Abstract
Objective:
The aim was to identify clinical predictors for survival and examine treatment outcome in patients with high-grade glioma (HGG).
Materials and Methods:
The authors retrospectively reviewed medical records of patients who was diagnosed HGG between January 2007 and December 2009. Demographic data, radiological data and treatment data of patients were reviewed and analyzed.
Results:
A total of 100 patients were analyzed. There was no difference in demographic data between Grade III and IV glioma. Patients with HGG had median survival time (MST) 18 months, The MST of patients with Grade III and IV glioma were 26 and 13 months, respectively. In this study, only anaplastic oligoastrocytoma and radiotherapy did impact strongly on survival of patients with HGG. In patients with Grade III and IV glioma, radiotherapy found to have influence on survival.
Conclusion:
Patients with HGG in Prasat Neurological Institute had short survival resemble to other previous study. The clinical predictors for survival of patients were identified on multivariate analysis.
Keywords: High-grade glioma, prognostic factor, survival
Introduction
Glioma was classified into 4 grades according to WHO classification of tumor of the central nervous system (CNS) on the basis of their degree of malignancy.[1] Grade III and IV glioma, anaplastic astrocytoma (AA), anaplastic oligodendroglioma (AO), mixed anaplastic oligoastrocytoma (AOA) and glioblastoma (GBM), were known as high-grade glioma (HGG),[2] which carried poor prognosis despite intensive treatments with surgery, radiotherapy, and chemotherapy.
Although the current standard treatment for HGG are maximal resection followed by radiotherapy with concomitant and adjuvant chemotherapy including temozolomide,[3,4,5] median survival time (MST) of GBM and AA were only <2 years and 2-5 years, respectively.[4,5,6,7]
There are several variables that could influence prognosis of patients with HGG such as age, performance status, tumor location, and extent of resection.[8,9,10,11,12,13,14] Therefore, assessment of patients by these variables may enable them receiving appropriate treatments and improve treatment outcome.
The purpose of this study was to identify clinical predictors of treatment outcome in HGG treated with combined modality approach in Prasat Neurological Institute and to examine the survival time of HGG patients at a single institute.
Materials and Methods
All patients who underwent surgery for diagnosed HGG (AA, AO, AOA and GBM) between January 2007 and December 2009 were included in this study. The authors reviewed medical records on patient characteristics and all of treatment modalities in each patient. The incomplete data on medical records were excluded.
Patient characteristics
Age, sex, Karnofsky performance status (KPS),[15] payment and neurological status in each patients were reviewed. The KPS was dichotomized at <70 based on Radiation Therapy Oncology Group (RTOG) Recursive Partitioning Analysis (RPA).[16]
Radiological data
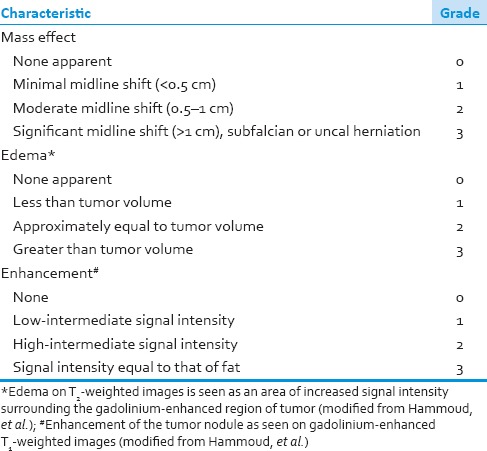
Tumor imaging characteristics were reviewed. Tumor size had a cut-off value at 4 cm because the previous studies have shown that it was significant for survival at this value.[10,17] Tumor location with regard to proximity to eloquent brain was characterized by functional grade as described by Sawaya, et al.[18] [Table 1]. Tumor necrosis [Figure 1], degree of mass effect, surrounding edema[11] and enhancement of tumor mass were also measured and recorded using the methods of Hammoud, et al.[19] [Table 2].
Table 1.
Grading of intraparenchymal tumors according to functional location*
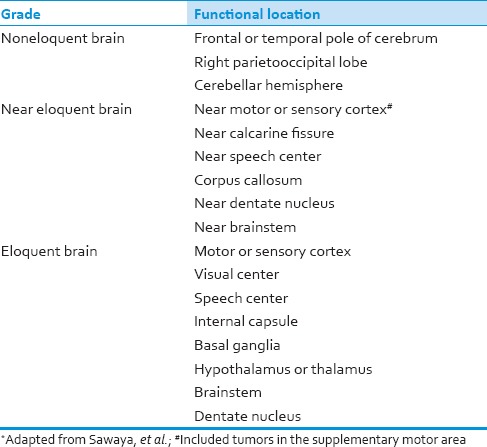
Figure 1.
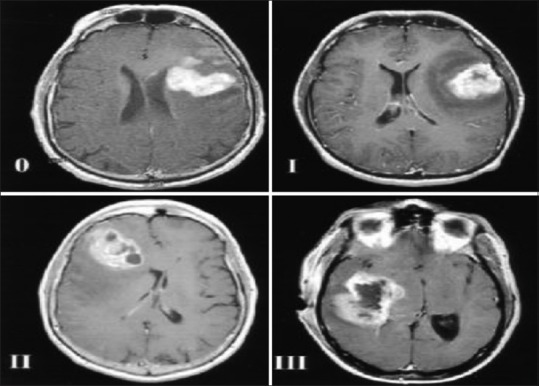
Grades of tumor necrosis adapted from Hammoud, et al. are demonstrated on magnetic resonance (MR) images. The amount of tumor necrosis, which appears as an area of decreased signal intensity on T1-weighted images, was divided into four grades as follow: Grade 0, no necrosis apparent on the MR images; Grade I, amount of necrosis <25% of the tumor volume; Grade II, amount of necrosis 25-50% of the tumor volume; and Grade III, amount of necrosis >50% of the tumor volume
Table 2.
Grading of tumor characteristic on preoperative MRI
Treatment data
All treatment modalities included surgery, radiotherapy and chemotherapy were reviewed. Extent of tumor resection were classified into total resection, which defined as no residual gross tumor intraoperatively and on postoperative image, subtotal resection and biopsy. The radiotherapy and chemotherapy data derived from the medical records. Duration from surgery to radiotherapy was also calculated in all patients.
Statistical analysis
Parametric data were expressed as means ± standard deviations and compared via the Student's t-test. Nonparametric data were expressed as median values (interquartile range) and compared via the Mann-Whitney U-test. Percentages were compared via the Chi-square test or Fisher exact test based on sample size. Survival time was calculated from the date of first treatment until the date of death. The record was ended at January 2012. Survival curves were analyzed using Kaplan-Meier method[20] and the log-rank test.[21] The univariate and multivariate analysis of prognostic factors for survival were performed using Cox proportional hazards model.[22] Hazard ratios and their 95% confidence intervals (CIs) were calculated. Statistical analyses were performed using the Statistical Package for the Social Sciences 19.0 (SPSS, Inc., Chicago, IL, USA). The author defined P value below 0.05 as significant.
Results
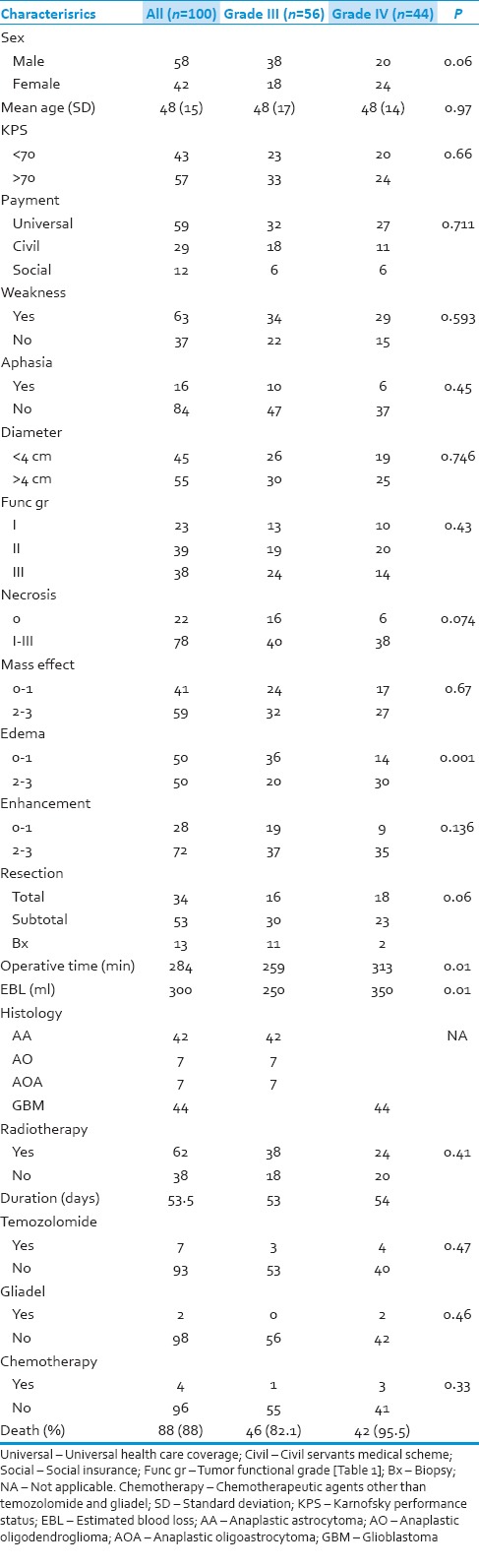
There were 121 patients who were diagnosed HGG during that time. Twenty one patients were excluded from this study because of incomplete data. Table 3 shows a demographic data of all 100 patients. Median follow-up time was 14 months (1-44 months). The data of each subgroups those are WHO Grade III and Grade IV glioma are also shown. There were no significant difference in age, sex, KPS, weakness, aphasia and imaging characteristics between the 2 subgroups. Surgical treatment was mostly resection rather than biopsy, especially in patients with Grade IV glioma (P = 0.06). Hence, the duration of operation and estimated blood loss in patients with Grade IV glioma were much more than in patients with Grade III glioma (P = 0.01, 0.01). No difference between patients in both Grade III and Grade IV glioma received the adjuvant therapy (temozolomide, gliadel and other chemotherapy) (P = 0.47, 0.46, 0.33, respectively).
Table 3.
Demographic data of 100 patients with high grade glioma
Survival time
The MST for all patients from the time of surgery was 18 (95% CI 13.4-22.6) months. The MST of patients with Grade III and IV glioma was 26 (95% CI 19-33) and 13 (95% CI 10.2-15.8) months, respectively. Figure 2 shows the survival curve of patients with Grade III and IV glioma. The log-rank test showed that patient with Grade III glioma had significantly longer survival time than Grade IV glioma (P = 0.004). The authors also analyzed the survival curve of patients with each histological type [Figure 3]. The log-rank test also confirmed that histological type did impact on survival of these patients (P = 0.004). It is shown that there were little patients diagnosed AO and AOA and they had better survival time than AA (P = 0.042). When compared the survival time between patients with AA (MST = 21 months, 95% CI = 17.7-24.3) and GBM [Figure 4], the log-rank test showed that the difference was not significant between these two groups (P = 0.85).
Figure 2.
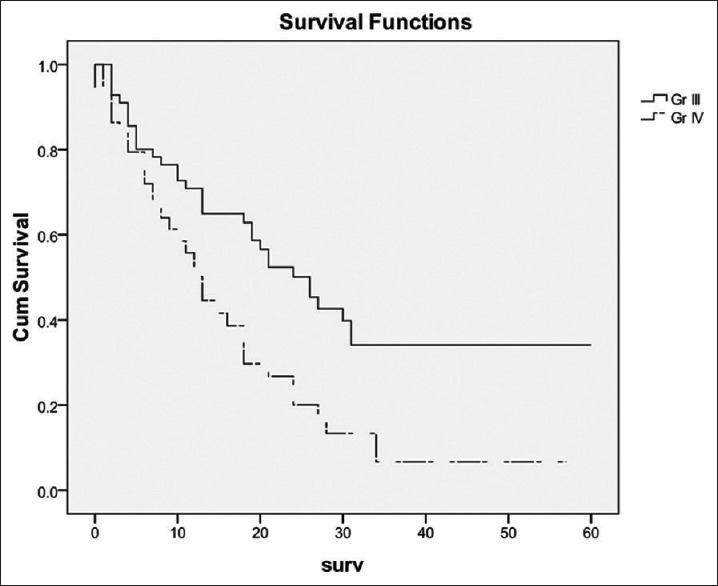
A comparison of survival times among patients with Grade III or IV glioma. Patients with Grade III glioma had significantly longer survival time than Grade IV glioma (P = 0.004)
Figure 3.
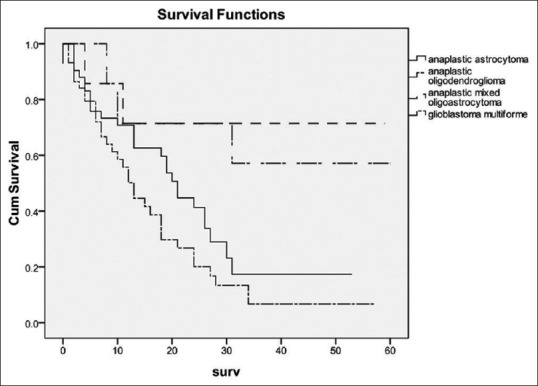
A comparison of survival times among tumor histology. Tumor histology did impact on survival of these patients (P = 0.004)
Figure 4.
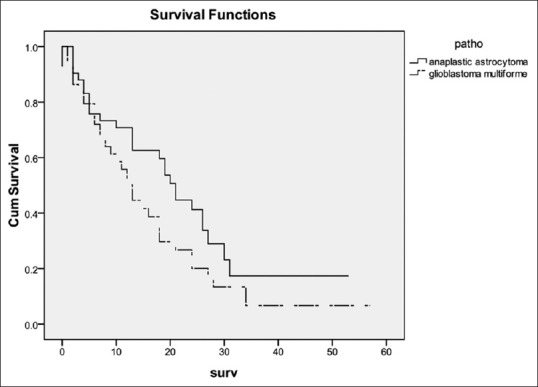
A comparison of survival times among patients with anaplastic astrocytoma or glioblastoma. The difference was not significant between these two groups (P = 0.85)
Univariate and multivariate analysis
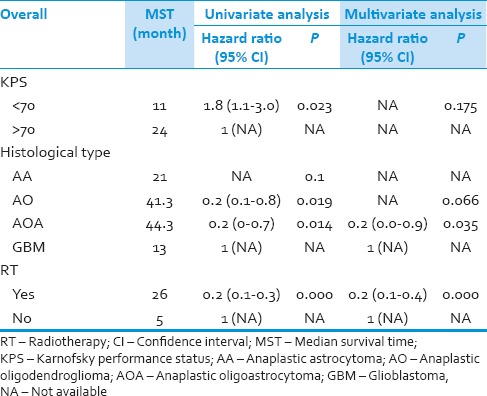
The clinical predictors for survival of all patients were analyzed by Cox proportional hazards model. On univariate analysis, KPS, histological type (those were AO and AOA) and radiotherapy had effect on survival (P = 0.023, 0.019 0.014, and 0.000, respectively). On multivariate analysis, only AOA and radiotherapy did impact on survival of these patients (P = 0.035 and 0.000, respectively) [Table 4].
Table 4.
Prognostic factors for survival of all patients
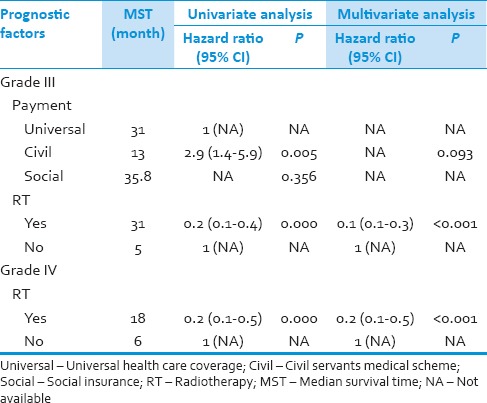
In patients with Grade IV glioma, there was only radiotherapy found to have influence on survival in both univariate and multivariate analysis (P = 0.000, <0.001). Finally, the Cox analysis was performed on patients with Grade III glioma. Patients with civil servants medical scheme and radiotherapy had influence on survival in univariate analysis (P = 0.005 and 0.000, respectively). However in multivariate analysis, only radiotherapy was the significant factor on survival (P < 0.001) [Table 5].
Table 5.
Prognostic factors for survival of patients with grade III and grade IV glioma
Discussion
High-grade glioma composed of WHO Grade III and Grade IV glioma which is the most common primary brain tumors. They represent 80% of malignant CNS tumors.[23] Although there are combination treatment between surgery, radiotherapy and chemotherapy, they carry poor prognosis and have short MST.[24]
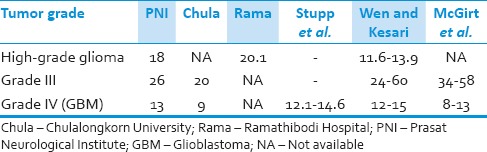
The previous studies have shown that MST of patients with Grade III and Grade IV glioma, on average, were 2-5 years and <2 years, respectively[4,7,25] In Thailand, Siangprasertkij and Navalitloha from Chulalongkorn University, reported the MST of Grade III and Grade IV glioma was 20 and 9 months, respectively.[26] Chansriwong and Sirisinha from Ramathibodi Hospital, Mahidol University, reported the overall survival time in patients with HGG was 604.04 days.[27] The treatment outcomes in this study are comparable to other previous studies [Table 6].
Table 6.
Survival time (months) of patients with high-grade glioma
There are many studies about clinical predictors for survival in patients with HGG. Most studies have been reported that age, performance status and tumor grade were independent important prognostic factors.[11,28,29] The RTOG used RPA to analyze survival in 1,578 patients with HGG. There are six prognostic classes that primarily used the variable of age, histology, mental status, KPS, symptom duration and extent of resection.[16] Laws et al. published data from the Glioma Outcome Project that resection instead of biopsy, age <60 and KPS >70 were all significantly correlated with outcome.[17] McGirt et al. have recently reported extent of resection was associated with improved survival independent of age, KPS, tumor grade, or adjuvant treatment for HGG.[13] The independent prognostic factors in this study are oligodendroglial component and radiotherapy.
The natural history of HGG is different according to tumor grade and histopathology. It has been universally accepted that the prognosis of Grade IV glioma is worse than Grade III glioma.[30] Furthermore, this study shows mixed oligoastrocytoma subtype had a better prognosis which is compatible with the previous studies.[25,29,30,31]
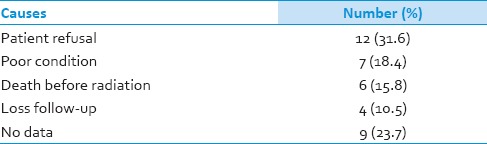
Radiotherapy has been well-documented as one of the advance treatment modalities for HGG.[1,4,32,33,34] It is strongly correlated with survival in HGG.[30] The multivariate Cox analysis in this study also reported it was a significant predictor for survival in patients with HGG. Patients in this study were received radiotherapy 62% (67.9% in Grade III, 54.5% in Grade IV). Median time from surgery to radiation was about 2 months. This seems to be less effective treatment and may have effect on survival.[35] Because there is no radiotherapy in our institute. All patients need to be transferred for radiation in the other hospitals. Hence, there may be some patients loss between the transferring. Furthermore, some patients refused to be treated with radiation due to their personal opinions. This is an important problem for the current health care system and have to be improved immediately [Table 7].
Table 7.
Causes of patients with high-grade glioma not receive radiation
It is important to note that there were some limitations to this study. This study is retrospective, thus potentially subject to sources of bias and variation. The number of patients is quite low. Data in this study were from medical records and population database of the ministry of public health. There was no detail in some aspects such as dose and technique of radiation, dose and duration of temozolomide and other chemotherapy. However, it is encouraging that the demographic characteristics of the patients and the overall survival data are very similar to those reported in other published studies.
Conclusions
This retrospective study at a single institute shows that patients with HGG had a short survival resemble to the other previous studies. The clinical predictors for survival of patients were identified on multivariate analysis. Patients should be encouraged to received suitable treatment by the new health care system.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN. Molecular pathology of malignant gliomas. Annu Rev Pathol. 2006;1:97–117. doi: 10.1146/annurev.pathol.1.110304.100043. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 6.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–23. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 7.Souhami L, Seiferheld W, Brachman D, Podgorsak EB, Werner-Wasik M, Lustig R, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: Report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853–60. doi: 10.1016/j.ijrobp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Chang SM, Barker FG., 2nd Marital status, treatment, and survival in patients with glioblastoma multiforme: A population based study. Cancer. 2005;104:1975–84. doi: 10.1002/cncr.21399. [DOI] [PubMed] [Google Scholar]
- 9.Chang SM, Parney IF, McDermott M, Barker FG, 2nd, Schmidt MH, Huang W, et al. Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg. 2003;98:1175–81. doi: 10.3171/jns.2003.98.6.1175. [DOI] [PubMed] [Google Scholar]
- 10.Jeremic B, Milicic B, Grujicic D, Dagovic A, Aleksandrovic J. Multivariate analysis of clinical prognostic factors in patients with glioblastoma multiforme treated with a combined modality approach. J Cancer Res Clin Oncol. 2003;129:477–84. doi: 10.1007/s00432-003-0471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–8. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 12.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: Recursive partitioning analysis. Neuro Oncol. 2004;6:227–35. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg. 2009;110:156–62. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 14.Nomiya T, Nemoto K, Kumabe T, Takai Y, Yamada S. Prognostic significance of surgery and radiation therapy in cases of anaplastic astrocytoma: Retrospective analysis of 170 cases. J Neurosurg. 2007;106:575–81. doi: 10.3171/jns.2007.106.4.575. [DOI] [PubMed] [Google Scholar]
- 15.Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer. 1984;53:2002–7. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 16.Curran WJ, Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–10. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 17.Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: Data from the Glioma Outcomes Project. J Neurosurg. 2003;99:467–73. doi: 10.3171/jns.2003.99.3.0467. [DOI] [PubMed] [Google Scholar]
- 18.Sawaya R, Hammoud M, Schoppa D, Hess KR, Wu SZ, Shi WM, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044–55. doi: 10.1097/00006123-199805000-00054. [DOI] [PubMed] [Google Scholar]
- 19.Hammoud MA, Sawaya R, Shi W, Thall PF, Leeds NE. Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neurooncol. 1996;27:65–73. doi: 10.1007/BF00146086. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 21.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70. [PubMed] [Google Scholar]
- 22.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 23.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 24.Brown PD, Ballman KV, Rummans TA, Maurer MJ, Sloan JA, Boeve BF, et al. Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. J Neurooncol. 2006;76:283–91. doi: 10.1007/s11060-005-7020-9. [DOI] [PubMed] [Google Scholar]
- 25.Hinsdale, Ill: CBTRUS; 2008. Central Brain Tumor Registry of the United States: 2007-2008 CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004-2006. [Google Scholar]
- 26.Siangprasertkij C, Navalitloha Y. A multivariate analysis of patients with glioma: A treatment outcome and prognostic factor for survival. J Med Assoc Thai. 2008;91:491–6. [PubMed] [Google Scholar]
- 27.Chansriwong P, Sirisinha T. Clinical features, management and outcomes of high-grade glioma patients in Ramathibodi Hospital. J Med Assoc Thai. 2010;93(Suppl 2):S68–73. [PubMed] [Google Scholar]
- 28.Burger PC, Green SB. Patient age, histologic features, and length of survival in patients with glioblastoma multiforme. Cancer. 1987;59:1617–25. doi: 10.1002/1097-0142(19870501)59:9<1617::aid-cncr2820590916>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 29.Quigley MR, Maroon JC. The relationship between survival and the extent of the resection in patients with supratentorial malignant gliomas. Neurosurgery. 1991;29:385–8. doi: 10.1097/00006123-199109000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Devaux BC, O’Fallon JR, Kelly PJ. Resection, biopsy, and survival in malignant glial neoplasms. A retrospective study of clinical parameters, therapy, and outcome. J Neurosurg. 1993;78:767–75. doi: 10.3171/jns.1993.78.5.0767. [DOI] [PubMed] [Google Scholar]
- 31.Tortosa A, Viñolas N, Villà S, Verger E, Gil JM, Brell M, et al. Prognostic implication of clinical, radiologic, and pathologic features in patients with anaplastic gliomas. Cancer. 2003;97:1063–71. doi: 10.1002/cncr.11120. [DOI] [PubMed] [Google Scholar]
- 32.Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345:1008–12. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 33.Valtonen S, Timonen U, Toivanen P, Kalimo H, Kivipelto L, Heiskanen O, et al. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: A randomized double-blind study. Neurosurgery. 1997;41:44–8. doi: 10.1097/00006123-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Westphal M, Ram Z, Riddle V, Hilt D, Bortey E. Executive Committee of the Gliadel Study Group. Gliadel wafer in initial surgery for malignant glioma: Long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien) 2006;148:269–75. doi: 10.1007/s00701-005-0707-z. [DOI] [PubMed] [Google Scholar]
- 35.Do V, Gebski V, Barton MB. The effect of waiting for radiotherapy for grade III/IV gliomas. Radiother Oncol. 2000;57:131–6. doi: 10.1016/s0167-8140(00)00257-7. [DOI] [PubMed] [Google Scholar]


