A conserved glutamic acid residue is thought to occupy three different conformations in the transport pathway of CLC H+/Cl− exchangers. Vien et al. provide functional evidence that the most central of these three positions is adopted by CLC-ec1 during transport and is stabilized by hydrogen bonds.
Abstract
The CLC proteins form a broad family of anion-selective transport proteins that includes both channels and exchangers. Despite extensive structural, functional, and computational studies, the transport mechanism of the CLC exchangers remains poorly understood. Several transport models have been proposed but have failed to capture all the key features of these transporters. Multiple CLC crystal structures have suggested that a conserved glutamic acid, Gluex, can adopt three conformations and that the interconversion of its side chain between these states underlies H+/Cl− exchange. One of these states, in which Gluex occupies the central binding site (Scen) while Cl− ions fill the internal and external sites (Sint and Sext), has only been observed in one homologue, the eukaryotic cmCLC. The existence of such a state in other CLCs has not been demonstrated. In this study, we find that during transport, the prototypical prokaryotic CLC exchanger, CLC-ec1, adopts a conformation with functional characteristics that match those predicted for a cmCLC-like state, with Gluex trapped in Scen between two Cl− ions. Transport by CLC-ec1 is reduced when [Cl−] is symmetrically increased on both sides of the membrane and mutations that disrupt the hydrogen bonds stabilizing Gluex in Scen destabilize this trapped state. Furthermore, inhibition of transport by high [Cl−] is abolished in the E148A mutant, in which the Gluex side chain is removed. Collectively, our results suggest that, during the CLC transport cycle, Gluex can occupy Scen as well as the Sext position in which it has been captured crystallographically and that hydrogen bonds with the side chains of residues that coordinate ion binding to Scen play a role in determining the equilibrium between these two conformations.
Introduction
The CLCs form a widespread family of ion channels and transporters. Although all family homologues mediate the transmembrane movement of anions across biological membranes, the two subclasses do so in mechanistically opposite ways: the channels mediate the passive passage of ions down an electrochemical gradient, whereas the transporters harness the energy stored in a H+ gradient to drive anion accumulation, or vice versa. Mutations in at least five of the nine CLC human genes cause genetically inherited disorders such as myotonia congenita, Dent’s disease, Barter syndrome, and osteopetrosis (Jentsch, 2008). These hereditary diseases shed light on the ubiquitous roles played by the CLC family members in physiology, from maintaining membrane potential to regulating transepithelial salt transport and controlling intravesicular pH.
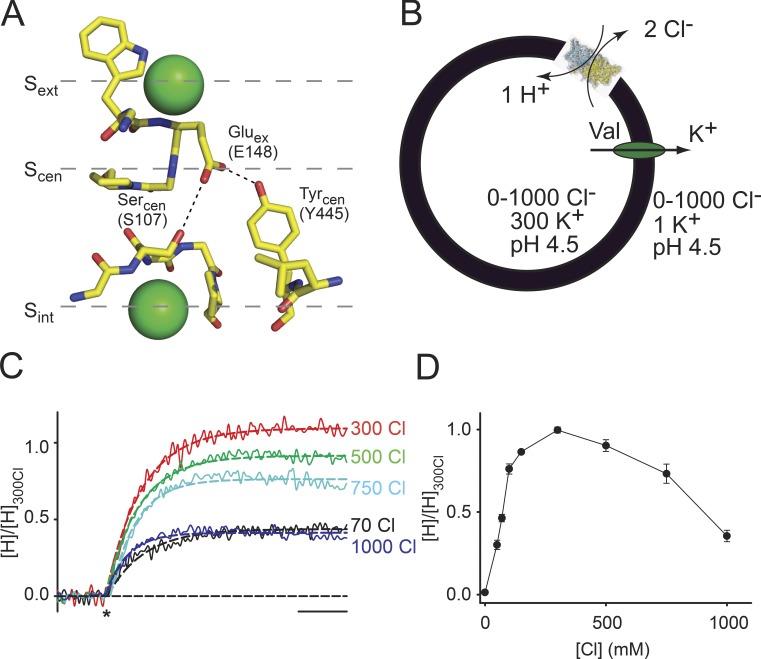
The structures of several prokaryotic and eukaryotic homologues define the overall CLC architecture: they are double-barreled homodimers, with each monomer forming independent ion permeation pathways (Dutzler et al., 2002). The anion permeation pathway is defined by three binding sites (Fig. 1 A) and is lined by the side chains of a triad of highly conserved residues: a glutamic acid, Gluex, which occludes the pathway toward the extracellular side, a serine, Sercen, and a tyrosine, Tyrcen, which constrict the pathway toward the intracellular side (Fig. 1 A). In the Escherichia coli homologue CLC-ec1, Gluex occupies the external binding site, Sext, while the central and internal sites, Scen and Sint, are occupied by Cl− ions (Dutzler et al., 2002, 2003). When Gluex is mutated to glutamine or becomes protonated, it moves out of the pathway, and all sites are occupied by Cl− ions (Dutzler et al., 2003). The structure of the eukaryotic homologue cmCLC, from the thermophilic alga Cyanidioschyzon merolae (Feng et al., 2010), revealed a third conformation of the pathway, where Gluex occupies Scen while Sint and Sext are occupied by Cl− ions (Fig. 1 A). The rearrangements of Gluex guided the design of numerous functional tests and resulted in the proposal of several hypotheses for the transport mechanism of the CLCs (Miller and Nguitragool, 2009; Accardi and Picollo, 2010; Feng et al., 2010; Basilio et al., 2014; Khantwal et al., 2016).
Figure 1.
Cl−-driven trapping of Gluex in Scen. (A) Close-up view of the Cl−-binding site in cmCLC. The numbering of Gluex, Sercen, and Tyrcen corresponds to CLC-ec1. Green spheres are the Cl− ions. (B) Schematic representation of the trapping experiments. (C) Normalized time course of H+ transport mediated by CLC-ec1 in the presence of 70, 300, 500, 750, or 1,000 mM symmetrical [Cl−]. The asterisk denotes the addition of valinomycin to initiate the experiment. The dashed line represents an exponential fit of the time courses. Bar, 10 s. (D) Mean steady-state H+ uptake in 1–1,000 mM symmetrical [Cl−] normalized to the value in 300 mM Cl. Values are reported in Table 1; error bars are the SEM of the number of experiments indicated in Table 1.
All models proposed so far share two key assumptions: that all crystallographic states are part of the transport cycle and that the constriction formed by Tyrcen and Sercen rate limits ion movement between Scen and Sint (Miller and Nguitragool, 2009; Feng et al., 2010; Basilio et al., 2014; Khantwal et al., 2016). Although extensive structure–function studies showed that the two states identified in CLC-ec1 are part of the CLC gating cycle, there is no evidence that a cmCLC-like state occurs during the exchange cycle. Several lines of evidence, stemming from computational (Bisset et al., 2005) and experimental work on CLC channels (Traverso et al., 2006) and transporters (Zifarelli et al., 2012), suggest that Gluex can occupy the central cavity. However, in the CLC-0 and -1 channels, interactions between Gluex and Tyrcen were proposed to occur (Bennetts and Parker, 2013) during the common gating process rather than during gating of the individual pores, as would be expected if this state occurred during the transport cycle. Therefore, the issue of whether a cmCLC-like state is part of the exchange cycle remains an open question. Here, we investigate how the activity of CLC-ec1 depends on [Cl−] and find that the transport activity of CLC-ec1 decreases when Sint and Sext become saturated by Cl− ions and trap Gluex in Scen, suggesting that during transport, CLC-ec1 adopts a cmCLC-like conformation.
Materials and methods
Protein purification
Expression and purification of WT and mutant CLC-ec1 was performed according to published protocols (Accardi et al., 2004, 2005, 2006). In some preparations, the detergent was exchanged from 5 mM DM (n-decyl-β-d-maltopyranoside) to 1 mM DMNG (2,2-dioctylpropane-1,3-bis-β-d-maltopyranoside). The eluted protein was run on a column (Superdex 200; GE Healthcare) preequilibrated in 100 mM NaCl, 20 mM HEPES, and 5 mM DM or 50 µM DMNG, pH 7.5 (buffer B), for liposome reconstitution and crystallography.
Liposome reconstitution
E. coli polar lipids (Avanti Polar Lipids, Inc.) were dried under N2, resuspended in pentane, and dried again. The dry lipids were suspended to a final concentration of 20 mg/ml in reconstitution buffer (buffer R): 300 mM KCl and 25 mM citric acid, adjusted to pH 7.0, with NaOH, to which 35 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate was added. The suspension was sonicated to clarity, and after a 1–2-h incubation, the purified protein was added to a 5-µg protein/milligram lipid ratio. Detergent was dialyzed out overnight in the desired buffer, and the resulting liposomes were fast frozen in ethanol/dry ice and stored at −80°C. For H+ transport, experiments in symmetrical Cl− liposomes were dialyzed in 0–1,000 mM KCl, 0–500 mM K2SO4, and 50 mM citric acid, pH 4.5, with [KCl] + 2[K2SO4] = 1,000. The external solution contained 1 mM KCl, 1 mM glutamic acid, 0–1,000 mM NaCl, and 0–500 mM Na2SO4, pH 4.5, with [NaCl] + 2[Na2SO4] = 1,000.
H+ fluxes
H+ flux measurements were performed as described previously (Accardi and Miller, 2004; Walden et al., 2007; Basilio et al., 2014). In brief, H+ influx was initiated by the addition of 1 µl of 1 mg/ml of the K+ ionophore valinomycin, which plays a dual role: it shunts the voltage established by the Cl− gradient and sets the membrane potential to the K+ equilibrium potential. The time course of H+ fluxes was monitored using a pH meter. H+ influx was terminated by the addition of 1 µl of 1 mg/ml of the H+ ionophore carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone.
36Cl− efflux
Proteoliposomes were reconstituted with WT or E148A CLC-ec1 at a 1.5 µg/mg protein/lipid ratio in buffer containing 300 or 1,000 mM KCl, 25 mM citric acid, and 25 mM K2HPO4, pH 4.5, with NaOH. The external solution was exchanged by spinning the liposomes through a G-50 column preswollen in 400 or 1,000 mM sorbitol, 25 mM citric acid, and 25 mM K2HPO4, pH 4.5, with NaOH. Uptake of 36Cl− was initiated by the addition of 5 µl of a 0.1-µCi/µl Na36Cl solution. Efflux was initiated by the addition of one third of the total volume of a stock solution containing 900 or 3,000 mM KCl, 25 mM citric acid, and 25 mM K2HPO4, pH 4.5, with NaOH. Individual efflux time courses were fitted to a decreasing single exponential function of the form C(t) = C0 + A · exp(−t/τ), where C(t) represents the fractional counts at time t, C0 is the equilibrium fraction of trapped radioactivity, t is time, and τ is the time constant of efflux. Individual time courses were normalized to the maximum counts to yield the normalized uptake and averaged among different experiments.
Results
Trapping Gluex in the central binding site
Our first goal is to determine whether the cmCLC filter conformation where Gluex in Scen is “sandwiched” between two Cl− ions in Sint and Sext is part of the conformational cycle of the CLC transporters. If this were the case, transport of these exchangers should have a bell-shaped dependence on Cl− concentration, with the initial rise in activity caused by the increased substrate availability and the subsequent decrease to the trapping of Gluex between the fully occupied Sext and Sint. To test this hypothesis, we monitored the activity of WT CLC-ec1 by measuring H+ transport as a function of [Cl−] while maintaining the pH and [Cl−] symmetry (Fig. 1 B). Transport was driven by a negative potential inside the liposomes generated by the application of the K+ ionophore valinomycin in the presence of a 300-fold K+ gradient, V(K+) ≅ −177 mV, which promotes H+ influx and Cl− efflux. As expected, in the absence of Cl−, there is no transport. As [Cl−] is raised symmetrically, H+ transport increases and peaks at ∼300 mM [Cl−]. However, instead of plateauing at its maximum, at [Cl−] > 300 mM, H+ transport decreases to ∼35% of its maximal value at 1 M [Cl−] (Fig. 1, C and D).
Table 1. Cl− dependence of the activity of CLC-ec1 measured by H+ transport.
| Protein | [Cl−] | [H]/[H]300Cl | n |
|---|---|---|---|
| mM | |||
| WT CLC-ec1 | 0 | 0.013 ± 0.001 | 3 |
| 50 | 0.30 ± 0.03 | 7 | |
| 70 | 0.46 ± 0.02 | 17 | |
| 100 | 0.76 ± 0.03 | 3 | |
| 150 | 0.86 ± 0.01 | 3 | |
| 300 | 1.00 ± 0.02 | 29 | |
| 500 | 0.90 ± 0.04 | 23 | |
| 750 | 0.73 ± 0.06 | 14 | |
| 1,000 | 0.35 ± 0.03 | 16 | |
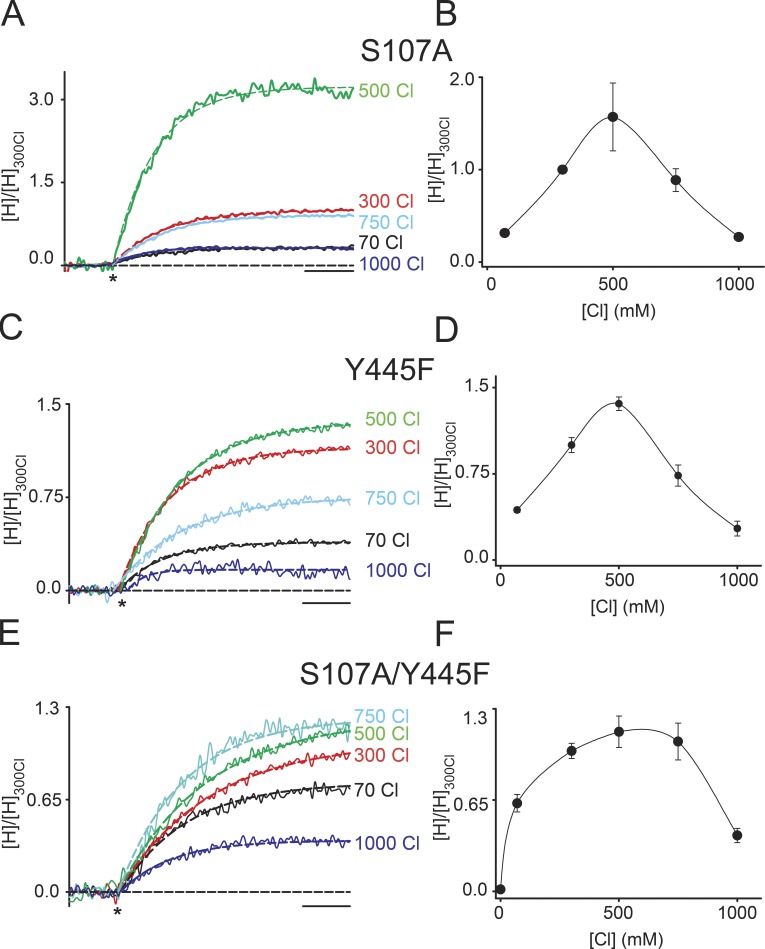
| S107A | 70 | 0.31 ± 0.01 | 8 |
| 300 | 1.00 ± 0.03 | 11 | |
| 500 | 1.57 ± 0.37 | 11 | |
| 750 | 0.89 ± 0.12 | 11 | |
| 1,000 | 0.27 ± 0.03 | 8 | |
| Y445F | 70 | 0.44 ± 0.02 | 9 |
| 300 | 1.00 ± 0.06 | 9 | |
| 500 | 1.36 ± 0.06 | 10 | |
| 750 | 0.73 ± 0.09 | 10 | |
| 1,000 | 0.27 ± 0.06 | 8 | |
| S107A/Y445F | 0 | 0.012 ± 0.001 | 3 |
| 70 | 0.63 ± 0.06 | 11 | |
| 300 | 1.00 ± 0.05 | 8 | |
| 500 | 1.13 ± 0.11 | 11 | |
| 750 | 1.07 ± 0.13 | 11 | |
| 1,000 | 0.40 ± 0.05 | 10 |
Data are reported as the mean ± SEM of n experiments from two to eight independent preparations.
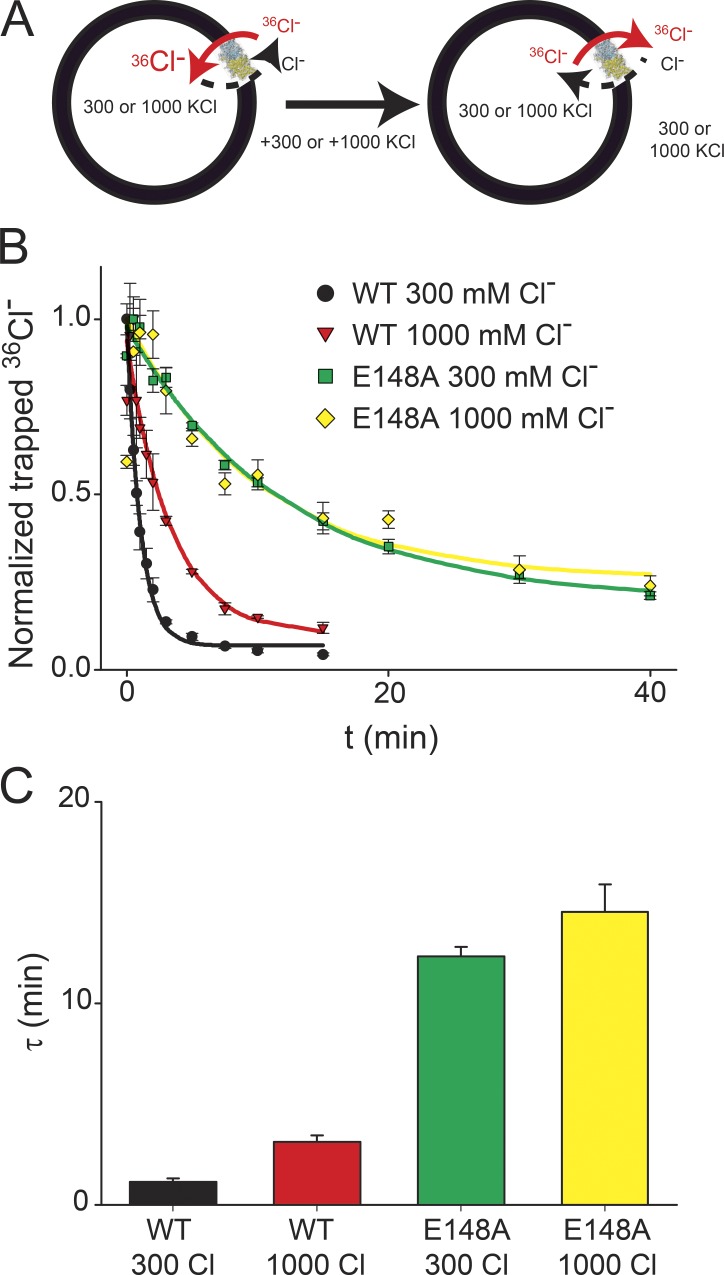
The simplest interpretation of these results is that at high salt concentrations, Gluex becomes trapped between the saturated Sint and Sext sites. However, alternative explanations exist. For example, saturation of only one site could be sufficient to inhibit transport. This could either be Sint, which has a low equilibrium affinity for Cl− (Lobet and Dutzler, 2006; Picollo et al., 2009), or an allosteric site outside the pathway, such as the computationally proposed Sext* site (Faraldo-Gómez and Roux, 2004; Yin et al., 2004). If the high salt inhibition reflects trapping of Gluex, introduction of the E148A mutation should abolish it. However, because this mutant does not transport H+ (Accardi and Miller, 2004), we used an independent approach to test this hypothesis (Fig. 2 A). We prepared proteoliposomes with 300 or 1,000 mM internal Cl− and loaded them with 36Cl− under uptake conditions (Fig. 2 A, left; Maduke et al., 1999). After waiting 15 min to let the uptake reaction reach steady state (Picollo et al., 2012), we added 300 or 1,000 mM Cl− to the external solution to annul the electrochemical gradient driving 36Cl− accumulation and release the accumulated radioisotope (Fig. 2 A, right). In liposomes reconstituted with WT CLC-ec1 at 300 mM Cl−, efflux occurs with a time constant of τWT(300 Cl) ≅ 1.1 min (Fig. 2, B and C). When the Cl− concentration is raised symmetrically to 1,000 mM, efflux slows down approximately threefold to τWT(1,000 Cl) ≅ 3.2 min (Fig. 2, B and C), in excellent agreement with the near threefold reduction in activity seen between the 300 and 1,000 mM Cl− conditions in the H+ transport measurements (Fig. 1 D). The inhibition is abolished in liposomes reconstituted with the E148A mutant (Fig. 2, B and C): 36Cl− efflux occurs with similar kinetics regardless of the salt concentrations (τE148A(300 Cl) ≅ 12.3 min and τE148A(1,000 Cl) ≅ 14.5 min). These results suggest that the high salt inhibition does not reflect saturation of a single Cl−-binding site, as in the E148A mutant, all sites can be occupied by ions but no decrease in activity is observed at maximal salt concentrations. Rather, our findings show that removal of the Gluex side chain is sufficient to abolish the high salt inhibition of transport, consistent with the hypothesis that this phenomenon reflects the trapping of Gluex between two fully occupied binding sites. It is interesting to note that efflux by E148A CLC-ec1 is ∼12-fold slower than that mediated by the WT transporter, consistent with the increased Cl− binding affinity of this mutant when measured in equilibrium conditions (Picollo et al., 2009).
Figure 2.
Mutation of Gluex relieves the high salt inhibition. (A) Schematic representation of the 36Cl− efflux experiments. (B and C) Time course of 36Cl− efflux from proteoliposomes reconstituted with WT CLC-ec1 at 300 mM Cl− or at 1,000 mM Cl− or with the E148A mutant at 300 mM Cl− or at 1,000 mM Cl−. Solid lines represent exponential fits of the time courses with time constants (shown in C) of τWT(300 Cl) = 1.15 ± 0.18 min, τWT(1,000 Cl) = 3.12 ± 0.32 min, τE148A (300 Cl) = 12.3 ± 0.5 min, and τE148A(1,000 Cl) = 14.5 ± 1.4 min. Symbols represent the mean from four independent experiments, and the error bars are the SEM.
Hydrogen bonds stabilize Gluex in the central site
Although consistent with our results, it remains to be demonstrated that the state stabilized by high Cl− is the one where Gluex occupies Scen while Sint and Sext are simultaneously occupied by Cl− ions. In the cmCLC structure, Gluex is within hydrogen bonding distance of the hydroxyls from Sercen and Tyrcen (Fig. 1 A), which correspond to S107 and Y445 in CLC-ec1. Therefore, we tested whether Gluex forms hydrogen bonds with either or both residues and how elimination of either hydroxyl moiety affects trapping. If the interactions between Gluex, Sercen, and Tyrcen stabilize the sandwiched state, their disruption should make the trapping more difficult. This is the case; the Cl− dependence of the activity of the S107A (Fig. 3, A and B) and Y445F (Fig. 3, C and D) mutants has the same bell shape as seen for the WT protein, but the peak is shifted to ∼500 mM [Cl−], indicating that more Cl− is required to trap Gluex in Scen. When both hydroxyls are removed in the S107A/Y445F double mutant, the peak broadens and activity begins to decrease only at [Cl−] ≥ 750 mM (Fig. 3, E and F). These results suggest that during the exchange cycle, Gluex does form hydrogen bonds with both Sercen and Tyrcen and that these interactions together with the Cl− occupancy of Sint and Sext stabilize Gluex in the Scen site. Alternatively, these results could also reflect weakened Cl− binding to Sint as a result of the mutations. However, this is unlikely to be the case, as these constructs mediate robust H+ transport, unlike the mutants at these positions that weaken ion binding (Accardi et al., 2006; Walden et al., 2007; Zifarelli and Pusch, 2009) but like the Y445F mutant that does not affect ion binding (Accardi et al., 2006). Interestingly, in low [Cl−], the relative activity of the double mutant is higher than that of the WT or single mutants (Fig. 3, B, D, and F), suggesting that these interactions might also play a role in other states visited by the transporter during the exchange cycle.
Figure 3.
Hydrogen bonds stabilize Gluex in the central site. (A and B) Normalized time course of H+ transport (A) and mean normalized steady-state H+ uptake (B) mediated by the S107A mutant in varying symmetrical [Cl−]. (C and D) Normalized time course of H+ transport (C) and mean normalized steady-state H+ uptake (D) mediated by the Y445F mutant in varying symmetrical [Cl−]. (E and F) Normalized time course of H+ transport (E) and mean normalized steady-state H+ uptake (F) mediated by the S107A/Y445F double mutant in varying symmetrical [Cl−]. The asterisks denote the addition of valinomycin to initiate the experiment. Dashed lines represent exponential fits of the time courses. Bars, 10 s. The values are reported in Table 1; error bars are the SEM of the number of experiments indicated in Table 1.
Discussion
Despite recent advances, the mechanism by which the CLC transporters catalyze the exchange of two Cl− for one H+ remains poorly understood. Combined crystallographic, functional, and computational studies have led to the proposal of several models (Miller and Nguitragool, 2009; Feng et al., 2010; Basilio et al., 2014; Khantwal et al., 2016) that, however, fail to capture all key features of these transporters. The crystal structures of WT and mutant CLC-ec1 and cmCLC suggested that a conserved glutamic acid, Gluex, can adopt three conformations (Dutzler et al., 2002, 2003; Feng et al., 2010) and that the interconversion of this side chain between these states underlies H+/Cl− exchange. In the structure of cmCLC, Gluex occupies the central binding site and is trapped by two Cl− ions in the internal and external sites (Fig. 1 A). The existence of such a state in other CLC homologues, however, has not been demonstrated. Here, we show that during transport, CLC-ec1, the prototypical CLC exchanger, visits a conformation whose functional characteristics closely match those predicted for a cmCLC-like state. We find that the activity of CLC-ec1 has a biphasic dependence on [Cl−]; it increases monotonically between 0 and 500 mM Cl− and then decreases, rather than plateauing as expected for a transporter that reaches substrate saturation. This decrease in activity is consistent with the idea that during transport, Gluex can reside in Scen, so that when both Sint and Sext are occupied by ions at very high chloride concentrations, it becomes trapped in a cmCLC-like state. This hypothesis is further corroborated by the finding that disrupting the hydrogen bonds that stabilize Gluex in Scen by mutating Tyrcen and Sercen destabilizes the trapped state.
Our results, in good agreement with past work (Lobet and Dutzler, 2006; Picollo et al., 2009), suggest that at least one of the binding sites of CLC-ec1, presumably Sint, has very low affinity for Cl−. However, in our measurements, the inhibition of CLC-ec1—which we hypothesize reflects the saturation of Sint—only manifests itself at Cl− concentrations >500 mM, whereas the isothermal titration calorimetry and crystallographic experiments placed the affinity of Sint in the 20–40-mM range. Although we do not have a definitive explanation for the discrepancy, we speculate that two issues mitigate this inconsistency. First, our experiments were performed during active transport and in the presence of a large applied voltage that varies during the course of the experiment. The presence of a large transbilayer voltage could affect the apparent Cl− affinity directly, as ions traverse different fractions of the electric field to reach the three binding sites, and indirectly, as the coupled movement of ions and of the Gluex side chain within the pore occurs within the electric field. In contrast, the isothermal titration calorimetry and crystallographic measurements were performed in equilibrium conditions and in the absence of an applied voltage. Second, the lack of orientation in our reconstitutions prevented us from extracting the apparent affinity of Cl− for the two sites, as the voltage used to drive transport has opposite effects on ion binding depending on the directionality of the insertion of CLC-ec1 in the liposomal membrane. It is worth noting that the affinity of the Sext site is too low to be detected in our experiments, as the lowest [Cl−] tested is 50 mM, at which point this site would be fully saturated (Picollo et al., 2009). At present, the concentrations at which the affinity of this site would be resolved are below our signal to noise detection limit. Collectively, our results suggest that during CLC gating, Gluex can occupy both Sext and Scen and that its interactions with Tyrcen and Sercen contribute to the determination of the equilibrium between these two conformations.
Acknowledgments
We wish to thank members of the Accardi laboratory for helpful discussions.
This work was supported by National Institutes of Health grant R01GM085232 to A. Accardi and an Irma T. Hirschl/Monique Weill-Caulier Scholar Award to A. Accardi.
The authors declare no competing financial interests.
Author contributions: M. Vien and D. Basilio carried out experiments and analyzed data. L. Leisle carried out experiments, and A. Accardi designed research, carried out experiments, and wrote the manuscript. All authors edited the manuscript.
Merritt C. Maduke served as editor.
References
- Accardi A., and Miller C.. 2004. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature. 427:803–807. 10.1038/nature02314 [DOI] [PubMed] [Google Scholar]
- Accardi A., and Picollo A.. 2010. CLC channels and transporters: proteins with borderline personalities. Biochim. Biophys. Acta. 1798:1457–1464. 10.1016/j.bbamem.2010.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accardi A., Kolmakova-Partensky L., Williams C., and Miller C.. 2004. Ionic currents mediated by a prokaryotic homologue of CLC Cl− channels. J. Gen. Physiol. 123:109–119. 10.1085/jgp.200308935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accardi A., Walden M., Nguitragool W., Jayaram H., Williams C., and Miller C.. 2005. Separate ion pathways in a Cl−/H+ exchanger. J. Gen. Physiol. 126:563–570. 10.1085/jgp.200509417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accardi A., Lobet S., Williams C., Miller C., and Dutzler R.. 2006. Synergism between halide binding and proton transport in a CLC-type exchanger. J. Mol. Biol. 362:691–699. 10.1016/j.jmb.2006.07.081 [DOI] [PubMed] [Google Scholar]
- Basilio D., Noack K., Picollo A., and Accardi A.. 2014. Conformational changes required for H+/Cl− exchange mediated by a CLC transporter. Nat. Struct. Mol. Biol. 21:456–463. 10.1038/nsmb.2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetts B., and Parker M.W.. 2013. Molecular determinants of common gating of a ClC chloride channel. Nat. Commun. 4:2507 10.1038/ncomms3507 [DOI] [PubMed] [Google Scholar]
- Bisset D., Corry B., and Chung S.H.. 2005. The fast gating mechanism in ClC-0 channels. Biophys. J. 89:179–186. 10.1529/biophysj.104.053447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzler R., Campbell E.B., Cadene M., Chait B.T., and MacKinnon R.. 2002. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature. 415:287–294. 10.1038/415287a [DOI] [PubMed] [Google Scholar]
- Dutzler R., Campbell E.B., and MacKinnon R.. 2003. Gating the selectivity filter in ClC chloride channels. Science. 300:108–112. 10.1126/science.1082708 [DOI] [PubMed] [Google Scholar]
- Faraldo-Gómez J.D., and Roux B.. 2004. Electrostatics of ion stabilization in a ClC chloride channel homologue from Escherichia coli. J. Mol. Biol. 339:981–1000. 10.1016/j.jmb.2004.04.023 [DOI] [PubMed] [Google Scholar]
- Feng L., Campbell E.B., Hsiung Y., and MacKinnon R.. 2010. Structure of a eukaryotic CLC transporter defines an intermediate state in the transport cycle. Science. 330:635–641. 10.1126/science.1195230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch T.J. 2008. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit. Rev. Biochem. Mol. Biol. 43:3–36. 10.1080/10409230701829110 [DOI] [PubMed] [Google Scholar]
- Khantwal C.M., Abraham S.J., Han W., Jiang T., Chavan T.S., Cheng R.C., Elvington S.M., Liu C.W., Mathews I.I., Stein R.A., et al. . 2016. Revealing an outward-facing open conformational state in a CLC Cl−/H+ exchange transporter. eLife. 5:e11189 10.7554/eLife.11189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobet S., and Dutzler R.. 2006. Ion-binding properties of the ClC chloride selectivity filter. EMBO J. 25:24–33. 10.1038/sj.emboj.7600909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduke M., Pheasant D.J., and Miller C.. 1999. High-level expression, functional reconstitution, and quaternary structure of a prokaryotic ClC-type chloride channel. J. Gen. Physiol. 114:713–722. 10.1085/jgp.114.5.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., and Nguitragool W.. 2009. A provisional transport mechanism for a chloride channel-type Cl−/H+ exchanger. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364:175–180. 10.1098/rstb.2008.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picollo A., Malvezzi M., Houtman J.C., and Accardi A.. 2009. Basis of substrate binding and conservation of selectivity in the CLC family of channels and transporters. Nat. Struct. Mol. Biol. 16:1294–1301. 10.1038/nsmb.1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picollo A., Xu Y., Johner N., Bernèche S., and Accardi A.. 2012. Synergistic substrate binding determines the stoichiometry of transport of a prokaryotic H+/Cl− exchanger. Nat. Struct. Mol. Biol. 19:525–531: S1. 10.1038/nsmb.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverso S., Zifarelli G., Aiello R., and Pusch M.. 2006. Proton sensing of CLC-0 mutant E166D. J. Gen. Physiol. 127:51–66. 10.1085/jgp.200509340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden M., Accardi A., Wu F., Xu C., Williams C., and Miller C.. 2007. Uncoupling and turnover in a Cl−/H+ exchange transporter. J. Gen. Physiol. 129:317–329. 10.1085/jgp.200709756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Kuang Z., Mahankali U., and Beck T.L.. 2004. Ion transit pathways and gating in ClC chloride channels. Proteins. 57:414–421. 10.1002/prot.20208 [DOI] [PubMed] [Google Scholar]
- Zifarelli G., and Pusch M.. 2009. Conversion of the 2 Cl−/1 H+ antiporter ClC-5 in a NO3−/H+ antiporter by a single point mutation. EMBO J. 28:175–182. 10.1038/emboj.2008.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zifarelli G., De Stefano S., Zanardi I., and Pusch M.. 2012. On the mechanism of gating charge movement of ClC-5, a human Cl−/H+ antiporter. Biophys. J. 102:2060–2069. 10.1016/j.bpj.2012.03.067 [DOI] [PMC free article] [PubMed] [Google Scholar]