 | 82.4 | 59.5 | 86.3 | 85.9 | 94.1 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
a p < 0.001, b p < 0.010 c p < 0.05 (two tailed) compared to other subgroups within a category using ANOVA; Dyslipidemia was diagnosed using NCEP ATP III guideline- hypercholesterolemia: total cholesterol (TC) ≥200 mg/dl; hypertriglyceridemia: triglycerides ≥150 mg/dl; low HDL cholesterol: HDL-C < 40 mg/dl (both males and females); high LDL cholesterol: LDL-C ≥ 100 mg/dl; high total cholesterol: HDL-C ratio ≥5; mixed dyslipidemia: when one or more of these lipid parameters were above the cut off values mentioned above
The results of bivariate correlation analysis are presented in Table 4. All correlations were significant at the level of p < 0.001. Both fasting plasma glucose and HbA1c showed significant (p < 0.001) positive correlations with TG, TC, LDL-C, TC/HDL-C ratio, non-HDL-C, ApoB and mixed dyslipidemia except for HDL-C in which case the correlation was negative. TC, LDL, TC/HDL-C ratio and non-HDL-C showed strong positive correlation with ApoB. TC, HDL-C and LDL-C also showed strong correlation with TC/HDL-C ratio. Non-HDL-C and ApoB showed the highest correlation with mixed dyslipidemia.
Table 4.
Correlation among FPG, HbA1c, lipid parameters and mixed dyslipidemia in diabetic patients
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. FPG | – | 0.574** | 0.279** | 0.258** | −0.274** | 0.223** | 0.264** | 0.274** | 0.270** | 0.150** |
| 2. HbA1c | – | 0.376** | 0.357** | −0.157** | 0.271** | 0.275** | 0.344** | 0.347** | 0.246** | |
| 3. TG | – | 0.446** | −0.309** | 0.205** | 0.392** | 0.452** | 0.440** | 0.362** | ||
| 4. TC | – | −0.481** | 0.954** | 0.857** | 0.986** | 0.990** | 0.468** | |||
| 5. HDL-C | – | −0.574** | −0.801** | −0.595** | −0.586** | −0.350** | ||||
| 6. LDL-C | – | 0.888** | 0.960** | 0.966** | 0.424** | |||||
| 7. TC/HDL-C | – | 0.913** | 0.903** | 0.370** | ||||||
| 8. Non-HDL-C | – | 0.994** | 0.480** | |||||||
| 9. ApoB | – | 0.489** | ||||||||
| 10. Mixed dyslipidemia | – |
**p value (two tailed) significant at the level of <0.001. The Pearson’s correlation coefficient (r) values of ±1 was interpreted as perfect correlation, r values between ±0.7 and ±0.9 as strong correlations, r values in the range ±0.4 to ±0.6 as moderate correlations, r values between ±0.1 and ±0.3 as weak correlations, and r value of 0 as no correlation
Multivariate logistic regression models were used to identify the independent predictors of dyslipidemia in the diabetic patients (Table 5). High LDL-C, high non-HDL-C, high ApoB and mixed dyslipidemia were associated with male gender. Only low HDL-C and high TC/HDL-C ratio were associated with central obesity. Likewise, only hypertriglyceridemia and high TC/HDL-C was associated with current smoking habit. Hypercholesterolemia, low HDL-C, high LDL-C, high TC/HDL-C, high non-HDL-C and high ApoB were associated with fasting hyperglycemia. Hypertriglyceridemia, hypercholesterolemia, high LDL-C, high TC/HDL-C, high non-HDL-C, high ApoB and mixed dyslipidemia were associated with poor glycemic control. Likewise, all forms of dyslipidemia except high LDL-C and mixed dyslipidemia were associated with hypertension. Only hypercholesterolemia was found to be associated with duration of hypertension. All associations between dyslipidemia and risk factors were positive except for duration of diabetes in which case the association was negative.
Table 5.
Association of co-variate risk factors independently associated with dyslipidemia in diabetic patients
| Risk factors | Hypertriglyceridemia | Hypercholesterolemia | Low HDL-C | High LDL-C | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | (OR) (95% CI) | p value | OR (95% CI) | p value | |
| Age (>55 year) | 064 (0.40–1.03) | 0.067 | 0.98 (0.63–1.54) | 0.941 | 1.13 (0.72–1.76) | 0.596 | 1.27 (0.77–2.09) | 0.357 |
| Male gender | 1.09 (0.69–1.72) | 0.720 | 1.29 (0.83–1.99) | 0.245 | 1.24 (0.81–1.90) | 0.324 | 1.65 (1.03–2.65) | 0.039 |
| BMI (>23 kg/m2) | 1.00 (0.64–1.60) | 0.978 | 1.05 (0.67–1.64) | 0.829 | 0.75 (0.48–1.17) | 0.204 | 1.04 (0.64–1.67) | 0.888 |
| Central obesity | 1.10 (0.57–2.10) | 0.781 | 1.14 (0.63–2.06) | 0.660 | 4.03 (2.09–7.77) | <0.001 | 0.83 (0.41–1.70) | 0.616 |
| Urban resident | 1.08 (0.67–1.73) | 0.764 | 0.92 (0.59–1.44) | 0.721 | 1.14 (0.73–1.78) | 0.561 | 0.83 (0.50–1.36) | 0.456 |
| Current smoking | 1.77 (1.03–3.02) | 0.037 | 1.07 (0.65–1.74) | 0.802 | 1.15 (0.71–1.87) | 0.566 | 1.07 (0.62–1.85) | 0.801 |
| Non-vegetarian diet | 1.10 (0.50–2.44) | 0.815 | 0.89 (0.41–1.93) | 0.774 | 0.86 (0.41–1.84) | 0.705 | 1.25 (0.56–2.74) | 0.585 |
| Fasting hyperglycemia | 1.27 (0.82–1.97) | 0.282 | 2.44 (1.57–3.81) | 0.000 | 3.00 (1.92–4.67) | <0.001 | 2.16 (1.39–3.37) | 0.001 |
| Poor glycemic control | 3.86 (2.11–7.07) | <0.001 | 1.74 (1.09–2.77) | 0.020 | 0.96 (0.60–1.54) | 0.874 | 2.10 (1.12–3.91) | 0.020 |
| DM duration(>10 year) | 0.65 (0.34–1.23) | 0.186 | 1.08 (0.59–1.97) | 0.813 | 1.12 (0.63–1.55) | 0.695 | 0.86 (0.44–1.69) | 0.663 |
| Hypertension (HTN) | 4.14 (2.40–7.16) | <0.001 | 1.97 (1.25–3.12) | 0.004 | 1.93 (1.21–3.07) | 0.006 | 1.46 (0.85–2.51) | 0.175 |
| Duration of HTN | 1.04 (0.96–1.12) | 0.383 | 1.09 (1.01–1.16) | 0.020 | 1.01 (0.95–1.08) | 0.690 | 1.05 (0.96–1.15) | 0.272 |
| Risk factors | High TC/HDL-C | High non-HDL-C | High ApoB | Mixed dyslipidemia | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Age (>55 year) | 1.29 (0.82–2.03) | 0.266 | 0.90 (0.53–1.54) | 0.708 | 0.91 (0.54-1.54) | 0.729 | 0.93 (0.47–1.86) | 0.846 |
| Male gender | 1.21 (0.79–1.87) | 0.383 | 1.70 (1.03–2.82) | 0.038 | 1.78 (1.08-2.93) | 0.023 | 2.18 (1.13–4.20) | 0.021 |
| BMI (>23 kg/m2) | 0.86 (0.55–1.34) | 0.500 | 0.85 (0.51–1.41) | 0.527 | 0.88 (0.54-1.45) | 0.626 | 1.14 ( 0.61–2.15) | 0.679 |
| Central obesity | 2.51 (1.35–4.69) | 0.004 | 0.71 (0.31–1.62) | 0.422 | 0.88 (0.41-1.91) | 0.750 | 1.48 (0.54–4.04) | 0.448 |
| Urban resident | 1.13 (0.72–1.77) | 0.602 | 0.85 (0.50–1.44) | 0.542 | 0.85 (0.50-1.43) | 0.530 | 0.85 (0.42–1.73) | 0.660 |
| Current smoking | 1.74 (1.06–2.85) | 0.029 | 1.00 (0.56–1.77) | 0.989 | 1.00 (0.56-1.75) | 0.985 | 0.80 (0.37–1.70) | 0.562 |
| Non-vegetarian diet | 1.12 (0.52–2.41) | 0.780 | 1.20 (0.53–2.76) | 0.660 | 1.21 (0.53-2.77) | 0.647 | 0.96 (0.34–2.74) | 0.941 |
| Fasting hyperglycemia | 2.92 (1.86–4.57) | <0.001 | 2.46 (1.55–3.89) | <0.001 | 2.40 (1.52-3.78) | <0.001 | 1.76 (0.97–3.16) | 0.061 |
| Poor glycemic control | 1.33 (0.83-2.15) | 0.236 | 3.92 (1.77–8.63) | 0.001 | 4.60 (2.08-10.01) | <0.001 | 5.22 (1.52–17.88) | 0.009 |
| DM duration (>10 year) | 1.04 (0.57–1.90) | 0.888 | 0.58 (0.29–1.15) | 0.119 | 0.51 (0.26-1.02) | 0.055 | 0.40 (0.17–0.91) | 0.030 |
| Hypertension (HTN) | 2.03 (1.28–3.24) | 0.003 | 1.85 (1.02–3.37) | 0.042 | 1.99 (1.11-3.60) | 0.022 | 2.18 (0.93–5.12) | 0.074 |
| Duration of HTN | 1.06 (0.99–1.14) | 0.081 | 1.09 (0.98–1.21) | 0.095 | 1.10 (1.0-1.22) | 0.056 | 1.12 (0.95–1.31) | 0.162 |
Values are presented as OR (95% CI). OR = Odds ratio, CI = Confidence Interval. Coding of categorical variables-Age groups: female = 0, male = 1; Sex: female = 0, male = 1; BMI: normal = 0, increased = 1; Central obesity: absent = 0, present = 1; Place of residence: village = 0, urban = 1; Smoking habit: non-smoker = 0, current smoker = 1; Diet: vegetarian = 0, non-vegetarian = 1, Glycemic control: good (HbA1c% <7)=0,poor (HbA1c% >7)=1; Duration of diabetes: <10 years = 0, >10 years, Blood pressure: normotensive = 0, hypertensive = 1. Fasting plasma glucose and duration of diabetes were continuous variables
Discussion
The aim of our study was to determine the prevalence, pattern and predictors of atherogenic dyslipidemia in a cohort of type 2 diabetic patients from a teaching hospital of Western hilly region of Nepal. We found that the majority of the patients had higher levels of serum TG, TC, non-HDL-C, ApoB and TC/HDL-C ratio and lower level of serum HDL-C than the cut off values recommended by the NCEP ATP III [2]. Abnormal lipid profiles in our diabetic patients were not surprising. Insulin resistance or deficiency leads to increased rate of lipolysis in adipocytes and influx of free fatty acids into the liver resulting into overproduction of triglyceride rich lipoproteins. Moreover there is delayed clearance of such lipoproteins due to decreased activity of the endothelial bound enzyme lipoprotein lipase [19]. There was no significant difference between the serum levels of these lipid parameters between males and females except for serum TG, which is in agreement with previous hospital and population based studies in Asian, African, European and North American type 2 diabetic populations [20–22]. Some studies have also shown higher levels of atherogenic lipid profile in women [22–24] but such different outcomes may have resulted from differences in age distribution, treatment status for diabetes and dyslipidemia, glycemic status, duration of diabetes and nature of study population.
Age is a non-modifiable risk factor for CVD [2]. We next analyzed the effect of age on serum lipid profile of our patients. We observed a rise in the serum levels of TC, LDL-C, non-HDL-C, ApoB and TC/HDL-C ratio with increasing age of patients and a gradual fall in serum TG and HDL-C levels. Several cross-sectional and longitudinal studies conducted elsewhere have also shown similar results [25–27]. The plasma level of lipids is determined by the balance between synthesis and removal of lipoprotein particles. Ageing causes increased TC and LDL-C levels due to impaired clearance from plasma through reduced expression of hepatic LDL-C receptor [28]. Similarly, age-associated rise in ApoB has been shown to be the result of an increased production of VLDL ApoB-100 and decreased clearance rate of LDL-C ApoB-100 [29]. Plasma TG levels were expected to be higher in older patients, but this was not observed in our study. The unexpected decline of plasma TG level with the advancing age could partly be due to masking effect of treatment of certain old age patients with insulin and lipid lowering drugs. Moreover, menopause has been shown to be an additional risk factor in older women that significantly decreases plasma HDL-C and increases LDL-C levels [30]. Age related decline in HDL-C levels likely results from insulin resistance, inflammation, hormonal decline, cellular senescence and ageing of the HDL-C particle itself, affecting HDL-C formation [31]. This explains the increased prevalence of atherogenic dyslipidemia and risk of CVD with age. Our study has confirmed previous findings that serum lipid parameters are highly correlated with fasting blood glucose and HbA1C, irrespective of the population studied. We further observed moderate to strong correlation of primary lipid parameters such as TC, HDL-C, and LDL-C with derived or secondary lipid parameters such as TC/HDL-C ratio, non-HDL-C and ApoB which are regarded as better predictors of insulin resistance, metabolic syndrome and CVDs [32].
There are some studies from other parts of Nepal that have reported varying prevalence and pattern of dyslipidemia in type 2 diabetic patients [20, 33, 34]. The latest prevalence of mixed dyslipidemia was 63.8% in eastern Nepal, 61.0% in central Nepal and 90.7% in mid-western Nepal. The most prevalent single dyslipidemia in both central and mid-western Nepal was low HDL-C. The least prevalent single dyslipidemia was hypercholesterolemia in central Nepal and high LDL-C in mid-western Nepal. Our study provides the first detailed report on the prevalence and pattern of dyslipidemia in diabetic population from the Dhaulagiri, Gandaki and Lumbini zones of western Nepal. We found high prevalence of dyslipidemia in our patients, with mixed dyslipidemia being the predominant type. The most prevalent primary single dyslipidemia was high LDL-C while hypercholesterolemia was the least prevalent. Three quarters of the patient population showed high non-HDL-C. High LDL-C was the only isolated dyslipidemia present in our patients. The typical atherogenic dyslipidemia was present in about half of the patients. Males had significantly higher prevalence of high LDL-C, high non-HDL-C, high ApoB and mixed dyslipidemia than females, while other lipid parameters were similar. This is in agreement with previous reports from Nepal and elsewhere [20, 35, 36]. We found age specific increase in prevalence of dyslipidemia only in males, with females showing stable or decreasing prevalence with age. Our findings are in agreement with previous reports [24]. The age-specific variation in the prevalence of dyslipidemia is believed to be due to age-related decline in sensitivity of peripheral tissues to insulin and increase in metabolic disorders of carbohydrates and lipids [37]. This effect could have been masked in our female patients because of better control of their diabetes.
We also analyzed the effect of other modifiable risk factors of CVD on the prevalence of dyslipidemia. We did not find significant difference in prevalence of dyslipidemia between smoker and non-smoker patients except for hypertriglyceridemia. This lack of difference could be due to inclusion of relatively low number of smokers (27.2%) as compared to non-smokers (72.8%). Another possibility is that the intensity of smoking was low in these smokers and some of the non-smokers may have been recent ex-smokers. Studies have shown that intensity of smoking is associated with small but significant increases in LDL-C and decreases in HDL-C while smoking cessation is associated with improvement in HDL-C, total HDL and large HDL particles, especially in women [38]. As expected, we found significantly higher prevalence of hypertriglyceridemia, low HDL-C, high LDL-C, high TG/HDL-C ratio and mixed dyslipidemia in patients with poor glycemic control and hypertension. Moreover, prevalence of low HDL-C and high TC/HDL-C ratio was also higher in patients with longer duration of diabetes. These findings are in agreement with the findings of many other studies conducted among diabetic patients in other populations [35, 39]. Type 2 diabetes is often associated with the cluster of several other risk factors of CVD such as older age, insulin resistance, obesity, hypertension, poor glycemic status, microalbuminuria, alterations in inflammatory, coagulation and thrombolytic markers in addition to the atherogenic dyslipidemia [40, 41]. The clustering of many of these risk factors often manifests as metabolic syndrome that precedes and then continues with the diabetes [42]. We have previously shown that Nepalese type 2 diabetic patients have high prevalence of metabolic syndrome and increased risk of CHD [43]. We also explored the covariate risk factors that were independently associated with dyslipidemia in our patients. Age group ≥55 year, current smoking habit, fasting hyperglycemia, poor glycemic control and hypertension were found to be strongly associated with hypertriglyceridemia. Fasting plasma glucose, hypertension and its duration were associated with hypercholesterolemia. Low HDL-C was associated with male gender, central obesity and fasting plasma glucose. High LDL-C was positively associated with male gender and fasting plasma glucose. High non-HDL-C and ApoB were independently associated with male gender, fasting plasma glucose, poor glycemic control and hypertension. High ApoB was also found to be associated with longer duration of hypertension. These risk factors are recognized by many international guidelines and remain the targets for preventing CVDs among diabetic patients. As dyslipidemia is a well-established risk factor for cardiovascular diseases, presence of other co-variate risk factors results significantly higher risk of future CVD. This is in agreement with our earlier studies which have estimated higher risk of 10-year CHD among Nepalese type diabetic patients [43]. These findings warrant extensive preventive approaches, both clinical and non-clinical, to treat all types of dyslipidemia to minimize the future risk of CVD in our patients. Government and other stakeholders of the health need to collaborate to increase public awareness about the impact of atherogenic dyslipidemia among both patients and health care providers and promote adoption of healthy diet and life-style.
Study limitations
The major limitation of our study is that it included only hospital based samples which may not truly represent the diabetic population of this region. Second, it did not analyze the types and effect of lipid lowering treatment in the dyslipidemic patients. Third, it also overlooked the analysis of the differences in diabetes treatment in patients with and without dyslipidemia. The findings of this study, therefore, should be interpreted within context, and may not be generalized to the whole diabetic population.
Conclusions
Our study provides the first detailed report of prevalence, pattern and predictors of atherogenic dyslipidemia in type 2 diabetic patients attending a tertiary care hospital of western hilly region of Nepal. It has shown an alarmingly high prevalence of dyslipidemia. Mixed dyslipidemia is more prevalent than combined or single dyslipidemia. The prevalence of dyslipidemia was found to be strongly associated with various co-variate risk factors of CVD such as old age, male gender, smoking, poor glycemic control, obesity and hypertension suggesting high risk of future CVD. Our study therefore contributes to the epidemiology of diabetic dyslipidemia from the Western hilly region of Nepal and serves as supportive data for health policy planners to formulate and implement policies that aim to increase public awareness about diabetic dyslipidemia, healthy diet and life-style among diabetic patients and health care providers. It also highlights the need of regular monitoring of blood glucose and lipid profile, aggressive lifestyle changes such as weight reduction and physical exercise and effective medication with anti-diabetic and lipid lowering drugs to obtain proper glycemic control and lipid profile. However, a population based nationwide survey is still warranted to reflect the actual epidemiology of diabetic dyslipidemia in Nepal as no such studies has been carried out so far.
Authors’ contributions
DRP designed the study, managed the data and wrote the manuscript. DK, MS and NKY contributed in the patient enrollment, data collection and management. RCK screened and confirmed the presence of type 2 diabetes and dyslipidemia in the study patients. SA provided help in the statistical analysis and interpretation of the analyzed data. RMS and TKS contributed in the critical reading, correction of the English and improvement of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank all patients who agreed to be the research subject of our study and technical staff of clinical biochemistry laboratory at Manipal Teaching Hospital for their invaluable support for generating data for this study. We would also like to thank Head, Department of Biochemistry for his constant encouragement and facilitation for this study.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All relevant data generated during this study are included in this manuscript.
Consent for publications
The authors provide their consent to publish the findings embodied in this study.
Ethical approval and consent to participate
The study protocol was approved by the institutional review committee (IRC) of MTH and written informed consent was obtained from all the enrolled study patients.
Funding
The authors declare that they did not receive any external funding for this study. It was carried out solely based on the local resources.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ANOVA
analysis of variance
- ApoB
apoliprotein B
- BMI
body mass index
- CHD
coronary heart disease
- CVD
cardiovascular disease
- EDTA
ethylene diamine tetraacetate
- HbA1c
glycated hemoglobin type A1c
- HDL-C
high density lipoprotein cholesterol
- IRC
institutional review committee
- LDL-C
low density lipoprotein cholesterol
- MTH
manipal teaching hospital
- NCEP ATP III
the national cholesterol education program adult treatment plan III
- SD
standard deviation
- SPSS
statistical package for the social sciences
- T2DM
type 2 diabetes mellitus
- TC
total cholesterol
- TG
triglycerides
- VLDL
very low density lipoprotein
Additional file
Additional file 1. Survey questionnaire and data collection form for screening dyslipidemia in Nepalese individuals with type 2 diabetes.
Contributor Information
Daya Ram Pokharel, Phone: +977 9847111258, Email: drpokharel09@gmail.com.
Dipendra Khadka, Email: dipen.khadka15@gmail.com.
Manoj Sigdel, Email: manoj.sigdel@hotmail.com.
Naval Kishor Yadav, Email: naval.rhythm@gmail.com.
Shreedhar Acharya, Email: sacharya@nosm.ca.
Ramchandra Kafle, Email: kafle30@hotmail.com.
Ravindra Mohan Sapkota, Email: ravindra.sapkota@gmail.com.
Tara Sigdel, Email: tara.sigdel@ucsf.edu.
References
- 1.Bhandari GP, Angdembe MR, Dhimal M, Neupane S, Bhusal C. State of non-communicable diseases in Nepal. BMC Public Health. 2014;14:23. doi: 10.1186/1471-2458-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 3.Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Type 2 diabetes as a “coronary heart disease equivalent”: an 18-year prospective population-based study in Finnish subjects. Diabetes Care. 2005;28:2901–2907. doi: 10.2337/diacare.28.12.2901. [DOI] [PubMed] [Google Scholar]
- 4.Mooradian AD. Cardiovascular disease in type 2 diabetes mellitus: current management guidelines. Arch Intern Med. 2003;163:33–40. doi: 10.1001/archinte.163.1.33. [DOI] [PubMed] [Google Scholar]
- 5.Krauss RM. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care. 2004;27:1496–1504. doi: 10.2337/diacare.27.6.1496. [DOI] [PubMed] [Google Scholar]
- 6.Reaven GM, Chen YD, Jeppesen J, Maheux P, Krauss RM. Insulin resistance and hyperinsulinemia in individuals with small, dense low density lipoprotein particles. J Clin Invest. 1993;92:141–146. doi: 10.1172/JCI116541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, Pugh K, Jenkins AJ, Klein RL, Liao Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52:453–462. doi: 10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]
- 8.Taskinen MR. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 2003;46:733–749. doi: 10.1007/s00125-003-1111-y. [DOI] [PubMed] [Google Scholar]
- 9.Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2009;5:150–159. doi: 10.1038/ncpendmet1066. [DOI] [PubMed] [Google Scholar]
- 10.Eliasson B, Cederholm J, Eeg-Olofsson K, Svensson AM, Zethelius B, Gudbjörnsdottir S, National Diabetes Register Clinical usefulness of different lipid measures for prediction of coronary heart disease in type 2 diabetes. Diabetes Care. 2011;34:2095–2100. doi: 10.2337/dc11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Betteridge DJ. Lipid control in patients with diabetes mellitus. Nat Rev Cardiol. 2011;8:278–290. doi: 10.1038/nrcardio.2011.23. [DOI] [PubMed] [Google Scholar]
- 12.Pokharel DR, Khadka D, Sigdel M, Yadav NK, Acharya S, Kafle RC, Shukla PS. Prevalence of metabolic syndrome in Nepalese type 2 diabetic patients according to WHO, NCEP ATP III, IDF and Harmonized criteria. J Diabetes Metab Disord. 2014;13:104. doi: 10.1186/s40200-014-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nepal Health Research Council (NHRC). Prevalence of non-communicable diseases in Nepal-Hospital based study. A study report published by NHRC, Ram Shah Path, Kathmandu, Nepal, December, 2010.
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 15.Hwang Y-C, Ahn H-Y, Lee WJ, Park C-Y, Park SW. An equation to estimate the concentration of serum apolipoprotein B. PLoS ONE. 2012;7:e51607. doi: 10.1371/journal.pone.0051607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization . Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: Report of a WHO/IDF Consultation. Geneva: World Health Organization; 2006. p. 39. [Google Scholar]
- 17.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National High Blood Pressure Education Program Coordinating Committee The Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 18.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 19.Taskinen M-R. Pathogenesis of dyslipidemia in type 2 diabetes. Exp Clin Endocrinol Diabetes. 2001;109:173–181. doi: 10.1055/s-2001-18580. [DOI] [PubMed] [Google Scholar]
- 20.Pandeya A, Sharma M, Regmi P, Basukala A, Lamsal M. Pattern of dyslipidemia and evaluation of non-HDL cholesterol as a marker of risk factor for cardiovascular disease in type 2 diabetes mellitus. Nepal Med Coll J. 2012;14:278–282. [PubMed] [Google Scholar]
- 21.Cook CB, Erdman DM, Ryan GJ, Greenlund KJ, Giles WH, Gallina DL, El-Kebbi IM, Ziemer DC, Ernst KL, Dunbar VG, Phillips LS. The pattern of dyslipidemia among urban African–Americans with type 2 diabetes. Diabetes Care. 2000;23:319–324. doi: 10.2337/diacare.23.3.319. [DOI] [PubMed] [Google Scholar]
- 22.Aljabri KS, Bokhari SA, Akl A. The relation between overweight, obesity and plasma lipids in Saudi adults with type 2 diabetes. J Health Spec. 2016;4:140–45. doi: 10.4103/1658-600X.179817. [DOI] [Google Scholar]
- 23.Chang JB, Chu NF, Syu JT, Hsieh AT, Hung YR. Advanced glycation end products (AGEs) in relation to atherosclerotic lipid profiles in middle-aged and elderly diabetic patients. Lipids Health Disease. 2011;10:228. doi: 10.1186/1476-511X-10-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali F, Jamil H, Anwar SS, Wajid N. Characterization of lipid parameters in diabetic and non-diabetic atherosclerotic patients. J Geriatr Cardiol. 2015;12:37–43. doi: 10.11909/j.issn.1671-5411.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson PWF, Anderson KM, Harris T, Kannel WB, Castelli WP. Determinants of change in total cholesterol and HDLC with age: the Framingham study. J Gerontol. 1994;49:M252–M257. doi: 10.1093/geronj/49.6.M252. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara A, Barrett-Connor E, Shan J. Total, LDL, and HDL cholesterol decrease with age in older men and women. The Rancho Bernardo Study 1984–1994. Circulation. 1997;96:37–43. doi: 10.1161/01.CIR.96.1.37. [DOI] [PubMed] [Google Scholar]
- 27.Heiss G, Tamir I, Davis CE, Tyroler HA, Rifkind BM, Schonfeld G, Jacobs D, Frantz ID. Lipoprotein-cholesterol distributions in selected North American populations: the Lipid Research Clinics Program Prevalence Study. Circulation. 1980;61:302–315. doi: 10.1161/01.CIR.61.2.302. [DOI] [PubMed] [Google Scholar]
- 28.Ericsson S, Eriksson M, Vitols S, Einarsson K, Berglund L, Angelin B. Influence of age on the metabolism of plasma low density lipoproteins in healthy males. J Clin Invest. 1991;87:591–596. doi: 10.1172/JCI115034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Millar JS, Lichtenstein AH, Cuchel M, Dolnikowski GG, Hachey DL, Cohn JS, Schaefer EJ. Impact of age on the metabolism of VLDL, IDL, and LDL apolipoprotein B-100 in men. J Lipid Res. 1995;36:1155–1167. [PubMed] [Google Scholar]
- 30.Stevenson JC, Crook D. Gods land IF. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis. 1993;98:83–90. doi: 10.1016/0021-9150(93)90225-J. [DOI] [PubMed] [Google Scholar]
- 31.Walter M. Interrelationships among HDL Metabolism, aging and atherosclerosis. Arterioscleroses Thrombi Vass Boil. 2009;29:1244–1250. doi: 10.1161/ATVBAHA.108.181438. [DOI] [PubMed] [Google Scholar]
- 32.Kimm H, Lee SW, Lee HS, Shim KW, Cho CY, Yun JE, Jee SH. Associations between lipid measures and metabolic syndrome, insulin resistance and adiponectin–usefulness of lipid ratios in Korean men and women. Circ J. 2010;2010(74):931–7. doi: 10.1253/circj.CJ-09-0571. [DOI] [PubMed] [Google Scholar]
- 33.Regmi P, Gyawali P, Shrestha R, Sigdel M, Mehta KD, Majhi S. Pattern of dyslipidemia in type 2 diabetic subjects in Eastern Nepal. J Nepal Assoc Med Lab Sci. 2009;10:11–3. [Google Scholar]
- 34.Shrewastwa MK, Thanpari C, Yadav NK, Mittal RK, Rohil V. Dyslipidemia in type 2 diabetes mellitus patients in western of Nepal: a hospital based study. Bali Med J. 2013;2:46–50. [Google Scholar]
- 35.Karim MN, Ahmed KR, Bukht MS, Akter J, Chowdhury HA, Hossain S, Anwar N, Selim S, Chowdhury SH, Hossain F, Ali L. Pattern and predictors of dyslipidemia in patients with type 2 diabetes mellitus. Diabetes Metab Syndr. 2013;7:95–100. doi: 10.1016/j.dsx.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Jayarama N, Reddy M, Lakshmaiah V. Prevalence and pattern of dyslipidemia in type 2 diabetes mellitus patients in a rural tertiary care centre, southern India. Glob J Med Public Health. 2012;1:24–7. [Google Scholar]
- 37.Rowe JW, Minaker KL, Pallotta JA, Flier JS. Characterization of the insulin resistance of aging. J Clin Invest. 1983;7:1581–7. doi: 10.1172/JCI110914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gepner AD, Piper ME, Johnson HM, Fiore MC, Baker TB, Stein JH. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J. 2011;161:145–51. doi: 10.1016/j.ahj.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang R, Schulze MB, Li T, Rifai N, Stampfer MJ, Rimm EB, Hu FB. Non-HDL cholesterol and apolipoprotein B predict cardiovascular disease events among men with type 2 diabetes. Diabetes Care. 2007;27:1991–7. doi: 10.2337/diacare.27.8.1991. [DOI] [PubMed] [Google Scholar]
- 40.Kalofoutis C, Piperi C, Kalofoutis A, Harris F, Phoenix D, Singh J. Type II diabetes mellitus and cardiovascular risk factors: current therapeutic approaches. Exp. Clin Cardiol. 2007;12:17–28. [PMC free article] [PubMed] [Google Scholar]
- 41.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 42.Gerich JE. Type 2 diabetes mellitus is associated with multiple cardiometabolic risk factors. Clin Cornerstone. 2007;8:53–68. doi: 10.1016/S1098-3597(07)80028-7. [DOI] [PubMed] [Google Scholar]
- 43.Pokharel DR, Khadka D, Sigdel M, Yadav NK, Sapkota LB, Kafle RC, Nepal S, Sapkota RM, Chaudhary N. Estimation of 10-year risk of coronary heart disease in Nepalese patients with type 2 diabetes: Framingham versus United Kingdom Prospective Diabetes Study. North Am J Med Sci. 2015;7:347–355. doi: 10.4103/1947-2714.163642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data generated during this study are included in this manuscript.


