Abstract
Background:
First-line maintenance with erlotinib in nonsmall cell lung cancer (NSCLC) patients without progression after four cycles of chemotherapy was well tolerated and significantly prolonged progression-free survival (PFS) compared with placebo.
Aim and Design:
This open-label, single arm, Phase IV, interventional study was designed to evaluate erlotinib as first-line maintenance after chemotherapy in Indian NSCLC patients. Primary efficacy objective was to evaluate PFS rate (PFSR) at week 52 and secondary objectives were determination of PFS, overall survival (OS), overall response rate (ORR), disease control rate, and safety.
Subjects and Methods:
Patients were treated with erlotinib until disease progression/death/unacceptable toxicity or end of study. Patients with disease progression underwent scheduled clinical assessments every 12 weeks thereafter. Kaplan–Meier estimates were used to evaluate PFSR, PFS, and OS. The ORR was summarized using number and percentage along with two-sided 95% Clopper–Pearson confidence interval. The incidence of adverse events (AEs) and serious AEs (SAEs) was tabulated according to severity, outcome, and relationship to erlotinib.
Results:
Of the 51 enrolled patients, 47 patients completed the study (2: Continuing treatment, 41: Disease progression, and 4: Death) and four patients discontinued treatment (3: Lost to follow-up; 1: Withdrew consent). PFSR was 22.5% at 12 months, median PFS 99 days (14.14 weeks), and median OS was 671 days (22 months). The probability of OS was 74.5% at 14 months. The ORR was 25.5%, and disease control rate was 55.3%. AEs were reported in 62.7% and SAE in 7.8% of patients. Common AEs were diarrhea and rash.
Conclusions:
Erlotinib was well tolerated by Indian patients in first-line maintenance setting and resulted in median PFS of 14 weeks and median OS of 22 months better than previously reported and with no new safety concerns in this population.
Key words: Epidermal growth factor receptor-tyrosine kinase inhibitor, first-line maintenance therapy, nonsmall cell lung cancer, progression-free survival rate, targeted therapy
Introduction
Lung cancer has been the most common cancer in the world for several decades. There are estimated 1.8 million new cases, 58% of which occur in the less developed regions of the world.[1]
The prevalence of lung cancer has reached epidemic proportions in India.[2] The estimated incidence of lung cancer in India was 6.9% (70,275 in both men and women) in 2012.[1] More than 85% of mortality is due to the nonsmall cell lung cancer (NSCLC). Up to one-third of NSCLC patients present with locally advanced NSCLC that is surgically unresectable.[3] Currently, chemotherapy with a platinum-doublet is the gold standard for advanced NSCLC without a known driver mutation.[4] The discovery of activating epidermal growth factor receptor (EGFR) mutations has revolutionized lung cancer management.
Epidermal growth factor and a number of other ligands bind to the EGFR, stimulating autophosphorylation of the intracellular tyrosine kinase domain of the receptor resulting in tumor cell proliferation. Targeted therapy with EGFR-tyrosine kinase inhibitors (EGFR-TKIs) has been developed and proven active in clinical studies.
Erlotinib hydrochloride is an orally active, potent, selective inhibitor of the EGFR tyrosine kinase. Initial studies with erlotinib showed its efficacy as first-line treatment in advanced NSCLC, achieving tumor response rates of 10%–20% and a median survival between 10.9 and 12.9 months in Phase II studies.[5,6] A study with erlotinib hydrochloride versus placebo in patients with locally advanced or metastatic NSCLC after the failure of first-line or second-line chemotherapy showed a survival benefit with the use of an EGFR-TKI.[7]
The Sequential Tarceva in Unresectable NSCLC (SATURN study), a Phase III randomized, double-blind, placebo-controlled trial evaluated erlotinib as switch maintenance therapy after four to six cycles of platinum-doublet chemotherapy and was shown to improve both progression-free survival (PFS) and OS compared with placebo irrespective of histology.[8]
The current study was undertaken on similar lines to evaluate the efficacy and safety of erlotinib in Indian patients with NSCLC, who had not progressed after four cycles of platinum-based chemotherapy.
Subjects and Methods
Subjects
Adult patients with histologically documented, locally advanced or recurrent (Stage IIIB) or metastatic (Stage IV) NSCLC, who had completed four cycles of acceptable, standard, platinum-based chemotherapy without progression or any unacceptable toxicity were enrolled. These patients had an Eastern Cooperative Oncology Group performance status of 0–1, granulocyte count ≥1500/mm3, platelet count >100,000/mm3, hemoglobin ≥9 g/dL, and normal serum calcium.
Patients excluded were those with prior exposure to agents directed at the human EGFR axis (e.g., gefitinib, cetuximab, and trastuzumab) or with any other monoclonal antibody therapy or surgery and/or localized irradiation. Patients who had undergone complete tumor resection after responding to platinum-based chemotherapy or had serum creatinine >1.5 upper limit of normality and/or creatinine clearance <60 mL/min were not enrolled. Patients with any other criteria that contraindicated the use of erlotinib as per local prescribing information or as per physicians' discretion were ineligible for participation.
Study design
This was an open-label, single arm, multi-center, Phase IV, interventional study (ClinicalTrials.gov number, NCT01230710) conducted in patients with NSCLC, who did not progress (i.e., patients with complete response [CR], partial response [PR], or stable disease) and without any unacceptable toxicity after completion of four cycles of first-line platinum-based chemotherapy and for whom erlotinib was prescribed as a part of the treatment strategy. The study was undertaken in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Fifty-one patients, who met the study eligibility criteria and provided signed informed consent, received erlotinib treatment as first-line maintenance therapy.
Patients visited the study center every 6 weeks during the treatment period until progression of disease/death/unacceptable toxicity or until the end of the study, i.e., 18 months after recruitment of the last patient. Patients with disease progression underwent scheduled clinical assessments every 12 weeks thereafter. Study medication was provided to all the patients who had completed 48 weeks of erlotinib treatment without disease progression and were willing to continue the study medication as a part of posttrial access.
Data collection overview
The study was carried out between March 2011 and September 2013. The primary efficacy objective was to evaluate PFS rate (PFSR) at week 52. The secondary objectives included evaluation of PFS, overall survival (OS), overall response rate (ORR) (CR + PR), and disease control rate (CR + PR + stable disease), and evaluation of the safety profile of erlotinib after standard platinum-based chemotherapy in the treatment of NSCLC.
Routine physical examinations, including an ophthalmologic examination if required, were done at every visit including safety follow-up, survival follow-up, and unscheduled visits. Clinical laboratory parameters such as hemoglobin, white blood cell count, neutrophils, lymphocytes, monocytes, eosinophils, basophils, platelet, creatinine clearance, and international normalized ratio/prothrombin time were measured and compared from baseline to week 90 in the study population.
Statistical analysis
All tables and listings were generated using Statistical Analysis Software (SAS®) version 9.2 (SAS Institute Inc., Cary, NC, USA). Demographic characteristics were summarized for all patients. The frequencies and percentages were given for categorical variables. The number (n), mean, median, standard deviation, first quartile, third quartile, minimum, and maximum were given for continuous variables.
Summary of each significant past or concomitant medical condition was provided. The descriptive statistics for all disease conditions, prior medical therapy other than the study, and concomitant medications were summarized. Frequency and duration of treatment were computed for all medications. History of prior cancer therapy, prior radiotherapy, and their response rate were summarized.
The PFSR was estimated as the proportion of patients who did not progress and were alive at a given time and was calculated from the date of enrollment until the date of progression or death from any cause. PFS was calculated in days from the start of treatment until the earliest date of disease progression or death from any cause; else, the participant was labeled as censored. Disease progression was defined according to the RECIST criteria (version 1.1). The investigator determined response and date of disease progression. OS was determined from the date of enrollment to the date of death irrespective of the cause of death. Patients who had not died at the time of the final analysis were censored at the date the patient was last known to be alive. The PFSR, PFS, and OS time with 95% confidence interval (CI) were estimated using the Kaplan–Meier method.
To have the best response as continued CR (for patients with CR at enrollment), the patient must have remained tumor free for at least 6 weeks postbaseline. To have a best response of CR or PR, patients were required to have follow-up measurements meeting the criteria for CR or PR at two consecutive visits at least 4 weeks apart at any time postbaseline. For best response stable disease, follow-up measurements must have met the stable disease criteria at least 6 weeks postbaseline. The ORR was summarized using number and percentage along with the two-sided 95% Clopper–Pearson CI.
The number and percentage of patients reporting adverse events (AEs) and serious AEs (SAEs) were tabulated, and severity, outcome, and relationship to the study medication were analyzed.
Results
Patient characteristics
Over a period of 12 months, 55 patients with NSCLC were screened, and 51 patients were enrolled across seven centers in India. All 51 patients were included in both the full analysis set (FAS) and the safety population for efficacy and safety analysis, respectively. Treatment was discontinued in three patients that were lost to follow-up and one patient who withdrew consent. Remaining patients completed the study and were censored at given time points due to the following reasons: Disease progression (41 patients [80.4%]), death (4 patients [7.8%]). Two patients (3.9%) continued treatment until the end of the study [Figure 1].
Figure 1.
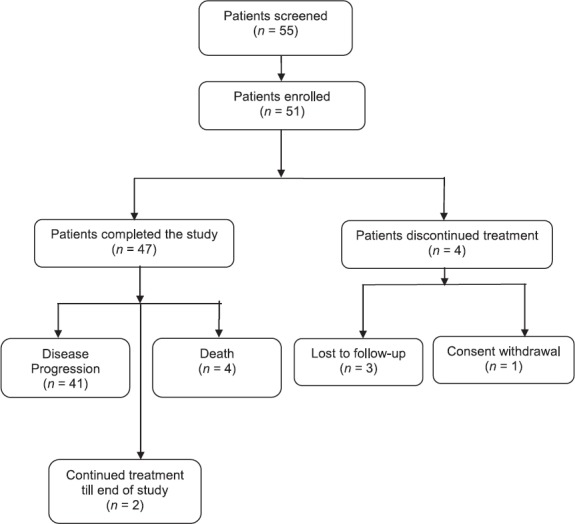
Patient disposition
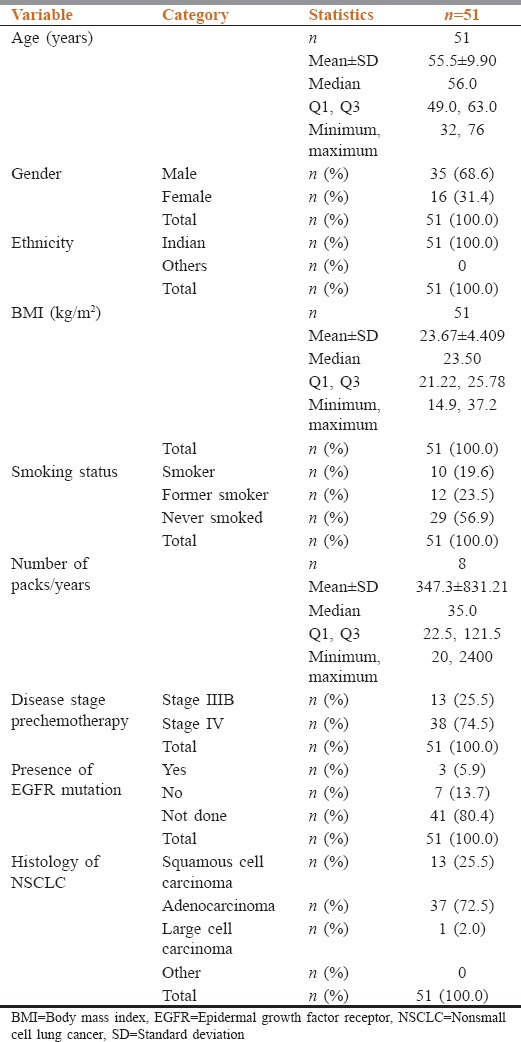
The study population comprised 35 males and 16 females with the mean age of 55.5 ± 9.90 years, the mean height of 159.7 ± 9.26 cm, and mean weight of 60.52 ± 12.925 kg [Table 1]. Thirty-seven patients (72.5%) had adenocarcinoma, 13 patients (25.5%) squamous cell carcinoma, and 1 patient (2.0%) had large cell carcinoma. Majority of patients were unresectable Stage IV (38 patients [74.5%]) and remaining were Stage IIIB (13 patients [25.5%]). Three patients were EGFR mutation positive, seven patients were negative, and the mutation status was not available for the remaining 41 patients.
Table 1.
Demographic characteristics
Clinically significant medical history was reported in four patients (13.3%) which included calcified renal cyst, chest pain, cough, diabetes mellitus, hypertension, and lumbosacral spondylosis.
Efficacy analysis
Median PFS in the FAS population was 99 days (95% CI: 53.0–292.0) or 14.14 weeks, and PFSR was 22.5% and 10.8% at 12 and 22 months, respectively [Table 2 and Figure 2]. As per Kaplan–Meier estimate, eight patients (15.7%) without disease progression or lost to follow were censored while 43 patients (84.3%) had either disease progression or had died.
Table 2.
Summary of progression-free survival using Kaplan-Meier estimate
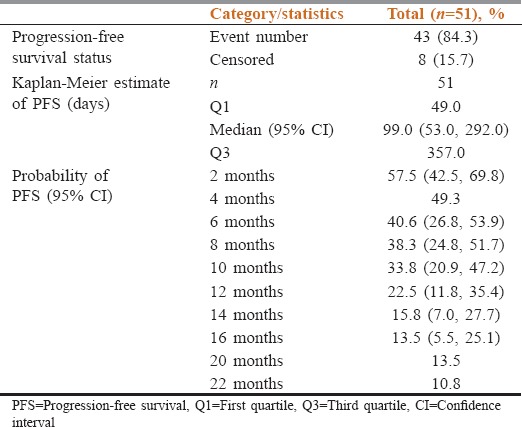
Figure 2.
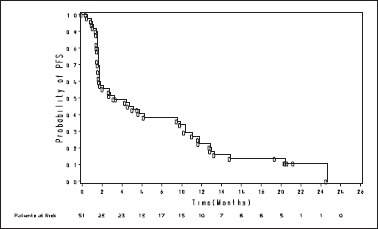
Kaplan–Meier analysis of progression-free survival. Depicts the Kaplan–Meier plot for probability of progression-free survival in months
Gender-wise median PFS was statistically significant (P = 0.0006) in female patients (376 days [53.7 weeks]) than in the male patients (51 days [7.2 weeks]). Probability of PFS at 12 months was 7.5% and 50% for male and female populations, respectively. Median PFS was 175 days (25 weeks) for nonsmokers and 47 days (6.7 weeks) for smokers which were statistically significant (P < 0.0001). The probability of PFS at 12 months was 27.1% for nonsmokers.
Median OS observed in this study was 671 days (22 months). The probability of OS was 74.5% at 14 months [Table 3 and Figure 3]. Gender-wise, median OS was 563 days (18 months) for male population and 671 days (22 months) for female population. The difference in OS between the two groups was not statistically significant. The probability of OS at 12 months was 72.1% and 90.9% for male and female populations, respectively.
Table 3.
Summary of overall survival using Kaplan-Meier estimate
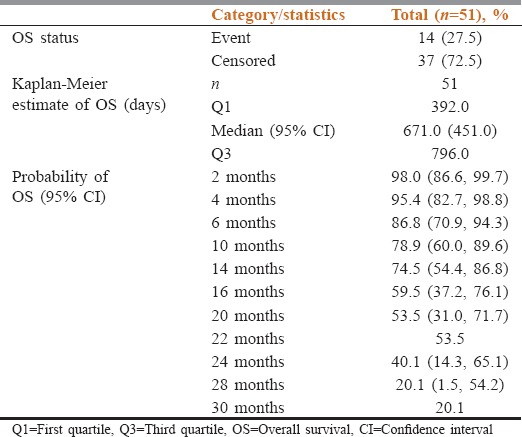
Figure 3.
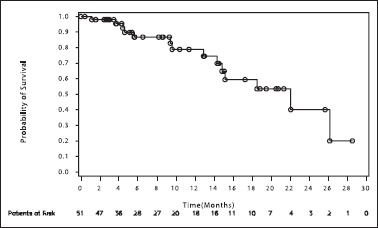
Kaplan–Meier analysis of overall survival. Depicts the Kaplan–Meier plot for probability of survival of the patients in months
Median OS was statistically significant (P < 0.0485) in nonsmokers (671 days [22 months]) as compared to smokers (286 days [9.4 months]). The probability of OS at 10 months was 45% for smokers and 83.1% for nonsmokers in this study.
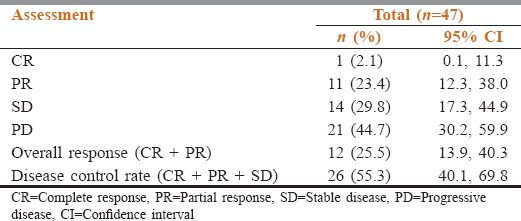
The ORR (CR + PR) was 25.5% (observed in 12 patients) and disease control rate was 55.3% (CR + PR + stable disease) (observed in 26 patients). The overall response and disease control was recorded for 47 patients out of which 1 patient (2.1%) reported CR, 11 patients (23.4%) had PR, 14 patients (29.8%) had stable disease, and 21 patients (44.7%) reported progressive disease [Table 4].
Table 4.
Summary of overall response and disease control rates
Safety evaluation
Of the 51 patients, 32 patients (62.7%) experienced a total of 114 AEs. Of these patients, 13 patients (25.5%) experienced one AE, 6 patients (11.8%) experienced two AEs each, and 13 patients (25.5%) experienced more than two AEs each. The most commonly reported AEs were diarrhea, acneiform rash, dry skin, and headache.
AEs of Grade 3 severity reported in 11.7% of patients were anemia, diarrhea, dyspnea, acneiform dermatitis, dry skin, rash, maculopapular rash, skin reaction, and skin ulcer. Reported AEs of Grade 4 severity (2.7%) were back pain, drug eruption, and erythema nodosum. In terms of outcome, 69 events (60.5%) recovered, 3 (2.6%) recovered with sequelae, 27 (23.7%) were on-going, and for 15 AEs (13.2%) the outcome was unknown.
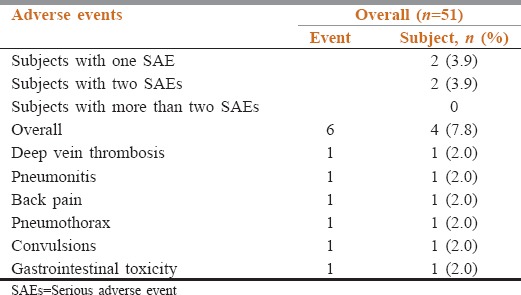
A total of six SAEs were reported in four patients (7.8%) [Table 5]. These were deep vein thrombosis, pneumonitis, back pain, pneumothorax, convulsions, and upper gastrointestinal toxicity. The event of gastrointestinal toxicity was considered related to the study medication, and the remaining five SAEs were considered not related to the study medication, erlotinib, by the investigator. The outcome of the event of convulsion was resolved, deep vein thrombosis resolved with sequelae, and gastrointestinal toxicity, back pain, pneumothorax, and pneumonitis were persisting in three patients in the study.
Table 5.
Incidence of serious adverse events (safety population)
Seventeen (33.3%) deaths were reported in the safety population. Of these, 13 patients died due to disease progression and were therefore not reported as SAEs. One patient discontinued treatment due disease progression and thereafter died due to myocardial infarction. Two patients discontinued treatment due to the progression of disease and during survival follow-up the patients did not report to the site for the scheduled visit. Subsequently, after repeated follow-ups by the site, deaths were reported due to an unknown cause. One (5.9%) patient died due to cardiorespiratory failure.
Physical examination was conducted, and vital signs were evaluated at every study visit and abnormal observations, if any, were recorded. Majority of abnormal physical examination observations were recorded under body systems of skin, lungs, and lymph nodes.
Discussion
Erlotinib, an oral EGFR-TKI, has shown both proapoptotic and antiproliferative effects.[9] The proapoptotic effects of erlotinib are beneficial in enhancing the antitumor effects of chemotherapy. The possible role of erlotinib monotherapy as sequential treatment, postchemotherapy regimen emerged from the data derived from the TALENT study.[10] Maintenance therapy can prolong survival by delaying progression.
The Phase III SATURN study[8] showed that erlotinib provided as switch maintenance therapy post four to six cycles of platinum-based chemotherapy significantly improved the PFS and OS of NSCLC patients. To the best of our knowledge, the current study is the first study to evaluate the safety and efficacy of erlotinib as first-line maintenance in the Indian population.
PFSR at 52 weeks was 22.5% and median PFS irrespective of EGFR status was 99 days or 14.14 weeks in patients on maintenance therapy with erlotinib. This was in line with that observed in the SATURN study in which patients on erlotinib had PFS of 12.3 weeks.[8]
Consistent with clinical data which suggest that tyrosine kinase inhibitors are more active in certain NSCLC histotypes such as in adenocarcinomas, never smokers, and in women,[11] the PFS for female population, nonsmokers and patients with adenocarcinoma were statistically significant as compared to males, smokers, and squamous cell carcinoma, respectively.
The OS observed in this study was 22 months which was longer than that reported in the SATURN study (12 months) and could be attributed to the difference in the follow-up period of the two studies. Patients were followed up for 18 months after the last patient was enrolled in this study and for median 11.5 months in the SATURN study. Similar to the PFS, OS was higher in females as compared to males and was statistically significant in nonsmokers as compared to smokers.
A higher ORR of 25.5% was observed in the current study in comparison to the 11.9% reported in the SATURN study while the disease control rate was 55.3% in the current study and 60.6% in SATURN study. Overall, despite the smaller size of the study population in this study, the efficacy of erlotinib as first-line maintenance therapy in Indian patients was comparable to the global data of the SATURN study.
EGFR mutation status was available for only ten patients of which three (30%) patients were mutation positive and seven (70%) patients were mutation negative. The small sample of EGFR positive patients in this study was inadequate to derive statistical conclusions in terms of the efficacy of erlotinib in this subset of the Indian population.
Recently, a randomized, double-blind, placebo-controlled Phase III (IUNO) study was conducted in 643 patients with advanced NSCLC whose tumors did not harbor an EGFR-activating mutation and who had not experienced disease progression after four cycles of platinum-based chemotherapy. The study did not meet its primary endpoint. OS of erlotinib in first-line maintenance was not superior to erlotinib as second-line treatment in patients whose tumor did not harbor an EGFR-activating mutation (hazard ratio = 1.02, 95% CI: 0.85–1.22, P = 0.82). Based on these data, erlotinib use is now no longer recommended for first-line maintenance treatment in patients without an EGFR activating mutation.[12]
However, at the start of our study, erlotinib was indicated for maintenance treatment irrespective of mutation status. The limitation of our study was that only three patients were known to be EGFR mutation positive. Further studies with a larger number of Indian patients with EGFR mutation are required to substantiate these findings.
Consistent with the SATURN trial the most commonly reported AEs were rash and diarrhea. The incidence of SAEs (7.8% vs. 11%) was slightly lower than that observed in the SATURN trial presumably due to the smaller sample size. No unexpected toxicities were noted in the study population.
Conclusions
Erlotinib was well tolerated by Indian patients in first-line maintenance setting and resulted in median PFS of 14 weeks and median OS of 22 months numerically better than what has been previously reported and with no new safety concerns in this population.
Financial support and sponsorship
This study was funded by Roche Products (India) Pvt. Ltd. (ClinicalTrials.gov number, NCT01230710 and Roche Protocol ID, ML25478).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to acknowledge the contribution of Dr. Ranjan Kumar Mohapatra, Oncologist, for conception, design, and data acquisition during this study, Dr. Ankita Jain for conception and design of the study and Dr. Amit Qamra for data acquisition and interpretation. We are grateful to Dr. Anil Kukreja, Medical Director, Roche Products (India) Pvt. Ltd., for his immense contribution toward conception and design of the study, as well as for his valuable inputs on the draft. We are also thankful to Shriram Vedapuri of Roche Products (India) Pvt. Ltd., for data acquisition and data interpretation and Priyanka Bhattacharya of Roche Products (India) Pvt. Ltd., for medical writing.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. [Last accessed on 2016 Jul 04]. Available from: http://www.globocan.iarc.fr . [Google Scholar]
- 2.Behera D, Balamugesh T. Lung cancer in India. Indian J Chest Dis Allied Sci. 2004;46:269–81. [PubMed] [Google Scholar]
- 3.Tyldesley S, Boyd C, Schulze K, Walker H, Mackillop WJ. Estimating the need for radiotherapy for lung cancer: An evidence-based, epidemiologic approach. Int J Radiat Oncol Biol Phys. 2001;49:973–85. doi: 10.1016/s0360-3016(00)01401-2. [DOI] [PubMed] [Google Scholar]
- 4.Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: Current standards and the promise of the future. Transl Lung Cancer Res. 2015;4:36–54. doi: 10.3978/j.issn.2218-6751.2014.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giaccone G, Gallegos Ruiz M, Le Chevalier T, Thatcher N, Smit E, Rodriguez JA, et al. Erlotinib for frontline treatment of advanced non-small cell lung cancer: A phase II study. Clin Cancer Res. 2006;12(20 Pt 1):6049–55. doi: 10.1158/1078-0432.CCR-06-0260. [DOI] [PubMed] [Google Scholar]
- 6.Jackman DM, Yeap BY, Lindeman NI, Fidias P, Rabin MS, Temel J, et al. Phase II clinical trial of chemotherapy-naive patients>or=70 years of age treated with erlotinib for advanced non-small-cell lung cancer. J Clin Oncol. 2007;25:760–6. doi: 10.1200/JCO.2006.07.5754. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 8.Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczésna A, Juhász E, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: A multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–9. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 9.Brand TM, Dunn EF, Iida M, Myers RA, Kostopoulos KT, Li C, et al. Erlotinib is a viable treatment for tumors with acquired resistance to cetuximab. Cancer Biol Ther. 2011;12:436–46. doi: 10.4161/cbt.12.5.16394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: The Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–52. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 11.Bareschino MA, Schettino C, Rossi A, Maione P, Sacco PC, Zeppa R, et al. Treatment of advanced non small cell lung cancer. J Thorac Dis. 2011;3:122–33. doi: 10.3978/j.issn.2072-1439.2010.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.EPAR Assessment Report Tarceva Procedure No. EMEA/H/C/000618/ II/0043. 2015. Dec 17, [Last accessed on 2016 Jul 05]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-Variation/human/000618/WC500203053.pdf .


