Dornier and Norman discuss the findings by Georgiadou et al. that show that AMPK activity regulates tensin expression, thereby modulating α5β1-integrin and fibrillar adhesion assembly.
Abstract
The regulation of integrin function is key to fundamental cellular processes, including cell migration and extracellular matrix (ECM) assembly. In this issue, Georgiadou et al. (2017. J. Cell Biol. https://doi.org/10.1083/jcb.201609066) report that the metabolic sensor adenosine monophosphate–activated protein kinase influences tensin production to regulate α5β1-integrin and fibrillar adhesion assembly and thus reveal an important connection between energy metabolism and ECM assembly.
The ECM is a principal structural component of tissues that performs many important roles, including directing tissue patterning during embryogenesis and maintaining compartmentalization and homeostasis in the adult. The epithelial basement membrane is a laminin- and collagen IV–rich ECM structure, synthesized primarily by epithelial cells, which contributes to the stability, structure, and regenerative capacity of epithelia as well as the barrier function of these tissues (Rowe and Weiss, 2008). However, a very different kind of fibrillar ECM—which is fibronectin and collagen I rich—is key to neural and vascular patterning during embryogenesis. In the adult, this fibronectin-rich ECM is commonly termed the “provisional matrix” and is primarily produced and assembled by fibroblasts during wound healing (Singh et al., 2010). Furthermore, the fibronectin-rich provisional-type ECM is assembled by carcinoma-associated fibroblasts, and this is known to be a key driver of cancer invasion and metastasis (Van Obberghen-Schilling et al., 2011).
Integrins are transmembrane receptors that form well-characterized physical links between the cytoskeleton and the ECM, and these transmit mechanical signals bidirectionally across the plasma membrane to influence ECM assembly. The cell’s principal fibronectin-binding integrin, the α5β1 heterodimer, controls fibronectin assembly. Thus, α5β1 engagement with fibronectin, and the manner in which this integrin manipulates the assembly of nascent fibronectin fibrils, will dictate the organization of the resulting provisional matrix (Yamada et al., 2003).
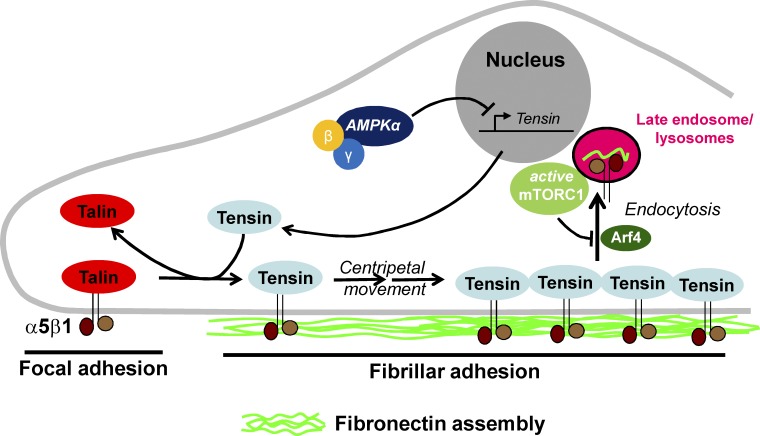
In 2000, Pankov et al. made a series of key observations that underpin how we now view the role played by fibroblasts in fibronectin ECM assembly. They reported that when α5β1 engages with fibronectin, this integrin is initially recruited to talin-rich focal adhesions (FAs) in the cell periphery. After this, α5β1 leaves the FAs and moves inward to populate fibrillar adhesions, and it is during this centripetal journey that the physical forces that promote fibronectin assembly are brought to bear (Fig. 1). In FAs, the β1-integrin cytodomain forges a well-characterized association with the phosphotyrosine binding (PTB)–like domain of talin, an actin-binding protein, and this connects the integrin to the actin cytoskeleton (Calderwood et al., 2013). However, as α5β1 moves centripetally out of FAs into fibrillar adhesions, it detaches from talin and associates with the PTB-like domain of another actin-binding protein called tensin (Pankov et al., 2000; McCleverty et al., 2007; Rainero et al., 2015). A subsequent study deployed tensin mutants, which are incapable of binding to β1-integrin, to demonstrate that α5β1 must swap allegiance from talin to tensin in order to undertake the inward journey that coordinates fibronectin assembly (Rainero et al., 2015). Finally, α5β1’s centripetal journey is terminated by its arrival at the cell center, whereupon it is internalized by an Arf4-dependent and clathrin-independent endocytic mechanism (Rainero et al., 2015). Thus, a picture is emerging in which fibronectin assembly is coordinated by a centripetal α5β1-integrin translocator operating between the cell periphery and the cell center, and this is a process in which tensin plays a pivotal role (Fig. 1).
Figure 1.
Control of centripetal α5β1 translocation and fibronectin assembly by AMPK and mTORC1. α5β1-Integrin is incorporated into FAs at the cell periphery and is associated with talin in these structures. Tensin competes with talin for binding to the β1-integrin cytodomain and displaces α5β1 from FAs. α5β1 then moves centripetally toward the cell center in association with tensin. Fibronectin is assembled as α5β1 undertakes this centripetal journey. Upon reaching the central region of the cell, which is under the nucleus, α5β1 is removed from the cell surface by an Arf4-dependent endocytic process. AMPK activity influences tensin levels by suppressing the expression of the mRNA that encodes tensin. mTORC1 suppresses the Arf4-dependent endocytosis of α5β1.
AMP-activated protein kinase (AMPK) is a key component of the cell’s metabolic sensing system. AMPK is activated by the increased levels of AMP that accompany various energy stresses and metabolic insults, and the phosphorylation of AMPK substrates, broadly speaking, leads to the activation of metabolic pathways that generate ATP (Hardie et al., 2012). It is now becoming clear that energy metabolism and nutrient sensing are linked to cell migration, and the likelihood that these links may be important to the acquisition of invasive and metastatic behavior in cancer has stimulated considerable interest in this area of cell biology. In this issue, Georgiadou et al. have shown that the key metabolic sensor AMPK controls β1-integrin activity and that its activity prevents integrin translocation and fibronectin remodeling in fibroblasts. The authors had previously developed functional RNAi approaches to screen for kinases that influenced integrin function, and this identified components of the AMPK complex as potential β1-integrin regulators (Rantala et al., 2011). Encouraged by this and by observations from both the Humphries (Horton et al., 2015) and Fassler (Schiller et al., 2013) laboratories showing that AMPK subunits are components of the integrin adhesome, the authors set out to determine how this metabolic sensing kinase might regulate β1-integrins and integrin-dependent cellular functions.
The authors manipulated AMPK levels and activity in fibroblasts in several ways. Principally, these include telomerase-immortalized human dermal fibroblasts (TIFs), in which AMPK subunits have been suppressed by siRNA in addition to embryonic fibroblasts from AMPKα1−/−,α2−/− or α1−/−/α2−/− knockout mice. To assess integrin activation in these cells, the authors used a portion of fibronectin containing the integrin-binding domain—the fibronectin repeats 7–10 (FN7–10)—in combination with conformation-specific antibodies, which only recognize extended α5β1-integrin heterodimers that are competent to bind to fibronectin. These data show clearly that although disruption of a single isoform of AMPK leads to modest up-regulation of integrin activation, a combined disruption of both AMPKα1 and AMPKα2 or a pharmacological blockade of these kinases robustly increases the ligand binding capacity of α5β1. Then, using immunofluorescence approaches, the authors show that AMPK depletion promotes the centripetal movement of activated α5β1 to fibrillar adhesions, where they colocalize with tensin. Consistently, fibroblasts from AMPK knockout mice assemble more fibronectin fibrils and display enhanced mechanotransduction and intracellular stiffness.
Given the role played by tensin in α5β1 translocation and fibronectin assembly, Georgiadou et al. (2017) asked whether AMPK might influence integrin function by controlling levels of this integrin-binding protein. In addressing this question, the authors found that AMPK controls tensin expression and that this is necessary for the kinase to influence integrin function. Indeed, mouse and human fibroblasts express all three of the known tensin isoforms (tensin1, 2, and 3), and, despite some variation in the relative contributions made by these isoforms in the cell type studied, AMPK depletion or inhibition clearly leads to increased tensin levels. Consistently, knockdown of the appropriate tensin isoforms represses the increased α5β1 activity, fibrillar adhesion formation, fibronectin assembly, and altered cell rheology that results from the suppression of AMPK. Conversely, overexpression of the appropriate tensin isoforms drives integrin activation, and, importantly, this requires tensin interaction with the β1 cytodomain, as tensin mutants deficient in β1-integrin binding are inactive in this regard.
It is interesting to discuss how this study aligns with another recent paper linking another nutrient-regulated kinase, mTORC1, to α5β1 dynamics (Rainero et al., 2015), and to hypothesize how these studies might illuminate the role of cell metabolism and nutrient availability in controlling the ECM microenvironment of tumors. Indeed, AMPK and mTORC1 activity are commonly reciprocally regulated, as not only do these two kinases respond in opposing ways to altered nutrient status, but AMPK is also well established to phosphorylate the mTORC1 component Raptor at sites that lead to its inhibition (Hardie, 2014). As mentioned above, α5β1-integrins leave FAs in the cell periphery and move centripetally in a tensin-dependent manner toward the cell center, and the characteristics of this inward journey are likely to dictate the nature of the resulting fibrillar fibronectin network (Fig. 1). The start of α5β1’s inward journey from FAs to the cell center would be expected to be promoted by an abundance of nutrients, leading to low AMPK activity and high tensin levels. Correspondingly, α5β1’s centripetal journey is terminated by an Arf4-dependent endocytic event, which is strongly inhibited by the nutrient-activated kinase mTORC1. Therefore, one might anticipate that under situations where the nutrient status of a cell is high, the combination of low AMPK, which promotes up-regulated integrin activity, and high mTORC1, which leads to reduced endocytosis, would result in increased levels of tensin-associated α5β1 to drive fibronectin assembly. In contrast, under conditions of nutrient starvation and metabolic stress, cells might be predicted to reduce fibronectin fibril assembly and increase the extent to which α5β1 can internalize and degrade the ECM.
Tumors are composed of a variety of different cell types, and the metabolic statuses of all of these will not be equivalent. Specifically, because tumor cells are rapidly proliferating, they will be more prone to metabolic stress and will be expected to have higher levels of AMPK (and lower levels of mTORC1) activity than carcinoma-associated fibroblasts, which are less proliferative. Thus, it is probable that this could generate a microenvironment in which high levels of ECM assembly by carcinoma-associated fibroblasts are supported, whereas the tumor cells endocytose and scavenge this ECM to try to satisfy their metabolic demands. Indeed, a recent study has shown that metabolically stressed epithelial cells uptake ECM that has been deposited by fibroblasts and that this helps these epithelial cells to survive under conditions of nutrient starvation (Muranen et al., 2017).
To conclude, this study by Georgiadou et al. (2017) provides mechanistic insight that will be key to understanding how energy metabolism and ECM production are coordinated in complex microenvironments and will facilitate the development of strategies to target these processes therapeutically.
Acknowledgements
We gratefully acknowledge Cancer Research UK and Breast Cancer Now for generously funding the research conducted in the Norman lab.
The authors declare no competing financial interests.
References
- Calderwood D.A., Campbell I.D., and Critchley D.R.. 2013. Talins and kindlins: partners in integrin-mediated adhesion. Nat. Rev. Mol. Cell Biol. 14:503–517. 10.1038/nrm3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadou M., Lilja J., Jacquemet G., Guzmán C., Rafaeva M., Alibert C., Yan Y., Sahgal P., Lerche M., Manneville J.-B., et al. . 2017. AMPK negatively regulates tensin-dependent integrin activity. J. Cell. Biol. 10.1083/jcb.201609066 [DOI] [PMC free article] [PubMed]
- Hardie D.G. 2014. AMPK—sensing energy while talking to other signaling pathways. Cell Metab. 20:939–952. 10.1016/j.cmet.2014.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie D.G., Ross F.A., and Hawley S.A.. 2012. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 13:251–262. 10.1038/nrm3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton E.R., Byron A., Askari J.A., Ng D.H., Millon-Frémillon A., Robertson J., Koper E.J., Paul N.R., Warwood S., Knight D., et al. . 2015. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat. Cell Biol. 17:1577–1587. 10.1038/ncb3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleverty C.J., Lin D.C., and Liddington R.C.. 2007. Structure of the PTB domain of tensin1 and a model for its recruitment to fibrillar adhesions. Protein Sci. 16:1223–1229. 10.1110/ps.072798707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranen T., Iwanicki M.P., Curry N.L., Hwang J., DuBois C.D., Coloff J.L., Hitchcock D.S., Clish C.B., Brugge J.S., and Kalaany N.Y.. 2017. Starved epithelial cells uptake extracellular matrix for survival. Nat. Commun. 8:13989 10.1038/ncomms13989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankov R., Cukierman E., Katz B.Z., Matsumoto K., Lin D.C., Lin S., Hahn C., and Yamada K.M.. 2000. Integrin dynamics and matrix assembly: tensin-dependent translocation of α5β1 integrins promotes early fibronectin fibrillogenesis. J. Cell Biol. 148:1075–1090. 10.1083/jcb.148.5.1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainero E., Howe J.D., Caswell P.T., Jamieson N.B., Anderson K., Critchley D.R., Machesky L., and Norman J.C.. 2015. Ligand-occupied integrin internalization links nutrient signaling to invasive migration. Cell Reports. 10:389–413. [DOI] [PubMed] [Google Scholar]
- Rantala J.K., Pouwels J., Pellinen T., Veltel S., Laasola P., Mattila E., Potter C.S., Duffy T., Sundberg J.P., Kallioniemi O., et al. . 2011. SHARPIN is an endogenous inhibitor of β1-integrin activation. Nat. Cell Biol. 13:1315–1324. 10.1038/ncb2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe R.G., and Weiss S.J.. 2008. Breaching the basement membrane: who, when and how? Trends Cell Biol. 18:560–574. 10.1016/j.tcb.2008.08.007 [DOI] [PubMed] [Google Scholar]
- Schiller H.B., Hermann M.R., Polleux J., Vignaud T., Zanivan S., Friedel C.C., Sun Z., Raducanu A., Gottschalk K.E., Théry M., et al. . 2013. β1- and αv-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat. Cell Biol. 15:625–636. 10.1038/ncb2747 [DOI] [PubMed] [Google Scholar]
- Singh P., Carraher C., and Schwarzbauer J.E.. 2010. Assembly of fibronectin extracellular matrix. Annu. Rev. Cell Dev. Biol. 26:397–419. 10.1146/annurev-cellbio-100109-104020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Tucker R.P., Saupe F., Gasser I., Cseh B., and Orend G.. 2011. Fibronectin and tenascin-C: accomplices in vascular morphogenesis during development and tumor growth. Int. J. Dev. Biol. 55:511–525. 10.1387/ijdb.103243eo [DOI] [PubMed] [Google Scholar]
- Yamada K.M., Pankov R., and Cukierman E.. 2003. Dimensions and dynamics in integrin function. Rev. Bras. Pesqui. Med. Biol. 36:959–966. [DOI] [PubMed] [Google Scholar]