Abstract
A progressive loss of dopamine neurons in the substantia nigra (SN) is considered the main feature of idiopathic Parkinson's disease (PD). Recent neuropathological evidence however suggests that the axons of the nigrostriatal dopaminergic system are the earliest target of α-synuclein accumulation in PD, thus the principal site for vulnerability. Whether this applies to in vivo PD, and also to the mesolimbic system has not been investigated yet.
We used [11C]FeCIT PET to measure presynaptic dopamine transporter (DAT) activity in both nigrostriatal and mesolimbic systems, in 36 early PD patients (mean disease duration in months ± SD 21.8 ± 10.7) and 14 healthy controls similar for age. We also performed anatomically-driven partial correlation analysis to evaluate possible changes in the connectivity within both the dopamine networks at an early clinical phase.
In the nigrostriatal system, we found a severe DAT reduction in the afferents to the dorsal putamen (DPU) (η2 = 0.84), whereas the SN was the less affected region (η2 = 0.31). DAT activity in the ventral tegmental area (VTA) and the ventral striatum (VST) were also reduced in the patient group, but to a lesser degree (VST η2 = 0.71 and VTA η2 = 0.31). In the PD patients compared to the controls, there was a marked decrease in dopamine network connectivity between SN and DPU nodes, supporting the significant derangement in the nigrostriatal pathway.
These results suggest that neurodegeneration in the dopamine pathways is initially more prominent in the afferent axons and more severe in the nigrostriatal system. Considering PD as a disconnection syndrome starting from the axons, it would justify neuroprotective interventions even if patients have already manifested clinical symptoms.
Abbreviations: SN, substantia nigra; VTA, ventral tegmental area; DPU, dorsal putamen; DCA, dorsal caudate; VST, ventral striatum; SUVr, standardized uptake value ratio; AI, asymmetry index; cAS, clinical asymmetry
Keywords: Parkinson's disease, Positron emission tomography, Dopamine transporter, Axonal damage, Molecular connectivity
Highlights
-
•
In vivo study of mesolimbic and nigrostriatal dopamine systems in early iPD
-
•
Evidence for a severe axonal damage with relative sparing of SN
-
•
Evidence for a moderate damage of the mesolimbic pathway in early iPD
-
•
Significant reduction of molecular connectivity between nigrostriatal nodes
-
•
Justification for neuroprotective interventions in early-iPD phase
1. Introduction
The substantia nigra (SN) axons project rostrally via the medial forebrain bundle to the dorsal striatum, forming the nigrostriatal dopaminergic pathway (Nieuwenhuys et al., 2013). A progressive neurodegeneration of the dopaminergic neurons in the SN pars compacta characterizes Parkinson's disease (PD) and it is the main cause of motor impairment (Fahn, 2003).
In addition, post mortem studies revealed variable levels of dopaminergic neural loss in ventral tegmental area (VTA) (Alberico et al., 2015, Surmeier and Sulzer, 2013). This system, however, has been associated to a series of psychopathological states, which often occur in PD and may precede motor disturbances by many years (Gustafsson et al., 2015). A damage to the dopaminergic mesolimbic system is considered the leading cause of the neuropsychiatric symptoms occurring in PD (see (Castrioto et al., 2016) for a recent review).
Even though dopaminergic therapies have improved PD patients' quality of life and also their life expectancy, to date we still lack drugs with disease-modifying effects, able to prevent and restore the damaged system (Olanow et al., 2008). One of the main reasons behind this is the uncertainty about the precise causes leading to cell death in PD, which consequently produces difficulties to select effective therapeutic targets (Olanow et al., 2008).
A recent pathological hypothesis suggests that at the time of disease onset there is a greater degeneration of dopaminergic axonal projections rather than SN cell bodies (Tagliaferro and Burke, 2016). Post mortem neurochemical studies reported that at the time of motor symptoms onset there is a greater degeneration of the dopaminergic pathway in the striatum compared to the SN. This suggests that, within the dopamine neurons, neurodegeneration involves the striatal axonal projections first, and subsequently spreads to cell bodies (see (Tagliaferro and Burke, 2016) for a recent review). When Kordower and colleagues assessed tyrosine hydroxylase (TH) and dopamine transporter (DAT) levels in 28 PD brains at different disease stages, they found that presynaptic terminals in dorsal striatum were affected by a progressive degeneration since the earliest disease phases. On the contrary, SN was less affected over the time, and a number of SN neurons maintained TH positivity even decades after diagnosis (Kordower et al., 2013).
Notably, α-synuclein accumulation, the pathological hallmark of PD, was predominantly found in axons and presynaptic spaces of neurons (Schulz-Schaeffer, 2010). Neurons of different systems presenting high vulnerability to α-synuclein pathology are characterized by long, highly branched axons, which may lead to the rapidly propagation of pathology, and by higher energy demand, which would enhance their vulnerability to α-synuclein-related mitochondrial dysfunction (Braak et al., 2004, Fu et al., 2016).
Our study aimed at characterizing presynaptic dopamine activity in early idiopathic PD patients by measuring the dopamine transporter (DAT) with [11C]FeCIT PET (Lucignani et al., 2002), in both nigrostriatal and mesolimbic pathways. The assessment of DAT availability with [11C]FeCIT PET is considered as a proxy measure of nigrostriatal integrity (see (Ba and Martin, 2015) for a recent review). The DAT is a membrane protein expressed exclusively in dopamine neurons, and localized on the plasma membrane of dopamine neurons in the dorsal and ventral striatum, as well as in SN and VTA (Miller et al., 1997). DAT is present on the plasma membrane in axons and to a lesser extent also in neuronal cell bodies and dendrites (Ciliax et al., 1999).
We measured [11C]FeCIT uptake in both nigrostriatal and mesolimbic dopaminergic structures in PD at the initial clinical stages, to specifically assess for different regional vulnerability, and to test in vivo the recent neuropathology hypothesis that considers synaptic axonal damage and dysfunction as a key feature of PD (Tagliaferro and Burke, 2016).
Moreover, in order to provide further insight into the dysfunctional communication within the two dopamine systems, we performed a dopamine network analysis. Recent studies applied such approach to different PET metabolic and neurotransmission targets that provided patterns of connectivity in healthy subjects and in neurodegenerative diseases (Caminiti et al., 2016, Cervenka et al., 2010, Huang et al., 2010, Premi et al., 2016, Titov et al., 2015, Tuominen et al., 2014). In particular, a recent report exploring the pattern of covariance with DATSCAN imaging, showed a specific loss of both inter- and intra-hemispheric dopamine connectivity in PD patients affected by impulse control disorder symptoms (Premi et al., 2016).
Our study is the first to investigate dopamine network connectivity in both nigrostriatal and mesolimbic pathways in early PD. The DAT activity measure in these dopamine system structures, together with the measure of their connectivity, represents a new integrated approach that might provide crucial in vivo biological insights in early PD axonal damage and system alterations.
2. Materials and methods
2.1. Subjects
We retrospectively evaluated 36 idiopathic PD patients (mean age in years ± SD 57.5 ± 12.6, mean disease duration in months ± SD 21.8 ± 10.7), who underwent [11C]FeCIT PET scans at San Raffaele Hospital to support diagnosis. The patients included in this study had Hoehn & Yahr (H&Y) Stage ≤ III at the first visit close to the PET scan (morning before medication intake), and a diagnostic confirmation at 3–5 years' follow-up. Diagnosis of PD was made according to UK Parkinson's Disease Society Brain Bank and the National Institute of Neurological Disorders and Stroke (Gelb et al., 1999). No patient was treated with anti-depressants at the time of PET acquisition. Moreover, they were free from dopamine agonists or L-DOPA at least 18 h before the PET scan.
The score of Unified Parkinson's disease Rating Scale (UPDRS) part III sub-scores (OFF state), and the Hoehn & Yahr staging were used to assess patients' motor impairment.
A group of 14 healthy volunteers (mean age ± SD 52.1 ± 14.6) entered the study as normal controls. They presented a negative medical history for neurological disease or other chronic illness and were not taking psychoactive medication.
All subjects signed a written informed consent according to the study protocol that was approved by the Ethical Committee of the Scientific Institute San Raffaele of Milan.
Demographic differences between groups were evaluated performing a Two-sample t-test and Chi-squared test.
2.2. PET acquisition and image processing
The synthesis and labeling with [11C]2β-carboxymethoxy-3β-{4-iodophenyl}tropane (β-CIT)-Fe PET (i.e. [11C]FeCIT PET) was performed following the method of Halldin et al. (Halldin et al., 1996). PET scans were acquired for 20 min starting 70 min after the injection of 3.7 MBq/kg of [11C]FeCIT. Acquisitions were performed using a General Electric (GE) Medical systems Advance PET. Thirty-five axial image slices (4.25 mm thick) covering the entire brain and cerebellum, were reconstructed using a filtered back projection algorithm (Shepp-Logan filter with a cut-off frequency of 5 mm), using the software provided on the acquisition console by the GE manufacturer. The reconstruction diameter was 20 cm and an image matrix of 128 × 128 was used, for a resulting pixel size of 1.56 × 1.56 × 4.25 mm. A more in depth description can be found in previous papers (Lucignani et al., 2002, Panzacchi et al., 2008).
For image normalization and co-registration, a [11C]FeCIT template was built averaging images of the above mentioned N = 14 healthy control subjects' scans normalized to a T1 weighted magnetic resonance image (T1 MRI), following the same procedures previously described by Lucignani and colleagues (Lucignani et al., 2002). Patients' images were spatially normalized to this template using statistical parametric mapping 5 normalization module (SPM, http://www.fil.ion.ucl.ac.uk/spm/software/spm5). The spatial smoothing was not applied in order to limit blurring or spill-over.
Parametric images were generated for each subject using the Image Calculator (ImCalc) function in SPM5. Specifically, DAT Specific Uptake Value ratio (SUVr), which was the parameter of interest, was obtained as follows (Guttman et al., 1997, Panzacchi et al., 2008):
The cerebellum was used as a reference region since it does not contain DAT sites (Guttman et al., 1997).
2.3. Region of interest analysis
The considered regions of interest (ROIs) were the right and left dorsal caudate (DCA), dorsal putamen (DPU), ventral striatum (VST), substantia nigra (SN) and ventral tegmental area (VTA). The ROIs were manually drawn in accordance to the guidelines proposed in literature (Ballard et al., 2011, Naidich, 2008, Tziortzi et al., 2011). The Automated Anatomical Labeling atlas was used as reference for the anatomical boundaries of the striatal regions (Tzourio-Mazoyer et al., 2002). Due to the small volume of brainstem regions and its proximity to other non-dopaminergic nuclei, we used a dopaminergic midbrain probabilistic atlas (Murty et al., 2014) to further improve the definition of the brainstem ROIs anatomical boundaries.
ROIs definition was supported by MriCron software http://www.mricron.com/mricron/install.html, using a standard high-resolution MRI T1 image as anatomical template.
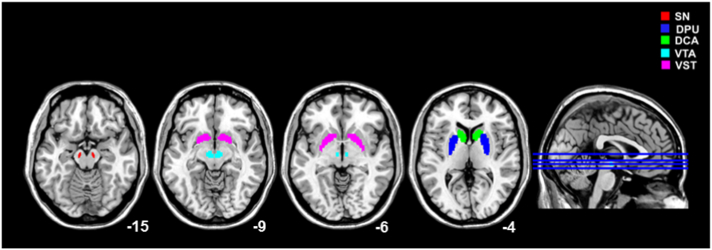
In addition, given the small size of the regions to be analysed compared to PET resolution, a strategy was adopted to minimize the impact of partial volume effect (PVE). Every ROI mask was convolved with a 3D 8 mm FWHM Gaussian kernel, representing the effective reconstructed resolution of the images. Only voxels that, after convolution, had a value > 50% were used for the analyses, as they can be expected to be the least affected by contamination from neighbouring structures (see Fig. 1).
Fig. 1.
Regions of Interest (ROIs) Definition.
ROIs of DPU (blue), DCA (green), VST (violet), SN (red) and VTA (cyan) over-imposed on a MNI-T1 template (see text for methodological details).
Abbreviations: SN = substantia nigra; DPU = dorsal putamen; DCA = dorsal caudate; VTA = ventral tegmental area; VST = ventral striatum.
We also applied the recovery coefficient for partial volume effect (PVE) correction to our data. The recovery coefficients were computed considering both the standard volume of each ROI and the effective spatial resolution of the PET tomograph used (Soret et al., 2007). Each recovery coefficient was applied to the uptake values, then transformed to SUVr by applying the equation previously described.
In order to identify the predominantly affected side in PD, we extracted mean SUVr values from left and right nigrostriatal regions, namely dorsal striatum and substantia nigra, and we computed the nigrostriatal asymmetry index (AI) between the two sides, adopting the following formula (Rabinovici et al., 2008):
Thus, negative outcomes indicated more severe [11C]FeCIT uptake reduction in the left hemisphere, while positive values reflected right lateralized asymmetry.
Mean [11C]FeCIT uptake within ROIs was extracted from each parametric image. The extracted values were then flipped ROI-by-ROI according to the Nigrostriatal-AI, in order to set the symptomatic hemisphere on the right side for all the patients.
Consistently with data reported in literature (van Dyck et al., 2002), our control group showed no hemispheric asymmetry. In this case, the flipping was arbitrarily assigned to the right side.
These ipsilateral and contralateral uptake values were then employed for the statistical analyses.
Statistical differences between PD and CTR in DAT levels in both nigrostriatal (SN, DCA, DPU) and mesolimbic (VTA and VST) regions were assessed performing a multivariate ANCOVA (MANCOVA), including mean SUVr obtained from each ROIs as dependent variables, and introducing age and gender as covariate.
Correlations between Nigrostriatal-AI and clinical asymmetry index (cAS) were investigated employing the Pearson correlation analysis. Considering UPDRS-IIII motor scores, clinical asymmetry index was defined as (Kaasinen, 2015):
CAS scores ≤ − 0.3 were considered as clinical left asymmetry and ≥ 0.3 as clinical right asymmetry.
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS 19).
2.4. Dopamine network analysis
In order to assess connectivity in both nigrostriatal and mesolimbic systems, we created two subject-by-Node/ROI matrices for each group (CTR and PD). The nigrostriatal and mesolimbic matrices contained the regional mean SUVr value derived for each subject in the specific ROIs, flipped according to the Nigrostriatal-AI. For each group, the partial correlation matrix was computed using the MATLAB (http://it.mathworks.com/products/matlab/) (Mathworks Inc., Sherborn, Mass., USA). Estimating partial correlations between regions of interest has been proposed to identify which regions directly relate to each other, when the contributions of other regions are already factored out (Huang et al., 2010). The number of edges grows like the square of the number of nodes, therefore, when many nodes are present, regularization techniques are used to estimate which partial correlation coefficients are non-zero (Huang et al., 2010). This procedure lacks information regarding the strength of the connection, and additional hypotheses and computations are required to obtain surrogate measures of connection strength (Titov et al., 2015). To assess the dopamine network connectivity, however, we selected N = 10 nodes, that constitute two separate pathways (mesolimbic and nigrostriatal). The first network has N = 6 nodes and the second one has N = 4 nodes. Therefore, the largest network has a theoretical number of 15 edges, while the smallest one only has 6 edges. Therefore, given the number of possible connections, it was possible to directly compute partial correlation coefficients to obtain a measure of the connectivity strength, without resorting to regularization techniques. The partial correlation coefficient determined the strength of dopamine network connectivity (i.e., edge). We computed the differences in the partial correlation coefficients among nodes-dopamine network between PD and CTR in order to quantify the specific reduction in strength. To better quantify which coefficients had significant differences between CTR and PD, we applied a resampling strategy. This method allows robust estimations of partial correlation coefficients and their errors, even with small sample sizes (Politis et al., 1999). Data samples were generated a total of 5000 times for each group. The Fisher's transformation was applied to each coefficient, to achieve a normal distribution. This allowed measuring changes in partial correlation coefficients using z-scores.
3. Results
Demographical data are reported in Table 1. PD patients and healthy controls were similar for age and gender.
Table 1.
Clinical and demographic data.
| Gender (M/F) | Age (SD) |
Age of disease onset (SD) |
Disease duration months (SD) |
UPDRS III-Motor (SD) |
UPDRS III-Left (SD) |
UPDRS III-Right (SD) |
H&Y (SD) |
|
|---|---|---|---|---|---|---|---|---|
| PD | 20/16 | 57.5(12.6) | 55.8(12.9) | 21.8(10.7) | 15.8(8.1) | 5.9(5) | 4.3(5) | 1.48(0.69) |
| CTR | 6/8 | 52.1(14.6) | – | – | – | – | – | – |
| ns | ns | – | – | – | – | – | – |
Abbreviations: M = male; F = female; SD = standard deviation; UPDRS = Unified Parkinson's disease Rating Scale; H&Y = Hoehn and Yahr; PD = Parkinson's disease patients; CTR = healthy control subjects.
3.1. Region of interest results
The MANCOVA performed to compare presynaptic DAT availability in PD patients and healthy controls showed significant differences in [11C]FeCIT SUVr, independent from age and gender, in all the considered ROIs.
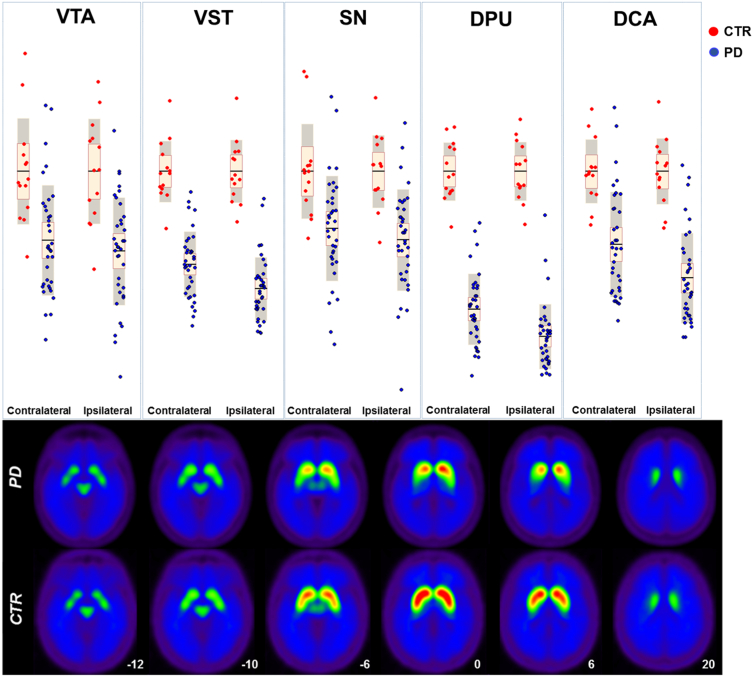
Nigrostriatal system: analysis revealed the major effect size (η2 = 0.84) in the dorsal putamen compared to controls; the dorsal caudate was less affected (DCA η2 = 0.52) and SN showed an even more limited reduction in [11C]FeCIT uptake levels (SN η2 = 0.30). Mesolimbic system: VST and VTA were both affected in comparison to normal controls, with a comparable pattern, being VST more affected (η2 = 0.71) than VTA (η2 = 0.31) (See Fig. 2 and Table 2). The effect size of the SUVr value reductions remained stable also applying recovery coefficients, suggesting that our results were independent from the ROIs volumes, and were not affected by the PVE.
Fig. 2.
Comparison between PD and CTR.
Top panel - Box-scatter plot representing SUVr in CTR and PD patients. The ROIs were defined contralateral or ipsilateral according to the Nigrostriatal-AI%. Bottom panel - Averaged [11C]FeCIT PET parametric images of PD patients and controls. Abbreviations: PD = Parkinson's disease patients; CTR = healthy control subjects; SN = substantia nigra; DPU = dorsal putamen; DCA = dorsal caudate; VTA = ventral tegmental area; VST = ventral striatum.
Table 2.
ROI-based statistical comparison between PD and CTR.
| PD | CTR | Effect Size (η2) | p-Value | |
|---|---|---|---|---|
| SN | 0.69 ± 0.17 | 0.92 ± 0.14 | 0.30 | 0.001 |
| DPU | 1.95 ± 0.61 | 4.79 ± 0.54 | 0.84 | < 0.001 |
| DCA | 2.45 ± 0.70 | 3.80 ± 0.48 | 0.52 | < 0.001 |
| VTA | 0.82 ± 0.23 | 1.16 ± 0.23 | 0.31 | 0.001 |
| VST | 2.68 ± 0.56 | 4.57 ± 0.55 | 0.71 | < 0.001 |
Results of MANCOVA analysis assessing differences between PD and CTR SUVr, and controlling for age and gender.
Abbreviations: PD = Parkinson's disease patients; CTR = healthy control subjects; SN = substantia nigra; DPU = dorsal putamen; DCA = dorsal caudate; VTA = ventral tegmental area; VST = ventral striatum.
Compared to SN, SUVr distribution in VTA showed a significant higher inter-subject variability in PD patients, as assessed with Levene's test for Equality of Variances (F = 4.10 p < 0.05), which was not significant for healthy control subjects.
The 60% of PD patients had unilateral Left and the 36% unilateral Right clinical signs, only the 4% showed a bilateral involvement as measured by UPDRS-III.
We found significant positive correlation (r = − 0.67; p < 0.001) between cAS and Nigrostriatal-AI. This means that in our PD group, the sides with higher motor impairment corresponded to lower contralateral [11C]FeCIT uptake.
3.2. Dopamine network analysis results
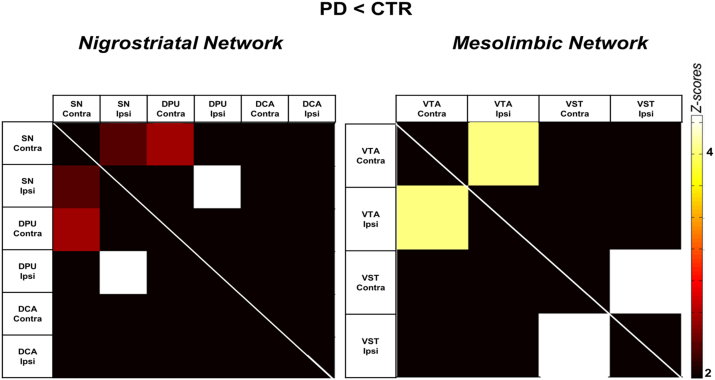
The nigrostriatal pathway analysis demonstrated a severe reduction in connectivity between ipsilateral SN and ipsilateral DPU in PD patients with respect to CTR (p < 0.0002), between contralateral SN and contralateral DPU (p < 0.008) and between ipsilateral and contralateral SN (p < 0.016) (see Fig. 3). Notably, the connectivity between the nigrostriatal regions and the DCA was spared.
Fig. 3.
Anatomically-driven dopamine network analysis.
The matrices represent the significant differences obtained when comparing correlation coefficients between PD and CTR in both nigrostriatal (left) and mesolimbic (right) pathways. The colour bar displays Z-scores (from white = high statistical significance to dark = no statistical significance). Abbreviations: PD = Parkinson's disease patients; CTR = healthy control subjects; SN = substantia nigra; DPU = dorsal putamen; DCA = dorsal caudate; VTA = ventral tegmental area; VST = ventral striatum; Ipsi = ipsilateral; Contra = contralateral.
Regarding the mesolimbic pathway, we found a severe disruption of local connectivity between ipsilateral and contralateral homotopic regions (p < 0.00001). The connectivity at long distance between VTA and VTS was not affected (Fig. 3).
4. Discussion
Neuroimaging approaches are crucial to define in vivo the nature of neurodegenerative changes, especially in prodromal and early stages. The advanced understanding of neurodegenerative processes, may also provide new imaging targets to facilitate early detection and disease staging (Weingarten et al., 2015). In particular, PET molecular neuroimaging studies are useful to track in vivo the dopaminergic involvement in different regions and at different disease stages in PD (see (Ba and Martin, 2015) for a recent review). In the present PET molecular study, regional DAT density, assessed in idiopathic PD patients at initial clinical phase, revealed significant decreases in the nigrostriatal dopamine system, but also in the mesolimbic system, even if to a lesser degree (Fig. 2 and Table 2). Within the nigrostriatal system, the presynaptic afferents to the DPU were the most significantly affected, and, notably, this loss of DAT activity largely exceeded that of SN (see Table 2 and Fig. 2).
A higher DAT impairment of the striatum than the SN was also described in other two studies using DAT or VMAT2 to target dopaminergic integrity. The first one, aiming at evaluating the characteristics of a new DAT PET radiotracer (i.e. 18F-FE-PE2I), reports a severe DAT loss in the dorsal putamen that was more limited in the SN, but in a smaller group of PD patients (N = 10) (Fazio et al., 2015). The second PET study by Hsiao and colleagues using 18F-DTBZ tracer for monoaminergic nerve ending vesicles (VMAT2), found a reduction more severe in the putamen than in SN, which correlates with disease severity in PD patients at an initial clinical stage (Hsiao et al., 2014).
Our new in vivo findings, pave the way for a strong support to the hypothesis of axonal degeneration occurring first in Parkinson's disease, thus confirming what previously described by post-mortem studies in early idiopathic PD. Specifically, it was reported about 70–80% of striatal nerve terminals loss with respect to 30–50% of SN neuron loss (see (Tagliaferro and Burke, 2016) for an overview). It has been proposed that α-synuclein-related synaptic dysfunction and the consequent axonal damage precede cell death in PD (Calo et al., 2016). The possible mechanism producing neurodegenerative effects from synapse to cell body remains to be clarified. Up to now, the proposed mechanisms are twofold, namely, the prolonged synaptic dysfunction in axonal transport, or the direct neurotoxic effect of α-synuclein aggregates, which spread from axons to cell body through retrograde transport (Calo et al., 2016).
Here we also showed in vivo the involvement of the mesolimbic system in early PD. In our group of patients, the VST was the second most affected region, after DPU (see Fig. 2 and Table 2). Notably also the VTA showed some impairment but less than the VST, supporting a comparable pattern in both the dopamine systems. The high inter-subject variability in VTA SUVr levels is consistent with previous post mortem evidence reporting high variability (i.e., 40–77%) in the levels of VTA neural loss as measured by TH expression in PD brains (Surmeier and Sulzer, 2013). Despite the α-synuclein pathology has been also reported in the VTA, its neurons seem to be more resistant to the overexpression of human A53T mutated α-synuclein than SN neurons (Maingay et al., 2006). The specific mechanisms protecting VTA DA neurons from neurodegeneration as compared to SN DA neurons are still unknown (Calo et al., 2016).
In addition, whether the impairment of the mesolimbic system is stochastic or occurs in specific clinical sub-populations of PD patients has not been clarified yet (Alberico et al., 2015, Maingay et al., 2006). In PD, impairment in the mesolimbic dopaminergic system has been associated with non-motor symptoms, in particular mood disorders, which may affect patients since or even before the initial clinical phase of PD (see (Castrioto et al., 2016) for a recent review). A recent review on previous neuropathological studies that directly quantified dopamine neurons in both the SN and VTA in PD patients suggests that the high VTA variability can explain the PD clinical non-motor heterogeneity, such as the occurrence of mood, cognition, and sleep disturbances (Alberico et al., 2015).
Contralateral asymmetry of motor symptoms in relation to the dopaminergic impairment is considered typical in the early stage of PD (Djaldetti et al., 2006). The majority of patients examined in this study presented an asymmetry of motor symptoms which correlated with the nigrostriatal asymmetry in DAT impairment. Nevertheless, the [11C]FeCIT SUVr was significantly lower also in the ipsilateral side. This observation supports the sensitivity of DAT measures in identifying the early involvement of the whole basal ganglia system.
Since the axonal damage seems to be the crucial neuropathological event affecting PD, studying dopamine network connectivity might be a further important step for understanding the pathophysiology of the disease. Here we applied anatomically-driven partial correlation analysis to test dopamine network connectivity since it is a common strategy to study connectivity with brain PET molecular images. Correlation analysis has already been applied in studies of brain metabolism (Caminiti et al., 2016, Huang et al., 2010, Titov et al., 2015) and in DAT, D2 receptor, serotonin transporter and μ-opioid receptor studies (Cervenka et al., 2010, Premi et al., 2016, Tuominen et al., 2014), in order to provide patterns of molecular connectivity in healthy subjects and in various neurodegenerative diseases.
In our study, adopting also this network perspective, we obtained support for the nigrostriatal axonal damage. Specifically, when the dopamine networks in PD and CTR groups were compared, we found a significant reduction of connectivity between SN and DPU nodes (see Fig. 3). Several anatomical MRI studies using diffusor tensor imaging reported PD-related reduction of structural connectivity between substantia nigra and striatum (Du et al., 2011, Hacker et al., 2012, Nilsson et al., 2007, Péran et al., 2010, Yoshikawa et al., 2004, Zhan et al., 2012, Y. Zhang et al., 2015, J. Zhang et al., 2015), which was also associated with the degree of motor deficits in PD patients (J. Zhang et al., 2015). In our study, the connectivity between DCA and the other nigrostriatal nodes was intact, the preserved connectivity was also reflected in the less severe dopamine impairment in the caudate relative to the putamen, as showed in the ROI-based analysis.
Less is known about the involvement of mesolimbic circuit in PD. So far, a single study investigated mesolimbic connectivity in early-stage PD patients, applying resting-state functional connectivity magnetic resonance imaging. For assessing mesolimbic circuit they selected amygdala as seed region, and they found reduced functional connectivity between seed and putamen in PD patients (Luo et al., 2014). Our connectivity approach, revealed an overall preserved dopamine network between VTA and VST mesolimbic nodes, which only suffered local disconnections (see Fig. 3). This might be explained by the lower vulnerability of VTA synapses to the α-synuclein-related axonal damage (Calo et al., 2016, Surmeier and Sulzer, 2013), which may preserve the functional communication in the mesolimbic system.
From a technical stand-point, the lack of high-resolution MRI for each patient may represent a limitation for the present study, since it would have provided a more accurate ROI segmentation and PVE correction. In the future, the application of integrated PET-MRI will facilitate the analyses, by providing simultaneous information (Catana et al., 2012).
5. Conclusion
This is the first comprehensive study addressing regional DAT analysis of the nigrostriatal and mesolimbic system structures and dopamine network connectivity in early PD. The results indicate a significant axonal presynaptic degeneration in early PD phases, more severe in the nigrostriatal, but to a lesser extent also in the mesolimbic pathway. The prevalent damage in the nigrostriatal system, was also supported by specific changes in dopamine network connectivity.
The severe DAT loss in the dopaminergic terminals projecting to the dorsal putamen is an early biomarker of nigrostriatal axonal pathology in PD, and the more spared SN neurons would justify neuroprotective interventions. A better clinical and molecular characterization in idiopathic PD is important for more effective prevention strategies in the early disease phase.
Fundings
This research was funded by EU FP7 INMIND Project (FP7-HEALTH-2013, grant agreement no. 278850), by CARIPLO Project “Evaluation of autonomic, genetic, imaging and biochemical markers for Parkinson-related dementia: longitudinal assessment of a PD cohort” 2016–2020 (grant agreement no. 2014-0832), and the IVASCOMAR project “Identificazione, validazione e sviluppo commerciale di nuovi biomarcatori diagnostici prognostici per malattie complesse” (grant agreement no. CTN01_00177_165430). All authors have approved the final article. None of the other authors has a conflict of interest to disclose.
Acknowledgements
We thank Dr. Valentino Bettinardi, Dr. Leonardo Iaccarino and Dr. Arianna Sala for their insightful suggestions.
References
- Alberico S.L., Cassell M.D., Narayanan N.S. The vulnerable ventral tegmental area in Parkinson's disease. Basal Ganglia. 2015;5:51–55. doi: 10.1016/j.baga.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba F., Martin W.R.W. Dopamine transporter imaging as a diagnostic tool for parkinsonism and related disorders in clinical practice. Parkinsonism Relat. Disord. 2015;21:87–94. doi: 10.1016/j.parkreldis.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Ballard I.C., Murty V.P., Carter R.M., MacInnes J.J., Huettel S.A., Adcock R.A. Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J. Neurosci. 2011;31:10340–10346. doi: 10.1523/JNEUROSCI.0895-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Ghebremedhin E., Rüb U., Bratzke H., Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Calo L., Wegrzynowicz M., Santivañez-Perez J., Grazia Spillantini M. Synaptic failure and α-synuclein. Mov. Disord. 2016;0 doi: 10.1002/mds.26479. (n/a-n/a) [DOI] [PubMed] [Google Scholar]
- Caminiti S.P., Tettamanti M., Sala A., Presotto L., Iannaccone S., Cappa S.F., Magnani G., Perani D. Metabolic connectomics targeting brain pathology in dementia with Lewy bodies. J. Cereb. Blood Flow Metab. 2016 doi: 10.1177/0271678X16654497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrioto A., Thobois S., Carnicella S., Maillet A., Krack P. Emotional manifestations of PD: neurobiological basis. Mov. Disord. 2016 doi: 10.1002/mds.26587. [DOI] [PubMed] [Google Scholar]
- Catana C., Drzezga A., Heiss W.-D., Rosen B.R. PET/MRI for neurologic applications. J. Nucl. Med. 2012;53:1916–1925. doi: 10.2967/jnumed.112.105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka S., Varrone A., Fransén E., Halldin C., Farde L. PET studies of D2-receptor binding in striatal and extrastriatal brain regions: biochemical support in vivo for separate dopaminergic systems in humans. Synapse. 2010;64:478–485. doi: 10.1002/syn.20765. [DOI] [PubMed] [Google Scholar]
- Ciliax B.J., Drash G.W., Staley J.K., Haber S., Mobley C.J., Miller G.W., Mufson E.J., Mash D.C., Levey A.I. Immunocytochemical localization of the dopamine transporter in human brain. J. Comp. Neurol. 1999;409:38–56. doi: 10.1002/(sici)1096-9861(19990621)409:1<38::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Djaldetti R., Ziv I., Melamed E. The mystery of motor asymmetry in Parkinson's disease. Lancet Neurol. 2006;5:796–802. doi: 10.1016/S1474-4422(06)70549-X. [DOI] [PubMed] [Google Scholar]
- Du G., Lewis M.M., Styner M., Shaffer M.L., Sen S., Yang Q.X., Huang X. Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson's disease. Mov. Disord. 2011;26:1627–1632. doi: 10.1002/mds.23643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dyck C.H., Seibyl J.P., Malison R.T., Laruelle M., Wallace E., Zoghbi S.S., Zea-Ponce Y., Baldwin R.M., Charney D.S., Hoffer P.B. Age-related decline in dopamine transporters: analysis of striatal subregions, nonlinear effects, and hemispheric asymmetries. Am. J. Psychiatry. 2002;10:36–43. [PubMed] [Google Scholar]
- Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann. N. Y. Acad. Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Fazio P., Svenningsson P., Forsberg A., Jönsson E.G., Amini N., Nakao R., Nag S., Halldin C., Farde L., Varrone A. Quantitative analysis of 18F-(E)-N-(3-iodoprop-2-enyl)-2β-carbofluoroethoxy-3β-(4′-methyl-phenyl) nortropane binding to the dopamine transporter in Parkinson disease. J. Nucl. Med. 2015;56:714–720. doi: 10.2967/jnumed.114.152421. [DOI] [PubMed] [Google Scholar]
- Fu Y., Paxinos G., Watson C., Halliday G.M. The substantia nigra and ventral tegmental dopaminergic neurons from development to degeneration. J. Chem. Neuroanat. 2016 doi: 10.1016/j.jchemneu.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Gelb D.J., Oliver E., Gilman S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- Gustafsson H., Nordström A., Nordström P. Depression and subsequent risk of Parkinson disease a nationwide cohort study. Neurology. 2015:1–8. doi: 10.1212/WNL.0000000000001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M., Burkholder J., Kish S.J., Hussey D., Wilson A., DaSilva J., Houle S. [11C] RTI-32 PET studies of the dopamine transporter in early dopa-naive Parkinson's disease implications for the symptomatic threshold. Neurology. 1997;48:1578–1583. doi: 10.1212/wnl.48.6.1578. [DOI] [PubMed] [Google Scholar]
- Hacker C.D., Perlmutter J.S., Criswell S.R., Ances B.M., Snyder A.Z. Resting state functional connectivity of the striatum in Parkinson's disease. Brain. 2012;135:3699–3711. doi: 10.1093/brain/aws281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldin C., Farde L., Lundkvist C., Ginovart N., Nakashima Y., Karlsson P., Swahn C. [11C] β-CIT-FE, a radioligand for quantitation of the dopamine transporter in the living brain using positron emission tomography. Synapse. 1996;22:386–390. doi: 10.1002/(SICI)1098-2396(199604)22:4<386::AID-SYN10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Hsiao T., Weng Y.-H., Hsieh C.-J., Lin W.-Y., Wey S.-P., Kung M.-P., Yen T.-C., Lu C.-S., Lin K.-J., Cravello L., Caltagirone C., Field A.S., Investigation O. Correlation of Parkinson disease severity and 18F-DTBZ positron emission tomography. JAMA Neurol. 2014;71:758–766. doi: 10.1001/jamaneurol.2014.290. [DOI] [PubMed] [Google Scholar]
- Huang S., Li J., Sun L., Ye J., Fleisher A., Wu T., Chen K., Reiman E. Learning brain connectivity of Alzheimer's disease by sparse inverse covariance estimation. NeuroImage. 2010;50:935–949. doi: 10.1016/j.neuroimage.2009.12.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V. Ipsilateral deficits of dopaminergic neurotransmission in Parkinson's disease. Ann. Clin. Transl. Neurol. 2015;21–26 doi: 10.1002/acn3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower J.H., Olanow C.W., Dodiya H.B., Chu Y., Beach T.G., Adler C.H., Halliday G.M., Bartus R.T. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013;136:2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucignani G., Gobbo C., Moresco R.M., Antonini A., Panzacchi A., Bonaldi L., Carpinelli A., Caraceni T., Fazio F., Milano Á., Sacco O.L. The feasibility of statistical parametric mapping for the analysis of positron emission tomography studies using 11 C-2-b-carbomethoxy-3-b-(4-fluorophenyl)-tropane in patients with movement disorders. Nucl. Med. Commun. 2002 doi: 10.1097/00006231-200211000-00003. [DOI] [PubMed] [Google Scholar]
- Luo C., Song W., Chen Q., Zheng Z., Chen K., Cao B., Yang J., Li J., Huang X., Gong Q. Reduced functional connectivity in early-stage drug-naive Parkinson's disease: a resting-state fMRI study. Neurobiol. Aging. 2014;35:431–441. doi: 10.1016/j.neurobiolaging.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Maingay M., Romero-Ramos M., Carta M., Kirik D. Ventral tegmental area dopamine neurons are resistant to human mutant alpha-synuclein overexpression. Neurobiol. Dis. 2006;23:522–532. doi: 10.1016/j.nbd.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Miller G.W., Staley J.K., Heilman C.J., Perez J.T., Mash D.C., Rye D.B., Levey A.I. Immunochemical analysis of dopamine transporter protein in Parkinson's disease. Ann. Neurol. 1997;41:530–539. doi: 10.1002/ana.410410417. [DOI] [PubMed] [Google Scholar]
- Murty V.P., Shermohammed M., Smith D.V., Carter R.M., Huettel S.A., Adcock R.A. Resting state networks distinguish human ventral tegmental area from substantia nigra. NeuroImage. 2014;100:580–589. doi: 10.1016/j.neuroimage.2014.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidich T.P. Duvernoy's atlas of the human brain stem and cerebellum: high-field MRI. Surf. Anatomy, Intern. Struct. Vasc. 2008;3 [Google Scholar]
- Nieuwenhuys R., Voogd J., van Huijzen C. Springer-Verlag; 2013. Das Zentralnervensystem des Menschen: ein Atlas mit Begleittext. [Google Scholar]
- Nilsson C., Bloch K.M., Brockstedt S., Lätt J., Widner H., Larsson E.-M. Tracking the neurodegeneration of parkinsonian disorders–a pilot study. Neuroradiology. 2007;49:111–119. doi: 10.1007/s00234-006-0165-1. [DOI] [PubMed] [Google Scholar]
- Olanow C.W., Kieburtz K., Schapira A.H.V. Why have we failed to achieve neuroprotection in Parkinson's disease? Ann. Neurol. 2008;64:101–110. doi: 10.1002/ana.21461. [DOI] [PubMed] [Google Scholar]
- Panzacchi A., Moresco R.M., Garibotto V., Antonini A., Gobbo C., Isaias I.U., Goldwurm S., Bonaldi L., Carpinelli A., Pezzoli G., Fazio F., Perani D. A voxel-based PET study of dopamine transporters in Parkinson's disease: relevance of age at onset. Neurobiol. Dis. 2008;31:102–109. doi: 10.1016/j.nbd.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Péran P., Cherubini A., Assogna F., Piras F., Quattrocchi C., Peppe A., Celsis P., Rascol O., Démonet J.-F., Stefani A. Magnetic resonance imaging markers of Parkinson's disease nigrostriatal signature. Brain. 2010 doi: 10.1093/brain/awq212. [DOI] [PubMed] [Google Scholar]
- Politis D.N., Romano J., Wolf M. Springer-Verlag; New York: 1999. Subsampling. [Google Scholar]
- Premi E., Pilotto A., Garibotto V., Bigni B., Turrone R., Alberici A., Cottini E., Poli L., Bianchi M., Formenti A., Cosseddu M., Gazzina S., Magoni M., Bertoli M., Paghera B., Borroni B., Padovani A. Parkinsonism and related disorders impulse control disorder in PD: a lateralized monoaminergic frontostriatal disconnection syndrome? Parkinsonism Relat. Disord. 2016;3–7 doi: 10.1016/j.parkreldis.2016.05.028. [DOI] [PubMed] [Google Scholar]
- Rabinovici G.D., Jagust W.J., Furst A.J., Ogar J.M., Racine C.A., Mormino E.C., O'Neil J.P., Lal R.A., Dronkers N.F., Miller B.L., Gorno-Tempini M.L. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann. Neurol. 2008;64:388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz-Schaeffer W.J. The synaptic pathology of α-synuclein aggregation in dementia with Lewy bodies, Parkinson's disease and Parkinson's disease dementia. Acta Neuropathol. 2010;120:131–143. doi: 10.1007/s00401-010-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soret M., Bacharach S.L., Buvat I. Partial-volume effect in PET tumor imaging. J. Nucl. Med. 2007;48:932–945. doi: 10.2967/jnumed.106.035774. [DOI] [PubMed] [Google Scholar]
- Surmeier D.J., Sulzer D. The pathology roadmap in Parkinson disease. Prion. 2013;7:85–91. doi: 10.4161/pri.23582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferro P., Burke R.E. Retrograde axonal degeneration in Parkinson disease. J. Parkinsons Dis. 2016;6:1–15. doi: 10.3233/JPD-150769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titov D., Diehl-Schmid J., Shi K., Perneczky R., Zou N., Grimmer T., Li J., Drzezga A., Yakushev I. Metabolic connectivity for differential diagnosis of dementing disorders. J. Cereb. Blood Flow Metab. 2015 doi: 10.1177/0271678X15622465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen L., Nummenmaa L., Keltikangas-Järvinen L., Raitakari O., Hietala J. Mapping neurotransmitter networks with PET: an example on serotonin and opioid systems. Hum. Brain Mapp. 2014;35:1875–1884. doi: 10.1002/hbm.22298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziortzi A.C., Searle G.E., Salinas C., Beaver J.D., Jenkinson M., Laruelle M., Rabiner E.A., Gunn R.N. NeuroImage imaging dopamine receptors in humans with [11 C]-(+)-PHNO: dissection of D3 signal and anatomy. NeuroImage. 2011;54:264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Vol. 289. 2002. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-subject Brain; pp. 273–289. [DOI] [PubMed] [Google Scholar]
- Weingarten C.P., Sundman M.H., Hickey P., Chen N. Neuroimaging of Parkinson's disease: expanding views. Neurosci. Biobehav. Rev. 2015;59:16–52. doi: 10.1016/j.neubiorev.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K., Nakata Y., Yamada K., Nakagawa M. Early pathological changes in the parkinsonian brain demonstrated by diffusion tensor MRI. J. Neurol. Neurosurg. Psychiatry. 2004;75:481–484. doi: 10.1136/jnnp.2003.021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan W., Kang G.A., Glass G.A., Zhang Y., Shirley C., Millin R., Possin K.L., Nezamzadeh M., Weiner M.W., Marks W.J. Regional alterations of brain microstructure in Parkinson's disease using diffusion tensor imaging. Mov. Disord. 2012;27:90–97. doi: 10.1002/mds.23917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wu I.-W., Buckley S., Coffey C.S., Foster E., Mendick S., Seibyl J., Schuff N. Diffusion tensor imaging of the nigrostriatal fibers in Parkinson's disease. Mov. Disord. 2015;0:1–8. doi: 10.1002/mds.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wei L., Hu X., Xie B., Zhang Y., Wu G.-R., Wang J. Akinetic-rigid and tremor-dominant Parkinson's disease patients show different patterns of intrinsic brain activity. Parkinsonism Relat. Disord. 2015;21:23–30. doi: 10.1016/j.parkreldis.2014.10.017. [DOI] [PubMed] [Google Scholar]