Abstract
Background
Paraquat ingestion is frequently fatal. While biomarkers of kidney damage increase during paraquat-induced acute kidney injury (AKI), significant concurrent proteinuria may alter diagnostic thresholds for diagnosis and prognosis to an unknown extent. This study evaluated the effect of albuminuria on biomarker cutoffs for diagnosis and outcome prediction.
Methods
This was a multi-centre prospective clinical study of patients following acute paraquat self-poisoning in 5 Sri Lankan hospitals. Biomarker concentrations were quantified using ELISA and microbead assays and correlated with urinary albumin. Functional-AKI was defined by the Acute Kidney Injury Network serum creatinine definition and alternatively by a ≥50% increase in serum cystatin C. Albuminuria was defined as albumin-creatinine ratio >30 mg/g. The study outcomes were compared with a retrospective analysis of a pre-clinical study of paraquat-induced nephrotoxicity with appropriate controls.
Results
Albuminuria was detected in 34 of 50 patients, and increased with functional-AKI severity. The concentrations of uNGAL, uCysC, uClusterin, uβ2M, and uKIM-1 were higher in albuminuric compared to non-albuminuric patients (p < 0.001). Albuminuria correlated with biomarker concentration (r > 0.6, p < 0.01) and was associated with death (p = 0.006). Optimal biomarker cutoffs for prediction of death were higher in the albuminuric group. Similar outcomes with more detailed analysis were obtained in experimental paraquat nephrotoxicity.
Conclusion
Albuminuria was associated with paraquat-induced nephrotoxicity and increased excretion of low-molecular weight protein biomarkers. AKI biomarker cutoffs for diagnosis, outcome prediction and AKI stratification increased in the presence of albuminuria. This may lead to over-diagnosis of AKI in conditions independently associated with proteinuria.
Electronic supplementary material
The online version of this article (doi:10.1186/s12882-017-0532-7) contains supplementary material, which is available to authorized users.
Keywords: Paraquat, Poisoning, Albuminuria, Biomarkers, Nephrotoxicity
Background
Acute kidney injury (AKI) is common and has diverse aetiology [1–3]. Nephrotoxic drugs are common contributory factors to AKI [4]. In Asia, purely nephrotoxic AKI (ToxAKI) is commonly seen following deliberate ingestion of agrochemicals [5–8].
AKI definitions have evolved around changes in creatinine or urine output [9, 10], with both measures lacking specificity and sensitivity for early AKI detection. Furthermore, plasma creatinine concentrations usually respond only slowly to kidney damage and may be altered by non-renal mechanisms [11, 12]. Alternative strategies for defining AKI with kidney-specific structural (injury) biomarkers have been proposed which may diagnose AKI earlier and with greater specificity and sensitivity than creatinine [13–17]. However, structural biomarker-based definitions also carry several challenges. One of these is low or absent biomarker concentrations in healthy populations. If these are normally absent, then the appearance of any biomarker should herald disease. Alternatively, reference ranges need to be defined for healthy populations and in the presence of co-morbidities. The majority of studies report biomarker reference ranges in heterogeneous ill subjects without AKI [18–20] and only a few studies define (some) biomarker concentrations in healthy populations [21, 22]. In addition, non-renal factors that increase structural biomarker concentrations independently of renal injury, have not yet been incorporated into AKI definitions [21–25]. In particular, proteinuria and albuminuria, both important biomarkers, increase excretion of urinary Neutrophil gelatinase-associated lipocalin (NGAL), and urinary cystatin C (uCysC) in critically ill patients [26].
Our observation of significant proteinuria following paraquat poisoning prompted analysis of the influence of proteinuria on the excretion of other renal biomarkers. We hypothesised that paraquat-induced albuminuria would increase the excretion of low molecular weight protein biomarkers subject to tubular reabsorption. This was examined in a prospective clinical study of patients following acute paraquat ingestion and then in a retrospective analysis of controlled data in an experimental rodent model of paraquat nephrotoxicity. These studies determined the effect of albuminuria on biomarker cutoffs for AKI diagnosis and outcome prediction.
Methods
Clinical study
This multi-centre prospective observational study was approved by the human research ethics committees of both the University of New South Wales (Sydney) and University of Peradeniya (Sri Lanka). The recruitment of healthy controls was carried out in 3 major provinces (North Central, Central and Southern Province of Sri Lanka) where the ‘Sinhala’ ethnic group is predominant; our patient cohort was also recruited from the hospitals located in these regions. These areas were selected since a high incidence of self-poisoning is reported from these regions. Healthy volunteers from several regions of Sri Lanka (outside the chronic kidney disease of unknown origin areas) were asked to volunteer for this study and informed written consent was obtained. All consenting volunteers underwent clinical screening and patients who had a history of any existing clinical conditions were excluded from this cohort. Single blood and urine samples were collected to quantify normal biomarker concentrations. Detail methods of patient recruitment to this cohort is described elsewhere [12, 27] and the patient cohort studied in this manuscript is identical to that discussed previously [12, 27]. Briefly, patients who presented to adult medical units of 5 study hospitals with a history of paraquat self-poisoning within 24 h of ingestion were consented for inclusion in the study. Patients who were young (age <15 years), or co-ingested another chemical were excluded. Informed written consent was obtained from all patients or their accompanying relatives. Paraquat ingestion was confirmed by a positive urine dithionate test four hours after ingestion. Demographic, clinical, and laboratory data were collected prospectively until discharge. Blood and urine sampling was scheduled at 4, 8, 16, 24 h after ingestion and then daily. Collected samples were immediately processed and stored in −70 °C freezers until batch-wise assays commenced.
Pre-clinical study
The rodent study was approved by the University Animal Ethics Committee (Health Sciences) of the University of Queensland and conducted as part of a doctoral study by one of the authors, KW at Therapeutic Research Centre, University of Queensland, Australia. Raw biomarker data [urinary albumin (uAlb), kidney injury molecule-1 (uKIM-1), cystatin C (uCysC), clusterin (uClu), osteopontin (uOstP), neutrophil gelatinase-associated lipocalin (uNGAL), beta-2-microglobulin (uβ2M)] from that study [28] was used to evaluate the influence of urinary albumin on the excretion of urinary biomarkers. Detailed methods including animal handling, paraquat dose, sample collection, biomarker assays, and histopathology of the original study are described elsewhere [28]. Briefly, male Wistar rats (200–250 g) from the Animal Resources Centre (Western Australia, Australia) were housed on a 12 h light/dark cycle. The animals were allowed free access to food (standard laboratory chow) and water. Rats were fasted overnight (12 h) before the experiments. Control rats (n = 6) were gavaged with water. The treatment group rats were randomly divided into the 4 dose groups with 6 rats in each group. These were dosed orally with 4 different doses (15, 30, 60 and 90 mg/kg) of paraquat dichloride solutions (Sigma-Aldrich, St. Louis, MO, USA). These doses were approximately 10, 25, 50, and 70% of the LD50 in rats [28]. After administration, rats were housed in individual metabolic cages and urine samples collected on dry ice at intervals of 0–8 h, 8–24 h and 24–48 h. Blood was collected from the tail vein at 8 and 24 h. At 48 h, the rats were sacrificed and blood was collected from the vena cava. Histopathology grades of 1 to 7 (1-no changes, 7-severe) were assigned for each rat [28] and represent the total numbers of necrotic cells and pyknotic nuclei in paraquat-treated rats normalised to total baseline necrotic cells and pyknotic nuclei counted in the control groups and assigned by KW under the supervision of two pathologists.
Biomarker assays
Serum creatinine (sCr) was measured using the Jaffe method (kinetic Jaffe reaction method, rate blank and compensated) on Roche Hitachi 912 automatic analyser. DuoSet ELISA development kits supplied by R&D systems® were used for quantifying uKIM-1, and uClu. Urinary interleukin-18 (IL-18) was measured using a commercially available ELISA kit (Bender MedSystems GmbH, Vienna, Austria). Intra and inter assay precision for ELISA was < 10%. Other AKI biomarkers such as uCysC, uAlb, trefoil factor-3 (uTFF3), uOstP, uβ2M and uNGAL were quantified on the same sample using Bio-Plex Pro™ RBM Human Kidney Toxicity Assays panel 2 on the Bio-Plex 200 system (BIO-RAD). Inter and intra assay precision estimates were <15% and <5% respectively. Rodent samples were assayed similarly as previously described [28].
Outcome definition and statistical analysis
Biomarker concentrations were reported as absolute concentrations and as normalised to urinary creatinine concentration. Albuminuria in patients was defined as urinary albumin creatinine ratio [(ACR) ≥30 mg/g (μg/mg)] [29, 30]. Albuminuria in the pre-clinical study was defined as ACR values ≥ 95th centile from control rats since cut-offs for albuminuria in rats were not available. Functional-AKI was defined by change in sCr according to the Acute Kidney Injury Network (AKIN) [10] criteria or ≥50% change in serum cystatin C [31]. Moderate to severe functional-AKI was defined as an increase in sCr of ≥200% (AKIN Stage 2) or 300% (AKIN Stage 3) respectively [32]. The 95th centile of each structural biomarker obtained in healthy volunteers was defined as the cutoff for structural-AKI. This definition was modelled on similar approaches to define healthy reference cutoff points of cardiac troponin for diagnosis and risk stratification of myocardial injury [33, 34].
Continuous variables were compared using the Wilcoxon rank sum test and reported as median and interquartile range. Categorical variables were reported as proportions and compared using Fisher’s exact test. Correlations were done using the Spearman rank-order. The prognostic performance of each biomarker for predicting death was evaluated by area under the receiver operating characteristic curves (AUC-ROC) stratifying to albuminuria and the optimal threshold for each biomarker was calculated. For each biomarker, the sensitivity and specificity of structural-AKI for predicting functional-AKI or death was calculated separately in the presence or absence of albuminuria. The statistical analyses were conducted using GraphPad Prism version 6 (GraphPad Software, San Diego, USA) and STATA IC10 (StataCorp, 2007).
Results
Clinical study findings
Biomarker concentrations in healthy volunteers
Plasma and urinary functional and urinary structural injury biomarker levels in urine and blood samples from 63 healthy young adult volunteers [median age 28 years (IQR 26–33), 70% male] are presented as absolute (Table 1) and normalised concentrations (Additional file 1: Table S1).
Table 1.
Serum and urinary functional and injury biomarkers in healthy subjects (absolute concentrations)
| Biomarkers | Median and IQR | Lower reference limit (5th centile) |
Upper reference limit (95th centile) |
|---|---|---|---|
| Serum biomarkers | |||
| Total protein (g/dl) | 6.5 (5.8–7.1) | 5.3 | 8.9 |
| Albumin (g/dl) | 4.1 (3.8–4.3) | 3.2 | 5.3 |
| Creatinine | |||
| mg/dl | 0.82 (0.72–0.94) | 0.56 | 1 |
| μmol/L | 72 (64–84) | 50 | 88 |
| Cystatin C (mg/l) | 0.84 (0.78–0.88) | 0.6 | 1 |
| Urinary biomarkers | |||
| Total protein (mg/L) | 196 (161–267) | 70 | 1870 |
| Creatinine | |||
| mg/dl | 123 (54–182) | 33 | 253 |
| mmol/L | 11 (5–16) | 3 | 22 |
| Urea | |||
| mg/dl | 1432 (822–2023) | 444 | 3206 |
| mmol/l | 511 (294–722) | 159 | 1144 |
| Cystatin C (ng/ml) | 24 (11–51) | 3.8 | 94 |
| Albumin (ng/ml) | 5700 (1700–9200) | 500 | 21000 |
| NGAL (ng/ml) | 14.8 (10.2–40.3) | 3.4 | 134 |
| KIM-1 (ng/ml) | 0.57 (0.33–1.23) | 0.02 | 2.8 |
| Clusterin (ng/ml) | 217 (104–393) | 28 | 798 |
| β2M (ng/ml) | 67 (36–139) | 14.5 | 250 |
| Osteopontin (ng/ml) | 1400 (400–0.2800) | 100 | 6800 |
| TFF3 (ng/ml) | 1400 500–1500) | 200 | 3200 |
| IL-18 (pg/ml) | 53.8 (39–80) | 39 | 136 |
Patient baseline demographics according to ACR
The 50 confirmed paraquat-poisoned patients were previously healthy young adults [median age, 24 years (IQR 19–32, range 15–56)], and 55% male. Baseline demographic and clinical characteristics were similar for albuminuric and non-albuminuric groups (Table 2) except for serum creatinine and paraquat concentrations. Thirty-four (70%) developed albuminuria within 16–24 h. Twenty-six patients developed moderate to severe functional-AKI (AKI stage 2, n = 7, or stage 3, n = 19). The incidence of moderate to severe functional-AKI was higher in albuminuric patients (68% compared with 25%; p < 0.01). Based on the serum cystatin C definition of functional-AKI, 19 patients developed functional-AKI, of these 17 had albuminuria. All 12 deaths occurred in patients with albuminuria (p < 0.01).
Table 2.
Baseline demographic and clinical characteristics
| Baseline characteristics | No-albuminuria (ACR < 30 mg/g) (n = 16) |
Albuminuria (ACR ≥ 30 mg/g) (n = 34) |
p |
|---|---|---|---|
| Age (years) | 23 (19–35) | 25 (19–32) | 0.97 |
| Male gender (%) | 60 | 50 | 0.5 |
| Weight (kg) | 50 (45–55) | 50 (39–60) | 0.65 |
| Volume ingested (ml) | 10 (5–30) | 20 (20–50) | 0.05 |
| Time to admission (hours) | 4 (2–6) | 3.5 (2–7.5) | 0.98 |
| Pulse (beats/min) | 80 (78–88) | 82 (80–89) | 0.50 |
| BP systolic (mm Hg) | 120 (110–120) | 110 (110–120) | 0.18 |
| BP diastolic | 80 (70–80) | 70 (70–80) | 0.52 |
| sCr (mg/dl) | 0.7 (0.5–0.8) | 0.9 (0.7–1.3) | 0.006 |
| sCysC (mg/l) | 0.7 (0.6–0.8) | 0.7 (0.6–0.8) | 0.53 |
| Maximum serum paraquat (ng/ml/24 h) | 20 (10–120) | 640 (140–1400) | 0.0006 |
| Functional-AKI (%) | 8 (50%) | 29 (85%) | 0.007 |
| Death (n) | 0 | 12 | 0.006 |
Albuminuria and functional AKI
ACR increased with functional AKI severity (p < 0.0001, Fig. 1) and was greater in patients with moderate to severe functional-AKI compared to the healthy controls (p < 0.0001). Albuminuria was also observed in 5 patients who did not develop functional-AKI (based on either sCr or sCysC definition) but did have increased structural biomarker concentrations. The median ACR was higher in paraquat-poisoned subjects without AKI compared to the healthy controls (p < 0.05) (Fig. 1).
Fig. 1.
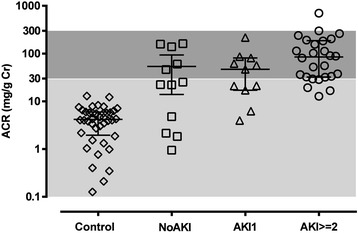
ACR in healthy controls and patients with or without functional-AKI. Albuminuria (dark grey shaded area; ACR ≥ 30 mg/g); normal ACR (light grey shaded area, <30 mg/g). AKI is defined based on AKIN classification
Biomarker concentrations stratified by ACR and AKI
Within 24 h of paraquat ingestion, maximum (normalised) biomarker concentrations were increased in the presence of albuminuria. In albuminuric patients, uCysC, uClu, uNGAL, uKIM-1, and uβ2M increased with increasing AKI severity (Additional file 2: Figure S1). Concentrations were lower in patients who did not develop albuminuria. The concentrations of filtered biomarkers, uCysC, uNGAL, uClu, uβ2M, uTFF3 and uKIM-1 were higher in albuminuric patients than healthy controls (p < 0.001) (Additional file 2: Figure S1).
Correlation of urinary biomarkers and albuminuria
Normalised uCysC and uClu correlated well with ACR (r = 0.7, p < 0.0001). Urinary NGAL, β2M, OstP and KIM-1 also correlated with ACR (r = 0.5, p < 0.01). Correlations with ACR for uIL-18 (r = 0.4, p < 0.05) and uTFF3 (r = 0.3, p > 0.05) were modest (Fig. 2). A similar correlation profile was obtained when the absolute urinary concentration of each biomarker was assessed against absolute uAlb (Additional file 2: Figure S2).
Fig. 2.
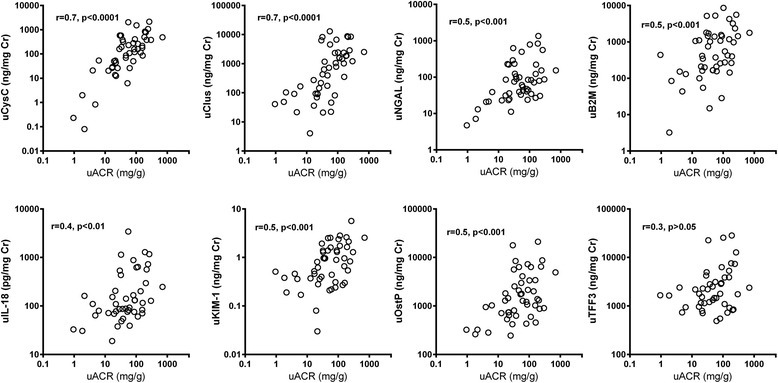
Correlation between normalised biomarker concentration and ACR following paraquat poisoning
Biomarker threshold for predicting death as an outcome in the presence of albuminuria
Twelve (n = 12) patients died during the hospital stay and all had developed albuminuria. No patient without albuminuria died (p < 0.01). Table 3 summarises the AUC-ROCs, specificity, sensitivity and the optimal cutoff for each biomarker to predict death in the albuminuric group (n = 34, 12 deaths) and for the entire patient cohort (n = 50, 12 deaths). There was an almost 2-fold increase in biomarker cut-offs for uCysC, uClu and uβ2M in the albuminuric cohort with smaller increases in the cutoffs for the other biomarkers (Table 3). In addition, biomarker performance was uniformly reduced in the presence of albuminuria.
Table 3.
Comparative diagnostic performance of renal biomarkers in predicting death in paraquat poisoning stratified by albuminuria
| All patients (n = 50) | Patients with albuminuria (n = 34) a | p ‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Biomarkers (ng/mg Cr) |
AUC-ROC (95% CI) |
Cut-off | Sensitivity (95% CI) |
Specificity (95% CI) |
AUC-ROC (95% CI) |
Cutoff | Sensitivity (95% CI) | Specificity (95% CI) |
|
| uCysC | 0.78 (0.62–0.94) |
>200 | 67 (35–90) | 67 (50–82) | 0.68 (0.49–0.88) |
>300 | 67 (35–90) | 66 (43–84) | 0.46 |
| uClu | 0.70 (0.52–0.87) |
>750 | 67 (34–90) | 60 (42–75) | 0.55 (0.34–0.76) |
>1600 | 58 (28–74) | 59 (36–80) | 0.29 |
| Uβ2M | 0.68 (0.48–0.88) |
>990 | 67 (35–90) | 68 (50–82) | 0.60 (0.39–0.82) |
>1280 | 67 (35–90) | 68 (45–86) | 0.62 |
| uNGAL | 0.81 (0.67–0.95) |
>80 | 67 (35–90) | 68 (50–82) | 0.75 (0.56–0.93) |
>90 | 67 (35–90) | 68 (45–86) | 0.60 |
| uKIM-1 | 0.75 (0.57–0.90) |
>0.96 | 75 (43–94) | 73 (56–85) | 0.61 (0.41–0.82) |
>1.3 | 67 (35–90) | 40–83) | 0.38 |
| uTFF3 | 0.85 (0.72–0.98) |
>2340 | 75 (43–94) | 71 (52–85) | 0.82 (0.67–0.97) |
>2830 | 75 (43–94) | 70 (46–88) | 0.74 |
| uOstP | 0.82 (0.68–0.97) | >1760 | 75 (42–94) | 70 (53–84) | 75 (57–94) | >2100 | 75 (42–94) | 68 (45–86) | 0.60 |
| uIL-18 (pg/mg Cr) |
64 (45–82) | >130 | 63 (31–89) | 60 (42–75) | 55 (34–76) | >130 | 63 (31–89) | 50 (28–72) | 0.54 |
a Albuminuria; ACR ≥ 30 mg/g, All normalised biomarker concentrations are presented in ng/mg Cr except uIL-18 (pg/mg Cr)
‡The AUC-ROC values were compared using Delong method
Sensitivity and specificity of structural biomarker based definition in diagnosis of AKI
The sensitivity of the 95th centile in the healthy volunteer group of structural biomarkers for diagnosis of moderate to severe functional-AKI was high amongst albuminuric patients for both uCysC (sensitivity 96%, CI 79-99%; diagnostic odds ratio = 8, CI 1–91) and uClu (sensitivity 91%, CI 73–98%; diagnostic odds ratio = 13, CI 2–82) (Table 4). Similarly, the sensitivity of structural AKI for predicting death was higher for both uCysC (sensitivity 92%, CI 64–98%; diagnostic odds ratio = 2, CI 0.2–18) and uClu (sensitivity 83%, CI 55–95%; diagnostic odds ratio = 2, CI 0.3–11) in patients with albuminuria (Fig. 3, Table 4). But, the lower end of the confidence interval for the odds ratios for AKI diagnosis using both biomarkers in predicting mortality was less than 1. Furthermore, the specificity of these two biomarkers in diagnosing functional AKI or predicting death was low (<30%) in patients with albuminuria. In contrast, the sensitivity for predicting death or diagnosing functional AKI by uCysC or uClu was low (1%) in non-albuminuric patients (Fig. 3, Table 4).
Table 4.
Sensitivity and specificity of 95th centile values of structural biomarker values from healthy volunteers in detecting functional-AKI
| Patients with albuminuria (n = 34) | No-albuminuria (n = 16) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biomarkers (ng/mg Cr) |
Sensitivitya | Specificitya | Positive likelihood ratioa | Negative likelihood ratioa | Diagnostic odds ratioa | Sensitivitya | Specificitya | Positive likelihood ratioa | Negative likelihood ratioa | Diagnostic odds ratioa |
| uCysC | 96 (79–99) | 27 (10–56) | 1.3 (0.9–1.9) | 0.16 (0–1.4) | 8.2 (0.7–91.2) | 0 (0–56) | 100 (75–100) | 0 | 1 (1–1) | 0 |
| uClu | 91 (73–98) | 54 (28–79) | 2 (1–3.4) | 0.2 (0–0.7) | 12.6 (1.9–82) | 0 (0–56) | 100 (75–100) | 0 | 1 (1–1) | 0 |
| Uβ2M | 91 (73–98) | 27 (9–56) | 1.2 (0.8–1.8) | 0.3 (0–1.6) | 3.9 (0.5–28) | 100 (43–100) | 66 (39–86) | 3 (1.3–6.7) | 0 | 0 |
| uNGAL | 48 (29–57) | 91 (62–98) | 5.3 (0.8–35.8) | 0.6 (0.4–0.9) | 9.2 (1–83) | 33 (6–79) | 92 (64 (98) | 4 (0.3–47) | 0.7 (0.3–16) |
5.5 (0.2–129) |
| uKIM-1 | 60 (40–78) | 63 (35–85) | 1.7 (0.7–3.9) | 0.6 (0.3–1.2) | 2.7 (0.6–12) | 0 (0–56) | 1 (75–100) | 0 | 1 (1–1) | 0 |
a Data presented with 95% CI
Serum creatinine ≥ 100% (AKI ≥ 2) is defined as functional-AKI while biomarker concentration >95th centile value in healthy volunteers (uCysC: 70 ng/mg Cr; uClu: 420 ng/mg Cr; uKIM-1 1.2 ng/mg Cr; uβ2M 166 ng/mg Cr and uNGAL: 120 ng/mg Cr) were used to define structural-AKI
Fig. 3.
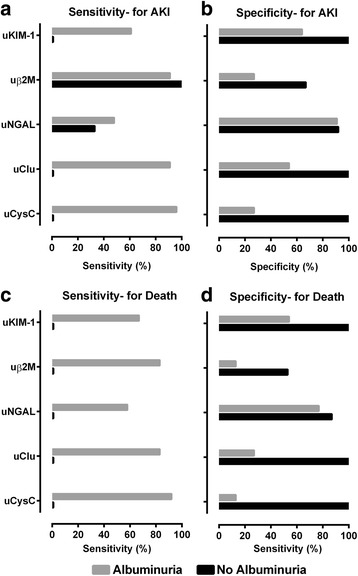
Sensitivity and specificity of 95th centile values of structural biomarker values from healthy volunteers in detecting functional-AKI or death. Serum creatinine ≥ 100% (AKI ≥ 2) is defined as functional-AKI while biomarker concentration >95th centile value in healthy volunteers (uCysC: 70 ng/mg Cr; uClu: 420 ng/mg Cr; uKIM-1 1.2 ng/mg Cr; uβ2M 166 ng/mg Cr and uNGAL: 120 ng/mg Cr) were used to define structural-AKI. Grey and black area on the chart depicts 'Albuminuria' and 'No albuminuria' respectively
Urinary β2-M structural AKI displayed excellent sensitivity for diagnosing functional-AKI in both albuminuric (sensitivity 91%, CI 73–98%; diagnostic odds ratio = 4, CI 0.5–21) and non-albuminuric subjects (sensitivity 100%, CI 44–100%). Among 5 albuminuric patients who did not develop functional-AKI (Fig. 3, Table 4), all had structural AKI based on sufficient increases in at least one damage biomarker.
Preclinical study findings
Albuminuria and biomarkers in paraquat treated rats
Urinary albumin concentration increased with severity of injury (i.e. increased histopathology grade) and with paraquat dose in rats (Fig. 4). Median urinary albumin concentrations at 24 h in 15, 30, and 60 mg/kg paraquat dose group were 35 (IQR 25–49), 45 (IQR 19–86) and 87 (IQR 26–117) μg/ml respectively.
Fig. 4.

Urinary albumin concentrations at different time points in controls and paraquat treated rats. Note that albumin concentration (y axis) values are actual raw values (not logarithmic values). Y axis is formatted on log scale to improve the visibility of data points. An increase urinary albumin concentration was observed as histopathology grades increased in paraquat treated rats. Each symbols indicate biomarker concentrations from individual rats at specific paraquat dose levels. The histopathology grades [28] are displayed on a scale of 0 (normal) to 7 (severe) [grade 1 (white), grade 2 (yellow), grade 3 (blue), grade 4 (green), grade 5 (red), grade 6 (purple) and grade 7 (black)]
Urinary albumin correlated strongly with uCysC (r = 0.9, p < 0.0001), well with uNGAL (r = 0.7, p < 0.0001), uKIM-1 (r = 0.6, p < 0.01), and uβ2M (r = 0.7, p < 0.001), but not with uOstP (r = 0.3, p < 0.05) or uClu (r = 0.3, p < 0.05) (Additional file 2: Figure S3). Correlations of similar magnitude were also observed after normalising the biomarker concentrations to urinary creatinine (Additional file 2: Figure S4).
Biomarker concentrations at 24 h in paraquat treated rats based on albuminuria
The 95th centile ACR from control rats used to define albuminuria was 115 μg/mg Cr. Albuminuria was associated with increased concentrations of uβ2M, uCysC, uOstP, and uKIM-1 in paraquat treated rats (Fig. 5; p < 0.05).
Fig. 5.

Urinary biomarker concentrations according to albuminuria in paraquat-induced nephrotoxicity in rats. This depicts urinary biomarker concentrations at 24 h stratified by albuminuria with respect to paraquat induced nephrotoxicity. Albuminuria was defined as ACR ≥115 (μg/mg Cr) which is the 95th centile values of ACR in control rats
Discussion
This study demonstrated that paraquat toxicity was associated with development of albuminuria in clinical and experimental nephrotoxicity. In turn, albuminuria was associated with increased excretion of renal biomarkers and modified biomarker sensitivity in outcome prediction. The data confirm that concentrations of the low molecular weight protein biomarkers, CysC, OstP, β2M, KIM-1 and NGAL increased in the presence of albuminuria. Albuminuria was also associated with mortality. To our knowledge, this is the first study to evaluate the influence of albuminuria on excretion of all Predictive Safety Testing Consortium (PSTC) qualified urinary biomarkers.
The influence of proteinuria and albuminuria on uNGAL, uCysC and uIL-18 concentration was previously evaluated in a heterogeneous intensive care unit patient population [26] where many fold increases in uNGAL and uCysC were seen in the presence of proteinuria or albuminuria. That study and others have highlighted that proteinuria and albuminuria result in competitive inhibition of megalin-cubulin mediated reabsorption of low molecular weight urinary proteins [14, 26, 35, 36]. Thus, the increase in filtered urinary biomarker concentrations, which result from AKI, receives a contribution from impaired absorption when albumin or other proteins are also present. Nearly all low molecular weight proteins are believed to be reabsorbed by megalin and cubulin-mediated endocytosis [36].
All 50 patients included in this analysis had ingested paraquat for deliberate self-harm and hence the change in biomarker concentration was assumed to be solely due to paraquat. In contrast to the present study, that of Nejat et al. [26] recruited patients who were older and were admitted to the intensive care unit with various clinical presentations. Not surprisingly, 20% of patients in that study had a prior history of CKD, which was further limited by the use of only semi-quantitative dipstick methods to determine proteinuria [26]. The present study amplifies those observations with better quantitation of albuminuria in a younger, presumably healthier, cohort, with implications for evolving AKI biomarker research [26, 35].
An additional novel approach in this study was the recruitment of healthy young adults from the same ethnic population to define normal biomarker reference ranges. A similar approach is used to define myocardial injury based on cutoff levels of cardiac troponin obtained from healthy reference data [33, 34]. Most previous novel kidney biomarker reference ranges have been established in control patients who were sick or exposed to similar noxious insults but who did not develop AKI [18–20]. Given that biomarker levels tend to be higher in patient controls without AKI undergoing cardiac catheterization or in the ICU compared to healthy volunteers [18, 21], using patient-based control groups to report normal levels might be misleading. Such patients might have other age or disease-specific co-morbidities [18] or have transient (formerly “pre-renal”) AKI, the mild end of a continuum of renal injury [37, 38].
Our clinical study recruited healthy young adults from the same ethnic population (median age = 24, range 15–56 years) with no previous history of chronic kidney disease (CKD) or other co-morbidities. The normal biomarker range in these healthy Sri Lankan volunteers (median age = 24, range 15–56 years, Table 1 and Additional file 1: Table S1) was similar to patients who didn’t develop AKI. The sensitivity of these cutoffs for diagnosing functional AKI or predicting death was examined in patients with and without albuminuria (Fig. 3, Table 4). Excellent sensitivity (>90%) was observed for uCysC, uClu and uβ2M in diagnosing AKI and predicting death in the presence of albuminuria. However, the sensitivity for uCysC and uClu in diagnosing functional-AKI in patients who didn’t develop albuminuria was less than 1%. In the rodent studies, we demonstrated that biomarker cutoffs for predicting histopathological change in paraquat-induced nephrotoxicity increased with increasing albuminuria (Figs. 4 and 5). These differences in biomarker concentrations between albuminuric and non-albuminuric groups confirm that renal biomarker excretion is increased in the presence of albuminuria and that the cut-offs for diagnosis of AKI may differ when albuminuria is present. Since creatinine-based criteria for defining and staging AKI may not be appropriate in situations where creatinine increases independently of glomerular filtration rate as occurs early after paraquat poisoning [12], alternative definitions based on structural biomarker levels may be needed [13–17].
Recently, we [14] defined AKI utilising both functional and structural markers and calculated cutoffs for defining AKI and subsequent AKI staging. Generalising such cutoffs to define AKI is challenging due to the different non-standardised assays currently used and if other factors, for example albuminuria, influence biomarker concentrations independently of AKI [21–25]. This study demonstrated that albuminuria increases the cutoff values for outcome prediction (Table 3). If biomarkers are used to define AKI, this study suggests that over- or under-estimation of AKI incidence may occur and that quantifying urinary albumin should be considered when defining biomarker cutoffs.
Albuminuria may result from paraquat-induced glomerular damage, increasing filtration of albumin, or from tubular injury, impairing reabsorption [39–41]. As we have shown, albuminuria itself is a good diagnostic and prognostic biomarker in paraquat-induced nephrotoxicity consistent with previous studies [42–45]. However, together with other studies, this suggests that albuminuria-increased biomarker excretion may lead to an increase in the false-positive diagnosis rate for structural AKI [42, 46]. Based on these pre-clinical and clinical studies, we propose that selection of specific biomarker cutoffs for AKI diagnosis, staging and for risk prediction should factor in the presence or absence of albuminuria. Although these observations are based on a cohort with paraquat poisoning which causes both glomerular [47] and tubular injury [28], the finding is likely to be particularly relevant to young patients with pre-existing albuminuria due to other aetiologies.
Strengths and limitations
This study has many strengths including that it is a multi-centre prospective study recruiting previously healthy young adults following a single nephrotoxic insult, with quantification of albuminuria and the establishment of biomarker ranges in healthy subjects. The study also has several limitations. The sample size of healthy control subjects was small (n = 63) and the 95th centile values used to define structural AKI need to be validated. Nevertheless, a sensitivity analysis using alternative cut offs (97.5 or 99%) didn’t change the conclusions. Thus healthy population cutoffs to define structural AKI may have clinical utility once validated in larger populations. Further studies are clearly warranted to examine this methodology in AKI biomarker research.
Conclusion
Albuminuria increased excretion of most low-molecular weight urinary protein biomarkers following paraquat poisoning and enhanced biomarker sensitivity in detection of functional AKI and in predicting poor outcome. Biomarker performance was reduced with altered sensitivities and increased cutoffs in the presence of albuminuria suggesting that diagnostic and predictive biomarker cutoffs need to be qualified in the presence of albuminuria.
Additional files
Urinary functional and injury biomarkers in healthy subjects. (DOCX 12 kb)
Biomarker profiles stratified by ACR. (DOCX 647 kb)
Acknowledgments
We thank the physicians, directors, medical and nursing staff of the study hospitals and SACTRC staff for their support. We also thank all the patients who took part in the study. Our special thanks to late Dr. Philip Peake whose immense guidance on this project. We dearly miss him.
Funding
The project was funded by NHMRC project grant 1011772. FM received an Endeavour Postgraduate Scholarship from the Australian Government (2241_2011). The funding body had no role in the design and execution of this study or interpretation of the data or decision to submit the manuscript.
Availability of data and materials
We have described all the data related to this study in the manuscript in detail and also provided more details in the supplementary data section. Our research group is also in the process of making these data in a public repository which will become available in near future.
Authors’ contributions
FM, NB, ZE conceived and designed the study. FM drafted the manuscript. KW performed rat studies. JWP helped in statistical analysis. SJ and IG supervise the clinical study. UC coordinated the clinical data collection. SD helped FM in lab work. All authors read and edited the manuscript and approved the final manuscript.
Competing interests
The authors of this manuscript declare that they have no competing interest. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Consent for publication
Not applicable. Only de-identified data are presented.
Ethics approval and consent to participate
The clinical study was approved by the human research ethics committees of both the University of New South Wales (Sydney) and University of Peradeniya (Sri Lanka). Informed written consent was obtained from all patients or their accompanying relatives. The pre-clinical study was approved by the University Animal Ethics Committee (Health Sciences) of the University of Queensland, Australia.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ACR
Albumin creatinine ratio
- AKI
Acute kidney injury
- AKIN
Acute Kidney Injury Network
- AUC-ROC
Area under the receiver operating characteristic curves
- IL-18
Interleukin-18
- LMW
Low-molecular weight
- NGAL
Neutrophil gelatinase-associated lipocalin
- ToxAKI
Nephrotoxic AKI
- uAlb
Albumin
- uClu
Clusterin
- uCysC
Cystatin C
- uKIM-1
Kidney injury molecule-1
- uOstP
Osteopontin
- uTFF3
Trefoil factor-3
- uβ2M
Beta-2-microglobulin
Contributor Information
Fahim Mohamed, Phone: +94812384556, Email: fahim.cader@gmail.com.
Nicholas A. Buckley, Email: nicholas.buckley@sydney.edu.au
John W. Pickering, Email: john.pickering@otago.ac.nz
Klintean Wunnapuk, Email: klintean.wunnapuk@uqconnect.edu.au.
Sandamali Dissanayake, Email: sanda_dissa@yahoo.com.
Umesh Chathuranga, Email: umeshchathu1@yahoo.com.
Indika Gawarammana, Email: indika@sactrc.org.
Shaluka Jayamanne, Email: shalukajaya@yahoo.com.
Zoltan H. Endre, Email: z.endre@unsw.edu.au
References
- 1.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53(6):961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 3.Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382(9887):170–179. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson MA, Vaidya VS, Bonventre JV. Biomarkers of nephrotoxic acute kidney injury. Toxicology. 2008;245(3):182–193. doi: 10.1016/j.tox.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gil HW, Yang JO, Lee EY, Hong SY. Clinical implication of urinary neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 in patients with acute paraquat intoxication. Clin Toxicol (Phila) 2009;47(9):870–875. doi: 10.3109/15563650903306651. [DOI] [PubMed] [Google Scholar]
- 6.Roberts DM, Buckley NA, Mohamed F, et al. A prospective observational study of the clinical toxicology of glyphosate-containing herbicides in adults with acute self-poisoning. Clin Toxicol (Phila) 2010;48(2):129–136. doi: 10.3109/15563650903476491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurjar M, Baronia AK, Azim A, Sharma K. Managing aluminum phosphide poisonings. J Emerg Trauma Shock. 2011;4(3):378–384. doi: 10.4103/0974-2700.83868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohamed F, Endre ZH, Buckley NA. Role of biomarkers of nephrotoxic acute kidney injury in deliberate poisoning and envenomation in less developed countries. Br J Clin Pharmacol. 2015;80(1):3–19. doi: 10.1111/bcp.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endre ZH, Pickering JW, Walker RJ. Clearance and beyond: the complementary roles of GFR measurement and injury biomarkers in acute kidney injury (AKI) Am J Physiol Renal Physiol. 2011;301(4):F697–F707. doi: 10.1152/ajprenal.00448.2010. [DOI] [PubMed] [Google Scholar]
- 12.Mohamed F, Endre Z, Jayamanne S, et al. Mechanisms underlying early rapid increases in creatinine in paraquat poisoning. Plos One. 2015;10(3):e0122357. doi: 10.1371/journal.pone.0122357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickering JW, Endre ZH. Linking injury to outcome in acute kidney injury: a matter of sensitivity. Plos One. 2013;8(4):e62691. doi: 10.1371/journal.pone.0062691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickering JW, Endre ZH. The clinical utility of plasma neutrophil gelatinase-associated lipocalin in acute kidney injury. Blood Purif. 2013;35(4):295–302. doi: 10.1159/000351542. [DOI] [PubMed] [Google Scholar]
- 15.Endre ZH, Kellum JA, Di Somma S, et al. Differential diagnosis of AKI in clinical practice by functional and damage biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2013;182:30–44. doi: 10.1159/000349964. [DOI] [PubMed] [Google Scholar]
- 16.Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol. 2012;23(1):13–21. doi: 10.1681/ASN.2010111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray PT, Mehta RL, Shaw A, et al. Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int. 2014;85(3):513–521. doi: 10.1038/ki.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaidya VS, Waikar SS, Ferguson MA, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1(3):200–208. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59(3):246–255. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royakkers AA, Korevaar JC, Van Suijlen JD, et al. Serum and urine cystatin C are poor biomarkers for acute kidney injury and renal replacement therapy. Intensive Care Med. 2011;37(3):493–501. doi: 10.1007/s00134-010-2087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Gibson B, Jr, Mori R, et al. Analytical and biological validation of a multiplex immunoassay for acute kidney injury biomarkers. Clin Chim Acta. 2012;415C:88–93. doi: 10.1016/j.cca.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Cullen MR, Murray PT, Fitzgibbon MC. Establishment of a reference interval for urinary neutrophil gelatinase-associated lipocalin. Ann Clin Biochem. 2012;49(Pt 2):190–193. doi: 10.1258/acb.2011.011105. [DOI] [PubMed] [Google Scholar]
- 23.Delanaye P, Rozet E, Krzesinski JM, Cavalier E. Urinary NGAL measurement: biological variation and ratio to creatinine. Clin Chim Acta. 2011;412(3–4):390. doi: 10.1016/j.cca.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Gracie JA, Robertson SE, Mcinnes IB. Interleukin-18. J Leukoc Biol. 2003;73(2):213–224. doi: 10.1189/jlb.0602313. [DOI] [PubMed] [Google Scholar]
- 25.Van Timmeren MM, Van Den Heuvel MC, Bailly V, Bakker SJ, Van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212(2):209–217. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- 26.Nejat M, Hill JV, Pickering JW, Edelstein CL, Devarajan P, Endre ZH. Albuminuria increases cystatin C excretion: implications for urinary biomarkers. Nephrol Dial Transplant. 2011;27(Suppl 3):iii96–iii103. doi: 10.1093/ndt/gfr222. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed F, Buckley NA, Jayamanne S, et al. Kidney damage biomarkers detect acute kidney injury but only functional markers predict mortality after paraquat ingestion. Toxicol Lett. 2015;237(2):140–150. doi: 10.1016/j.toxlet.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Wunnapuk K, Liu X, Peake P, et al. Renal biomarkers predict nephrotoxicity after paraquat. Toxicol Lett. 2013;222(3):280–288. doi: 10.1016/j.toxlet.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Sacks DB, Arnold M, Bakris GL, et al. Albuminuria (formerly microalbuminuria) Guidel Recomm Lab Anal Diagn Manag Diab Mellitus. 2011;12:39–42. [Google Scholar]
- 30.Sacks DB, Arnold M, Bakris GL, et al. Executive summary: guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2011;57(6):793–798. doi: 10.1373/clinchem.2011.163634. [DOI] [PubMed] [Google Scholar]
- 31.Nejat M, Pickering JW, Walker RJ, Endre ZH. Rapid detection of acute kidney injury by plasma cystatin C in the intensive care unit. Nephrol Dial Transplant. 2010;25(10):3283–3289. doi: 10.1093/ndt/gfq176. [DOI] [PubMed] [Google Scholar]
- 32.Basu RK, Wong HR, Krawczeski CD, et al. Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol. 2014;64(25):2753–2762. doi: 10.1016/j.jacc.2014.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrow DA, Cannon CP, Jesse RL, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem. 2007;53(4):552–574. doi: 10.1373/clinchem.2006.084194. [DOI] [PubMed] [Google Scholar]
- 34.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959–969. doi: 10.1016/S0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 35.Thielemans N, Lauwerys R, Bernard A. Competition between albumin and low-molecular-weight proteins for renal tubular uptake in experimental nephropathies. Nephron. 1994;66(4):453–458. doi: 10.1159/000187863. [DOI] [PubMed] [Google Scholar]
- 36.Gekle M. Renal tubule albumin transport. Annu Rev Physiol. 2005;67:573–594. doi: 10.1146/annurev.physiol.67.031103.154845. [DOI] [PubMed] [Google Scholar]
- 37.Nejat M, Pickering JW, Devarajan P, et al. Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int. 2012;81(12):1254–1262. doi: 10.1038/ki.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doi K, Katagiri D, Negishi K, et al. Mild elevation of urinary biomarkers in prerenal acute kidney injury. Kidney Int. 2012;82(10):1114–1120. doi: 10.1038/ki.2012.266. [DOI] [PubMed] [Google Scholar]
- 39.Ware LB, Johnson AC, Zager RA. Renal cortical albumin gene induction and urinary albumin excretion in response to acute kidney injury. Am J Physiol Renal Physiol. 2011;300(3):F628–F638. doi: 10.1152/ajprenal.00654.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol. 2011;72(5):745–757. doi: 10.1111/j.1365-2125.2011.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dinis-Oliveira RJ, Duarte JA, Sanchez-Navarro A, Remiao F, Bastos ML, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38(1):13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 42.Yu Y, Jin H, Holder D, et al. Urinary biomarkers trefoil factor 3 and albumin enable early detection of kidney tubular injury. Nat Biotechnol. 2010;28(5):470–477. doi: 10.1038/nbt.1624. [DOI] [PubMed] [Google Scholar]
- 43.Coca SG, Jammalamadaka D, Sint K, et al. Preoperative proteinuria predicts acute kidney injury in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2012;143(2):495–502. doi: 10.1016/j.jtcvs.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molnar AO, Parikh CR, Sint K, et al. Association of postoperative proteinuria with AKI after cardiac surgery among patients at high risk. Clin J Am Soc Nephrol. 2012;7(11):1749–1760. doi: 10.2215/CJN.13421211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zappitelli M, Coca SG, Garg AX, et al. The association of albumin/creatinine ratio with postoperative AKI in children undergoing cardiac surgery. Clin J Am Soc Nephrol. 2012;7(11):1761–1769. doi: 10.2215/CJN.12751211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson DW, Jones GR, Mathew TH, et al. Chronic kidney disease and measurement of albuminuria or proteinuria: a position statement. Med J Aust. 2012;197(4):224–225. doi: 10.5694/mja11.11468. [DOI] [PubMed] [Google Scholar]
- 47.Ben Rejeb A, Maillet M, Bescol-Liversaac J, Guillam-Megnin C. Ultrastructure of the kidney in paraquat-poisoned rats. Comparative study with literature data on man and animal. Arch Anat Cytol Pathol. 1997;45(4):199–207. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Urinary functional and injury biomarkers in healthy subjects. (DOCX 12 kb)
Biomarker profiles stratified by ACR. (DOCX 647 kb)
Data Availability Statement
We have described all the data related to this study in the manuscript in detail and also provided more details in the supplementary data section. Our research group is also in the process of making these data in a public repository which will become available in near future.


