Abstract
These NCCN Guidelines Insights discuss the major recent updates to the NCCN Guidelines for Bladder Cancer based on the review of the evidence in conjunction with the expert opinion of the panel. Recent updates include (1) refining the recommendation of intravesical bacillus Calmette-Guérin, (2) strengthening the recommendations for perioperative systemic chemotherapy, and (3) incorporating immunotherapy into second-line therapy for locally advanced or metastatic disease. These NCCN Guidelines Insights further discuss factors that affect integration of these recommendations into clinical practice.
Overview
An estimated 76,960 new cases (58,950 men and 18,010 women) of urinary bladder cancer will be diagnosed in the United States in 2016, with approximately 16,390 deaths (11,820 men and 4,570 women) occurring during this same period.1 Bladder cancer, the fifth most common cancer in the United States, is 4 times more prevalent in men than in women. Bladder cancers are rarely diagnosed in individuals younger than 40 years. Given that the median age at diagnosis is 65 years, medical comorbidities are a frequent consideration in patient management. Management of bladder cancer is based on the pathologic findings of the biopsy specimen, with attention to histology, grade, and depth of invasion. These factors are used to estimate the probability of recurrence and progression to a more advanced stage.
Transurethral resection of the bladder tumor (TURBT) with examination under anesthesia is the initial treatment for bladder cancer. The goal of TURBT is to correctly identify the clinical stage and grade of disease while completely resecting all visible tumor; therefore, an adequate sample that includes bladder muscle (ie, muscularis propria) must be in the resection specimen. For non-muscle- invasive tumors, intravesical therapy or radical cystectomy may be recommended based on the estimated probability of recurrence and progression to a more advanced stage. In patients with muscle-invasive tumors, treatment after initial TURBT is required. Different treatment modalities may be used, including radical cystectomy with or without neoadjuvant or adjuvant therapy, partial cystectomy, bladder-preserving approaches, and systemic therapy for advanced disease. Clinical trials investigating new treatments and further evidence to support current recommendations are reflected in the update of these guidelines. To view the most recent and complete version of these guidelines, visit NCCN.org.
Intravesical Bacillus Calmette-Guérin
Intravesical therapy is used in 2 general settings: most commonly as prophylactic or adjuvant therapy after a complete endoscopic resection, or, rarely, as adjuvant therapy with the goal of eradicating residual disease that could not be completely resected. This distinction is important, because most published data reflect the former, with the goal of preventing recurrence or delaying progression to a higher grade or stage. In many cases, intravesical therapy may be overused if given to patients who have a low probability of recurrence or progression. When given prophylactically, bacillus Calmette-Guérin (BCG) has been shown to prevent bladder cancer recurrences after TURBT. There are 4 meta-analyses demonstrating that BCG after TURBT is superior to TURBT alone or TURBT and chemotherapy in preventing recurrences of highgrade Ta and T1 tumors.2–5 A meta-analysis including 9 trials of 2,820 patients with non-muscle-invasive bladder cancer reported that mitomycin C was superior to BCG without maintenance in preventing recurrence, but inferior to BCG in trials with maintenance.6 Using the SEER database, a reduction in mortality of 23% was reported in patients receiving BCG therapy. However, only 22% of patients who met the eligibility criteria for BCG were given this treatment, suggesting that BCG therapy is underused.7
There are concerns regarding potentially severe local and systemic side effects and the inconsistent availability of BCG. BCG induces a systemic non-specific immunostimulatory response leading to secretion of pro inflammatory cytokines. An early response from innate immune cells triggers chemokine and cytokine production that initiates a cellular immune response, including neutrophils, monocytes and macrophages, T cells, and natural killer (NK) cells. This causes patients to experience flu-like symptoms that may last 48 to 72 hours.8 Installation of BCG into the bladder also mimics a urinary tract infection and may produce intense local discomfort. The side effects of treatment have translated to patient refusal of BCG therapy. Local dysuria has been reported in 60% of patients in clinical trials.8 Symptom management with single-dose, short-term quinolones and/or anticholinergics have been reported to reduce adverse events.9,10 A reduced (one-third) dose of BCG has been evaluated for the possible reduction of side effects. In a phase III study, 1,316 patients with intermediate- or high-risk Ta, T1 papillary carcinoma of the bladder were randomized to receive either reduced- or full-dose BCG for either 1 or 3 years of maintenance.11 Among all 4 groups, the percentage of patients with 1 or more side effect was similar (P=.41). Although the one-third dose of BCG was effective, side effects were not reduced. Conversely, other publications suggest that the one-third dose may reduce side effects.12–14 Full-dose BCG is recommended by the panel until more data are available to evaluate the low-dose BCG regimen. However, dose reduction may be used if there are substantial local symptoms during maintenance.
Timing and duration of maintenance BCG remain a subject of debate. BCG therapy is commonly given once a week for 6 weeks, followed by a rest period of 4 to 6 weeks, with a full re-evaluation at week 12 (ie, 3 months) after the start of therapy.15 There is less agreement for the optimal schedule of maintenance BCG. The controversy over the effectiveness of BCG maintenance reflects the wide array of schedules and conflicting reports of efficacy. Quarterly and monthly installations and 3- and 6-week schedules have been evaluated. To date, the strongest data support the 3-week BCG regimen used in the SWOG trial, which demonstrated reduced disease progression and metastasis.16 The 3-week timing of BCG has shown improved outcomes compared with epirubicin17 or isoniazid.18 Most patients receive maintenance BCG for 1 to 3 years. In an evaluation of randomized controlled trials and meta-analyses, limited evidence was found for 1 year of BCG maintenance.19 Conversely, a study of 1,355 patients with a median follow-up of 7.1 years found no benefit with 3 years of maintenance BCG compared with 1 year for intermediate-risk patients.20 In high-risk patients, 3-year maintenance BCG reduced recurrence compared with 1-year maintenance, but did not impact progression or survival. These data suggest that 1 year of maintenance may be suitable for patients at intermediate risk. It should also be noted that duration of treatment may be limited by toxicity and patient refusal to continue.
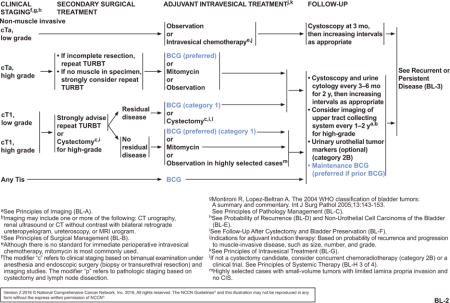
BCG is not recommended for low-grade Ta tumors due to the low risk of disease progression. The NCCN Guidelines for Bladder Cancer generally manage Tis, high-grade Ta, or T1 tumors with intravesical BCG therapy or cystectomy (see BL-2, page 1215). Primary Tis is a high-grade lesion that is managed with resection followed by intravesical therapy with BCG. Patients with high-grade Ta tumors may be treated with intravesical BCG (preferred) or mitomycin C. A repeat TURBT is strongly advised for patients with T1 disease. As long as no muscle-invasive disease is found after the second resection, intravesical therapy with BCG (preferred; category 1) or mitomycin C (category 2A) is recommended. Observation may be reasonable in highly select cases where small-volume tumors had limited lamina propria invasion and no Tis.21,22 If residual disease is found, treatment should consist of BCG (category 1) or cystectomy (category 2A). Within T1 disease, a particularly high-risk stratum can be identified: multifocal lesions, tumors associated with carcinoma in situ or lymphovascular invasion, micropapillary tumors, or lesions that recur after BCG treatment. There are data suggesting that early cystectomy may be preferred if residual disease is found because of the high risk for progression to a more advanced stage.23
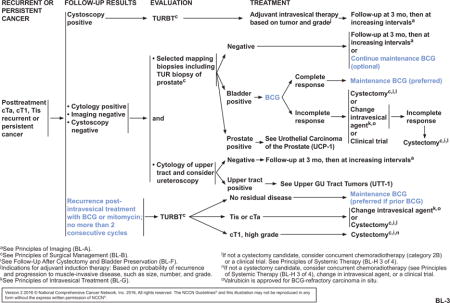
After the initial intravesical treatment and 12-week evaluation, patients with persistent cTa, cT1, or Tis disease tumors can be given a second induction course of BCG induction therapy (see BL-3, page 1216); no more than 2 consecutive induction courses should be given. If a second course of BCG is given, TURBT is performed to determine the presence of residual disease at the second 12-week follow-up. If no residual disease is found, maintenance BCG is recommended and preferred for patients who received prior BCG. If residual disease is seen after TURBT, patients with persistent high-grade cT1 tumors are recommended to proceed to cystectomy. Patients with persistent Tis or cTa disease after TURBT may be treated with intravesical therapy using a different intravesical agent or cystectomy. For patients with disease that does not respond to BCG or that shows an incomplete response, subsequent management options include cystectomy, change in intravesical agent, and participation in a clinical trial. Although a few NCCN Member Institutions do not routinely administer maintenance BCG, panel consensus is to recommend maintenance BCG if prior BCG was given. This recommendation is based on findings that an induction course of BCG intravesical therapy followed by a maintenance regimen produced better outcomes than intravesical chemotherapy.2,3,16,24–26
Perioperative Chemotherapy
One of the most noteworthy issues in the treatment of bladder cancer is the optimal use of perioperative chemotherapy for muscle-invasive disease. Data support the role of neoadjuvant chemotherapy before cystectomy for T2, T3, and T4a lesions.27–32 In a SWOG randomized trial of 307 patients with muscle-invasive disease that compared radical cystectomy alone versus 3 cycles of neoadjuvant methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) followed by radical cystectomy, neoadjuvant chemotherapy increased median survival (77 vs 46 months; P=.06) and lowered the rate of residual disease (15% vs 38%; P<.001) with no apparent increase in treatment-related morbidity or mortality.27 Another trial randomized 196 patients with invasive bladder cancer to 2 cycles of neoadjuvant MVAC before radical cystectomy or cystectomy only.33 Neoadjuvant chemotherapy resulted in more patients achieving pT0 than cystectomy alone (34% vs 9%; P<.01). Overall survival (OS) favored the neoadjuvant group, although it did not reach statistical significance.33 In a meta-analysis of 11 trials involving 3,005 patients, cisplatin-based neoadjuvant chemotherapy was associated with improved 5-year OS and disease-free survival (DFS) (5% and 9% absolute improvement, respectively).34
Since the neoadjuvant trial with MVAC, the use of dose-dense MVAC (ddMVAC) with growth factor support in the metastatic setting has been shown to have good comparable tolerance, with an increased complete response rate compared with standard dosing of MVAC (11% vs 25%; 2-sided P=.006).35 Based on these findings, ddMVAC has also been investigated in the neoadjuvant setting. In a multicenter prospective phase II trial, patients with cT2 to cT4a tumor staging and N0 or N1 muscle-invasive bladder cancer (n=44) were given 3 cycles of ddMVAC with pegfilgrastim followed by radical cystectomy and lymph node dissection.36 ddMVAC was anticipated to have a safer profile, a shorter time to surgery, and a similar pathologic complete response rate compared with historical control data for neoadjuvant MVAC chemotherapy given in previous studies. Patients receiving ddMVAC had no grade 3 or 4 renal toxicities and no toxicity-related deaths. Grade 1 or 2 treatment-related toxicities were seen in 82% of patients. The median time to cystectomy was 9.7 weeks from the start of chemotherapy.36 A separate single-arm phase II study also reported pathologic downstaging in 49% of patients receiving neoadjuvant ddMVAC with a similar safety profile.37 An additional neoadjuvant clinical trial of ddMVAC with bevacizumab reported 5-year survival outcomes of 63% and 64% (OS and disease-specific survival, respectively; median follow-up, 49 months), with pT0N0 and less than or equal to pT1N0 downstaging rates of 38% and 53%, respectively.38 Bevacizumab had no definitive impact on overall outcomes.
Despite evidence that neoadjuvant chemotherapy targets micrometastases and improves patient outcomes, clinical practice has been slow to incorporate this combination therapy. A survey of physicians reported the underuse of neoadjuvant chemotherapy by 30% to 57%.39 Common physician concerns included patient age or presence of comorbidities, delay of definitive treatment, and a presumed lack of evidence to support neoadjuvant chemotherapy.39,40 Although the NCCN Bladder Cancer Panel recognizes the potential for increased morbidity with chemotherapy and the potential delay in definitive treatment, the substantial benefits outweigh the risks when used appropriately. In addition to the benefit from early reduction of micrometastases, there may be improved drug delivery and patient tolerance in the preoperative setting.
Data are less clear regarding the role of adjuvant systemic chemotherapy in invasive bladder cancer. Studies have shown that adjuvant chemotherapy may delay recurrences and improve OS,41–43 but no randomized comparisons of adequate sample size have definitively shown a survival benefit, in large part due to the poor accrual.44 Clinical trials of adjuvant chemotherapy with cyclophosphamide, doxorubicin, and cisplatin (CAP); MVAC; and methotrexate, vinblastine, epirubicin, and cisplatin (MVEC) regimens have each suggested a survival advantage.45–47 However, methodologic issues call into question the applicability of these studies to all patients with urothelial tumors. In the MVEC trial, patients who experienced relapse in the control arm did not undergo chemotherapy, which is not typical of more contemporary series. Many trials were not randomized, raising the question of selection bias in the analysis of outcomes. A meta-analysis of 6 trials found a 25% mortality reduction with adjuvant chemotherapy, but the authors pointed out several limitations of the data and concluded that evidence is insufficient for treatment decisions.48 Interestingly, the follow-up analysis included 3 more studies, for a total of 9 trials (N=945 patients).43 A 23% risk reduction for death was observed in the updated analysis (hazard ratio [HR], 0.77; 95% CI, 0.59–0.99; P=.049) and improved DFS was achieved (HR, 0.66; 95% CI, 0.45–0.91; P=.014). Patients with nodepositive disease had an even greater DFS benefit.43 A recent observational study evaluated 5,653 patients, of which 23% received adjuvant chemotherapy postcystectomy.42 Patients who received adjuvant chemotherapy had an improved OS (HR, 0.70; 95% CI, 0.06–0.76).42 Although evidence for adjuvant therapy is not as strong as for neoadjuvant therapy, the growing body of data support the administration of adjuvant chemotherapy for patients with a high risk for relapse who did not receive neoadjuvant therapy.
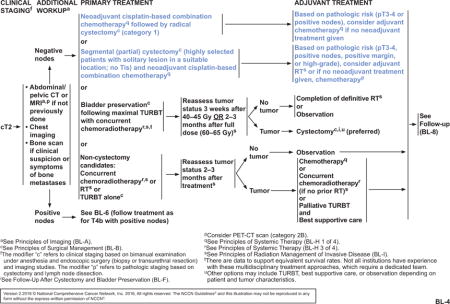
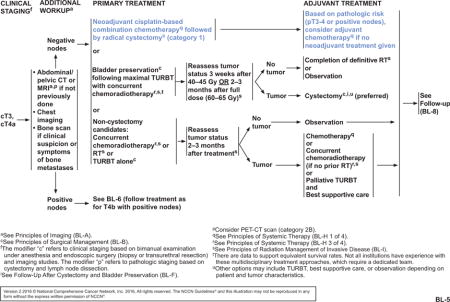
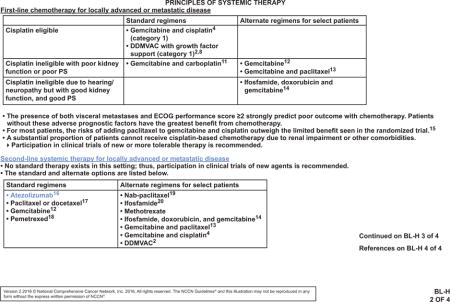
The NCCN Bladder Cancer Panel strengthened the recommendations for neoadjuvant chemotherapy for patients with cT2, cT3, and cT4a bladder cancer, and for adjuvant chemotherapy for patients with pT3 or pT4 disease or positive nodes (see BL-4 and BL-5, pages 1217 and 1218). Neoadjuvant chemotherapy followed by radical cystectomy is a category 1 recommendation by the panel. Patients with hearing loss or neuropathy, poor performance status, or renal insufficiency may not be eligible for cisplatin-based neoadjuvant chemotherapy. For patients with borderline renal function or minimal dysfunction, a split-dose administration of cisplatin may be considered (category 2B). Although split-dose is a safer alternative, the relative efficacy remains undefined. Adjuvant chemotherapy may be given to patients with high-risk pathology who did not receive neoadjuvant chemotherapy (category 2A). For highly select patients who undergo a partial cystectomy, neoadjuvant chemotherapy is a category 2A recommendation, with the option of adjuvant chemotherapy for patients who did not receive neoadjuvant chemotherapy (see BL-4, page 1217). A minimum of 3 cycles of a cisplatin-based combination, such as ddMVAC, gemcitabine plus cisplatin, or CMV (cisplatin, methotrexate, and vinblastine), may be used in patients undergoing perioperative chemotherapy. Regimen and dosing recommendations are mainly based on studies in advanced disease.27,32,49–51 Carboplatin has not demonstrated a survival benefit and should not be substituted for cisplatin in the perioperative setting. It should be noted that patients with tumors that are pT2 or less and have no nodal involvement or lymphovascular invasion after cystectomy are considered to have lower risk and are not recommended to receive adjuvant chemotherapy. Data are limited to support perioperative chemotherapy for nonurothelial carcinomas; however, neoadjuvant chemotherapy may have benefit in patients with small cell carcinoma of the bladder, and is recommended by the panel for any patient with small-cell component histology with localized disease, regardless of stage.52–55
Targeted Immunotherapy
Platinum-based chemotherapy has been the standard of care in patients with metastatic disease with an OS of 9 to 15 months.56,57 However, in patients with disease that relapses after this type of chemotherapy, the median survival is reduced to 5 to 7 months.58 Several new agents for the treatment of metastatic urothelial carcinoma are being advanced in clinical trials, and early data suggest improved outcomes compared with standard therapies.
PD-1 and PD-L1 checkpoint inhibitors have garnered attention recently based on clinical trial data and the FDA approval of the PD-L1 inhibitor atezolizumab. Atezolizumab is approved for the treatment of locally advanced or metastatic urothelial cell carcinoma that has progressed during or after platinum-based chemotherapy or that has progressed within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy, regardless of PD-L1 expression levels. In a single-arm, multicenter, phase II trial in 310 patients with metastatic urothelial carcinoma that progressed after treatment with platinum-based chemotherapy, data showed a significantly improved objective response rate compared with historical controls (15% vs 10%; P=.0058).59 Notably and consistent to observation of checkpoint inhibitors in other cancer types, responses tended to be durable, with ongoing responses recorded in 38 of 45 responders (84%) with a median follow-up of 11.7 months. Although a similar response rate was seen regardless of PD-L1 status of tumor cells, a greater response was associated with increased PD-L1 expression status on infiltrating immune cells in the tumor microenvironment. Grade 3 or 4 treatment-related or immune-mediated adverse events occurred in 16% and 5% of patients, respectively. Furthermore, there were no treatment-related deaths in this trial, suggesting good tolerability. Atezolizumab marks the first immunotherapy to be approved for patients with advanced urothelial carcinoma, a setting that has had a dearth of new therapies.
Durvalumab and avelumab are 2 other PD-L1 inhibitors in clinical trials to evaluate their activity in the treatment of bladder cancer. Early results from a phase I/II multicenter study of 61 patients has led to FDA breakthrough therapy designation of durvalumab for patients with PD-L1–positive inoperable or metastatic urothelial bladder cancer with a tumor that has progressed during or after one standard platinum-based regimen. In this study, 46.4% of patients who were PD-L1-positive experienced a response to treatment; no response was seen in those who were PD-L1-negative.60 Median duration of response for 12 of the 13 patients was not yet reached at the time of publication (range, 4.1–49.3 weeks). Results from the phase Ib trial for patients with platinum-refractory disease or who are ineligible for cisplatin-based chemotherapy demonstrated an overall response rate (ORR) of 18.2% that consisted of 2 complete responses and 6 partial responses after treatment with avelumab.61 A higher progression-free survival was seen in patients with positive PD-L1 tumor cells versus patients that did not express PD-L1 (58.3% vs 16.6% at 24 weeks), although some patients who were PD-L1-negative did experience a response to treatment.
Nivolumab and pembrolizumab are antibodies directed towards PD-1. Nivolumab is indicated for the treatment of several advanced and metastatic cancers and has been granted FDA breakthrough status for the treatment of advanced bladder cancer. In a phase I/II trial in patients with locally advanced or metastatic urothelial carcinoma that progressed after treatment with at least one platinum-containing regimen, early data showed an objective response rate of 24% (95% CI, 15.3%−35.4%) that was unaffected by PD-1 tumor status.62 Of the 78 patients enrolled in the study, 2 experienced grade 5 treatment-related adverse events, and grade 3 or 4 treatment-related adverse events were reported in 22% of patients.62 Pembrolizumab has been evaluated as second-line therapy for patients with bladder cancer who previously received platinum-based therapy and who subsequently experienced disease progression or metastasis.63,64 The ORR was 25% in this trial, with 7 of the 22 patients reporting a complete or partial response.65 Grade 3 to 4 adverse events occurred in 15% of patients.65
Emerging data are encouraging for the effectiveness of checkpoint inhibitors in the treatment of urothelial carcinoma. Cancers with higher rates of somatic mutations have been shown to respond better to checkpoint inhibitors.66–71 Data from The Cancer Genome Atlas rank bladder cancer as the third highest mutated cancer,72,73 suggesting that checkpoint inhibitors may have a substantial impact as a treatment option for this cancer. The value of checkpoint inhibitors is reflected in the unanimous decision by the NCCN panel to include atezolizumab as a second-line systemic therapy for locally advanced or metastatic disease after platinum-based therapy (see BL-H 2 of 4, page 1219).
Conclusions
The advancement of treatment options for bladder cancer is illustrated by the array of treatment modalities and clinical trials. It has been 40 years since immunotherapy was first implemented as a therapy in bladder cancer. BCG has become the backbone of treatment for high-grade, non-muscle-invasive bladder cancer. Checkpoint inhibitors have emerged as a second immunotherapy option for urothelial carcinoma. In addition, neoadjuvant and adjuvant therapies have gained wider acceptance. Experts surmise that the treatment of urothelial tumors will evolve rapidly over the next few years, with improved outcomes for patients at all stages of disease. Although advances are expected, improvement of patient outcomes will only be achieved with the universal implementation of these changes in clinical practice.
NCCN: Continuing Education.
Accreditation Statement
This activity has been designed to meet the educational needs of physicians, nurses, and pharmacists involved in the management of patients with cancer. There is no fee for this article. The National Comprehensive Cancer Network (NCCN) is accredited by the ACCME to provide continuing medical education for physicians. NCCN designates this journal-based CE activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
NCCN is accredited as a provider of continuing nursing education by the American Nurses Credentialing Center’s Commission on Accreditation.
NCCN designates this educational activity for a maximum of 1.0 contact hour. Accreditation as a provider refers to recognition of educational activities only; accredited status does not imply endorsement by NCCN or ANCC of any commercial products discussed/displayed in conjunction with the educational activity. Kristina M. Gregory, RN, MSN, OCN, is our nurse planner for this educational activity.
National Comprehensive Cancer Network is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. NCCN designates this continuing education activity for 1.0 contact hour(s) (0.1 CEUs) of continuing education credit in states that recognize ACPE accredited providers. This is a knowledge-based activity. UAN: 0836-0000-16-010-H01-P
All clinicians completing this activity will be issued a certificate of participation. To participate in this journal CE activity: 1) review the learning objectives and author disclosures; 2) study the education content; 3) take the posttest with a 66% minimum passing score and complete the evaluation at http://education.nccn.org/node/79636; and 4) view/print certificate.
Learning Objectives
Upon completion of this activity, participants will be able to:
Integrate into professional practice the updates to NCCN Guidelines for Bladder Cancer
Describe the rationale behind the decision-making process for developing the NCCN Guidelines for Bladder Cancer
NCCN Categories of Evidence and Consensus.
Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2B: Based upon lower-level evidence, there is NCCN consensus that the intervention is appropriate.
Category 3: Based upon any level of evidence, there is major NCCN disagreement that the intervention is appropriate.
All recommendations are category 2A unless otherwise noted.
Clinical trials: NCCN believes that the best management for any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
Instructions for Completion.
To participate in this journal CE activity: 1) review the learning objectives and author disclosures; 2) study the education content; 3) take the posttest with a 66% minimum passing score and complete the evaluation at http://education.nccn.org/node/79636; and 4) view/print certificate. After reading the article, you should be able to answer the following multiple-choice questions. Credit cannot be obtained for tests completed on paper. You must be a registered user on NCCN.org. If you are not registered on NCCN.org, click on “New Member? Sign up here” link on the left hand side of the Web site to register. Only one answer is correct for each question. Once you successfully answer all posttest questions you will be able to view and/or print your certificate. Software requirements: Internet
Posttest Questions.
- Which immunotherapy or immunotherapies is/are FDA-approved for use in patients with bladder cancer?
- BCG
- Atezolizumab
- Durvalumab
- Avelumab
- 2
- 1 and 2
- 2 and 3
- All of the above
- Which statement about BCG is false?
- Early cystectomy may be preferred to BCG in patients with high-risk T1 disease
- Duration of BCG treatment may be limited by toxicity
- Tis, Ta, or T1 tumors are all generally managed with intravesical BCG therapy or cystectomy
- The most common timing for BCG maintenance is the 3-week schedule
True or False: Patients who are cisplatin-ineligible can consider carboplatin-based chemotherapy in the perioperative setting.
Acknowledgments
This activity is supported by educational grants from AstraZeneca, Bayer Healthcare Pharmaceuticals Inc., Bristol-Myers Squibb, Clovis Oncology, Foundation Medicine, Genentech, Novartis Oncology, Otsuka America Pharmaceutical, Inc., Seattle Genetics, Inc., and Takeda Oncology; support provided by Actelion Pharmaceuticals US, Inc.; and by an independent educational grant from Astellas and Medivation, Inc.
Disclosure of Relevant Financial Relationships
Editor
Kerrin M. Green, MA, Assistant Managing Editor, JNCCN—Journal of the National Comprehensive Cancer Network, has disclosed that she has no relevant financial relationships.
CE Authors
Deborah J. Moonan, RN, BSN, Director, Continuing Education, NCCN, has disclosed that she has no relevant financial relationships.
Kristina M. Gregory, RN, MSN, OCN, Vice President, Clinical Information Operations, NCCN, has disclosed that she has no relevant financial relationships.
Rashmi Kumar, PhD, Senior Manager, Clinical Content, NCCN, has disclosed that she has no relevant financial relationships.
Individuals Who Provided Content Development and/or Authorship Assistance:
Peter E. Clark, MD, Panel Chair, has disclosed that he is a scientific advisor for Genentech, Inc.
Philippe E. Spiess, MD, MS, has disclosed that he receives consulting fees and/or honoraria from Janssen Pharmaceutica Products, LP, and Prometheus Laboratories Inc.
Rick Bangs, MBA, Panel Member, has disclosed that he has no relevant financial relationships.
Stephen A. Boorjian, MD, Panel Member, has disclosed that he has no relevant financial relationships.
Thomas W. Flaig, MD, Panel Member, has disclosed that he is the founder of Aurora Oncology, and that he receives honoraria from GTx, Inc. and BN ImmunoTherapeutics, Inc. He also receives grant/research support from from GTx, Inc.; BN ImmunoTherapeutics, Inc.; Novartis Pharmaceuticals Corporation; Dendreon Corporation; Janssen Pharmaceutica Products, LP/Cougar Biotechnology; Pfizer Inc.; Medivation, Inc.; sanofi-aventis; Bristol-Myers Squibb Company; Roche/Genentech, Inc.; Exelixis Inc.; Aragon Pharmaceuticals Inc.; SOTIO, LLC; Tokai Pharmaceuticals, Inc.; AstraZeneca Pharmaceuticals LP; Eli Lilly and Company; and Astellas US LLC.
Noah Hahn, MD, Panel Member, has disclosed that he receives consulting fees from Genentech, Inc.; AstraZeneca Pharmaceuticals/MedImmune, LLC; Inovio Pharmaceuticals, Inc.; Principia Biopharma Inc.; and Merck & Co., Inc. He also receives research support from Novartis Pharmaceuticals Corporation; Heat Biologics, Inc.; Genentech, Inc.; Bristol-Myers Squibb Company; AstraZeneca Pharmaceuticals/MedImmune, LLC; Merck & Co., Inc.; Acerta Pharma; and Principia Biopharma Inc.
A. Karim Kader, MD, PhD, Panel Member, has disclosed that he is a consultant for Pellficure Pharmaceuticals, Inc., and is a founder, board member, and officer for SNP Bio, Inc.
Joshua J. Meeks, MD, PhD, Panel Member, has disclosed that he has no relevant financial relationships.
Lance C. Pagliaro, MD, Panel Member, has disclosed that he receives honoraria from sanofi-aventis and Novartis Pharmaceuticals Corporation.
Elizabeth R. Plimack, MD, MS, Panel Member, has disclosed that she is a scientific advisor for Acceleron Pharma, Inc., AstraZeneca Pharmaceuticals LP, Bristol- Myers Squibb Company, Genentech, Inc., Eli Lilly and Company, Novartis Pharmaceuticals Corporation, Pfizer Inc., Roche, and SynerGene Therapeutics; and receives grant/research support from Acceleron Pharma, Inc., AstraZeneca Pharmaceuticals LP, AVEO Pharmaceuticals, Inc., Bristol-Myers Squibb Company, Dendreon Corporation, Glaxo SmithKline, Eli Lilly and Company, Merck & Co., Inc., Peloton Therapeutics, Inc., and Pfizer Inc.
Wade J. Sexton, MD, Panel Member, has disclosed that he is on the advisory board for Altor BioScience Corporation.
Arlene O. Siefker-Radtke, MD, Panel Member, has disclosed that she is a member of the advisory board for Janssen Pharmaceuticals, Inc.; Eisai Inc.; Genen-tech, Inc.; and AstraZeneca Pharmaceuticals LP.
Mary A. Dwyer, MS, CGC, Senior Manager, Guidelines, NCCN, has disclosed that she has no relevant financial relationships.
Courtney Smith, PhD, MT(ASCP), Oncology Scientist/Medical Writer, NCCN, has disclosed that she has no relevant financial relationships.
Footnotes
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) are a statement of consensus of the authors regarding their views of currently accepted approaches to treatment. The NCCN Guidelines® Insights highlight important changes to the NCCN Guidelines® recommendations from previous versions. Colored markings in the algorithm show changes and the discussion aims to further the understanding of these changes by summarizing salient portions of the NCCN Guideline Panel discussion, including the literature reviewed.
These NCCN Guidelines Insights do not represent the full NCCN Guidelines; further, the National Comprehensive Cancer Network® (NCCN®) makes no representation or warranties of any kind regarding the content, use, or application of the NCCN Guidelines and NCCN Guidelines Insights and disclaims any responsibility for their applications or use in any way.
The full and most current version of these NCCN Guidelines are available at NCCN.org.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Bohle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169:90–95. doi: 10.1016/S0022-5347(05)64043-8. [DOI] [PubMed] [Google Scholar]
- 3.Han RF, Pan JG. Can intravesical bacillus Calmette-Guerin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology. 2006;67:1216–1223. doi: 10.1016/j.urology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Shelley MD, Kynaston H, Court J, et al. A systematic review of intravesical bacillus Calmette-Guerin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int. 2001;88:209–216. doi: 10.1046/j.1464-410x.2001.02306.x. [DOI] [PubMed] [Google Scholar]
- 5.Shelley MD, Wilt TJ, Court J, et al. Intravesical bacillus Calmette-Guerin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: a meta-analysis of randomized trials. BJU Int. 2004;93:485–490. doi: 10.1111/j.1464-410x.2003.04655.x. [DOI] [PubMed] [Google Scholar]
- 6.Malmstrom PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle- invasive bladder cancer. Eur Urol. 2009;56:247–256. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 7.Spencer BA, McBride RB, Hershman DL, et al. Adjuvant intravesical bacillus calmette-guerin therapy and survival among elderly patients with non-muscle- invasive bladder cancer. J Oncol Pract. 2013;9:92–98. doi: 10.1200/JOP.2011.000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.TICE (BCG live), for intravesical use [prescribing information] Durham, NC: OrganonTeknika Corporation LLC; 2009. [Google Scholar]
- 9.Colombel M, Saint F, Chopin D, et al. The effect of ofloxacin on bacillus calmette-guerin induced toxicity in patients with superficial bladder cancer: results of a randomized, prospective, double-blind, placebo controlled, multicenter study. J Urol. 2006;176:935–939. doi: 10.1016/j.juro.2006.04.104. [DOI] [PubMed] [Google Scholar]
- 10.Damiano R, De Sio M, Quarto G, et al. Short-term administration of prulifloxacin in patients with nonmuscle-invasive bladder cancer: an effective option for the prevention of bacillus Calmette-Guerin-induced toxicity? BJU Int. 2009;104:633–639. doi: 10.1111/j.1464-410X.2009.08469.x. [DOI] [PubMed] [Google Scholar]
- 11.Brausi M, Oddens J, Sylvester R, et al. Side effects of Bacillus Calmette-Guerin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol. 2014;65:69–76. doi: 10.1016/j.eururo.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Lebret T, Bohin D, Kassardjian Z, et al. Recurrence, progression and success in stage Ta grade 3 bladder tumors treated with low dose bacillus Calmette-Guerin instillations. J Urol. 2000;163:63–67. [PubMed] [Google Scholar]
- 13.Martinez-Pineiro JA, Martinez-Pineiro L, Solsona E, et al. Has a 3-fold decreased dose of bacillus Calmette-Guerin the same efficacy against recurrences and progression of T1G3 and Tis bladder tumors than the standard dose? Results of a prospective randomized trial. J Urol. 2005;174:1242–1247. doi: 10.1097/01.ju.0000173919.28835.aa. [DOI] [PubMed] [Google Scholar]
- 14.Mugiya S, Ozono S, Nagata M, et al. Long-term outcome of a low-dose intravesical bacillus Calmette-Guerin therapy for carcinoma in situ of the bladder: results after six successive instillations of 40 mg BCG. Jpn J Clin Oncol. 2005;35:395–399. doi: 10.1093/jjco/hyi111. [DOI] [PubMed] [Google Scholar]
- 15.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 16.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163:1124–1129. [PubMed] [Google Scholar]
- 17.Duchek M, Johansson R, Jahnson S, et al. Bacillus Calmette-Guerin is superior to a combination of epirubicin and interferon-alpha2b in the intravesical treatment of patients with stage T1 urinary bladder cancer. A prospective, randomized, Nordic study. Eur Urol. 2010;57:25–31. doi: 10.1016/j.eururo.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 18.Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette- Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57:766–773. doi: 10.1016/j.eururo.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehdaie B, Sylvester R, Herr HW. Maintenance bacillus Calmette-Guerin treatment of non-muscle-invasive bladder cancer: a critical evaluation of the evidence. Eur Urol. 2013;64:579–585. doi: 10.1016/j.eururo.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 20.Oddens J, Brausi M, Sylvester R, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guerin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. 2013;63:462–472. doi: 10.1016/j.eururo.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 21.Gofrit ON, Pode D, Lazar A, et al. Watchful waiting policy in recurrent Ta G1 bladder tumors. Eur Urol. 2006;49:303–306. doi: 10.1016/j.eururo.2005.12.029. discussion 306–307. [DOI] [PubMed] [Google Scholar]
- 22.Soloway MS, Bruck DS, Kim SS. Expectant management of small, recurrent, noninvasive papillary bladder tumors. J Urol. 2003;170:438–441. doi: 10.1097/01.ju.0000076621.71247.6c. [DOI] [PubMed] [Google Scholar]
- 23.Herr HW, Sogani PC. Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? J Urol. 2001;166:1296–1299. [PubMed] [Google Scholar]
- 24.Bohle A, Bock PR. Intravesical bacille Calmette-Guerin versus mitomycin C in superficial bladder cancer: formal meta-analysis of comparative studies on tumor progression. Urology. 2004;63:682–686. doi: 10.1016/j.urology.2003.11.049. discussion 686–687. [DOI] [PubMed] [Google Scholar]
- 25.Sylvester RJ, van der MA, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–1970. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 26.Sylvester RJ, van der Meijden AP, Witjes JA, Kurth K. Bacillus calmette-guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol. 2005;174:86–91. doi: 10.1097/01.ju.0000162059.64886.1c. discussion 91–92. [DOI] [PubMed] [Google Scholar]
- 27.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 28.Sherif A, Holmberg L, Rintala E, et al. Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two Nordic studies. Eur Urol. 2004;45:297–303. doi: 10.1016/j.eururo.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Winquist E, Kirchner TS, Segal R, et al. Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review and metaanalysis. J Urol. 2004;171:561–569. doi: 10.1097/01.ju.0000090967.08622.33. [DOI] [PubMed] [Google Scholar]
- 30.Vashistha V, Quinn DI, Dorff TB, Daneshmand S. Current and recent clinical trials for perioperative systemic therapy for muscle invasive bladder cancer: a systematic review. BMC Cancer. 2014;14:966. doi: 10.1186/1471-2407-14-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) metaanalysis collaboration. Eur Urol. 2005;48:202–205. doi: 10.1016/j.eururo.2005.04.006. discussion 205–206. [DOI] [PubMed] [Google Scholar]
- 32.Griffiths G, Hall R, Sylvester R, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29:2171–2177. doi: 10.1200/JCO.2010.32.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitamura H, Tsukamoto T, Shibata T, et al. Randomised phase III study of neoadjuvant chemotherapy with methotrexate, doxorubicin, vinblastine and cisplatin followed by radical cystectomy compared with radical cystectomy alone for muscle-invasive bladder cancer: Japan Clinical Oncology Group study JCOG0209. Ann Oncol. 2014;25:1192–1198. doi: 10.1093/annonc/mdu126. [DOI] [PubMed] [Google Scholar]
- 34.Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–205. doi: 10.1016/j.eururo.2005.04.006. discussion 205–206. [DOI] [PubMed] [Google Scholar]
- 35.Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer. 2006;42:50–54. doi: 10.1016/j.ejca.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 36.Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol. 2014;32:1895–1901. doi: 10.1200/JCO.2013.53.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choueiri TK, Jacobus S, Bellmunt J, et al. Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol. 2014;32:1889–1894. doi: 10.1200/JCO.2013.52.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McConkey DJ, Choi W, Shen Y, et al. A prognostic gene expression signature in the molecular classification of chemotherapy-naive urothelial cancer is predictive of clinical outcomes from neoadjuvant chemotherapy: a phase 2 trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with bevacizumab in urothelial cancer. Eur Urol. 2016;69:855–862. doi: 10.1016/j.eururo.2015.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cowan NG, Chen Y, Downs TM, et al. Neoadjuvant chemotherapy use in bladder cancer: a survey of current practice and opinions. Adv Urol. 2014;2014:746298. doi: 10.1155/2014/746298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burger M, Mulders P, Witjes W. Use of neoadjuvant chemotherapy for muscle- invasive bladder cancer is low among major European centres: results of a feasibility questionnaire. Eur Urol. 2012;61:1070–1071. doi: 10.1016/j.eururo.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 41.Millikan R, Dinney C, Swanson D, et al. Integrated therapy for locally advanced bladder cancer: final report of a randomized trial of cystectomy plus adjuvant M-VAC versus cystectomy with both preoperative and postoperative M-VAC. J Clin Oncol. 2001;49:4005–4013. doi: 10.1200/JCO.2001.19.20.4005. [DOI] [PubMed] [Google Scholar]
- 42.Galsky MD, Stensland KD, Moshier E, et al. Effectiveness of adjuvant chemotherapy for locally advanced bladder cancer. J Clin Oncol. 2016;34:825–832. doi: 10.1200/JCO.2015.64.1076. [DOI] [PubMed] [Google Scholar]
- 43.Leow JJ, Martin-Doyle W, Rajagopal PS, et al. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol. 2014;66:42–54. doi: 10.1016/j.eururo.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 44.Hussain MH, Wood DP, Bajorin DF, et al. Bladder cancer: narrowing the gap between evidence and practice. J Clin Oncol. 2009;27:5680–5684. doi: 10.1200/JCO.2009.23.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lehmann J, Franzaring L, Thuroff J, et al. Complete long-term survival data from a trial of adjuvant chemotherapy vs control after radical cystectomy for locally advanced bladder cancer. BJU Int. 2006;97:42–47. doi: 10.1111/j.1464-410X.2006.05859.x. [DOI] [PubMed] [Google Scholar]
- 46.Stockle M, Wellek S, Meyenburg W, et al. Radical cystectomy with or without adjuvant polychemotherapy for non-organ-confined transitional cell carcinoma of the urinary bladder: prognostic impact of lymph node involvement. Urology. 1996;48:868–875. doi: 10.1016/s0090-4295(96)00299-3. [DOI] [PubMed] [Google Scholar]
- 47.Skinner DG, Daniels JR, Russell CA, et al. The role of adjuvant chemotherapy following cystectomy for invasive bladder cancer: a prospective comparative trial. J Urol. 1991;145:459–464. doi: 10.1016/s0022-5347(17)38368-4. [DOI] [PubMed] [Google Scholar]
- 48.Adjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis of individual patient data Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur Urol. 2005;48:189–199. doi: 10.1016/j.eururo.2005.04.005. discussion 199–201. [DOI] [PubMed] [Google Scholar]
- 49.Sternberg CN, de Mulder PH, Schornagel JH, et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony- stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol. 2001;19:2638–2646. doi: 10.1200/JCO.2001.19.10.2638. [DOI] [PubMed] [Google Scholar]
- 50.Dash A, Pettus JA, IV, Herr HW, et al. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: a retrospective experience. Cancer. 2008;113:2471–2477. doi: 10.1002/cncr.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 52.Ismaili N. A rare bladder cancer–small cell carcinoma: review and update. Orphanet J Rare Dis. 2011;6:75. doi: 10.1186/1750-1172-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siefker-Radtke AO, Dinney CP, Abrahams NA, et al. Evidence supporting preoperative chemotherapy for small cell carcinoma of the bladder: a retrospective review of the M. D. Anderson cancer experience. J Urol. 2004;172:481–484. doi: 10.1097/01.ju.0000132413.85866.fc. [DOI] [PubMed] [Google Scholar]
- 54.Siefker-Radtke AO, Kamat AM, Grossman HB, et al. Phase II clinical trial of neoadjuvant alternating doublet chemotherapy with ifosfamide/doxorubicin and etoposide/cisplatin in small-cell urothelial cancer. J Clin Oncol. 2009;27:2592–2597. doi: 10.1200/JCO.2008.19.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaushik D, Frank I, Boorjian SA, et al. Long-term results of radical cystectomy and role of adjuvant chemotherapy for small cell carcinoma of the bladder. Int J Urol. 2015;22:549–554. doi: 10.1111/iju.12729. [DOI] [PubMed] [Google Scholar]
- 56.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 57.De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30:191–199. doi: 10.1200/JCO.2011.37.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum- containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27:4454–4461. doi: 10.1200/JCO.2008.20.5534. [DOI] [PubMed] [Google Scholar]
- 59.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer [published online ahead of print June 6, 2016] J Clin Oncol. doi: 10.1200/JCO.2016.67.9761. pii: JCO679761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Apolo AB, Infante JR, Hamid O, et al. Avelumab (MSB0010718C; anti- PD-L1) in patients with metastatic urothelial carcinoma from the JAVELIN solid tumor phase 1b trial: Analysis of safety, clinical activity, and PD-L1 expression [abstract] J Clin Oncol. 2016;34 Abstract 4514. [Google Scholar]
- 62.Sharma P, Bono P, Kim JW, et al. Efficacy and safety of nivolumab monotherapy in metastatic urothelial cancer: results from the phase I/II CheckMate 032 study. Oral presentation at the 2016 ASCO Annual Meeting; June 3–7, 2016; Chicago, Illinois. [Google Scholar]
- 63.Bajorin DF, Plimack ER, Siefker-Radtke AO, et al. KEYNOTE-052: phase 2 study of pembrolizumab (MK-3475) as first-line therapy for patients (pts) with unresectable or metastatic urothelial cancer ineligible for cisplatin-based therapy [abstract] J Clin Oncol. 2015;33(Suppl) Abstract TPS4572. [Google Scholar]
- 64.Bellmunt J, Sonpavde G, De Wit R, et al. KEYNOTE-045: randomized phase 3 trial of pembrolizumab (MK-3475) versus paclitaxel, docetaxel, or vinflunine for previously treated metastatic urothelial cancer [abstract] J Clin Oncol. 2015;33 Abstract TPS4571. [Google Scholar]
- 65.Plimack ER, Bellmunt J, Gupta S, et al. Pembrolizumab (MK-3475) for advanced urothelial cancer: updated results and biomarker analysis from KEYNOTE-012 [abstract] J Clin Oncol. 2015;33(Suppl) Abstract 4502. [Google Scholar]
- 66.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of nonsmall-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 67.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch- repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]


