Abstract
Background
Very sensitive measurements of serum estrogens and testosterone are important in adult and pediatric endocrinology and immunoassays are known to lack the required performance at very low levels. Our aim was to develop a sensitive HPLC-MS/MS assay for both estradiol (E2) and testosterone (Te) in serum without the need for chemical derivatization and using commercially available calibrators.
Methods
Serum samples were prepared by the addition of internal standards followed by extraction using hexane:ethyl acetate. Chromatographic separation was achieved using a C18 column and mass spectrometry was performed in both positive and negative ion modes.
Results
The lower limits of quantitation (LLOQs) of E2 and Te were 5 pg/mL and 1 ng/dL, respectively. The analytical measurement range (AMR) for E2 was 5–600 pg/mL and 1–1,170 ng/dL for Te. Assay accuracy was determined both by comparison with a LC-MS/MS method performed at a national laboratory and the CDC HoSt program. Comparison with samples analysed by both methods showed excellent correlation. Within-day (N=10) and between-day (N=20) CVs at concentrations spanning the AMR were less than 7% for both analytes.
Conclusion
We have developed an accurate and highly sensitive assay to measure E2 and Te levels in serum by HPLC-MS/MS without chemical derivatization and using commercially available calibrators.
Keywords: testosterone, estradiol, mass spectrometry, liquid chromatography, method correlation, CDC HoSt, NIST SRM 971
1. Introduction
Very sensitive measurements of serum estrogens and androgens are important in adult and pediatric endocrinology and oncology. Sensitive measurements of estradiol (E2) are needed for determination of menopausal status, estrogen deficiency, estrogen measurements in men, during antiestrogen treatment and in the diagnosis of other sex-hormone-related disorders. E2 levels in postmenopausal women, men and children are typically < 20 pg/mL (1). Very low level testosterone (Te) measurements are needed for adult women, whose values are routinely < 50 ng/dL, in children, and men undergoing antiandrogen therapy whose values are usually < 10 ng/dL (2).
The most commonly used methods for steroid analysis are immunoassays because they are rapid and sensitive enough for most routine applications, however immunoassays do not meet the sensitivity requirements for E2 in post menopausal women undergoing aromatase inhibition, men, and children. In addition, Te immunoassays lack the sensitivity requirements for chemically castrated males, women and children. Many immunoassays lack specificity and accuracy as they may show cross reactivity with structurally similar compounds (3–6). In addition, most steroid immunoassays are not standardized against internationally recognized standards and none are certified by the Centers for Disease Control and Prevention (CDC) Hormone Standardization (HoSt) Program (7,8).
For these reasons a number of sensitive and specific assays for mass spectrometry have been described for E2 & Te. While sensitive assays by mass spectrometry for Te usually do not require chemical modification, the same is not true for E2. To increase the sensitivity of E2 assays by mass spectrometry most previous reports have resorted to chemical derivatization (1, 9–11) which introduces an additional step that increases complexity and may introduce variability. Recently some E2 methods have been reported that do not require chemical modification; however, these assays either have not been validated by standardization to reference materials (12, 13) or they include estrogens only and do not incorporate testosterone into the assay (14–16).
While there are a number of excellent steroid assays described by mass spectrometry, none were sufficient to meet our needs and overcome the limitations described above. Our aim was to develop a very sensitive HPLC-MS/MS assay for both E2 and Te in serum in a single analysis without chemical derivatization and extended extraction protocols. We also chose to use commercially available calibrators and quality controls and ensured their accuracy by comparison with internationally accepted reference standards and a direct comparison with a reference assay.
2. Materials and methods
2.1. Materials and chemicals
E2 and Te were from Sigma-Aldrich (Saint-Louis, MO, USA). Estriol, estrone, and deuterated internal standards, 17-β estradiol (E2-D5) and testosterone (Te-D3) were from Cerilliant (Round Rock, TX, USA). All compounds had a purity greater than 99%. 17-OH-progesterone, aldosterone, androstenedione, corticosterone, cortisol, cortisone, 11-deoxycortisol, dehydroepiandrosterone, dehydroepiandrosterone sulfate, dihydrotestosterone, and progesterone were from Chromsystems Steroid Panel I & II kits (Grafelfing, Germany). Water, methanol, acetonitrile, 2-propanol, acetone, and acetic acid (LCMS grade) were from Fisher Scientific (Fair Lawn, NJ, USA). Calibrators and quality controls were from Chromsystems Steroid Panel II kit previously mentioned. Ultra-low steroid human serum (DC Mass Spect Gold) was from Golden West Biologicals, Inc. (Temecula, CA, USA) and is certified to contain < 0.03 pg/mL and < 0.025 ng/dL E2 and Te, respectively. Serum separator and clot activator blood collection tubes were obtained from Beckton Dickenson (Franklin Lakes, NJ, USA).
2.2. Calibrator and quality control material
The Chromsystems calibrators were human based, lyophilized material and contained six levels and a blank which span the range from 0–5,110 pg/mL for E2 and 0–1,170 ng/dL for Te.
Working calibrators and QC material were reconstituted according to manufacture specifications and stored at −80°C. For assessment of assay precision and recovery (including LLOQ) serum pools of patient specimen collected into serum separator tubes were prepared at various concentrations, aliquoted, and stored at −80°C until ready to assay. E2 and Te levels for the recovery experiment were prepared by adding the working stock solution (1 μg/mL) into patient pooled serum to give final concentrations of 500, 250, and 50 pg/mL for E2 and 750, 250, and 25 ng/dL for Te. It is also important to note that all patient pools were extracted in quintuplet prior to the addition of E2 and Te so that a baseline steroid concentration could be determined.
The internal standard working solution (500 pg/mL and 37.5 ng/dL) were prepared in methanol from the Cerilliant stock solutions for E2-D5 & Te-D3, respectively.
2.3. Sample preparation
The use of patient specimens was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center. Specimens were prepared for analysis by transferring 500 μL of calibrator, control, or patient sample to a 13 × 100 mm glass culture tube to which 50 μL of internal standard solution was added. A liquid-liquid extraction was performed by the addition of 2.5 mL of hexane:ethyl acetate (9:1, v/v). The samples were vigorously vortexed at 2,000 RPM for two minutes and then frozen at −80°C for fifteen minutes. The organic layer was decanted into an additional 13 × 100 mm glass culture tube and evaporated to dryness at 35°C under a stream of nitrogen and reconstituted in 150 μL of methanol:water (1:1, v/v). Due to the volume or organic solvents used, all of the above steps were carried out in a chemical fume hood. The samples were transferred to autosampler vials containing inserts and 40 μL was injected into the system and analyzed by HPLC-MS/MS.
2.4. HPLC-MS/MS conditions
An Aria TLX-2 system (Thermo Scientific, San Jose, CA), which is a two-channel, two-dimension liquid chromatography system with a CTC Pal autosampler, was operated in one-dimension or “LX” mode in accordance with manufacturers recommendations. Analytes from injected samples were separated by reversed-phase chromatography and eluted to the mass spectrometer as a binary eluting pump delivered a solvent gradient.
The system was controlled with Aria (Thermo Scientific) and Analyst (Sciex) software, versions 1.6.3 and 1.6.2, respectively. The mobile phases consisted of ammonium fluoride in water and acetonitrile. The analytical column used was an Accucore C18 and the temperature was maintained using a column heater. This column was chosen because C18 reverse phase columns are widely used in steroid separations and acknowledged by CLSI for androgen and estrogen separations [17]. In addition we also compared the performance of the Accucore pentafluorophenyl (PFP) column under similar chromagraphic conditions.
The triple quadrupole mass spectrometer was a Sciex 6500 (Framingham, MA, USA) equipped with a heated electrospray ionization probe maintained at 750°C. Analyses were performed in both positive and negative ionization modes simultaneously with a spray voltage of 4,500 V and cycle time of 0.5 seconds. Nitrogen was used as the ion source gas 1, ion source gas 2, curtain gas and collision gas at 70, 90, 35 and 12 psi, respectively. The system was operated in the multiple reaction monitoring (MRM) low mass mode.
2.5. Method validation
The method validation was adopted from guidelines from the U.S. Food and Drug Administration (FDA) [18], Clinical and Laboratory Standard Institute (CLSI) [19], and CLIA. The assay was fully validated for linearity, accuracy, precision, selectivity, carryover, recovery and matrix effects.
2.5.1. Specificity
In order to rule out interferences from isobaric and structurally related compounds [21], an HPLC method was developed that allowed for baseline separation of 17-OH-progesterone, aldosterone, androstenedione, corticosterone, cortisol, cortisone, 11-deoxycortisol, dehydroepiandrosterone, dehydroepiandrosterone sulfate, dihydrotestosterone, estriol, estrone and progesterone from both estradiol and testosterone. To accomplish this control level III of the Chromsystems Steroid Panel 1 and Panel 2 were combined. The remaining steroids, estriol and estrone, were spiked into the mixture at 14 ng/mL and 300 pg/mL respectively. Additionally, an interference study was carried out to evaluate the effect of hemolyzed, lipemic and icteric samples on quantification of E2 and Te. This interference study was conducted by adding a known concentration of E2 and Te into hemolyzed, icteric, and lipemic patient specimens. The percent recovery was calculated to see if hemoglobin (5.9 g/dL), bilirubin (38.2 mg/dL), and triglycerides (3,180 mg/dL) had any impact on the calculated concentrations of E2 and Te. It should be noted that all unadulterated patient samples were extracted in triplicate prior to the addition of E2 and Te so that a baseline steroid concentration could be determined. To rule out potential interferences from the gel present in serum separator tubes, specimens were obtained from 6 male and 6 female subjects and collected both in serum separator and clot activator tubes. The specimens were allowed to remain on the gel separator for 5 hours after centrifugation and analyzed for E2 and Te and the results compared.
2.5.2. Linearity
The linearity of this method was evaluated by 6-point calibration curves spanning AMR for E2 and Te by extracting each sample in triplicate. The quantitation software, MultiQuant (Sciex) calculated the concentrations of the calibrators using a weighted linear regression analysis with weights inversely proportional to the X values. The results were then entered into the linearity function of EP Evaluator (South Burlington, VT, USA) for the determination of the clinical linearity. EP Evaluator determines linearity according to the method described in CLSI EP-6A [20].
2.5.3. Accuracy and precision
Between-day assay imprecision was evaluated over a twenty-day period from patient pooled serum with three levels that span the AMR and a level at the LLOQ extracted in triplicate daily. The average concentration of the specimen and standard deviation were determined over the validation period for both analytes. The CV was not to exceed 15% for the levels, except LLOQ, over the duration of the imprecision study [19].
The accuracy of E2 and Te were evaluated by four different complementary methods. (1) Comparison with reference materials: (a) for E2 comparison with the European Commission Institute Reference Materials and Measurements (IRMM) certified reference material (CRM) BCR-576, 577 and 578; (b) For Te comparison with the National Institute of Standards and Technology (NIST) standard reference material (SRM) 971 F and 971 M; (2) comparison with 40 samples obtained from the CDC H+t program; (3) an alternate LC-MS/MS assay performed at a national reference laboratory [1, 22]; and (4) through recovery studies using pooled patient serum. The LLOQ was defined as the lowest concentration of analyte where the coefficient of variation (CV) was below 20% as stated in the FDA guideline of Bioanalytical Method Validation [18].
Since concentrations can exceed 600 pg/mL and 1,170 ng/dL for E2 and Te, respectively a dilution protocol was validated for 1:2 and 1:5 fold dilutions. The dilution protocol was as follows: For a 1:2 dilution 250 μL of ultra-low steroid serum and specimen was added to a 13 × 100 mm glass culture tube and vortex mixed briefly. For a 1:5 dilution 400 μL of ultra-low steroid serum and 100 μL of specimen was added to a 13 × 100 mm glass culture tube, vortex mixed briefly and extracted per protocol.
2.5.4. Matrix interference and ion suppression
To evaluate matrix effects a tee-fitting was placed between the outlet of the HPLC column and the infusion pump on the front of the MS. A standard mix solution containing both deuterated internal standards was infused into the eluent stream and the MRM for E2-D5 and Te-D3 was monitored. After a steady baseline was achieved, six patient extracts were injected into the HPLC-MS/MS system. Any eluting compound that interfered with the ionization of the target analyte would lead to an elevation or depression of the baseline which would reflect a matrix effect. Detector response due to matrix variability should not exceed 15% [19].
2.5.5. Quantitation of sample carryover
Sample carryover was evaluated by running a serum blank after the highest calibrator on all calibration curves during the validation period. The percent carryover was required to be < 25% of the LLOQ for both analytes [19].
3. Results
3.1. HPLC-MS/MS
The liquid chromatography parameters were optimized to maximize sensitivity and separation for both analytes. An Accucore C18 column (3.0 × 50 mm, 2.6 μm) produced adequate retention and separation of both compounds (Figure 1 & Figure 2) and produced better separation and reduced band width compared with the Accucore PFP (data not shown). Various mobile phase compositions, flow rates and profiles were evaluated. The desired sensitivity was achieved by using water with 0.2 mM ammonium fluoride in water and acetonitrile as the aqueous and organic solvents, respectively. The analytes were loaded on the analytical column in 85% mobile phase A and eluted with 100% mobile phase B using a 100 μL injection loop (Table 1). The optimal injection volume was 40 μL and the analytical column was maintained at 70°C.
Figure 1.
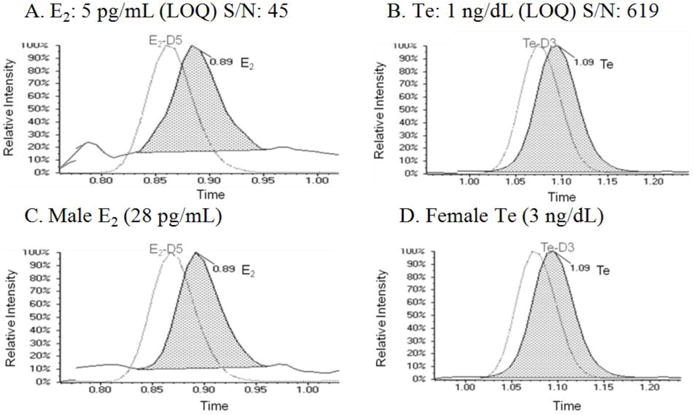
HPLC-MS/MS ion chromatograms depicting estradiol and testosterone LLOQ’s and specimen collected from male (estradiol) and female (testosterone) patients.
Figure 2.
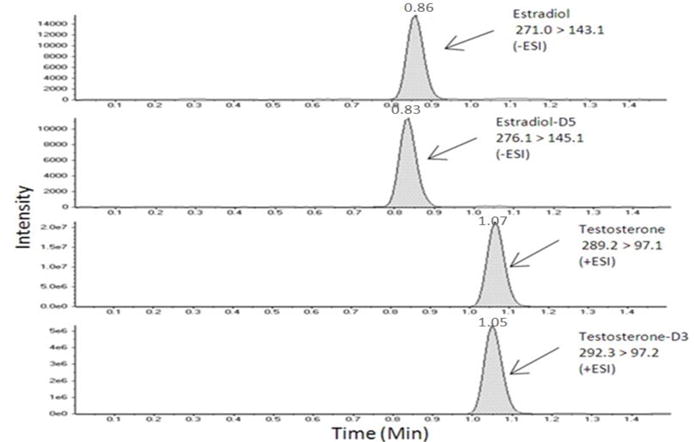
HPLC-MS/MS ion chromatogram of E2, E2-D5, Te, and Te-D3 product ions in a serum sample.
Table 1.
Gradient Used For The HPLC-MS/MS Analysis of Estradiol and Testosterone HPLC Parameters
| Step | Time (s) | Flow (mL/Min) | Gradient | %A | %B |
|---|---|---|---|---|---|
| 1 | 30 | 0.5 | Step | 85 | 15 |
| 2 | 60 | 0.5 | Ramp | 67.5 | 32.5 |
| 31 | 135 | 0.5 | Ramp | 50 | 50 |
| 4 | 60 | 0.5 | Step | 0 | 100 |
| 5 | 60 | 0.5 | Step | 85 | 15 |
Data Acquisition window: 1.75–3.25 minutes.
The LC-MS/MS analysis was carried out using the conditions as previously described in Section 2.4. The optimization of the MRM parameters was performed by direct infusion of the standards using alternating positive and negative electrospray ionization. The transitions monitored in MRM mode for E2 were 271.0 > 143.1 and 271.0 > 145.1 m/z for its 1° and 2° ions, respectively and 276.1 > 145.1 and 276.1 > 147.1 m/z for its internal standard. The transitions monitored in MRM mode for Te were 289.2 > 97.1 and 289.2. > 109.1 m/z for its 1° and 2° ions, respectively and 292.3 > 97.2 and 292.3 > 109.0 m/z for its internal standard. Collision-induced dissociation mass spectra were recorded for each analyte. The optimal DP, EP, and CXP values were −140 V and 95 V, −11 V and 10 V, and −10 V and 10 V for E2 and Te, respectively. The CE was 30 V for all Te and Te-D3 transitions while E2 and E2-D5 had CE values ranging from −53 to −70 V. The scan time for each transition was 50 msec and operated in unit resolution. Data was processed using MultiQuant software version 2.1.
3.2. Method validation
3.2.1. Specificity
Baseline separation of 17-OH-progesterone, aldosterone, androstenedione, corticosterone, cortisol, cortisone, 11-deoxycortisol, dehydroepiandrosterone, dehydroepiandrosterone sulfate, dihydrotestosterone, estriol, estrone and progesterone from both estradiol and testosterone where achieved using the LC method described in this paper (See Figure 1 in supplemental data). In the experiment to evaluate possible interferences from hemolysis, icterus, and lipemia no interferences were observed from hemoglobin (4.6 g/dL), bilirubin (34.9 mg/dL), or triglycerides (926 mg/dL). To assess any potential interferences from serum separator gel tubes, neither testosterone nor estradiol from the test subjects (6 males and 6 females) showed any significant differences compared with specimens collected in clot activator tubes after five hours on the gel (data not shown).
3.2.2. Linearity
A linear relationship was found between analyte concentrations and peak area ratios within the AMR of each compound as determined by the linearity function in EP Evaluator software version 11.0, which determines linearity according to the method described in CLSI EP6-A [20]. The slopes obtained from the linearity summary were 1.00 and 1.01 for E2 and Te, respectively. The assay was linear from 6–600 pg/mL and 1–1170 ng/dL for E2 and Te, respectively.
3.2.3. Accuracy and precision
The CV was < 7% for all patient pools with the exception of the LLOQ over the duration of the twenty day imprecision study (Table 2). The LLOQs were determined to be 5 pg/mL for E2 and 1 ng/dL for Te. The lowest signal-to-noise ratio (S/N) of all analytes at the LLOQs was E2 which had a value greater than 60; more than three times the minimum S/N requirement [19]. The accuracy of E2 and Te were evaluated by four different complementary methods. For the comparison of E2 with IRMM CRM BCR 576, 577 and 578 the analysis yielded a percent bias of less than 2% and CVs less than 2.5% for all CRMs (Table 3). The Te comparison with NIST SRM 971 F and 971 M yielded a percent bias of less than 0.5% and CVs of less than 2.5% for both SRMs (Table 3). A comparison with 40 samples obtained from the CDC HoSt program showed that E2 had a regression slope of 1.008 and a R2=0.993 while Te had a regression slope of 1.089 and a R2=0.997 (Figure 3). Additionally, a comparison to an alternate LC-MS/MS assay performed at a CLIA certified national reference laboratory relieved that E2 had a regression slope of 0.920 and a R2=0.984 while Te had a regression slope of 1.052 and a R2=0.986 (Figure 4). These statistical evaluations express an excellent method correlation to multiple reference materials. The accuracy of the assay was also evaluated by performing a recovery experiment for E2 and Te. The recovery experiment was performed (Table 4) with three concentrations of each analyte spanning the AMR with recoveries of 100.2 – 107.3 and 97.5 – 102.8%, for E2 and Te, respectively.
Table 2.
Within and Between-Day Imprecision of Analytes in Pooled Patient Serum
| Sample ID | Mean Within-Day | Within-Day CV (%) N=10 | Mean Between-Day | Between-Day CV (%) N=20 |
|---|---|---|---|---|
| E2, LLOQ | 5.1 pg/mL | 16.1 | 5.4 pg/mL | 19.3 |
| E2, Level 1 | 64.0 pg/mL | 3.7 | 61.9 pg/mL | 7.4 |
| E2, Level 2 | 278.1 pg/mL | 2.2 | 277.1 pg/mL | 3.4 |
| E2, Level 3 | 527.0 pg/mL | 1.2 | 518.2 pg/mL | 2.9 |
| Te, LLOQ | 1.3 ng/dL | 15.1 | 1.2 ng/dL | 19.4 |
| Te, Level 1 | 25.3 ng/dL | 1.3 | 25.3 ng/dL | 3.4 |
| Te, Level 2 | 242.7 ng/dL | 1.5 | 248.6 ng/dL | 3.5 |
| Te, Level 3 | 709.1 ng/dL | 1.7 | 721.2 ng/dL | 2.7 |
Table 3.
Standard Reference Material Comparison
| Estradiol | ||||
|---|---|---|---|---|
| Sample ID | Certified Value | Measures value (N = 3) | % Bias | CV |
| BCR 576 | 31.1 pg/mL | 31.3 (95% CI: 29.6–32.9) | 0.7 | 0.6 |
| BCR 577 | 188.0 pg/mL | 186.7 (95% CI: 181.7–191.6) | −0.7 | 2.3 |
| BCR 578 | 365.0 pg/mL | 358.8 (95% CI: 350.3–367.3) | −1.7 | 2.1 |
| Testosterone | ||||
|---|---|---|---|---|
| Sample ID | Certified Value | Measures value (N = 3) | % Bias | CV |
| SRM 971 F | 27.7 ng/dL | 27.6 (95% CI: 27.1–28.1) | −0.4 | 1.5 |
| SRM 971 | M 642.9 ng/dL | 640.6 (95% CI: 624.8–656.4) | −0.4 | 2.2 |
Figure 3.
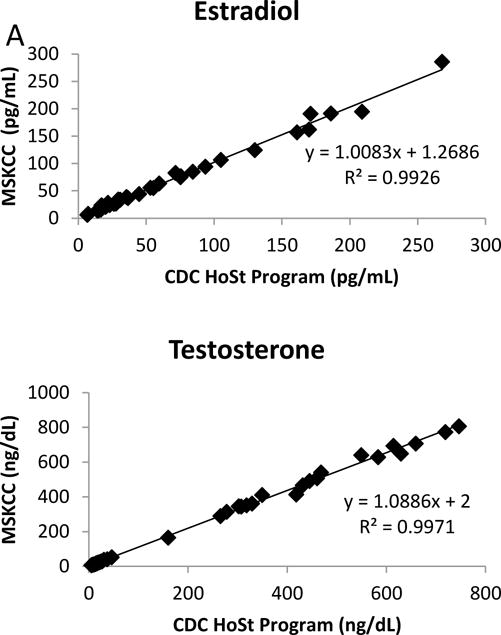
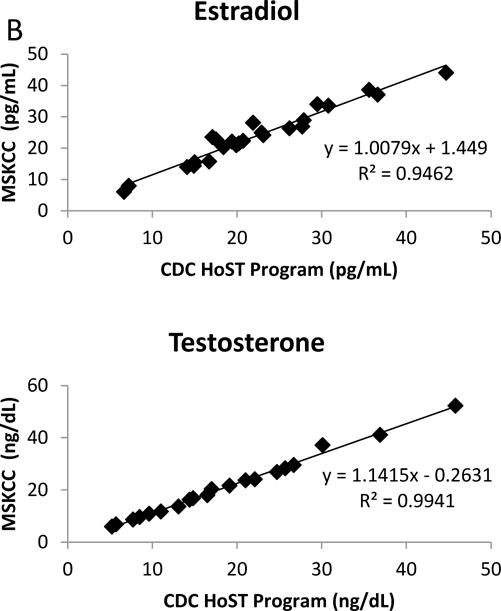
(A) Correlation between the new HPLC-MS/MS assay and the CDC HoSt standardization program; (B) Correlation between the new HPLC-MS/MS assay and the CDC HoSt standardization program at the low end of the AMR from the above data.
Figure 4.
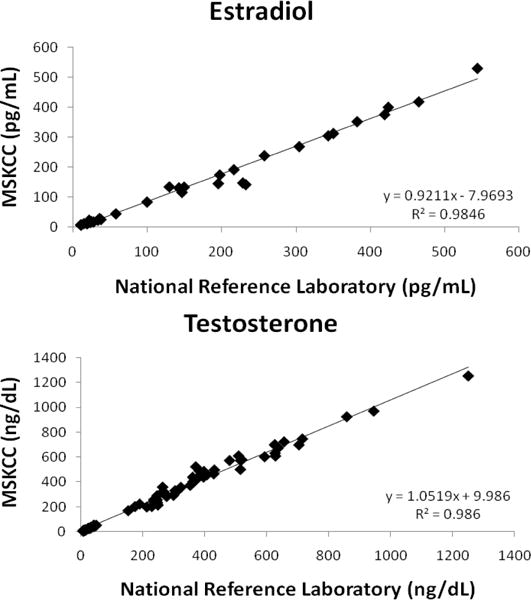
Correlation between the new HPLC-MS/MS assay and a national reference laboratory.
Table 4.
Assay Accuracy Determined by a Recovery Experiment
| Sample ID | N | Unit | Target Value (nmol/L) | Measure Value | % Recovery (measured/target) |
|---|---|---|---|---|---|
| Estradiol, Level 1 | 5 | pg/mL | 50 | 51.4 ± 1.4 | 102.9 ± 2.8 |
| Estradiol, Level 2 | 5 | pg/mL | 250 | 268.2 ± 10.0 | 107.3 ± 4.0 |
| Estradiol, Level 3 | 5 | pg/Ml | 500 | 501.2 ± 14.9 | 100.2 ± 3.0 |
| Testosterone, Level 1 | 5 | ng/dL | 25 | 25.7 ± 0.9 | 102.8 ± 3.6 |
| Testosterone, Level 2 | 5 | ng/dL | 250 | 250.3 ± 19.5 | 100.1 ± 7.8 |
| Testosterone, Level 3 | 5 | ng/dL | 750 | 731.6 ± 33.5 | 97.5 ± 4.5 |
In order to evaluate our dilution protocol, concentrations above the AMR were diluted in a 1:2 and 1:5 ratios for both analytes to bring the values within the AMR. These values were 4,930 pg/mL and 1,170 ng/dL for E2 and Te, respectively. All samples were extracted in quintuplet and each dilution scheme yielded a percent difference within 4% of the expected concentration.
3.2.4. Matrix interference and ion suppression
The results of the tee-infusion experiment indicated there was no ion suppression or enhancement (data not shown). This demonstrated that the extraction matrix had little co-eluting endogenous substances that could influence the ionization of the analytes and I.S.
3.2.5. Quantitation of sample carryover
Sample carryover was evaluated by running a serum blank after the highest calibrator on all calibration curves during the validation period. The average carryover was < 8% of the calculated response of the LLOQ for both target compounds.
4. Discussion
The objective of this study was to develop an analytical method to accurately and precisely quantitate E2 and Te in serum from both chemically castrated males and pre/post-menopausal females that did not require chemical derivatization. Therefore, this method needed to have a wide AMR and be capable of quantitation at very low concentrations to support our specific patient demographic.
We have developed a HPLC-MS/MS assay for the simultaneous quantification of E2 and Te from 500 μL serum without derivitization and using commercially available calibrators. The Chromsystems calibrators are lyophilized, human serum based and contain E2 and Te as well as eleven other steroids and are prepared for use by the simple addition of water. The commercially prepared lyophilized calibrators greatly simplified assay development, validation and routine clinical use. As of this writing we have analyzed several lots of this material and found no significant differences for either steroid. The method is specific, sensitive and accurate for both steroids over the entire AMR. This assay is superior to immunoassays in that E2 and Te can be analysed in the same specimen and the LLOQ is much lower than those of immunoassays. Our method showed an excellent sensitivity for both compounds. The LLOQ was determined to be 5 pg/mL and 1 ng/dL for E2 and Te, respectively. The verified linear range for E2 and Te was 5–600 pg/mL and 1–1,170 ng/dL, respectively. Therefore, this assay will be useful in the management of postmenopausal women and males with prostate cancer being treated with antiandrogen drugs.
The lack of sufficient accuracy and standardization of E2 and Te assays is a major concern for the clinical and public health communities [8]. The CDC has begun to address this concern through its HoSt program which provides laboratories with specimens spanning their AMR that have been previously analysed using their reference method [7]. Forty samples for each analyte were obtained and evaluated and did not show a significant difference between our method and the established reference methods. E2 had a regression slope of 1.008, R2=0.993 while Te had a regression slope of 1.089, R2=0.997. In addition, we obtained standard and certified reference materials from NIST (SRM 971F and SRM 971M) for Te and from IRMM (BCR 576–578) for E2. These specimens were analyzed and resulted in differences from target values of less than 2% and CVs less than 2.5% for all SRMs and CRMs (Table 3). Additionally, correlation to a national reference laboratory and recovery experiments both confirmed the excellent accuracy of our newly developed HPLC-MS/MS assay (Table 4 & Figure 4).
Extensive validation has proven that this assay is consistently precise and accurate, not affected by matrix interferences, free of sample carryover and isomers of target species and structurally similar compounds showed either baseline separation or no interference (data not shown). The lack of chemical derivatization minimizes complexity and facilitates automation. Limitations of this method include the use of organic solvents which have a relatively high disposal cost and require many of the steps to be carried out in a chemical fume hood. Additionally, since this method is a relatively manual process the sample throughput is modest and suitable for small to medium-sized institutions. Despite the drawbacks of using a liquid-liquid extraction step with strong solvents, it was deemed necessary to attain the very low LOQ for both analytes, especially for underivatized E2 which ionizes relatively poorly in the negative ion mode. In this study we desired to develop assays with reference method performance. Indeed, both candidate reference methods for Te and E2 incorporate very similar extraction steps using hexane and ethyl acetate [23,24]. Extensive sample clean-up with strong solvents is also recommended by CLSI [17] for steroid measurements using mass spectrometry.
5. Conclusion
We have developed an accurate and highly sensitive assay to simultaneously measure E2 and Te levels in serum by HPLC-MS/MS without the need for chemical derivatization. Unlike many immunoassays this method is free of cross reactivity from structurally similar analogs and offers a distinct advantage over alternative quantitative methods such as chemiluminescent and radioactive immunoassays.
Highlights.
Liquid chromatography tandem mass spectrometry was used for the determination of estradiol and testosterone in human serum without derivatization.
The assay was developed using commercially available calibrator and quality control materials.
This assay showed excellent correlation to the CDC HoSt Standardization Program for Te and E2, NIST 971 for Te, BCR 576–578 for E2, and an alternate LC-MS/MS assay.
The method is accurate, reliable and sensitive.
Acknowledgments
This work was supported by the Department of Laboratory Medicine of Memorial Sloan Kettering Cancer Center and through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Abbreviations
- CDC
Centers for Disease Control and Prevention
- CE
Collision energy
- CLIA
Clinical Laboratory Improvement Amendments
- CLSI
Clinical and Laboratory Standards Institute
- CXP
Collision cell exit potential
- DP
Declustering potential
- E2
Estradiol
- E2-D5
Deuterated estradiol
- EP
Entrance potential
- HoSt
Hormone Standardization Program
- Te
Testosterone
- Te-D3
Deuterated testosterone
- VIM
Valve interface module
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nelson R, Grebe SK, O’Kane DJ, Singh RJ. Liquid chromatography–tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50(2):373–384. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 2.Kushnir MM, Blamires T, Rockwood AL, Roberts WL, Yue B, Erdogan E, Bunker AM, Meikle AW. Liquid chromatography-tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clin Chem. 2010;56(7):1138–1147. doi: 10.1373/clinchem.2010.143222. [DOI] [PubMed] [Google Scholar]
- 3.Herold DA, Fitzgerald RL. Immunoassays for testosterone in women: better than a guess? Clin Chem. 2003;49(8):1250–1251. doi: 10.1373/49.8.1250. [DOI] [PubMed] [Google Scholar]
- 4.Demers LM. Testosterone and estradiol assays: current and future trends. Steroids. 2008;73(13):1333–1338. doi: 10.1016/j.steroids.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Leung YS, Dees K, Cyr R, Schoegel L, Kao PC. Falsely increased serum estradiol results reported in direct estradiol assays. Clin Chem. 1997;43(7):1250–1251. [PubMed] [Google Scholar]
- 6.Jaque J, Macdonald H, Brueggmann D, Patel SK, Azen C, Clarke N, Stanczyk FZ. Deficiencies in immunoassay methods used to monitor serum Estradiol levels during aromatase inhibitor treatment in postmenopausal breast cancer patients. Springer Plus. 2013;2(1):1–6. doi: 10.1186/2193-1801-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vesper HW, Botelho JC, Wang Y. Challenges and improvements in testosterone and estradiol testing. Asian J Androl. 2014;16(2):178. doi: 10.4103/1008-682X.122338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosner W, Vesper H. Toward excellence in testosterone testing: a consensus statement. J Clin Endocrinol Metab. 2010;95(10):4542–4548. doi: 10.1210/jc.2010-1314. [DOI] [PubMed] [Google Scholar]
- 9.Tai SSC, Welch MJ. Development and evaluation of a reference measurement procedure for the determination of estradiol-17β in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2005;77(19):6359–6363. doi: 10.1021/ac050837i. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita K, Okuyama M, Watanabe Y, Honma S, Kobayashi S, Numazawa M. Highly sensitive determination of estrone and estradiol in human serum by liquid chromatography–electrospray ionization tandem mass spectrometry. Steroids. 2007;72(11):819–827. doi: 10.1016/j.steroids.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79(20):7813–7821. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- 12.Harwood DT, Handelsman DJ. Development and validation of a sensitive liquid chromatography–tandem mass spectrometry assay to simultaneously measure androgens and estrogens in serum without derivatization. Clin Chim Acta. 2009;409(1):78–84. doi: 10.1016/j.cca.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Wooding KM, Hankin JA, Johnson CA, Chosich JD, Baek SW, Bradford AP, Murphy RC, Santoro N. Measurement of estradiol, estrone, and testosterone in postmenopausal human serum by isotope dilution liquid chromatography tandem mass spectrometry without derivatization. Steroids. 2015;96:89–94. doi: 10.1016/j.steroids.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiers T, Casetta B, Bernaert B, Vandersypt E, Debock M, Kaufman JM. Development of a highly sensitive method for the quantification of estrone and estradiol in serum by liquid chromatography tandem mass spectrometry without derivatization. J Chromatogr B. 2012;893:57–62. doi: 10.1016/j.jchromb.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Pauwels S, Antonio L, Jans I, Lintermans A, Neven P, Claessens F, Decallonne B, Billen J, Vanderschueren D, Vermeersch P. Sensitive routine liquid chromatography–tandem mass spectrometry method for serum estradiol and estrone without derivatization. Anal Bioanal Chem. 2013;405(26):8569–8577. doi: 10.1007/s00216-013-7259-5. [DOI] [PubMed] [Google Scholar]
- 16.Owen LJ, Wu FC, Keevil BG. A rapid direct assay for the routine measurement of oestradiol and oestrone by liquid chromatography tandem mass spectrometry. Ann Clin Biochem. 2014;51(3):360–367. doi: 10.1177/0004563213501478. [DOI] [PubMed] [Google Scholar]
- 17.CLSI. CLSI document C57. Wayne, PA: Clinical and Laboratory Standards Institute; 2015. Mass Spectrometry for Androgen and Estrogen Measurements in Serum; Approved Guideline. [Google Scholar]
- 18.FDA guidance for industry bioanalytical method validation. US department of health and human service, Food and Drug Administration, Center for drug evaluation and research (CDER); 2001. [Google Scholar]
- 19.CLSI. CLSI document C62-A. Wayne, PA: Clinical and Laboratory Standards Institute; 2013. Liquid Chromatography-Mass Spectrometry Methods; Approved Guideline. [Google Scholar]
- 20.CLSI. CLSI document EP6-A. Wayne, PA: Clinical and Laboratory Standards Institute; 2013. Evaluation of the Linearity of Quantitative Measurement Procedures: A Statistical Approach; Approved Guideline. [Google Scholar]
- 21.Hall TG, McKearn D, Smukste I, Bresciano KR, Savage RE, Wang Y. Identifying and overcoming matrix effects in drug discovery and development. INTECH Open Access Publisher; 2012. [Google Scholar]
- 22.Singh RJ. Validation of a high throughput method for serum/plasma testosterone using liquid chromatography tandem mass spectrometry (LC–MS/MS) Steroids. 2008;73(13):1339–1344. doi: 10.1016/j.steroids.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Botelho JC, Shacklady C, White J, Vesper HW. Development of a candidate reference measurement procedure for the determination of estradiol in human serum. Clin Chem. 2011;57(10):A180. [Google Scholar]
- 24.Botelho JC, Shacklady C, Cooper HC, Tai SS, Van Uytfanghe K, Thienpont LM, Vesper HW. Isotope-dilution liquid chromatography–tandem mass spectrometry candidate reference method for total testosterone in human serum. Clin Chem. 2013;59(2):372–80. doi: 10.1373/clinchem.2012.190934. [DOI] [PubMed] [Google Scholar]


