Abstract
Objective:
To report the main syndrome of dipeptidyl-peptidase–like protein 6 (DPPX) antibody–associated encephalitis, immunoglobulin G (IgG) subclass, and the antibody effects on DPPX/Kv4.2 potassium channels.
Methods:
A retrospective analysis of new patients and cases reported since 2013 was performed. IgG subclass and effects of antibodies on cultured neurons were determined with described techniques.
Results:
Nine new patients were identified (median age 57 years, range 36–69 years). All developed severe prodromal weight loss or diarrhea followed by cognitive dysfunction (9), memory deficits (5), CNS hyperexcitability (8; hyperekplexia, myoclonus, tremor, or seizures), or brainstem or cerebellar dysfunction (7). The peak of the disease was reached 8 months (range 1–54 months) after onset. All patients had both IgG4 and IgG1 DPPX antibodies. In cultured neurons, the antibodies caused a decrease of DPPX clusters and Kv4.2 protein that was reversible on removal of the antibodies. Considering the current series and previously reported cases (total 39), 67% developed the triad: weight loss (median 20 kg; range 8–53 kg)/gastrointestinal symptoms, cognitive-mental dysfunction, and CNS hyperexcitability. Outcome was available from 35 patients (8 not treated with immunotherapy): 60% had substantial or moderate improvement, 23% had no improvement (most of them not treated), and 17% died. Relapses occurred in 8 of 35 patients (23%) and were responsive to immunotherapy.
Conclusions:
DPPX antibodies are predominantly IgG1 and IgG4 and associate with cognitive-mental deficits and symptoms of CNS hyperexcitability that are usually preceded by diarrhea, other gastrointestinal symptoms, and weight loss. The disorder is responsive to immunotherapy, and this is supported by the reversibility of the antibody effects in cultured neurons.
In 2013, we described 4 patients with a disorder that occurs with antibodies against dipeptidyl-peptidase–like protein 6 (DPPX), a regulatory protein of the Kv4.2 potassium channels that are involved in somatodendritic signal integration and attenuation of back-propagation of action potentials.1 The clinical picture was consistent with a syndrome of CNS hyperexcitability including hyperekplexia, myoclonus, tremor, or seizures that in 3 patients were preceded by unexplained weight loss and diarrhea. Subsequent studies confirmed and expanded these findings, suggesting that the course of the disease can be protracted and that in some patients the syndrome may occur in association with systemic lymphoma.2 Less frequently, the presence of myoclonus and hyperekplexia was found to be associated with a syndrome resembling progressive encephalomyelitis with rigidity and myoclonus (PERM),3 but the frequency of this presentation and potential symptom similarity between the main syndrome related to DPPX antibodies and PERM could not be investigated because of the small number of cases. Additional studies showed that patients' antibodies increased neuronal excitability in preparations of myenteric neurons and reduced cell membrane protein levels of DPPX/Kv4.2 in cultured neurons4; the potential reversibility of these effects was not investigated.
Here, we report 9 additional patients and review all previously reported cases to determine whether the anti-DPPX syndrome can be recognized clinically and discerned from PERM. In addition, we have determined the main immunoglobulin G (IgG) subclass, the antibody effects on neuronal cell-surface clusters of DPPX and protein levels of Kv4.2 channels, and whether the antibody effects are reversible.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study was approved by the institutional review board of the Hospital Clinic (Barcelona, Spain). All patients gave written informed consent for use of serum, CSF, and clinical information for research purposes.
Patients and serologic testing.
Patients investigated in the laboratory of Clinical and Experimental Neuroimmunology (Hospital Clinic, University of Barcelona) and University of Pennsylvania (Philadelphia) whose serum and CSF were found positive for DPPX antibodies were included in the study. The study period includes all new patients identified after the initial report of 2013 until May 30, 2016. During this time 9,798 patients were studied for encephalitis and a variety of disorders of the CNS suspected to be autoimmune, including among others 121 patients with stiff-person syndrome spectrum disorders.5 Criteria for the presence of DPPX antibodies included brain tissue immunostaining similar to that reported for human DPPX antibodies1 and cell-based assay with human embryonic kidney 293 cells transfected with DPPX, as reported.1 The presence of other antibodies was determined with in house cell-based assay specific for NMDA receptor (NMDAR),6 α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic receptor (AMPAR),7 GABAA receptor,8 GABAB receptor,9 LGI1,10 CASPR2,10 glycine receptor,5 mGluR1,11 mGluR5,11 IgLON5,12 and neurexin-3α.13
Clinical information was obtained from the authors or referring physicians through a structured written questionnaire. Neurologic disability was measured with the modified Rankin Scale (mRS), and treatment effect was assessed with the mRS score.14
Cultures of neurons, antibody effects on DPPX and Kv4.2, and confocal microscopy.
Details of the methods used to determine a mode of action of the antibodies on cultured neurons are provided in appendix e-1 at Neurology.org. Briefly, patients' IgG (including IgG1 and IgG4) antibodies were purified from serum with protein A/G Sepharose columns. Hippocampal neurons were prepared from isolated rat hippocampi of E18 embryos, and cultured neurons were treated with purified patient or control IgG (final concentration 50 μg/mL media) for 3 days to assess the antibody effects on cell-surface DPPX clusters and concentration of Kv4.2 channels. In parallel experiments, neurons similarly treated were washed and allowed to recover using media without DPPX antibodies for 4 or 7 days. The changes in surface DPPX and Kv4.2 were quantitatively analyzed with confocal microscopy and immunoblot of biotinylated surface proteins, respectively.
Review of previously reported patients with DPPX antibodies.
To assess the spectrum of symptoms, response to treatment, presence of associated tumors, and outcome of patients with DPPX antibodies, we reviewed the current data along with all previously reported cases with these antibodies.1–4,15,16
Statistical analysis.
Confocal DPPX cluster density and quantitative immunoblot analysis among IgG-treated groups were given as the median with interquartile range and the mean with SEM, respectively. Statistical significance was analyzed with the Kruskal-Wallis test followed by the Dunn post hoc test for nonnormally distributed data. A value of p < 0.05 was considered significant in post hoc testing after correction for multiple comparisons (Dunn test). All tests were done with GraphPad Prism version 7 (GraphPad Software Inc, La Jolla, CA).
RESULTS
Symptoms associated with DPPX antibodies.
A summary of the clinical information of the 9 patients identified since 2013 is shown in the table. Eight were male, and the median age at onset was 57 years (range 36–69 years). All 9 patients had prodromal weight loss (median 20 kg, range 8–53 kg), and 7 had severe diarrhea that preceded the development of neurologic symptoms in a median of 4 months (range 2–60 months). Four of these patients also had mood changes or depression preceding other neurologic symptoms (median 5 months, range 3–60 months).
Table.
Demographic, clinical, and immunologic data in 9 new patients with anti-DPPX antibodies
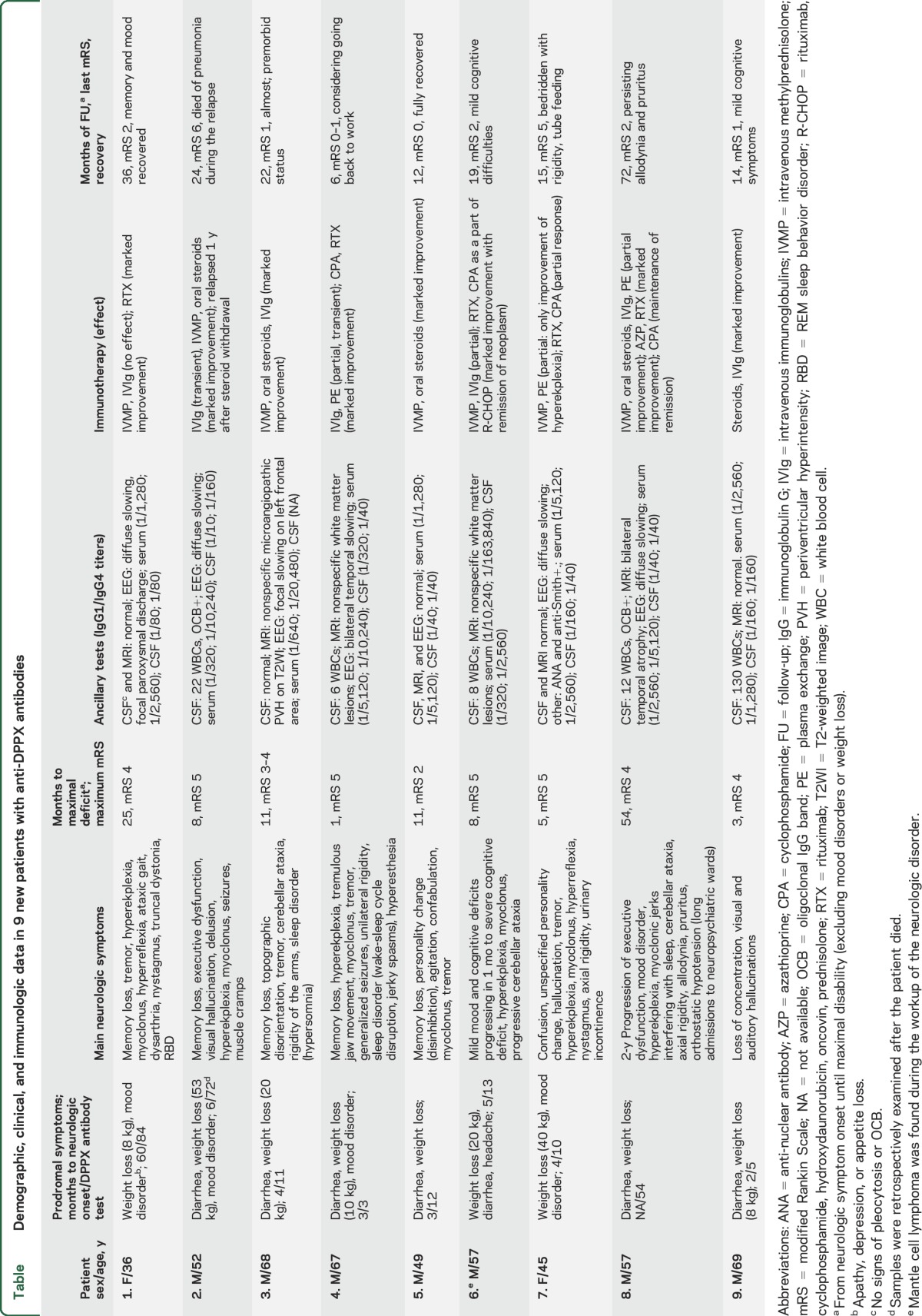
The neurologic disorder progressed for a median of 8 months (range 1–54 months) before reaching the peak of the disease, and in one patient (case 4), the symptom progression was subacute, reaching the maximum disability in 1 month. The progression of neurologic symptoms included cognitive dysfunction or memory loss (all patients) accompanied by mood or personality disorders in 4 and psychosis in 3. Symptoms of CNS hyperexcitability occurred in 8 patients, including hyperekplexia (6), myoclonus (7), tremor (5), muscle rigidity or stiffness (4), or seizures (2). Additionally, 7 patients developed brainstem or cerebellar dysfunction, 4 had sleep disorders, and 3 developed sensory symptoms (2 dysesthesia and 1 pruritus). During the course of the disease, 2 patients developed orthostatic hypotension or urinary incontinence; none of the 9 patients had cardiac dysrhythmia.
CSF, brain MRI, and EEG findings are shown in the table. Pleocytosis (median 12 white blood cells/mm3, range, 6–130 white blood cells/mm3) occurred in 5, and intrathecal IgG synthesis was confirmed in 2 of 5 patients. Brain MRI showed nonspecific T2/fluid-attenuated inversion recovery white matter abnormalities in 3 patients, indicated atrophy of the temporal lobes in 1 patient, and was normal in the other 5 patients. EEG was available from 7 patients, showing slow background activity in 5 patients, epileptiform discharges in 1 patient, and normal findings in 1 patient. Tumor screening revealed a Mantle cell lymphoma in 1 patient (case 6).
Treatment and outcomes.
All patients were treated with immunotherapy. After a median follow-up of 19 months (range 6–72 months), 4 had substantial recovery (mRS score 0–1), 3 had mild disability (mRS score 2), 1 patient did not improve (remained bedbound; mRS score 5), and 1 patient died (table, see below for treatment outcome for the whole series of patients).
Anti-DPPX syndrome spectrum and response to treatment.
The number of patients reported with anti-DPPX encephalitis since the discovery of this disorder is 30.1–4,15,16 Combining these patients with the current series (total 39 patients), the median age was 52 years (range 13–76 years), and 27 (69%) were male. Only 2 patients were <18 years old (13 and 15 years). Prodromal weight loss, diarrhea, or other gastrointestinal symptoms occurred in 30 patients (77%). Cognitive, memory, or mental alterations were identified in 36 patients (92%) and signs or symptoms of hyperexcitability in 30 (77%). Only 3 patients (8%) had a clinical picture compatible with PERM (muscle rigidity, brainstem symptoms, and myoclonus with absent or mild and late cognitive alteration). Overall the triad of prodromal weight loss or gastrointestinal symptoms, altered cognitive or mental functions, and CNS hyperexcitability (myoclonus, hyperekplexia, tremor, or seizures) was identified in 26 patients (67%). The presence of an underlying tumor was identified in 3 patients (all B-cell neoplasms: 1 Mantle-cell lymphoma, 1 B-cell lymphoma, and 1 B-cell chronic lymphocytic leukemia). Immunotherapy was used in 29 patients (74%). The outcome was available for 35 patients, including 8 who did not receive immunotherapy: 16 (46%) had substantial improvement, 5 (14%) had moderate improvement, 8 (23%, 6 of them not treated with immunotherapy) had no improvement or disease progression, and 6 (17%, 2 of them not treated with immunotherapy) died.
Eight of the 35 patients (23%) had clinical relapses, in 7 of them during immunotherapy withdrawal. Three of these 8 patients did not receive additional immunotherapy and had unfavorable outcome (2 died, 1 clinical progression). Among the other 5 patients, outcome was assessable for 4 (1 treated with rituximab; 1 plasma exchange and rituximab; 1 plasma exchange, rituximab, and cyclophosphamide; and 1 steroids and plasma exchange), and all showed clinical improvement (3 substantial and 1 moderate).
Anti-DPPX IgG antibodies are predominantly IgG1 and IgG4.
Serum was available from all 9 newly studied patients and CSF from 7, including paired serum/CSF samples in 7 cases. All samples had IgG1 and IgG4 DPPX antibodies (figure 1). Additionally, IgG2 antibodies were identified in 7 of 9 sera and 6 of 6 CSF samples; none of the patients had IgG3 antibodies. The IgG antibody subclass was similar in serum and CSF of all cases examined (5 of 5, not enough CSF sample was available from the other 2 patients).
Figure 1. Demonstration of DPPX antibodies and IgG subclasses in a cell-based assay.
(A) Serum of a representative case (patient 4) showing reactivity (green, A.a) with human embryonic kidney (HEK) cells expressing dipeptidyl-peptidase–like protein 6 (DPPX). The reactivity of a commercial antibody against DPPX (red, A.b) colocalizes with that of the patient's serum (yellow, A.c). Note that a control serum is negative (A.d–A.f). (B) Determination of immunoglobulin G (IgG) antibody subclasses in 2 cases (patients 4 and 1). Patients' antibodies bound to HEK cells expressing DPPX are demonstrated with secondary anti-human antibodies specific for the indicated subclasses. Patient 4 has DPXX antibodies of the IgG1, IgG2, and IgG4 subclasses (B.a–B.c), whereas patient 1 has IgG1 and IgG4 subclass antibodies (B.d–B.f). Nuclei counterstained with 4′, 6-diamino-2-phenylindole (A and B). Scale bars = 10 μm.
Patients' antibodies decrease the density of surface DPPX and concentration of cell-surface Kv4.2 protein level.
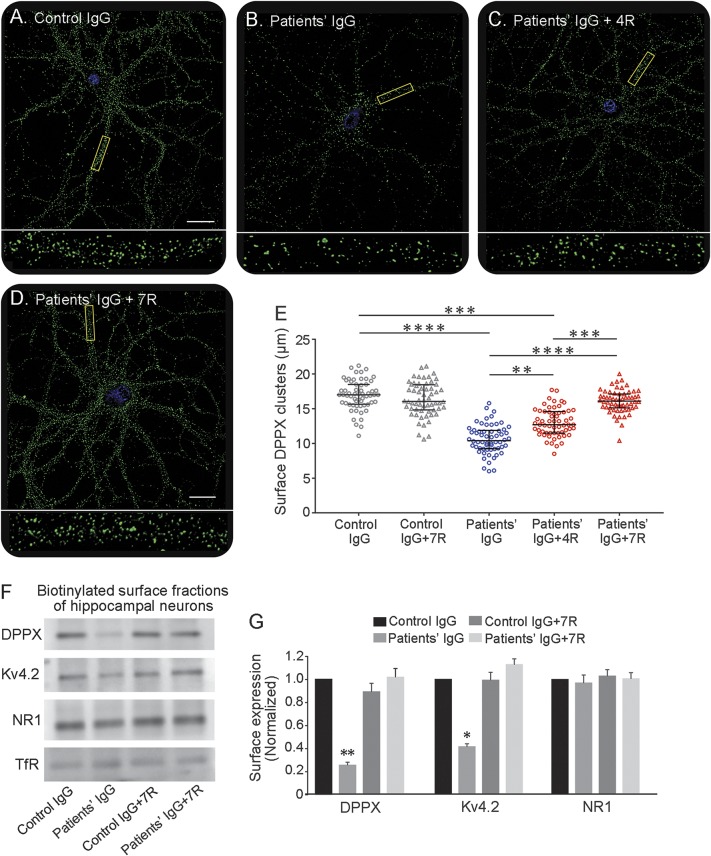
Cultured hippocampal neurons treated for 3 days with purified patients' IgG containing DPPX antibodies showed a significant decrease of the density of surface DPPX clusters compared with neurons treated with control IgG (figure e-1, A–D). Because 2 of 3 commercial antibodies against Kv4.2 are raised against intracellular epitopes and the other commercial antibody produced suboptimal live neuronal cell-surface immunostaining (data not shown), we used quantitative immunoblot analysis of cell-surface biotinylated proteins to determine the effects of patients antibodies on Kv4.2. These studies showed a significant reduction of surface protein levels of Kv4.2 and DPPX in neurons treated with patients IgG compared with control IgG (figure e-1, E and F). In parallel experiments, neurons were similarly treated with patients' or control IgG for 3 days and subsequently washed and allowed to recover for 4 or 7 days in media free of antibodies (figure 2). This experiment showed that the levels of cell-surface DPPX clusters were progressively restored, with normal baseline levels reached 7 days after removal of patients' antibodies (figure 2, A–E). Similarly, quantitative immunoblot studies showed that the cell-surface concentrations of Kv4.2 and DPPX were also restored to normal baseline concentrations (figure 2, F and G). Given that patients' antibodies did not react with Kv4.2 (figure e-2), the effects on this receptor are postulated to be caused by the antibody-mediated reduction of DPPX.
Figure 2. Effects of patients' antibodies on DPPX and Kv4.2 are reversible.
(A–D) Clusters of dipeptidyl-peptidase–like protein 6 (DPPX) in cultured neurons incubated for 3 days with pooled control immunoglobulin G (IgG), pooled patients’ (cases 4 and 7) IgG without recovery, and pooled patients’ (cases 4 and 7) IgG for 3 days followed by 4 or 7 days of recovery (fresh media without antibodies). Scale bars = 10 μm. Boxed dendrites are shown at higher magnification below (×100/1.3 numerical aperture oil objective). Note the reduction of clusters of DPPX caused by 3 days’ exposure to patients' antibodies and the progressive restoration of the density of DPPX clusters after neurons are allowed to recover for 4 to 7 days. (E) Graphic representation of the density of DPPX clusters (median with interquartile range) after treatment for 3 days with control or patients' IgG and after 4 to 7 days of recovery (4 independent experiments, 15 neurons per experiment in each condition). (F) An immunoblot of biotinylated surface proteins of hippocampal neurons treated with pooled control IgG and pooled patients’ IgG for 3 days without or with recovery for 4 to 7 days. The protein bands are demonstrated with commercial antibodies specific for DPPX and Kv4.2; NR1 and transferrin receptor (TfR) are used as loading controls. Note that patients' antibodies cause a substantial decrease of levels of DPPX and Kv4.2 after a 3-day incubation and that the levels of cell-surface proteins are progressively restored after 4 to 7 days. (G) Quantitative densitometry analysis of panel F; the data were normalized to the values of control IgG and are represented as median with SEM of 3 independent experiments (for each experiment, immunoblots were repeated, n = 6). All statistical analyses: Kruskal-Wallis test followed by Dunn test, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
DISCUSSION
We report our experience with 9 new patients with anti-DPPX encephalitis and review all previously reported cases, providing the main clinical features that should raise suspicion for this disorder and the profile of symptoms that differentiate it from PERM. We also confirm that symptom progression can be chronic and that a few patients may have an underlying tumor, usually a B-cell neoplasm. In this study we show that the antibodies are predominantly IgG1 and IgG4 and that their pathogenic effects on DPPX and Kv4.2 in cultured neurons are reversible on removal of the antibodies from the media.
The enriched expression of DPPX in myenteric plexus may explain the frequent gastrointestinal problems, usually accompanied by weight loss.1 In our experience, this weight loss is not subtle (median 20 kg in the current patients), and it is one of the first symptoms of the disease. Considering the current series and all previously reported cases in which clinical information was assessable (total 39 patients),1–4,15,16 67% of all patients had weight loss/gastrointestinal symptoms, cognitive or mental dysfunction, and symptoms of CNS hyperexcitability. This triad of symptoms is unusual in other forms of paraneoplastic or antibody-associated encephalitis (except for anti-Hu associated gastroparesis and encephalitis)17,18; therefore, in the context of encephalitis of unclear etiology, it should raise concern for DPPX antibody–associated encephalitis. None of our patients developed cardiac dysrhythmias, but a study in which 3 of 20 patients had ventricular tachycardia (1 case associated with cardiac arrest) suggested that these symptoms could be related to the important role of DPPX in the normal generation of Kv4.3-dependent cardiac rhythms.2
The neurologic syndrome associated with DPPX antibodies may resemble that of PERM, a syndrome that was described in the 1970s as a polioencephalomyelitis predominantly involving lower brainstem and spinal cord.19–22 Recent studies support the concept that PERM is mediated by immune responses against proteins of the GABAergic synapses such as the glycine receptor and, less frequently, amphiphysin or glutamic acid decarboxylase 65.5,23 The core symptoms of PERM (regardless of antibody association) include prominent muscle stiffness/rigidity in limbs and trunk accompanied by abnormal postures, generalized myoclonus, brainstem dysfunction, and dysautonomia. The clinical presentation is usually slowly progressive, over months, and unlike most patients with DPPX antibody–associated symptoms, patients with PERM do not present with prodromal gastrointestinal symptoms, severe loss of weight, or early and prominent cognitive or mental alterations.19–22 A previous report described 3 patients with DPPX antibodies who developed a clinical syndrome of PERM,3 but a subsequent study of 20 patients with DPPX antibodies showed that none developed PERM although 6 had stiffness and rigidity.2 We did not find this syndrome in any of the patients of the present series or our previous study,1 which combined include 13 patients. Twelve of these patients had early cognitive or mental changes, and although hyperekplexia was found to be a prominent symptom in 7, only 4 of them had muscle stiffness/rigidity. Nevertheless, case 8 of the current series shows points of convergence between both disorders and the possibility of misdiagnosis. This patient was initially described as having PERM in a cohort of 121 patients with stiff-person syndrome spectrum disorders5; however, he had prodromal diarrhea, weight loss, and early executive dysfunction, which are unusual for PERM.19–22
In pathologic studies of PERM,19,24,25 the inflammatory infiltrates predominated in the brainstem and spinal cord; in rare instances in which supratentorial inflammatory infiltrates were identified, they were mild and none of the patients developed cognitive or mental alterations. In contrast, the autopsy (limited to brain) of one of our patients with DPPX antibodies who died of pneumonia during a relapse showed prominent inflammatory infiltrates in the hippocampus, amygdala, cingulum, and temporo-occipital cortex and milder involvement of the pons, cerebellum, and medulla.16
Most patients of our series (7 of 9) and 67% of patients from previous studies2–4,15,16 responded to immunotherapy regardless of the duration of symptoms (range 0–177 months),2 suggesting that early diagnosis and treatment may further improve outcome. The observation that 9 (4 current and 5 previous1–3) of 12 patients who failed first-line immunotherapies responded to rituximab alone or combined with other therapies (5 cyclophosphamide, 1 azathioprine) emphasizes the importance of second-line immunotherapies. Moreover, 7 of 8 patients (1 current and 7 previous1–3,16) who developed clinical relapses had not been previously treated with rituximab, and the only case who had received this treatment developed the relapse while treatment was being discontinued. Future studies will clarify the relative contribution of each individual drug, but rituximab was the most frequently used second-line immunotherapy during the initial episode or at relapses, resulting in clinical improvement in 10 of 13 patients (77%). Rituximab has been reported to be highly effective in a number of autoimmune disorders associated with IgG4 antibodies,26–28 as occurred in our patients.
In addition to IgG4 DPPX antibodies, all our patients had IgG1 antibodies. The distribution of antibody subclass did not change in serum and CSF. Serum IgG (containing both IgG1 and IgG4) from representative patients showed a significant decrease of both cell-surface DPPX and Kv4.2 potassium channels, confirming the findings of a previous study.4 In addition, our study shows that these effects are reversible on removal of the antibodies from the media, resembling the mode of action of IgG1 antibodies against ion channels (NMDAR,29 AMPAR7). For DPPX/Kv4.2, the process of recovery of cell-surface levels of proteins took longer (7 days) than that observed for antibody-internalized NMDAR (<4 days),29 suggesting that the underlying mechanisms leading to cell-surface reinsertion of target proteins may be different (e.g., transcriptional for DPPX vs recycling for NMDAR30). The limited amount of samples did not allow further studies examining whether isolated IgG4 DPPX had the same effects on decreasing the levels of DPPX/Kv4.2. Given that IgG4 has the unique feature of being hetero-bispecific (continuously undergoing half-antibody exchange) and less effective than IgG1 in crosslinking and internalizing the target antigen,31,32 we postulate that the observed effects were mediated predominantly by IgG1, causing internalization and reduction of levels of DPPX that in turn regulate the cell-surface levels of Kv4.2.33,34 The internalization of a protein not directly targeted by the antibodies (e.g., Kv4.2) but that interacts with the antigen (DPPX) resembles the effects of LGI1 antibody–associated encephalitis in which the antibodies are specific for LGI1 but cause a decrease of density of the interacting AMPARs.35
A limitation of the study is the retrospective assessment of symptoms, but all information (current patients and the 9,798 cases studied since 2013) is similarly collected with a structured questionnaire. Future studies should include EMG evaluation to determine whether patients with DPPX develop continuous motor unit dischargers (such as in PERM), to determine the frequency of other autonomic symptoms, mainly cardiac dysrhythmias, and to confirm the beneficial effect of rituximab. The exact mechanism causing the decrease of DPPX/Kv4.2 and restoration of the levels of these proteins needs to be determined, as well as the individual contributions of IgG1 and IgG4 to the pathogenic effects.
Supplementary Material
GLOSSARY
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic receptor
- DPPX
dipeptidyl-peptidase–like protein 6
- IgG
immunoglobulin G
- mRS
modified Rankin Scale
- NMDAR
NMDA receptor
- PERM
progressive encephalomyelitis with rigidity and myoclonus
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Design/conceptualization of the study, analysis and interpretation of the data: M.H., M.R.R., F.G., J.D.; data collection: M.H., H.A., M.P.-P., L.S., M.J.T., E.M.-H.; statistical analysis: M.H.; figure development: M.H., J.D.; drafting of the manuscript M.H., M.R.R., F.G., J.D.
STUDY FUNDING
This study was supported in part by Instituto Carlos III/FEDER (FIS PI15/00377, F.G.; FIS PI14/00203, J.D.; CD14/001555, E.M.-H.), Biomedical Research Networking Centre for Rare Diseases (CB15/00010, J.D.; NIH RO1NS077851, J.D.), the Netherlands Organisation for Scientific Research (NWO; Memorabel Fellowship, M.J.T.; Agaur SGR93, J.D.), CERCA Program, Generalitat Catalunya (J.D.), and Fundació CELLEX (J.D.).
DISCLOSURE
M. Hara, H. Ariño, M. Petit-Pedrol, and L. Sabater report no disclosures relevant to the manuscript. M. Titulaer received research funds for serving on a scientific advisory board of MedImmune LLC. E. Martinez-Hernandez and M. Schreurs report no disclosures relevant to the manuscript. M. Rosenfeld receives royalties from Athena Diagnostics for the use of Ma2 as an autoantibody test and from Euroimmun for the use of NMDAR as an autoantibody test. F. Graus received a licensing fee from Euroimmun for the use of IgLON5 as an autoantibody test. J. Dalmau receives royalties from Athena Diagnostics for the use of Ma2 as an autoantibody test and from Euroimmun for the use of NMDA, GABAB receptor, GABAA receptor, DPPX, and IgLON5 as autoantibody tests; he has received an unrestricted research grant from Euroimmun. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Boronat A, Gelfand JM, Gresa-Arribas N, et al. Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv4.2 potassium channels. Ann Neurol 2013;73:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tobin WO, Lennon VA, Komorowski L, et al. DPPX potassium channel antibody: frequency, clinical accompaniments, and outcomes in 20 patients. Neurology 2014;83:1797–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balint B, Jarius S, Nagel S, et al. Progressive encephalomyelitis with rigidity and myoclonus: a new variant with DPPX antibodies. Neurology 2014;82:1521–1528. [DOI] [PubMed] [Google Scholar]
- 4.Piepgras J, Holtje M, Michel K, et al. Anti-DPPX encephalitis: pathogenic effects of antibodies on gut and brain neurons. Neurology 2015;85:890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Hernandez E, Arino H, McKeon A, et al. Clinical and immunologic investigations in patients with stiff-person spectrum disorder. JAMA Neurol 2016;73:714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol 2009;65:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petit-Pedrol M, Armangue T, Peng X, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol 2014;13:276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol 2010;9:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai M, Huijbers MG, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol 2010;9:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology 2011;77:1698–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabater L, Gaig C, Gelpi E, et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol 2014;13:575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gresa-Arribas N, Planaguma J, Petit-Pedrol M, et al. Human neurexin-3alpha antibodies associate with encephalitis and alter synapse development. Neurology 2016;86:2235–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin Scale: implications for stroke clinical trials: a literature review and synthesis. Stroke 2007;38:1091–1096. [DOI] [PubMed] [Google Scholar]
- 15.Stoeck K, Carstens PO, Jarius S, et al. Prednisolone and azathioprine are effective in DPPX antibody-positive autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm 2015;2:e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stokin GB, Popovic M, Gelpi E, Kogoj A, Dalmau J, Graus F. Neuropathologic features of anti-dipeptidyl-peptidase-like protein-6 antibody encephalitis. Neurology 2015;84:430–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lennon VA, Sas DF, Busk MF, et al. Enteric neuronal autoantibodies in pseudoobstruction with small-cell lung carcinoma. Gastroenterology 1991;100:137–142. [DOI] [PubMed] [Google Scholar]
- 18.Condom E, Vidal A, Rota R, Graus F, Dalmau J, Ferrer I. Paraneoplastic intestinal pseudo-obstruction associated with high titres of Hu autoantibodies. Virchows Arch A Pathol Anat Histopathol 1993;423:507–511. [DOI] [PubMed] [Google Scholar]
- 19.Kasperek S, Zebrowski S. Stiff-man syndrome and encephalomyelitis: report of a case. Arch Neurol 1971;24:22–30. [DOI] [PubMed] [Google Scholar]
- 20.Lhermitte F, Chain F, Escourolle R, Chedru F, Guilleminault C, Francoual M. A further case of tetanus-like contracture distinct from the stiff man syndrome: pharmacological and neuropathological study of a case of predominantly spinal encephalomyelitis [in French]. Rev Neurol (Paris) 1973;128:3–21. [PubMed] [Google Scholar]
- 21.Whiteley AM, Swash M, Urich H. Progressive encephalomyelitis with rigidity. Brain 1976;99:27–42. [DOI] [PubMed] [Google Scholar]
- 22.Howell DA, Lees AJ, Toghill PJ. Spinal internuncial neurones in progressive encephalomyelitis with rigidity. J Neurol Neurosurg Psychiatry 1979;42:773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carvajal-Gonzalez A, Leite MI, Waters P, et al. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain 2014;137:2178–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armon C, Swanson JW, McLean JM, et al. Subacute encephalomyelitis presenting as stiff-person syndrome: clinical, polygraphic, and pathologic correlations. Mov Disord 1996;11:701–709. [DOI] [PubMed] [Google Scholar]
- 25.Barker RA, Revesz T, Thom M, Marsden CD, Brown P. Review of 23 patients affected by the stiff man syndrome: clinical subdivision into stiff trunk (man) syndrome, stiff limb syndrome, and progressive encephalomyelitis with rigidity. J Neurol Neurosurg Psychiatry 1998;65:633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huijbers MG, Querol LA, Niks EH, et al. The expanding field of IgG4-mediated neurological autoimmune disorders. Eur J Neurol 2015;22:1151–1161. [DOI] [PubMed] [Google Scholar]
- 27.Mahajan VS, Mattoo H, Deshpande V, Pillai SS, Stone JH. IgG4-related disease. Annu Rev Pathol 2014;9:315–347. [DOI] [PubMed] [Google Scholar]
- 28.Arino H, Armangue T, Petit-Pedrol M, et al. Anti-LGI1-associated cognitive impairment: presentation and long-term outcome. Neurology 2016;87:759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci 2010;30:5866–5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moscato EH, Peng X, Jain A, Parsons TD, Dalmau J, Balice-Gordon RJ. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol 2014;76:108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Neut Kolfschoten M, Schuurman J, Losen M, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007;317:1554–1557. [DOI] [PubMed] [Google Scholar]
- 32.Schuurman J, Van Ree R, Perdok GJ, Van Doorn HR, Tan KY, Aalberse RC. Normal human immunoglobulin G4 is bispecific: it has two different antigen-combining sites. Immunology 1999;97:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nadal MS, Ozaita A, Amarillo Y, et al. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron 2003;37:449–461. [DOI] [PubMed] [Google Scholar]
- 34.Foeger NC, Norris AJ, Wren LM, Nerbonne JM. Augmentation of Kv4.2-encoded currents by accessory dipeptidyl peptidase 6 and 10 subunits reflects selective cell surface Kv4.2 protein stabilization. J Biol Chem 2012;287:9640–9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohkawa T, Fukata Y, Yamasaki M, et al. Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors. J Neurosci 2013;33:18161–18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.