Abstract
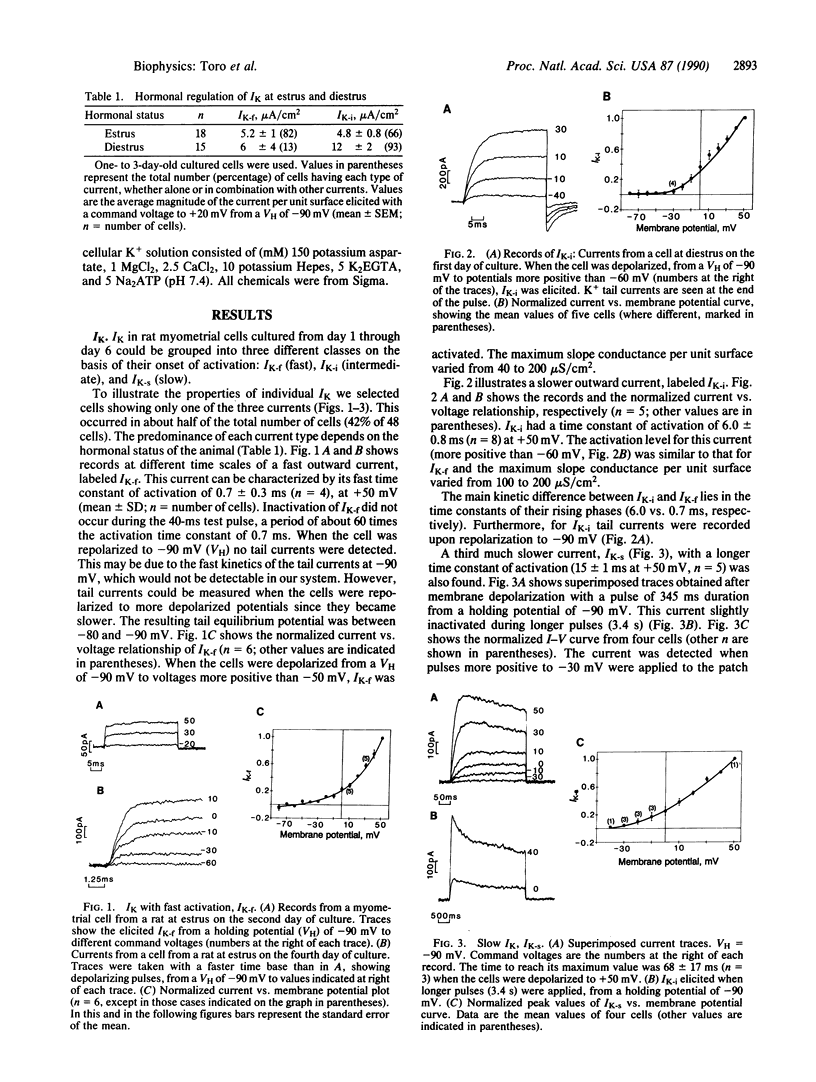
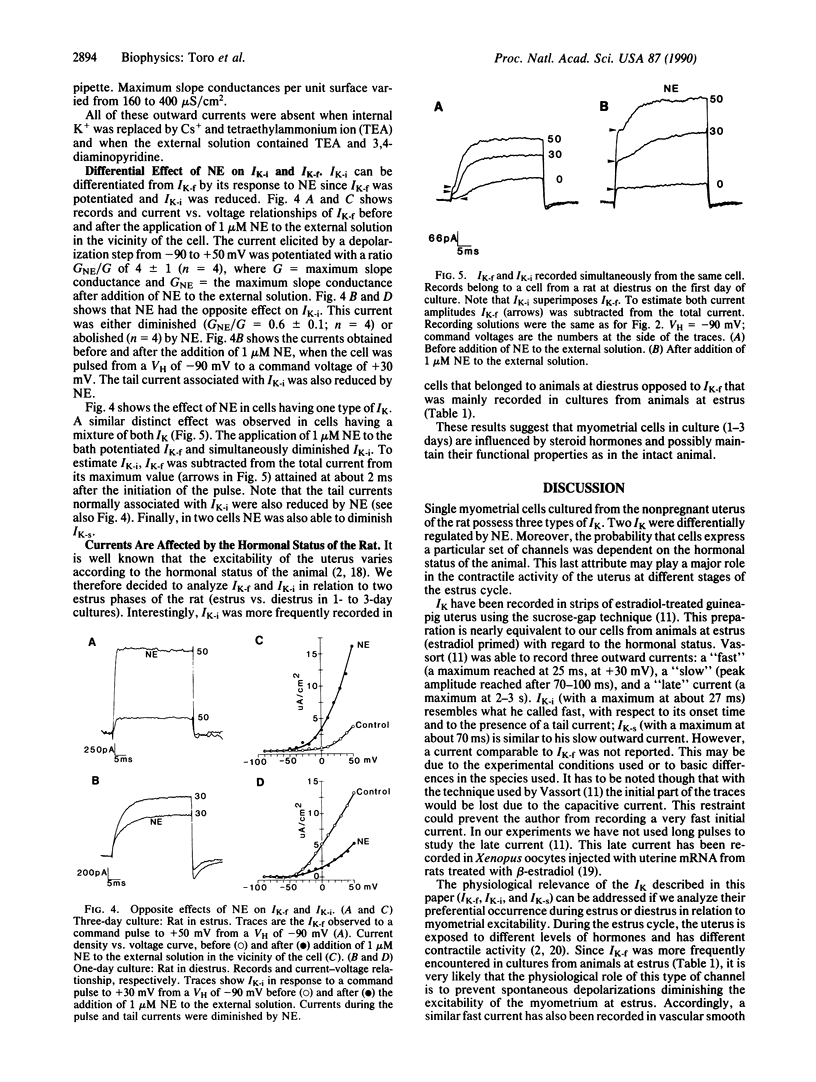
Three potassium currents (IK) were recorded from myometrial cells isolated from the uterus of rats at estrus and diestrus and kept in culture for 1-6 days. IK were differentiated by their modulation with norepinephrine and/or by their onset kinetics. At +50 mV the activation time constants were about 0.7 ms, 6 ms, and 15 ms for the fast, the intermediate, and the slow IK, respectively. Norepinephrine (1 microM) potentiated the fast IK and reduced the intermediate IK. In addition, differences were found with respect to cells from animals at estrus and diestrus. The fast IK was preferentially expressed in cultures from animals at estrus, whereas the intermediate IK was more frequent in cells from rats at diestrus. These results indicate that K+ channels from myometrial cells are multiregulated. Regulation may occur by short-term signals (neurotransmitters) and/or by preferentially expressing distinct types of channels depending on the hormonal status of the animal.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amédée T., Mironneau C., Mironneau J. The calcium channel current of pregnant rat single myometrial cells in short-term primary culture. J Physiol. 1987 Nov;392:253–272. doi: 10.1113/jphysiol.1987.sp016779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkon M., Nerbonne J. M. Alpha 1-adrenergic agonists selectively suppress voltage-dependent K+ current in rat ventricular myocytes. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8756–8760. doi: 10.1073/pnas.85.22.8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra S., Bengtsson B. Effects of diethylstilboestrol and ovarian steroids on the contractile responses and calcium movements in rat uterine smooth muscle. J Physiol. 1978 Mar;276:329–342. doi: 10.1113/jphysiol.1978.sp012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M. B., Azhderian E. M., MacLusky N. J., Naftolin F., Kaczmarek L. K. Xenopus oocytes injected with rat uterine RNA express very slowly activating potassium currents. Science. 1987 Mar 6;235(4793):1221–1224. doi: 10.1126/science.2434999. [DOI] [PubMed] [Google Scholar]
- Franchi A. M., Chaud M., Borda E. S., Gimeno M. F., Lazzari M. A., Gimeno A. L. Spontaneous motility and prostaglandin generation in rat uterine horns isolated during the estrous cycle. Prostaglandins. 1981 Oct;22(4):637–642. doi: 10.1016/0090-6980(81)90073-3. [DOI] [PubMed] [Google Scholar]
- Krall J. F., Mori H., Tuck M. L., LeShon S. L., Korenman S. G. Demonstration of adrenergic catecholamine receptors in rat myometrium and their regulation by sex steroid hormones. Life Sci. 1978 Sep 11;23(10):1073–1081. doi: 10.1016/0024-3205(78)90669-0. [DOI] [PubMed] [Google Scholar]
- Kroeger E. A., Marshall J. M. Beta-adrenergic effects on rat myometrium: mechanisms of membrane hyperpolarization. Am J Physiol. 1973 Dec;225(6):1339–1345. doi: 10.1152/ajplegacy.1973.225.6.1339. [DOI] [PubMed] [Google Scholar]
- Kume H., Takai A., Tokuno H., Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989 Sep 14;341(6238):152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Suzuki H. Changes in electrical properties of rat myometrium during gestation and following hormonal treatments. J Physiol. 1976 Sep;260(2):315–333. doi: 10.1113/jphysiol.1976.sp011517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J., Savineau J. P. Effects of calcium ions on outward membrane currents in rat uterine smooth muscle. J Physiol. 1980 May;302:411–425. doi: 10.1113/jphysiol.1980.sp013253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollard P., Mironneau J., Amedee T., Mironneau C. Electrophysiological characterization of single pregnant rat myometrial cells in short-term primary culture. Am J Physiol. 1986 Jan;250(1 Pt 1):C47–C54. doi: 10.1152/ajpcell.1986.250.1.C47. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Sperelakis N. Fast Na+ and slow Ca2+ channels in single uterine muscle cells from pregnant rats. Am J Physiol. 1989 Aug;257(2 Pt 1):C408–C412. doi: 10.1152/ajpcell.1989.257.2.C408. [DOI] [PubMed] [Google Scholar]
- Osa T., Maruta K. Comparative effects of Mg, Ca, Sr, and verapamil on the uterine longitudinal muscle of spayed and estrogen-treated rats. Jpn J Physiol. 1986;36(5):871–889. doi: 10.2170/jjphysiol.36.871. [DOI] [PubMed] [Google Scholar]
- Pacaud P., Loirand G., Mironneau C., Mironneau J. Opposing effects of noradrenaline on the two classes of voltage-dependent calcium channels of single vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1987 Nov;410(4-5):557–559. doi: 10.1007/BF00586539. [DOI] [PubMed] [Google Scholar]
- Tomita T. Ionic channels in smooth muscle studied with patch-clamp methods. Jpn J Physiol. 1988;38(1):1–18. doi: 10.2170/jjphysiol.38.1. [DOI] [PubMed] [Google Scholar]
- Toro L., González-Robles A., Stefani E. Electrical properties and morphology of single vascular smooth muscle cells in culture. Am J Physiol. 1986 Nov;251(5 Pt 1):C763–C773. doi: 10.1152/ajpcell.1986.251.5.C763. [DOI] [PubMed] [Google Scholar]
- Vassort G. Voltage-clamp analysis of transmembrane ionic currents in guinea-pig myometrium: evidence for an initial potassium activation triggered by calcium influx. J Physiol. 1975 Nov;252(3):713–734. doi: 10.1113/jphysiol.1975.sp011167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K. B., Begenisich T. B., Kass R. S. Beta-adrenergic modulation of cardiac ion channels. Differential temperature sensitivity of potassium and calcium currents. J Gen Physiol. 1989 May;93(5):841–854. doi: 10.1085/jgp.93.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Honeyman T. W., Fay F. S. Beta-adrenergic actions on membrane electrical properties of dissociated smooth muscle cells. Am J Physiol. 1988 Mar;254(3 Pt 1):C423–C431. doi: 10.1152/ajpcell.1988.254.3.C423. [DOI] [PubMed] [Google Scholar]



