Abstract
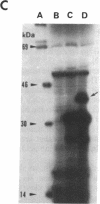
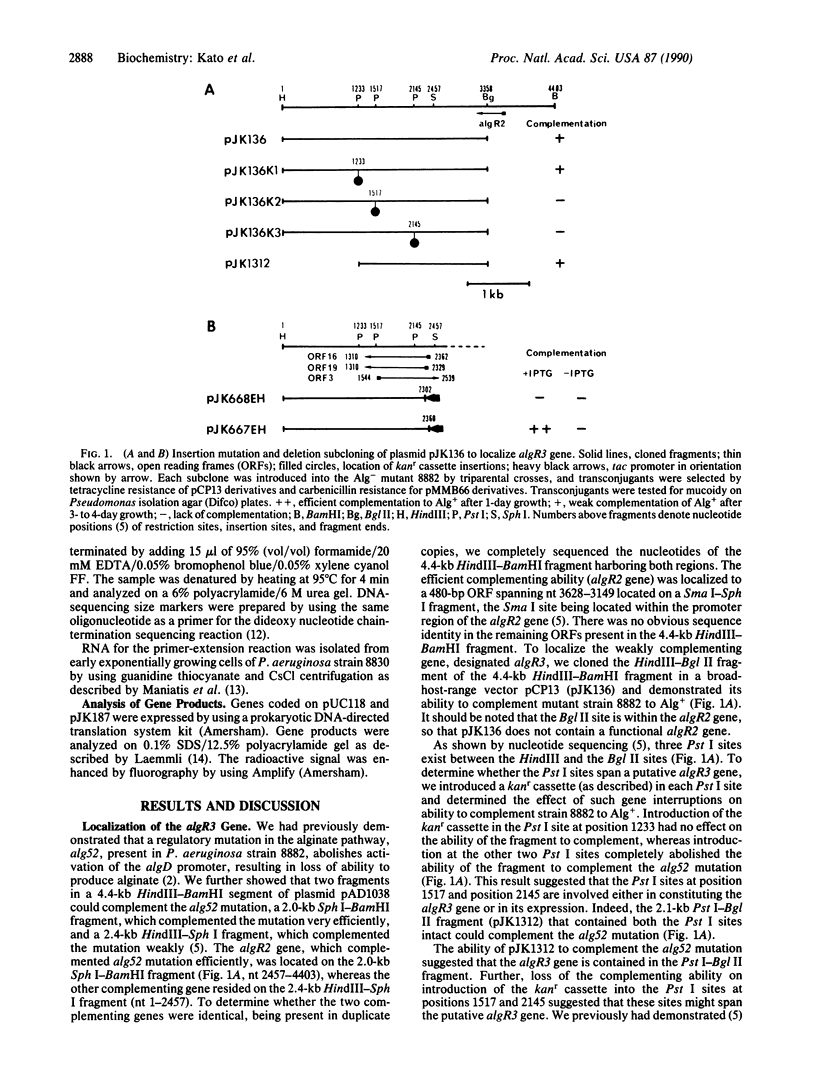
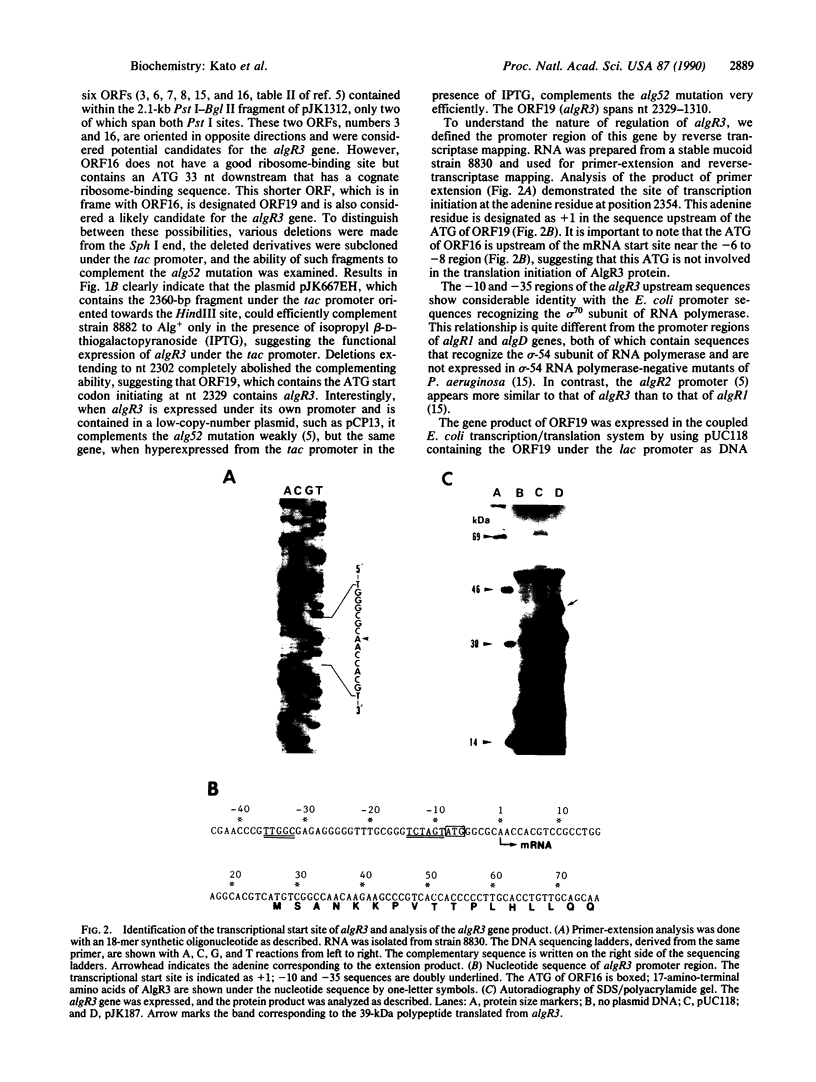
A regulatory mutation (alg52) in a Pseudomonas aeruginosa alginate-negative mutant (strain 8882) is complemented efficiently by the gene algR2 and somewhat inefficiently by a second gene termed algR3. algR3 and algR2 are located on a 4.4-kilobase-pair HindIII-BamHI fragment, which has been completely sequenced. algR2 has previously been characterized. Introduction of kanamycin-resistance cassettes and deletion-subcloning experiments involving various open reading frames in the HindIII-BamHI fragment have localized the algR3 gene, which encodes a 340-amino acid polypeptide. This highly basic regulatory protein contains 17% lysine and 36% alanine. The predicted amino acid sequence shows no significant similarity with any bacterial proteins and yet is highly similar to the sea urchin Lytechinus pictus histone H1 subtype of protein. Promoter localization by reverse transcriptase mapping of the algR3 gene shows the presence of Escherichia coli sigma 70 recognition sequences, and coupled transcription/translation experiments in E. coli demonstrate the presence of a 39-kDa polypeptide encoded by the cloned algR3 gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry A., DeVault J. D., Chakrabarty A. M. High osmolarity is a signal for enhanced algD transcription in mucoid and nonmucoid Pseudomonas aeruginosa strains. J Bacteriol. 1989 May;171(5):2312–2317. doi: 10.1128/jb.171.5.2312-2317.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bustin M., Cole R. D. Species and organ specificity in very lysine-rich histones. J Biol Chem. 1968 Sep 10;243(17):4500–4505. [PubMed] [Google Scholar]
- Darzins A., Chakrabarty A. M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol. 1984 Jul;159(1):9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V., Dikshit R., Konyecsni W. M., Chakrabarty A. M., Misra T. K. The algR gene, which regulates mucoidy in Pseudomonas aeruginosa, belongs to a class of environmentally responsive genes. J Bacteriol. 1989 Mar;171(3):1278–1283. doi: 10.1128/jb.171.3.1278-1283.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K., Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Hartman P. G., Chapman G. E., Moss T., Bradbury E. M. Studies on the role and mode of operation of the very-lysine-rich histone H1 in eukaryote chromatin. The three structural regions of the histone H1 molecule. Eur J Biochem. 1977 Jul 1;77(1):45–51. doi: 10.1111/j.1432-1033.1977.tb11639.x. [DOI] [PubMed] [Google Scholar]
- Kato J., Chu L., Kitano K., DeVault J. D., Kimbara K., Chakrabarty A. M., Misra T. K. Nucleotide sequence of a regulatory region controlling alginate synthesis in Pseudomonas aeruginosa: characterization of the algR2 gene. Gene. 1989 Dec 7;84(1):31–38. doi: 10.1016/0378-1119(89)90136-4. [DOI] [PubMed] [Google Scholar]
- Kimbara K., Chakrabarty A. M. Control of alginate synthesis in Pseudomonas aeruginosa: regulation of the algR1 gene. Biochem Biophys Res Commun. 1989 Oct 31;164(2):601–608. doi: 10.1016/0006-291x(89)91502-7. [DOI] [PubMed] [Google Scholar]
- Knowles J. A., Childs G. J. Comparison of the late H1 histone genes of the sea urchins Lytechinus pictus and Strongelocentrotus purpuratus. Nucleic Acids Res. 1986 Oct 24;14(20):8121–8133. doi: 10.1093/nar/14.20.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]