Takahashi et al. find that epithelial cell–conditional knockdown of transcription factor Fli1 in mice drives systemic autoimmunity derived from thymic defects as well as selective tissue fibrosis in the skin and esophagus, mimicking human scleroderma. This study unravels the unanswered question about the origin of autoimmunity and selective tissue fibrosis in this disease.
Abstract
Systemic sclerosis (SSc), or scleroderma, is a multisystem autoimmune disorder characterized by vasculopathy and fibrosis in the skin and internal organs, most frequently in the esophagus and lungs. Hitherto, studies on SSc pathogenesis centered on immune cells, vascular cells, and fibroblasts. Although dysregulated keratinocytes in SSc have been recently reported, the contribution of epithelial cells to pathogenesis remains unexplored. In this study, we demonstrated the induction of SSc-like molecular phenotype in keratinocytes by gene silencing of transcription factor Friend leukemia virus integration 1 (Fli1), the deficiency of which is implicated in SSc pathogenesis. Keratin 14–expressing epithelial cell–specific Fli1 knockout mice spontaneously developed dermal and esophageal fibrosis with epithelial activation. Furthermore, they developed remarkable autoimmunity with interstitial lung disease derived from thymic defects with down-regulation of autoimmune regulator (Aire). Importantly, Fli1 directly regulated Aire expression in epithelial cells. Collectively, epithelial Fli1 deficiency might be involved in the systemic autoimmunity and selective organ fibrosis in SSc. This study uncovers unidentified roles of dysregulated epithelial cells in SSc pathogenesis.
Introduction
Systemic sclerosis (SSc), or scleroderma, is a chronic connective tissue disease characterized by three cardinal features: autoimmunity/inflammation, vasculopathy, and fibrosis in the skin and various internal organs (Asano, 2010; Asano and Sato, 2015). Although SSc pathogenesis remains elusive, genetic studies have demonstrated that most of the susceptibility genes for SSc are HLA haplotypes and non-HLA genes related to immunity and inflammation, suggesting the central role of immune abnormalities in SSc development (Agarwal and Reveille, 2010). Indeed, during the early and sclerotic phases, the infiltration of activated T cells and macrophages and the degranulation of mast cells are observed in the affected skin, which correlate with the severity of skin thickening (Fleischmajer et al., 1977). With regard to CD4+ T cells, the T helper type 1 cell (Th1 cell)/Th2 cell and Th17 cell/regulatory T cell (T reg cell) balances shift to Th2 and Th17 lineage dominance, respectively (O’Reilly et al., 2012). In particular, the increased expression of several Th2 cytokines, such as IL-6 and IL-13, contributes to fibroblast activation (Khan et al., 2012). In addition, despite the rarity of B cell infiltration in the skin, a cluster of B cell–related genes is strongly expressed in lesional and nonlesional skin of SSc patients (Whitfield et al., 2003). SSc B cells are constitutively activated, as represented by the increased expression of CD19, a critical positive response regulator (Sato et al., 2004), and produce various autoantibodies, including disease-specific antinuclear antibodies (ANAs) and other pathogenic antibodies against disease-related molecules (Sato et al., 2000; Hamaguchi, 2010). In interstitial lung disease (ILD) associated with SSc (SSc-ILD), activated B cells characteristically form aggregates in the lungs (Lafyatis et al., 2007). Supporting the critical role of B cells in SSc-ILD, rituximab, an anti-CD20 antibody, has proven efficacious in controlling ILD in a subset of patients (Lafyatis et al., 2009; Jordan et al., 2015). The initial immune activation and autoimmunity lead to the structural and functional abnormalities of vasculature and the constitutive activation of fibroblasts of SSc in various organs (Asano and Sato, 2015). However, the initial triggers of the dysregulated immune homeostasis and the origin of autoimmunity in this disease remain obscure (Harris and Rosen, 2003; Boin and Rosen, 2007; Joseph et al., 2014). Furthermore, although tissue fibrosis most commonly affects the skin, esophagus, and lungs in SSc (Gabrielli et al., 2009), a convincing explanation for this distinct target organ specificity is currently lacking. Hence, these unresolved key questions in this disease remain to be addressed.
Reflecting the main disease manifestations, the majority of previous studies on SSc have centered on immune cells, vascular endothelial cells, and fibroblasts. However, more recent studies have demonstrated anomalous phenotypes of the skin epithelium, or keratinocytes, in SSc (Leask, 2009; Aden et al., 2010; Nikitorowicz-Buniak et al., 2014, 2015; Suwara et al., 2014; Assassi et al., 2015). For example, SSc keratinocytes persistently express wound-associated keratins keratin 6 (K6) and K16 not only in the sclerotic skin, but also in the nonlesional skin (Aden et al., 2010), suggesting that the altered epithelial phenotype manifests early in this disease. Besides, SSc keratinocytes stimulate fibroblasts in cell culture with excessively secreted IL-1α (Aden et al., 2010), which is a major alarmin released from the epithelial cells triggering an inflammatory response in fibroblasts (Suwara et al., 2014). Increased expression of the key profibrotic growth factor connective tissue growth factor (CTGF) is also evident in SSc epidermis (Leask, 2009; Nikitorowicz-Buniak et al., 2014). Additionally, epithelial–mesenchymal transition (EMT), a central mechanism in fibrosis development driven by TGF-β1 (Nieto et al., 2016), is enhanced in SSc epidermis with the increased expression of its cardinal regulator SNAI1 (Nakamura and Tokura, 2011; Wei et al., 2011; Nikitorowicz-Buniak et al., 2015). Of particular relevance is a recent study on global gene profiling of SSc lesional skin describing a correlation between specific keratin expression signatures and the presence of ILD (Assassi et al., 2015). Therefore, it seems that the epithelial phenotype is not merely related directly to dermal fibrosis but, more profoundly, associated with SSc development itself.
Numerous studies from our laboratory and others have demonstrated the critical role of Friend leukemia virus integration 1 (Fli1), a member of the Ets transcription factor family, in SSc pathogenesis. Fli1 is constitutively suppressed in dermal fibroblasts, dermal microvascular endothelial cells, and perivascular inflammatory cells not only in the lesional skin, but also in the nonlesional skin of SSc patients, at least in part, by an epigenetic mechanism (Kubo et al., 2003; Wang et al., 2006; Asano, 2015). Fli1 deficiency reproduces SSc-like phenotypes in dermal fibroblasts and dermal microvascular endothelial cells at molecular levels in vitro and in vivo (Asano et al., 2009; Noda et al., 2012, 2013; van Bon et al., 2014; Akamata et al., 2015a; Ichimura et al., 2015; Saigusa et al., 2015; Takahashi et al., 2015b; Romano et al., 2016). Consistently, bleomycin injection induces more prominent SSc-like phenotypes in Fli1+/− mice compared with wild-type mice (Taniguchi et al., 2015), whereas endothelial cell–specific Fli1 knockout mice reproduce functional and structural vascular abnormalities characteristic of SSc vasculopathy (Asano et al., 2010a; Akamata et al., 2015b). Most importantly, double heterozygous mice for Fli1 and Krüppel-like factor 5 (Klf5), another transcription factor epigenetically suppressed in SSc dermal fibroblasts, spontaneously develop three cardinal features of SSc, including autoimmunity/inflammation, vasculopathy, and tissue fibrosis of the skin and lungs, with the disease-specific pathological cascade (Noda et al., 2014). Thus, Fli1 deficiency is now considered as a key predisposing factor in SSc pathogenesis.
In the course of our study, we have consistently observed a substantial decrease of Fli1 expression also in keratinocytes. Collectively with the recent studies on SSc keratinocytes, this preliminary observation encouraged us to investigate the potential role of Fli1-deficient keratinocytes in SSc. Notably, our findings indicate that Fli1 deficiency in epithelial cells drives dermal and esophageal fibrosis as well as ILD through the induction of epithelial cell activation and immune self-intolerance caused by thymic defects, suggesting a critical role of epithelial Fli1 deficiency in the development of autoimmunity and selective organ fibrosis in SSc. Now, we believe that this might be a new clue to resolve the unaddressed key questions in this disease.
Results
Fli1 down-regulation leads to the induction of SSc-like gene expression profiles in keratinocytes
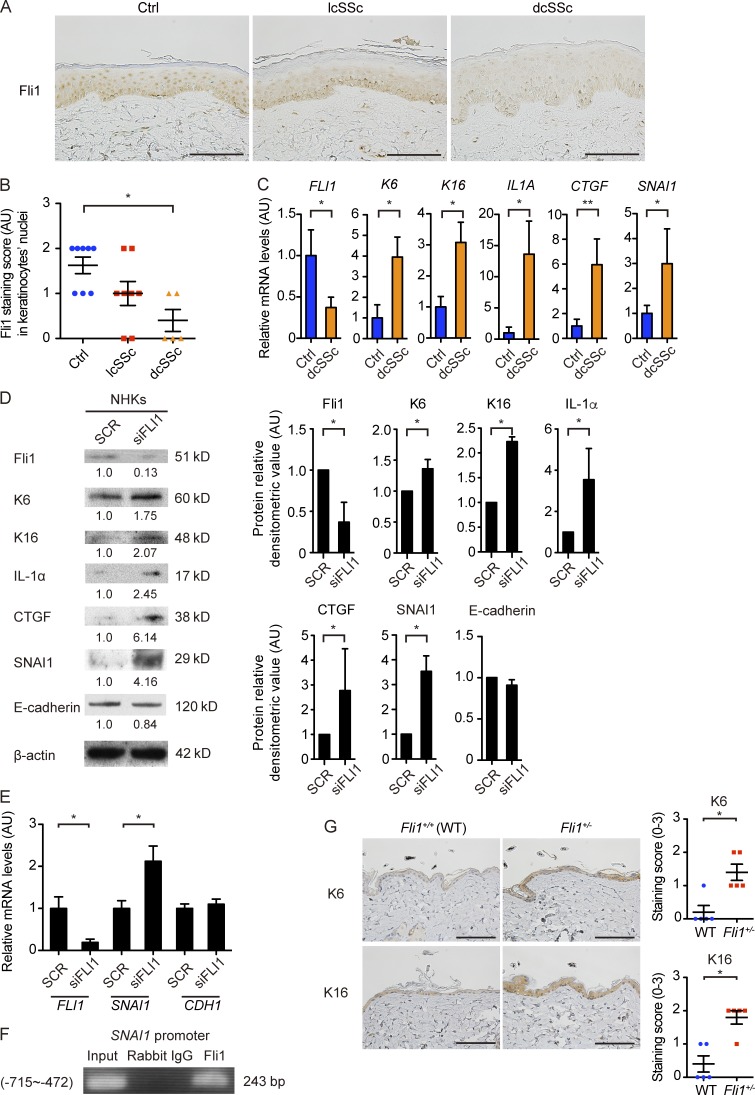
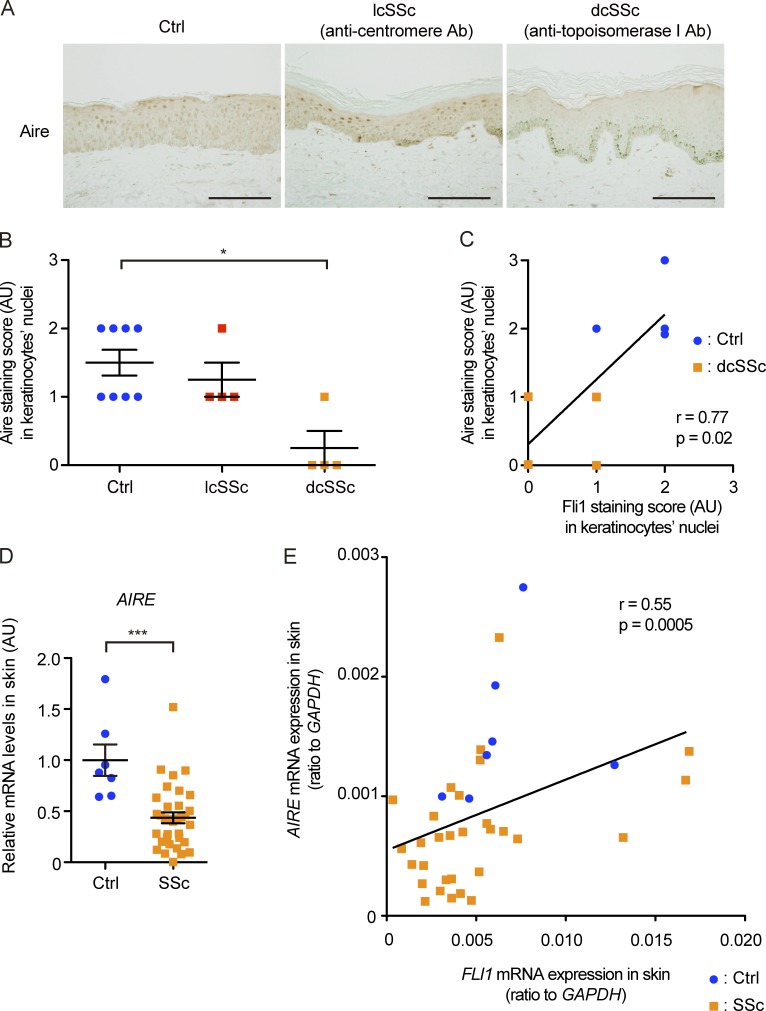
We initially confirmed our unpublished observation that Fli1 expression is decreased in SSc keratinocytes. The immunostaining verified marked down-regulation of Fli1 in keratinocytes in addition to the previously reported deficiency in dermal fibroblasts and dermal microvascular endothelial cells in SSc patients (Fig. 1 A; Asano et al., 2010a). Because SSc is classified into two distinct subtypes, namely, diffuse cutaneous SSc (dcSSc) with skin sclerosis extending proximal to the elbow and limited cutaneous SSc (lcSSc) with skin sclerosis only distal to the elbow (LeRoy et al., 1988), the signal intensity was evaluated by a four-point grading scale in these subtypes. Of note, the decreased signal intensity in keratinocytes reached a statistical significance in dcSSc patients but not in lcSSc patients, compared with closely age- and sex-matched healthy controls (Fig. 1 B). In the epidermal sheets, consistently, FLI1 mRNA was significantly down-regulated in dcSSc patients compared with healthy controls, whereas the increase in K6, K16, IL1A, CTGF, and SNAI1 mRNAs was evident in dcSSc patients, some of which are known to be up-regulated in SSc epidermis (Fig. 1 C; Nikitorowicz-Buniak et al., 2014, 2015).
Figure 1.
Gene silencing of Fli1 leads to the induction of SSc-like protein and gene expression profiles in keratinocytes. (A) Immunohistochemistry for Fli1 on the skin sections of healthy controls (n = 8), lcSSc patients with anticentromere antibody (n = 8), and dcSSc patients with anti–topoisomerase I antibody (n = 5). Representative images are shown. Bars, 100 µm. (B) The results of Fli1 staining intensity in A were semiquantitatively evaluated with a four-point grading scale. Kruskal-Wallis test followed by Dunn’s posthoc test was used. (C) mRNA was isolated from skin epidermal sheets from healthy controls (n = 4) and dcSSc patients (n = 6), and gene expressions were analyzed by qRT-PCR. (D) Whole cell lysates from cultured NHKs treated with control nonsilencing SCR or FLI1 siRNA (siFLI1) were subjected to immunoblotting. The values below each blot represent the relative levels of target molecules normalized by controls with densitometry. Representative images of the blots of four independent experiments are shown. (Right) The bar graphs summarize the relative values of the density from these four experiments. (E) The mRNA expression of FLI1, SNAI1, and CDH1 in NHKs treated with SCR or siFLI1 was evaluated. Relative expression levels from four independent experiments were normalized to the expression levels treated with SCR. (F) ChIP assay in NHKs was performed with anti-Fli1 antibody and the primers specific for the designated area of the SNAI1 gene promoter. (G) The back skin taken from Fli1+/− mice and their littermate wild-type (Fli1+/+) mice were immunohistochemically stained for K6 and K16. n = 5 for each group. (Left) Representative images of immunohistochemistry are shown. Bars, 100 µm. (Right) The graphs show the results of semiquantitative scoring of the staining intensity. Data are shown as mean ± SEM. *, P < 0.05; **, P < 0.01; by two-tailed Mann-Whitney U test, unless otherwise indicated. The results are from representative experiments that have been repeated twice independently or are otherwise indicated. AU, arbitrary units; Ctrl, control.
Next, we studied the impact of Fli1 deficiency on protein expression profiles of normal human keratinocytes (NHKs) in vitro. When transfected with FLI1 siRNA, the expressions of several molecules associated with the altered phenotype of SSc keratinocytes, including keratins K6 and K16, as well as IL-1α, CTGF, and SNAI1 (Aden et al., 2010; Nikitorowicz-Buniak et al., 2014, 2015) were increased in NHKs (Fig. 1 D). However, Fli1 knockdown did not affect E-cadherin expression, whose down-regulation is another indicator of EMT as well as SNAI1 (Fig. 1 D). In line with these data, the increased expression of SNAI1 mRNA but not CDH1 mRNA was confirmed in NHKs transfected with FLI1 siRNA (Fig. 1 E), and Fli1 occupied the SNAI1 promoter as shown by chromatin immunoprecipitation (ChIP; Fig. 1 F). Consistent with these in vitro observations, K6 and K16 were highly expressed in keratinocytes of Fli1+/− mice compared with those of wild-type (Fli1+/+) mice in vivo (Fig. 1 G). Collectively, these results indicate the critical role of Fli1 deficiency in the induction of SSc-like properties in keratinocytes.
Conditional deletion of Fli1 in K14-expressing epithelial cells recapitulates the phenotype of SSc epidermis in vivo
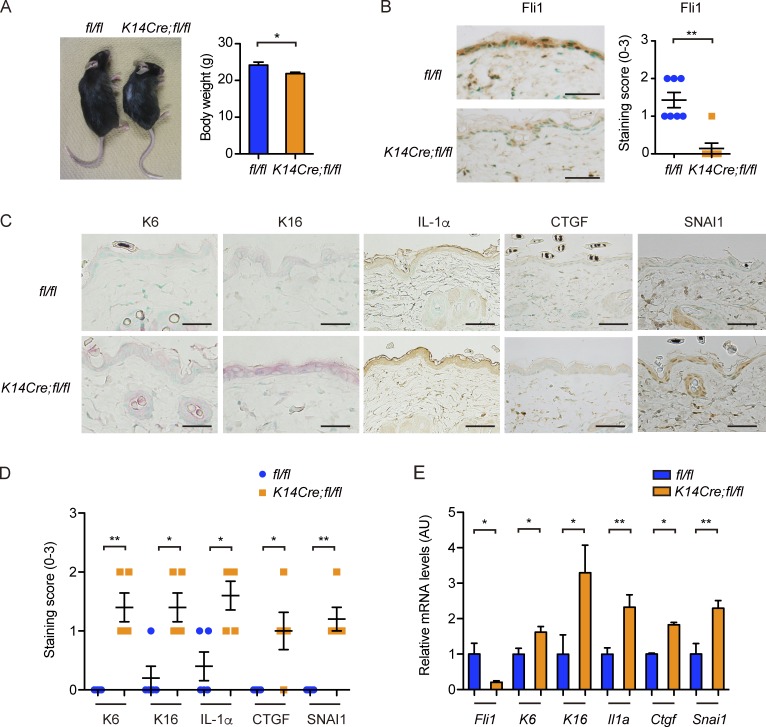
To investigate the potential role of Fli1-deficient epithelial cells in SSc pathogenesis, we generated conditional Fli1 knockout mice using the Cre-loxP system. We crossed Fli1flox/flox mice (Asano et al., 2010b) with mice expressing Cre recombinase under the control of the K14 gene promoter (K14Cre mice), widely used to create keratinocyte-specific conditional knockout mice. Of note, Cre recombinase is also expressed in esophageal stratified squamous epithelia and medullary thymic epithelial cells (TECs; mTECs) of K14Cre mice (Qiu et al., 2011). Fli1flox/floxK14Cre/− mice (K14Cre;fl/fl mice) and their littermate Fli1flox/flox mice (fl/fl mice) were used in this study.
K14Cre;fl/fl mice were born according to the normal Mendelian ratio and grew up without obvious abnormality, except that these mice were generally smaller in size than their littermate fl/fl mice (Fig. 2 A). The efficient down-regulation of Fli1 was confirmed by immunostaining in epidermal and follicular keratinocytes in K14Cre;fl/fl mice (Fig. 2 B). Concerning the epidermal phenotype, K6, K16, IL-1α, CTGF, and SNAI1 expressions in keratinocytes were significantly up-regulated in K14Cre;fl/fl mice compared with fl/fl mice (Fig. 2, C and D). Importantly, the expressions of IL-1α and CTGF were augmented also in dermal fibroblasts of K14Cre;fl/fl mice, as reported in SSc (Fig. 2 C; Kawaguchi et al., 2004; Noda et al., 2014). Furthermore, the analysis on gene expression profiles in epidermal sheets revealed increased mRNA expressions of the K6, K16, Il1a, Ctgf, and Snai1 genes as well as significantly suppressed Fli1 mRNA expression, mimicking the results of dcSSc skin epidermis (Fig. 2 E). These results indicate that epithelial Fli1 deficiency largely recapitulates the epidermal phenotype characteristic of dcSSc in vivo.
Figure 2.
K14Cre;fl/fl mice exhibit SSc-like epidermal phenotypes. (A, left) A representative image of mice at ∼4 mo of age. (Right) The comparison of body weight between two strains of mice. n = 7 per genotype. (B, left) Immunohistochemistry for Fli1 in the back skin of mice. Bars, 50 µm. (Right) The graph summarizes the result of semiquantitative scoring of the staining intensity. n = 7 per genotype. (C) Immunohistochemistry for K6, K16, IL-1α, CTGF, and SNAI1 in the back skin of mice. n = 5 per genotype. Bars, 50 µm. (D) The results of staining intensity in the epidermis as shown in C were evaluated with semiquantitative scoring. n = 5 per genotype. (E) mRNA expressions of these key molecules were determined by qRT-PCR in the epidermal sheets prepared from the back skin of mice. n = 5 per genotype. Data are shown as mean ± SEM. *, P < 0.05; **, P < 0.01; by two-tailed Mann-Whitney U test. The results are from representative experiments that have been repeated twice in different pairs of mice with similar results. Representative images of immunohistochemistry are shown. AU, arbitrary units.
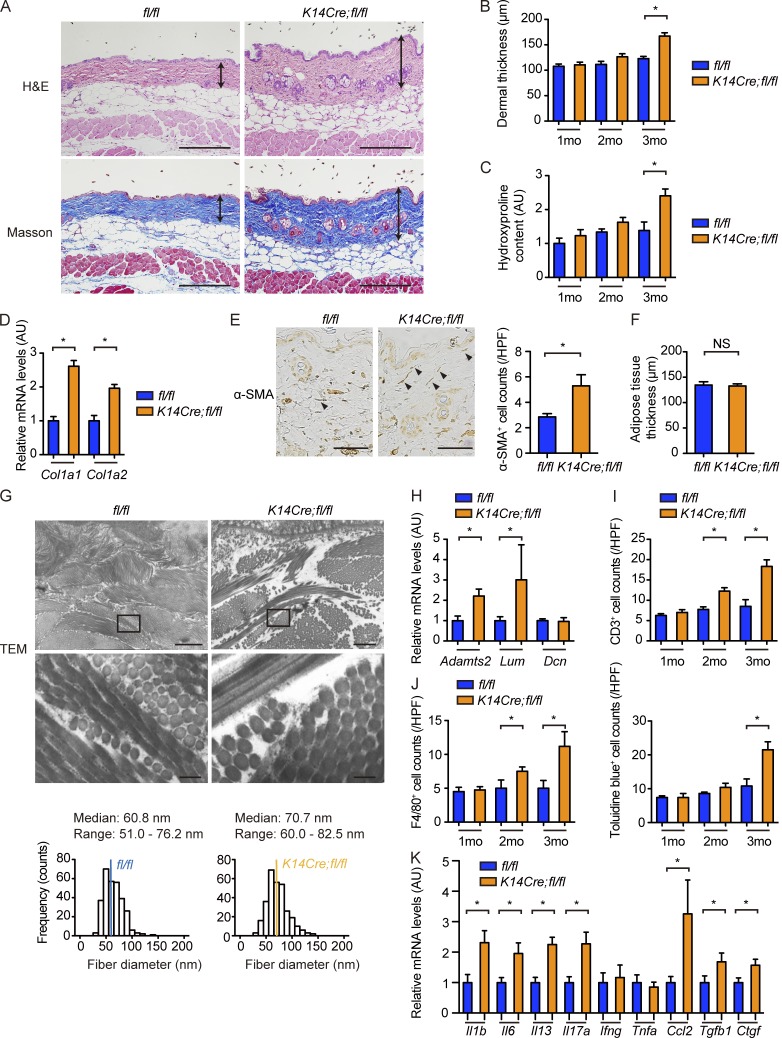
K14Cre;fl/fl mice spontaneously develop dermal fibrosis similar to that of SSc
We proceeded to analyze the skin as a whole including the dermis. Dermal fibrosis with a thickened dermis, more densely packed collagen bundles, and enhanced signal intensity of Masson-Trichrome staining was observed in K14Cre;fl/fl mice, becoming evident at the age of 3 mo (Fig. 3, A and B). Fibrosis was homogenous throughout the dermis without predominance of the subepithelial upper dermis (Fig. 3 A). As well, total collagen content was elevated in the skin of K14Cre;fl/fl mice, reaching a statistical significance at the age of 3 mo (Fig. 3 C). Moreover, Col1a1 and Col1a2 mRNA expressions and the number of myofibroblasts were also increased in the skin of K14Cre;fl/fl mice at the age of 3 mo (Fig. 3, D and E). However, unlike human SSc and its several mouse models (Marangoni et al., 2015), there was no evident subcutaneous adipose tissue atrophy in K14Cre;fl/fl mice (Fig. 3 F). Further analyses using electron microscopy delineated mildly increased thickness and diameter variability in the collagen fibrils in K14Cre;fl/fl mice (Fig. 3 G), consistent with the impairment of collagen fibrillogenesis as represented by the up-regulation of fibrillogenesis-related molecules such as ADAMTS2 (a disintegrin and metalloproteinase with thrombospondin motif 2) and lumican (Fig. 3 H). These alterations related to collage fibrillogenesis are reminiscent of the previously reported changes in SSc dermis (Perlish et al., 1988; Noda et al., 2014).
Figure 3.
K14Cre;fl/fl mice spontaneously develop SSc-like dermal fibrosis. (A) H&E and Masson-Trichrome staining of the back skin of 3-mo-old mice. Double-headed arrows represent dermal thickness. Bars, 200 µm. (B) The dermal thickness was evaluated at the ages of 1, 2, and 3 mo in each group of mice. (C) Hydroxyproline contents of the back skin were compared between two strains of mice at the ages of 1, 2, and 3 mo. The values are normalized to the value of 1-mo-old fl/fl mice. (D) mRNA expressions of the Col1a1 and Col1a2 genes were measured by qRT-PCR in the back skin of 3-mo-old mice. (E) Skin sections from 3-mo-old mice were stained for α-SMA. (Left) Representative images of the staining, with arrowheads pointing to α-SMA–positive myofibroblasts. Bars, 50 µm. (Right) The bar graph shows the number of α-SMA–positive myofibroblasts per HPF. (F) Adipose tissue thickness in each group of mice was evaluated at the age of 3 mo. (G) Transverse and longitudinal sections of collagen fiber alignment in the superficial dermis were examined by transmission electron microscopy (TEM). (Middle) Higher magnification images of the regions indicated by squares in the top panels. Bars: (top) 1 µm; (middle) 100 nm. (Bottom) Median, range, and frequency distribution profiles obtained by manually measuring the smallest diameter of 300 collagen fibrils are summarized. Colored bars in the histogram indicate the median of each group. n = 3 mice per genotype. (H) mRNA expressions of genes related to collagen fibrillogenesis were determined by qRT-PCR in the back skin of 3-mo-old mice. (I and J) Inflammatory cell infiltrations in the back skin at the ages of 1, 2, and 3 mo were assessed per HPF. Immunostaining for CD3 (I) and F4/80 (J, left) and toluidine blue staining (J, right) were performed to count T cells, macrophages, and mast cells, respectively. (K) mRNA levels of key cytokines, chemokines, and growth factors were assessed by qRT-PCR in the back skin of 3-mo-old mice. Data are shown as mean ± SEM. *, P < 0.05 by two-tailed Mann-Whitney U test. n = 4 – 5 mice per genotype, unless otherwise indicated. The results are from representative experiments that have been repeated three times in different pairs of mice with similar results. Representative images of H&E, Masson-Trichrome, and α-SMA staining and transmission electron microscopy are shown. AU, arbitrary units.
With respect to inflammatory components, the perivascular infiltration of T cells, macrophages, and mast cells was augmented throughout the whole dermis in K14Cre;fl/fl mice by the age of 2 mo, without evidence of interface dermatitis (Fig. 3, I and J). The analysis of the expression profiles of several key profibrotic/proinflammatory cytokines, chemokines, and growth factors disclosed the heightened expression of Il1b, Il6, Il13, Il17a, Ccl2, Tgfb1, and Ctgf mRNAs in the skin of K14Cre;fl/fl mice (Fig. 3 K), also simulating the expression profiles in SSc lesional skin. Collectively, these results indicate that epithelial Fli1 deficiency is sufficient to induce a pathological dermal fibrosis similar to that of SSc at histological, morphological, and molecular levels in mice.
K14Cre;fl/fl mice recapitulate esophageal involvement of SSc
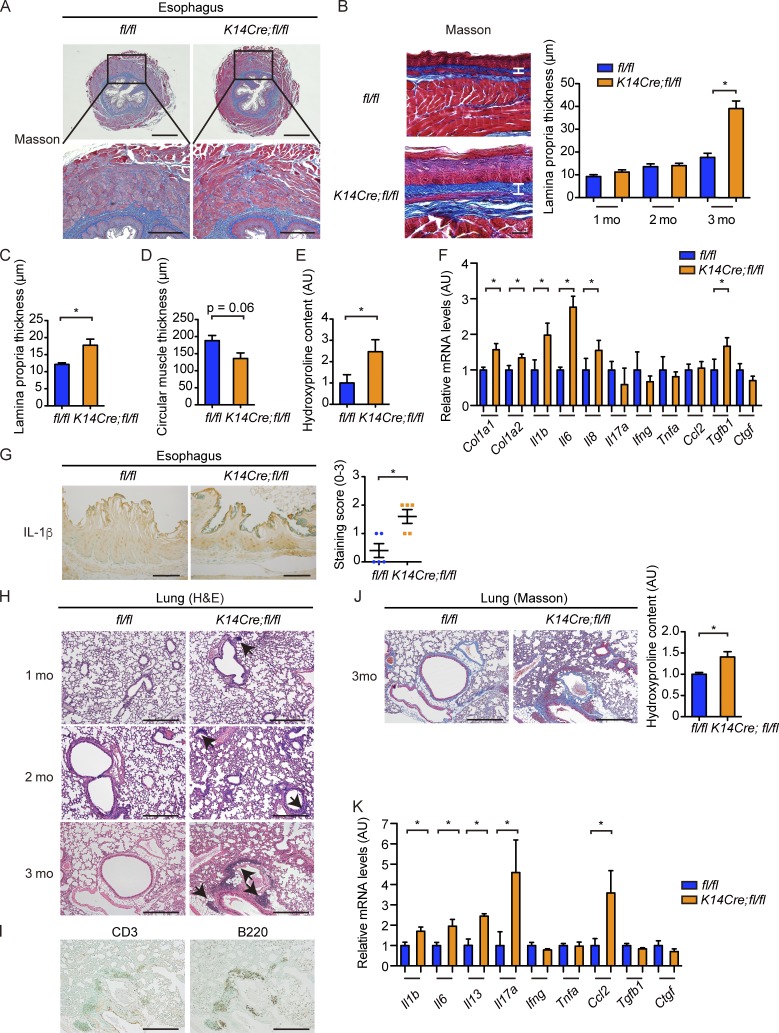
We further examined the esophagus, the second most frequently affected organ in SSc, because it has stratified squamous epithelia expressing K14 (Gabrielli et al., 2009). Augmented collagen deposition as represented by enhanced signal intensity of Masson-Trichrome staining was evident in the subepithelial lamina propria in the lower esophagus of aged K14Cre;fl/fl mice, and the increase in the thickness of this layer, which was reminiscent of esophageal and gastric wall alteration in SSc (Manetti et al., 2007; Taroni et al., 2015), became overt by the age of 3 mo (Fig. 4, A and B). The increased lamina propria thickness was also observed in the upper esophagus above the esophageal hiatus in K14Cre;fl/fl mice (Fig. 4 C). In contrast, the thickness of the circular muscle layer tended to be decreased in the lower esophagus of K14Cre;fl/fl mice with augmented collagen deposition between the muscular fibers (Fig. 4, A and D), resembling SSc esophagus (Roberts et al., 2006). Increased collagen deposition in the esophagus of K14Cre;fl/fl mice was even more supported by the results of hydroxyproline assay (Fig. 4 E). Moreover, gene expressions of fibrosis-related molecules, cytokines, and growth factors, such as Col1a1, Col1a2, Il1b, Il6, Il8, and Tgfb1, were up-regulated in the lower esophagus of K14Cre;fl/fl mice (Fig. 4 F). The augmented expression of IL-1β was also immunohistochemically confirmed in the esophageal epithelial cells of K14Cre;fl/fl mice (Fig. 4 G), further supporting the activation of esophageal epithelial cells. Considering the up-regulation of IL1B, IL6, and IL8 mRNAs in SSc esophagus (Rieder et al., 2010; Taroni et al., 2015), these results suggest that K14Cre;fl/fl mice recapitulate the histological and molecular features of SSc esophageal disease.
Figure 4.
K14Cre;fl/fl mice develop esophageal fibrosis and ILD. (A) Masson-Trichrome staining of the lower esophagus of 3-mo-old fl/fl and K14Cre; fl/fl mice. (Bottom) Higher magnification images of the regions indicated by squares in the top panels. Bars: (top) 500 µm; (bottom) 200 µm. (B) The lower esophagi were cut along the longitudinal direction, spread on sheets, fixed in formalin, and embedded in the paraffin. Masson-Trichrome staining was performed, and the thickness of the lamina propria was evaluated at the ages of 1, 2, and 3 mo. Vertical bars represent lamina propria thickness. Bars, 50 µm. (C) The thickness of lamina propria at upper (thoracic) esophagus was evaluated at the age of 3 mo. (D) The thickness of the circular muscle layer was evaluated in the sections of 3-mo-old mice. (E) Hydroxyproline contents of the lower esophagus were compared between these two strains of mice at the age of 3 mo. (F) mRNA levels of type I collagen, cytokines, chemokines, and growth factors in the lower esophagi of 3-mo-old mice were assessed. (G) The lower esophagus sections from 3-mo-old mice were immunohistochemically stained for IL-1β. Bars, 50 µm. (Right) The graph shows the result of semiquantitative scoring of the staining intensity. (H) H&E staining of the lungs from 1-, 2-, and 3-mo-old fl/fl and K14Cre;fl/fl mice. Arrows indicate the peribronchial lymphocyte aggregates. Bars, 200 µm. (I) Infiltrating inflammatory cells were immunohistochemically stained for CD3 and B220 in the lungs of 3-mo-old K14Cre;fl/fl mice. Bars, 200 µm. (J, left) The Masson-Trichrome staining of the lungs from 3-mo-old fl/fl and K14Cre;fl/fl mice. Bars, 200 µm. (Right) Collagen contents of the total left lung from these mice were measured by hydroxyproline assay. (K) mRNA levels of cytokines, chemokines, and growth factors in the lung of 3-mo-old mice were assessed by qRT-PCR. Data are shown as mean ± SEM. *, P < 0.05 by two-tailed Mann-Whitney U test. n = 4–5 mice per each genotype in all experiments. The results are from representative experiments that have been repeated three times in different pairs of mice with similar results. Representative images of H&E and Masson-Trichrome staining and immunohistochemistry are shown. AU, arbitrary units.
K14Cre;fl/fl mice spontaneously develop ILD
Further examination of histological changes in various internal organs revealed enhanced mononuclear cell infiltration into the lung interstitium (Fig. 4 H), whereas other internal organs, such as the heart, liver, kidney, stomach, small intestine, and large intestine, did not show evident fibrosis or inflammation (not depicted). The inflammatory cell infiltration in the lungs was enhanced to the slightly noticeable level already at the age of 1 mo and became more prominent along with aging especially in the peribronchial areas in K14Cre;fl/fl mice (Fig. 4 H). By the age of 3 mo, several mice with severe disease developed peribronchial lymphocyte aggregates or lymphoid structures composed of T and B cells, resembling inducible bronchus-associated lymphoid tissues (Fig. 4, H and I), which occur in the settings of chronic inflammation, including ILD, in human autoimmune diseases (Rangel-Moreno et al., 2006). Total collagen content was mildly increased, and interstitial fibrosis was particularly salient in the peribronchial interstitium as shown by Masson-Trichrome staining (Fig. 4 J). Furthermore, expression of IL-1β, IL-6, IL-13, IL-17A, and CCL2, known to play important roles in the development of ILD (Wilson and Wynn, 2009), was up-regulated at the mRNA level (Fig. 4 K), suggesting that Th2/Th17 cell–skewed immune polarization is involved in ILD in K14Cre;fl/fl mice. In aggregate, the emergence of dermal and esophageal fibrosis together with ILD in K14Cre;fl/fl mice indicated striking similarities of the fibrotic and autoimmune phenotypes between these mice and human SSc.
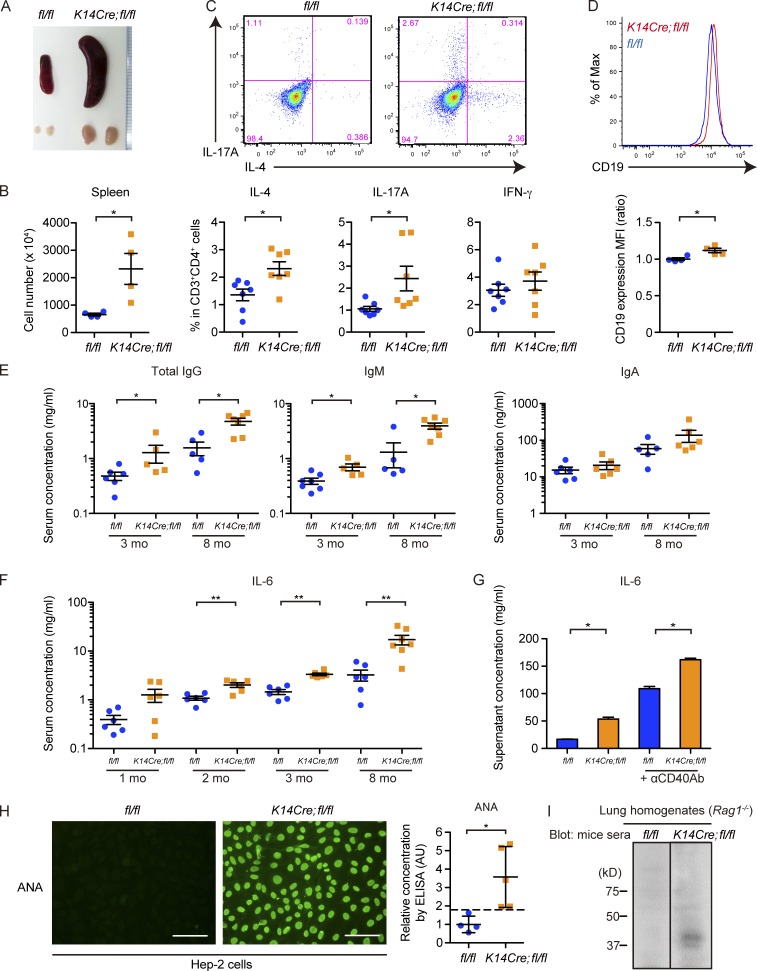
K14Cre;fl/fl mice exhibit prominent Th2/Th17 cell polarization and abnormal B cell activation together with the emergence of autoantibodies
The immune changes in the affected organs of K14Cre;fl/fl mice suggested systemic immune activation polarized toward the Th2/Th17 axis. Further analyses of mutant mice revealed splenomegaly and lymphadenopathy together with the increased number of splenocytes (Fig. 5, A and B). Intracellular cytokine staining of CD4+ T cells isolated from lymph nodes showed enhanced proportion of IL-4/IL-17A–positive cells in 3-mo-old K14Cre;fl/fl mice, supporting the presence of Th2/Th17 cell–skewed immune polarization (Fig. 5 C). Under the same condition, CD19 expression was augmented ∼12% on splenic B cells (B220+CD19+ cells; Fig. 5 D), possibly reflecting their abnormal activation because an ∼20% increase in CD19 expression was observed in activated B cells from SSc patients and human CD19 transgenic mice producing excessive amount of autoantibodies (Saito et al., 2002). Consistently, serum levels of total IgG and IgM were elevated in 3-mo-old K14Cre;fl/fl mice, which became more evident in older mice (Fig. 5 E). Serum concentration of IL-6, a key cytokine in SSc pathogenesis (O’Reilly et al., 2013), was significantly higher in K14Cre;fl/fl mice as early as 2 mo of age, and its elevation got more remarkable along with aging (Fig. 5 F). B cell activation in K14Cre;fl/fl mice was further supported by the increased IL-6 concentration in the culture supernatant of isolated B cells (Fig. 5 G). Moreover, ANA was detected in K14Cre;fl/fl mice as early as 2 mo of age (two out of eight mice in 2-mo-old mice, six out of eight mice in 3-mo-old mice, and seven out of eight mice in 8-mo-old mice), whereas not at all in control fl/fl mice (n = 8 at each time point; Fig. 5 H). Elevated ANA titers in K14Cre;fl/fl mice were also confirmed by ELISA (Fig. 5 H). To more strictly confirm the presence of systemic autoimmune response, autoantibodies against self-proteins were assessed by immunoblotting using homogenates from the lungs of Rag1−/− mice, which lack their intrinsic immunoglobulins. The blotting of electrophoresed Rag1−/− mice lung homogenates with sera from K14Cre;fl/fl mice disclosed the presence of autoantibodies against certain lung proteins in these mice (Fig. 5 I). Collectively, these findings suggest the emergence of robust systemic autoimmunity in K14Cre;fl/fl mice, characterized by the prominent Th2/Th17 cell polarization and abnormal B cell activation with autoantibody production.
Figure 5.
K14Cre;fl/fl mice exhibit prominent autoimmunity with Th2/Th17 cell polarization and abnormal B cell activation leading to autoantibody production. (A) A representative image of the gross appearance of spleens and inguinal lymph nodes from 3-mo-old fl/fl and K14Cre;fl/fl mice. (B) The number of splenocytes in 3-mo-old fl/fl and K14Cre;fl/fl mice was counted. n = 4 per each group. (C) Lymphocytes isolated from inguinal lymph nodes were stained for CD3 and CD4 and intracellularly stained for IL-4, IL-17A, and IFN-γ. (Top) Representative two-dimensional plots for IL-4 and IL-17A in CD3+CD4+ cells. (Bottom) The percentages of cells positive for IL-4, IL-17A, and IFN-γ are summarized. n = 7 mice per each group. (D) Splenocytes from 3-mo-old fl/fl and K14Cre;fl/fl mice were stained for B220 and CD19. B220+CD19+ cells were analyzed for CD19 expression. A representative histogram of mean fluorescence intensity (MFI; top) and the comparison of mean fluorescence intensities (bottom) are shown. n = 4 mice per genotype. (E) Sera from 3- and 8-mo-old mice were analyzed for the concentrations of total IgG, IgM, and IgA. n = 5–7 mice per genotype. Note that the results are shown with log scale. (F) Sera from 1-, 2-, 3-, and 8-mo-old mice were analyzed for the concentration of IL-6. n = 6–7 mice per genotype. (G) B cells were isolated from splenocytes of 3-mo-old mice and cultured for 48 h, either unstimulated or stimulated with anti-CD40 antibody (αCD40Ab). The culture supernatants were subject to the measurement of IL-6 concentration. n = 4 mice per each group. (H) The presence of ANA in the sera was assessed by indirect immunofluorescence with Hep-2 cells. (Left) The photographs show representative immunofluorescence images with sera from 2-mo-old mice. n = 8 mice per genotype. Bars, 100 µm. (Right) Titers of ANA in the sera were assessed with a specific ELISA. The dashed line indicates the mean + 2 SD value of fl/fl mice. n = 4–5 mice per genotype. (I) Immunoblotting of lung tissue lysates from Rag1−/− mice with sera from fl/fl and K14Cre;fl/fl mice. n = 3 mice sera per group. For photographs, immunofluorescence, and immunoblotting, representative results are shown. Data are shown as mean ± SEM. *, P < 0.05; **, P < 0.01; by two-tailed Mann-Whitney U test. The results are from representative experiments that have been repeated three times in different pairs of mice. AU, arbitrary units; MFI, mean fluorescence intensity.
mTECs robustly express Fli1
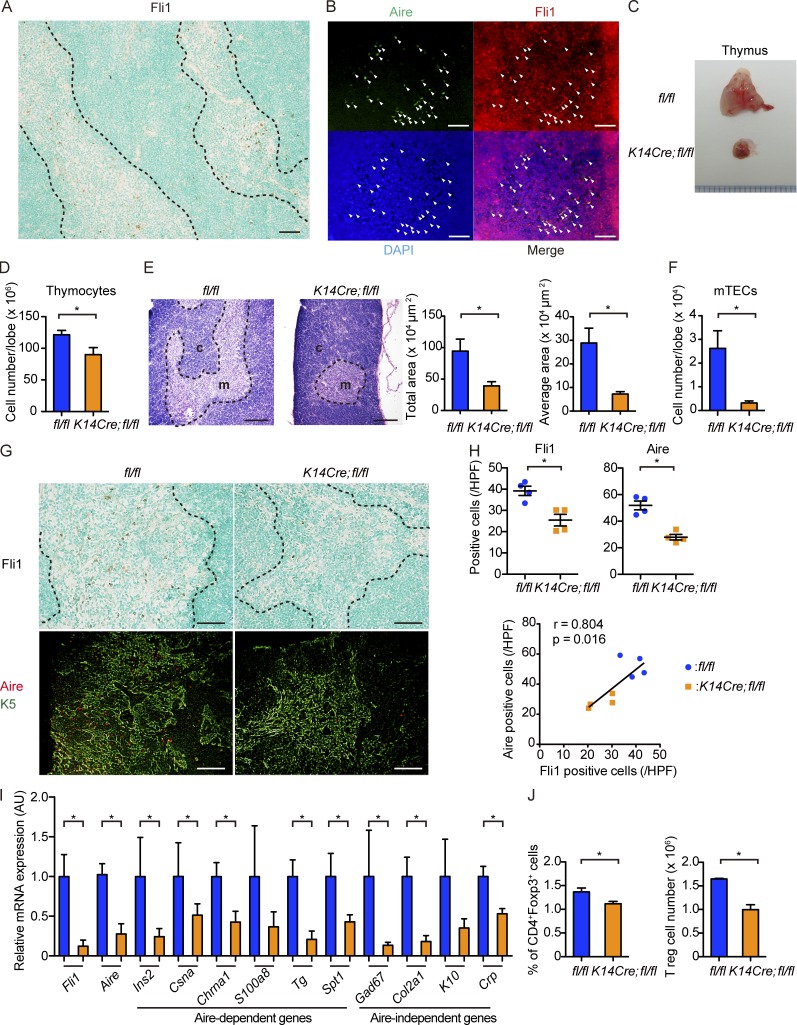
The autoimmune phenotype in K14Cre;fl/fl mice prompted us to focus on the thymus, the central organ for immune self-tolerance consisting of thymocytes and two kinds of TECs, cortical TECs and mTECs. Because mTECs abundantly express K5 and K14, Cre recombinase under the control of K5 or K14 promoter was used to create mTEC-specific conditional knockout mice (Sano et al., 2001; Sukseree et al., 2012). Various tissue-specific self-antigens (TSAs) are expressed by mTECs under the control of master regulators, including autoimmune regulator (Aire), resulting in the elimination of autoreactive T cells by apoptosis and the development of T reg cells in the thymus (Anderson et al., 2002; Mathis and Benoist, 2009). Importantly, the decrease in Aire expression suppresses the expression of TSAs dose dependently, allowing escape of autoreactive T cells from negative selection (Liston et al., 2004; Kont et al., 2008), and affects the development and differentiation of mTECs (Matsumoto, 2011). Given that Fli1 is abundantly expressed in the thymus (Mélet et al., 1996; Qiu et al., 2011), we hypothesized that immune activation and autoimmunity of K14Cre;fl/fl mice might be caused by the thymic abnormalities. To address this issue, we analyzed Fli1 expression in the thymuses of wild-type (C57BL/6) mice. Indeed, the immunostaining of Fli1 in the thymuses of wild-type mice illustrated scattered strong positivity in the nuclei of the cells almost exclusively confined to the thymic medulla (Fig. 6 A). Supportively, double immunofluorescence revealed the weak signal intensity of Fli1 in thymocytes and strong signal intensity of Fli1 in the medullary stromal cells expressing Aire, indicating that mTECs abundantly express Fli1 (Fig. 6 B). Therefore, we hypothesized that K14Cre;fl/fl mice develop autoimmunity because of a Fli1 deficiency–dependent defect of the thymus, or especially of the mTECs, and proceeded to further investigations of the thymus.
Figure 6.
mTECs robustly express Fli1, and K14Cre;fl/fl mice show thymic hypotrophy with decreased Aire expression. (A and B) Immunohistochemistry for Fli1 (A) and double immunofluorescence for Aire and Fli1 (B) were performed with thymuses of 1-mo-old wild-type (C57BL/6) mice. In immunofluorescence, Aire, Fli1, and nuclei were stained with fluorescein isothiocyanate (green), Alexa Flour 555 (red), and DAPI (blue), respectively. Arrowheads indicate Aire-positive mTECs expressing Fli1. n = 5 mice. Bars, 50 µm. (C) Gross appearance of thymuses from 2-mo-old fl/fl and K14Cre;fl/fl mice. (D) The number of thymocytes was counted per lobe. n = 4 mice per genotype. (E) H&E staining of thymuses from 2-mo-old fl/fl and K14Cre;fl/fl mice. c, cortex; m, medulla. (Right) The total area of the medulla and the mean area per islet in each group. n = 4 per each group of mice. Bars, 200 µm. (F) Thymuses from 2-mo-old mice were subjected to flow cytometric analysis. The number of mTECs, defined as CD45−EpCAM+Ly51− cells, was calculated per lobe. n = 4 per group of mice. (G) Immunohistochemistry for Fli1 and double immunofluorescence for Aire and K5 with thymuses from 8-wk-old mice. K5-positive areas indicate the thymic medulla. Bars, 50 µm. (H, top) Fli1- and Aire-positive cells per HPF were counted. (Bottom) The correlation between the numbers of Aire- and Fli1-positive cells was assessed by Spearman’s correlation analysis. The solid line represents the regression line. n = 4 per group. (I) mRNA expression analysis for Fli1, Aire, and representative Aire-dependent and Aire-independent genes in the sorted mTECs defined as CD45−EpCAM+Ly51− cells. n = 4 per group. AU, arbitrary units. (J) Analysis on thymic T reg cells. Gates were set serially on singlets, and CD3+ and CD4+Foxp3+ cells were analyzed. The proportion of CD4+Foxp3+ cells in CD3+ cells (left) and the number of these cells (right) are shown. n = 4 mice per group. (A, E, and G) Dashed lines indicate the border between medulla and cortex. For immunofluorescence, immunohistochemistry, and H&E staining, representative images of five independent experiments are shown. Results of other experiments are from representative experiments that have been repeated three times in different pairs of mice yielding similar results. Data are shown as mean ± SEM. *, P < 0.05 by two-tailed Mann-Whitney U test.
Thymuses of K14Cre;fl/fl mice exhibit prominent atrophy with decreased expression of Aire
In line with our hypothesis, we found salient atrophy of thymuses with the significantly decreased number of thymocytes in K14Cre;fl/fl mice (Fig. 6, C and D). Hematoxylin and eosin (H&E) staining showed the reduced size of bright areas corresponding to thymic medulla and total medullary area and area per islet were significantly decreased (Fig. 6 E). Correspondingly, flow cytometric analyses of the thymic stromal cells revealed a significantly smaller number of mTECs (defined as CD45−epithelial cell adhesion molecule [EpCAM]+Ly51− cells) in K14Cre;fl/fl mice (Fig. 6 F). In immunostaining, both Fli1-positive cells and Aire-positive cells were notably decreased with a strong correlation in the thymic medulla of K14Cre;fl/fl mice (Fig. 6, G and H). Consistent with these findings, the analyses of mRNA expression in sorted mTECs demonstrated significantly suppressed Fli1 and Aire gene expressions in K14Cre;fl/fl mice (Fig. 6 I). Also observed was the down-regulation of Aire-dependent as well as Aire-independent genes, such as Ins2, Chrna1, Sp1, Col2a1, and Crp, suggesting the defective presentation of self-antigens regulated by Aire and other transcription factors in their thymuses (Fig. 6 I). Because the presentation of self-antigens in mTECs plays an essential part in T reg cell development (Mathis and Benoist, 2009), we also assessed T reg cells in the thymuses. As expected, the proportion and number of thymic T reg cells (defined as CD3+CD4+Foxp3+ cells) were significantly decreased in K14Cre;fl/fl mice (Fig. 6 J). Collectively, these results indicate that the thymuses of K14Cre;fl/fl mice exhibit an abnormality in mTEC development, including the suppressed expression of self-antigens by loss of Aire, and possibly other transcription factors, contributing to the development of the deletion-escaped autoreactive T cells underlying autoimmunity.
Development of ILD, but not dermal and esophageal fibrosis, in K14Cre;fl/fl mice is dependent on their autoreactive T cells
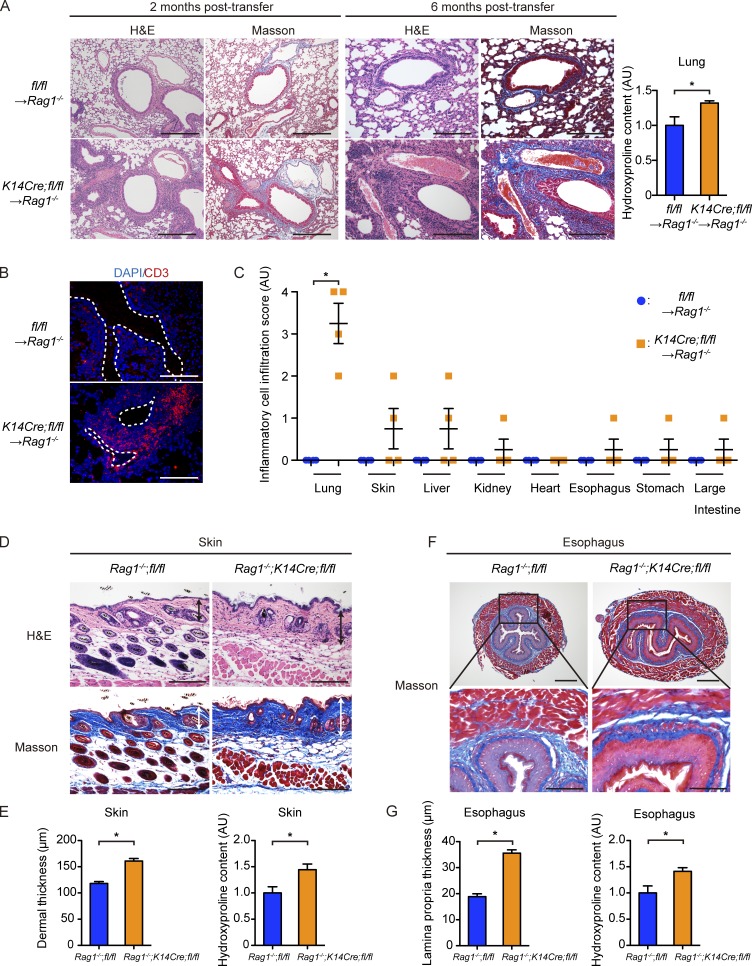
To verify whether deletion-escaped autoreactive T cells are responsible for autoimmunity in K14Cre;fl/fl mice, we transferred isolated T cells into Rag1−/− mice lacking intrinsic mature lymphocytes. As expected, transfer of NK1.1−CD3+ T cells isolated from K14Cre;fl/fl mice to Rag1−/− mice reproduced the prominent interstitial inflammatory cell infiltration, mainly composed of CD3+ T cells, in the lungs with increased collagen deposition in the interstitium, whereas transfer from the littermate fl/fl mice did not (Fig. 7, A and B). These changes were seen as early as 2 mo after transfer and strikingly aggravated at 6 mo after transfer (Fig. 7 A). The analysis of various internal organs of recipient Rag1−/− mice at 6 mo after transfer demonstrated that such a prominent inflammatory phenotype was almost exclusive to the lungs, except some mice exhibiting moderately increased inflammatory cell infiltration in several organs, including the skin, liver, kidney, and esophagus (Fig. 7 C). Importantly, fibrosis was totally absent in the skin and esophagus even at 6 mo after transfer, confirmed with histological observation and measurements of collagen contents (not depicted). These results indicated that ILD development in K14Cre;fl/fl mice is primarily caused by autoreactive T cells, whereas dermal and esophageal fibrosis could not be caused by these cells alone.
Figure 7.
Development of ILD in K14Cre;fl/fl mice is dependent on their autoreactive T cells, whereas skin and esophageal fibrosis is not. (A) Representative H&E and Masson-Trichrome staining of the lungs of recipient Rag1−/− mice to which 106 NK1.1−CD3+ T cells from fl/fl mice or K14Cre;fl/fl mice were transferred 2 (left) and 6 (right) mo before. Bars, 200 µm. (Right) Collagen contents of the total left lung from the recipient mice (2 mo after transfer) were measured by hydroxyproline assay. n = 4–7 mice per group. (B) Immunofluorescence with anti-CD3 antibody and DAPI was performed using the lungs of mice under the same conditions (2 mo after transfer). White, dashed lines indicate the bronchi. Bar, 200 µm. (C) Histological evaluation of inflammatory cell infiltration to various internal organs of recipient Rag1−/− mice 6 mo after transfer. (D) H&E and Masson-Trichrome staining of the back skin of 3-mo-old Rag1−/−;K14Cre;fl/fl and Rag1−/−;fl/fl mice. Double-headed arrows represent dermal thickness. Bars, 200 µm. (E, left) Dermal thickness was evaluated at the age of 3 mo in each group of mice. (Right) Hydroxyproline contents of the back skin were compared between these two groups of mice. (F, top) Masson-Trichrome staining of the lower esophagus of 3-mo-old Rag1−/−;K14Cre;fl/fl and Rag1−/−;fl/fl mice. Bars, 500 µm. (Bottom) Higher magnification images of the regions indicated by squares in the top panels. Bars, 200 µm. (G, left) Lamina propria thickness was evaluated at the age of 3 mo in each group of mice. (Right) Hydroxyproline contents of the lower esophagus were compared between these two groups of mice. n = 4 mice per group, unless otherwise indicated. Data in graphs are shown as mean ± SEM. *, P < 0.05 by two-tailed Mann-Whitney U test. For histological analyses, representative images of three independent experiments are shown. Results of other experiments are from representative experiments that have been repeated twice in different pairs of mice yielding similar results. AU, arbitrary units.
To further analyze whether dermal and esophageal fibrosis is driven by their epithelia and/or systemic autoimmunity, we generated Rag1−/−;K14Cre;fl/fl mice by crossing Rag1−/− mice and K14Cre;fl/fl mice. These mice lack mature lymphocytes, whereas the epithelial phenotype in the skin and esophagus is considered to be identical to that of K14Cre;fl/fl mice. We analyzed Rag1−/−;K14Cre;fl/fl mice at the age of 3 mo, with their littermate Rag1−/−;fl/fl mice as controls. Of note, the skin and esophagus of Rag1−/−;K14Cre; fl/fl mice showed a significant increase in the thickness of dermis and lamina propria, respectively, compared with Rag1−/−;fl/fl mice (Fig. 7, D–G). In accordance with the observation in K14Cre;fl/fl mice, inflammatory cell infiltration was enhanced in the skin of Rag1−/−;K14Cre;fl/fl mice (Fig. 7 D), whereas ILD was totally absent (not depicted). In addition, collagen content in the skin and esophagus of Rag1−/−;K14Cre;fl/fl mice was significantly increased compared with controls, although the ratio of increase was smaller than that between K14Cre;fl/fl mice and fl/fl mice (Fig. 7, E and G).
Next, we conducted splenocyte transfer from fl/fl mice to Rag1−/−;K14Cre;fl/fl mice to analyze whether T cell–mediated autoimmunity in K14Cre;fl/fl mice is truly derived from the thymic abnormalities or, alternatively, from the systemic skin abnormality. If the autoimmunity is derived from the skin, the autoimmune phenotype should be reproduced in the recipient Rag1−/−;K14Cre;fl/fl mice by the transferred lymphocytes, which are not originally autoreactive. We transferred 107 splenocytes from 3-mo-old fl/fl mice to 1-mo-old Rag1−/−;K14Cre;fl/fl mice, sacrificed them 3 mo later, and analyzed the presence of serum ANA and ILD in the recipient mice, as these traits seem to be the hallmarks of the autoimmune phenotypes of K14Cre;fl/fl mice. The results showed negativity of ANA and ILD in all recipient Rag1−/−;K14Cre;fl/fl mice (10 out of 10; not depicted). Collectively, these results suggest that tissue fibrosis in the skin and esophagus of K14Cre;fl/fl mice is principally driven by their epithelia, whereas the T cell–mediated autoimmunity leading to ILD depends on the thymic abnormality.
Fli1 serves as a potent transcriptional activator of the AIRE gene in epithelial cells
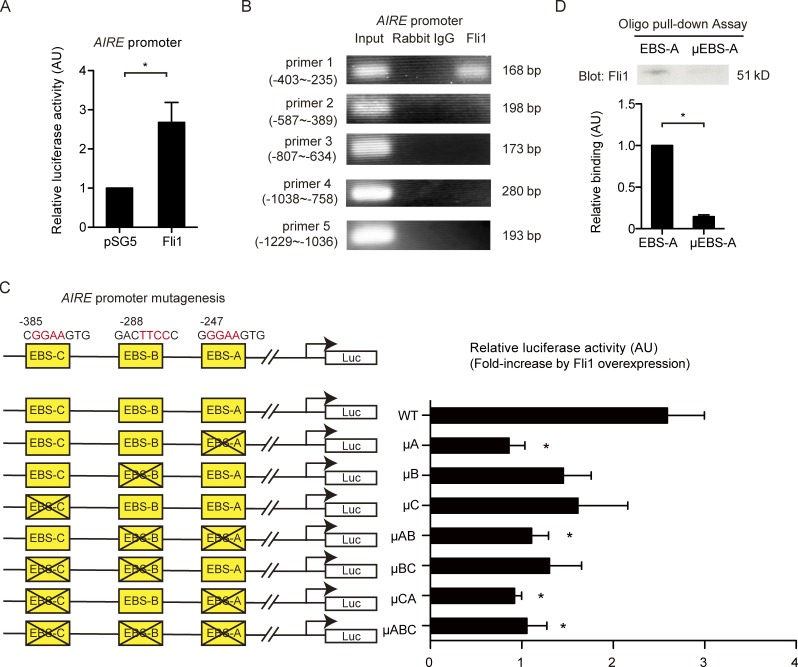
The thymic defects, including its atrophy and suppressed expression of Aire in K14Cre;fl/fl, mice prompted us to investigate whether Fli1 directly regulates Aire expression. Indeed, several Ets transcription factors, such as Ets1, Ets2, and ESE-1, have positive effects on AIRE transcription (Murumägi et al., 2006). Because of the difficulties in isolating and culturing mTECs, we used NHKs because Aire is also expressed in keratinocytes (Kumar et al., 2011). The analysis with the luciferase promoter construct revealed a markedly enhanced activity of the AIRE promoter in response to Fli1 overexpression (Fig. 8 A). ChIP assay displayed the binding of Fli1 to the AIRE promoter at −403/−235 bp upstream of the transcription start site (Fig. 8 B). This region contains three putative Ets consensus binding sites (EBS; GGAA/TTCC; Fig. 8 C). We mutated these EBSs (EBS-A/-B/-C) individually and assessed luciferase activity. The results showed particular importance of EBS-A (Fig. 8 C). Furthermore, the oligonucleotide pull-down assay verified the sequence-specific binding of Fli1 to EBS-A (Fig. 8 D). Thus, Fli1 serves as a potent transcriptional activator of the AIRE gene in epithelial cells.
Figure 8.
Fli1 directly regulates transcription of the AIRE gene. (A) Luciferase activities were measured in NHKs transfected with the AIRE promoter luciferase construct and either Fli1 expression vector or empty vector (pSG5). n = 4 per group. (B) ChIP in NHKs was performed with anti-Fli1 antibody and the primers specific for the designated areas of the AIRE gene promoter, covering all the EBSs between −1235 and 1. (C) Three putative Fli1 binding sites were mutated as shown in the illustration. Luciferase (Luc) activities were measured in NHKs transfected with wild-type or mutated promoter constructs together with Fli1 expression vector. Fli1-dependent induction of luciferase activities, which is normalized to luciferase activities induced by pSG5 transfection, is shown for each construct. n = 4 per group. The differences were compared with the results of the wild-type promoter construct. (D) The oligonucleotide pull-down assay was conducted using oligonucleotides with wild-type EBS-A or mutated EBS-A (µEBS-A). (Top) A representative blot of three independent experiments. (Bottom) The band density of each blot was measured by densitometry and summarized in the graph. In all the graphs, data are shown as mean ± SEM. *, P < 0.05 by two-tailed unpaired Student’s t test. The results are from representative experiments that have been repeated twice independently, unless otherwise indicated. AU, arbitrary units.
Aire expression is decreased in SSc keratinocytes
Finally, we investigated the expression of Aire in SSc patients. Because of the limited availability of human thymus samples, we used human skin samples instead because Aire has been reported to be expressed in human keratinocytes in cell culture (Clark et al., 2005). The immunohistochemical analysis with the skin samples showed the expression of Aire abundantly in the nuclei of normal keratinocytes but moderately or weakly in the nuclei of SSc keratinocytes (Fig. 9 A). Notably, the expression was uniformly attenuated in almost all of the keratinocytes in dcSSc patients with anti–topoisomerase I antibody, which typically exhibit systemic autoimmunity and chronic inflammation resulting in multiple organ fibrosis, most frequent in the lungs and esophagus (Gabrielli et al., 2009). However, in lcSSc patients with anticentromere antibody, who manifest relatively milder or little fibrotic involvement of internal organs, the decrease in Aire expression was moderate to none, which was variable in individual keratinocytes. When evaluated by a four-point grading scale, the signal intensity was significantly decreased in dcSSc patients but not in lcSSc patients, compared with healthy controls (Fig. 9 B). Importantly, the staining intensity of Aire and Fli1 in the nuclei of keratinocytes revealed a significant positive correlation in the specimens from dcSSc patients and age- and sex-matched healthy controls in which both of these two molecules were analyzed together (Fig. 9 C). Consistent with these data, AIRE mRNA expression was significantly suppressed in SSc lesional skin compared with healthy control skin (Fig. 9 D) and positively correlated with FLI1 mRNA expression (Fig. 9 E). Additionally, we failed to detect the correlation of Aire expression with inflammatory cytokines IL-6 and TNFα at the mRNA level, indicating the negative causality of inflammation in AIRE down-regulation (not depicted). We also performed Aire staining in the skin of mice, as well as in the thymuses of wild-type mice as positive controls, to examine whether relevant findings were confirmed in mice. The result showed negativity of Aire protein expression in keratinocytes or other cells in the skin of both groups of mice (not depicted). This result was in line with a previous study that demonstrated very low Aire expression in normal mice skin, whereas its expression is to some extent induced in certain situations such as skin tumors (Hobbs et al., 2015). Collectively, although the significance of Aire expression in human skin remains to be resolved, these findings seem to support the close relationship between Aire and Fli1 in humans.
Figure 9.
Aire is expressed in the nuclei of human keratinocytes in vivo, and its expression is significantly down-regulated in SSc patients. (A) Representative images of immunohistochemistry for Aire in healthy controls (n = 8) and lcSSc (n = 4) and dcSSc (n = 4) patients. Bars, 200 µm. (B) The signal intensities of Aire staining in the keratinocytes in A were semiquantitatively evaluated with a four-point grading scale. Kruskal-Wallis test followed by Dunn’s posthoc test was used. (C) Correlation between signal intensities of Aire and Fli1 in the nuclei of keratinocytes in four dcSSc patients and four closely matched healthy controls was analyzed. (D) mRNA expression of the AIRE gene in skin samples from 7 healthy controls and 33 SSc patients was assessed by qRT-PCR. Two-tailed Mann-Whitney U test was used. (E) Correlation analysis of AIRE and FLI1 mRNA expressions in the skin samples used in D (7 healthy controls and 33 SSc patients). (C and E) The solid lines indicate the regression lines. Data are shown as mean ± SEM. *, P < 0.05; ***, P < 0.0001. Ab, antibody; AU, arbitrary units; Ctrl, control.
Discussion
In the field of SSc research, few previous studies have addressed the following simple question: why is SSc an autoimmune disease? Our present study might give a possible answer to this fundamental question: it is the epithelial Fli1 deficiency that is implicated in the development of immune activation toward self through defective thymic epithelial development together with down-regulation of the AIRE gene. Additionally, our study might provide a clue to another basic question on the characteristic organ specificity in this disease: why are the skin and esophagus the two most frequent targets of fibrosis in SSc? The answer might be the epithelial Fli1 deficiency that leads to the altered epithelial phenotype and subsequent fibrosis in these organs. Thus, the down-regulation of single transcription factor Fli1 provides a useful clue to solve these two unanswered fundamental questions in SSc pathogenesis, further supporting the notion that Fli1 deficiency serves as a predisposing factor of SSc by integrating the induction of SSc-related gene programs in various types of cells (Asano, 2015).
We demonstrate that K14Cre;fl/fl mice develop spontaneous organ fibrosis in the skin and esophagus. The skin change of K14Cre;fl/fl mice reproduced SSc-like phenotype with the increased dermal thickness and the impaired collagen fibril structure together with SSc-like alterations of fibrosis- and fibrillogenesis-related gene programs (Perlish et al., 1988). Furthermore, the expression profiles of cytokines and chemokines and the pattern of inflammatory cell infiltration were quite similar between K14Cre;fl/fl mice and human SSc. In addition, the esophagus of K14Cre;fl/fl mice recapitulated the histological features of SSc esophagus, such as increased collagen content and thicker subepithelial lamina propria, as well as the gene expression profiles of cytokines and growth factors. Importantly, the induction of SSc-like phenotype was confirmed in keratinocytes and esophageal stratified squamous epithelia by immunostaining and/or quantitative RT-PCR (qRT-PCR). Indeed, there certainly exist some differences between dermal fibrosis in K14Cre;fl/fl mice and human SSc. For example, K14Cre;fl/fl mice did not exhibit adipose tissue loss, which is an important feature of skin fibrosis in SSc and its mouse models (Marangoni et al., 2015). Furthermore, alteration of collagen fibril ultrastructure was milder than in the skin of human SSc and its mouse models (Noda et al., 2014). However, the similarity of the histological and molecular features of the skin and esophagus of K14Cre;fl/fl mice and human SSc strongly suggests that Fli1 deficiency–dependent activation of epithelial cells contributes to fibrosis development in these two most frequently affected organs in this disease (Gabrielli et al., 2009).
Similar to human SSc, K14Cre;fl/fl mice spontaneously developed ILD characterized by Th2/Th17 cytokine predominance, suggesting the common pathological background between them. Because K14 is not expressed in the lungs of K14Cre;fl/fl mice (unpublished data), lung epithelial cells are not directly subjected to Fli1 deletion by the Cre-LoxP system. Therefore, ILD likely results from the autoimmune reaction in these mice, as represented by the presence of autoantibodies against lung antigens. Because ILD is frequently caused by the impairment of central tolerance caused by defective thymus and/or Aire loss in mice (Shum et al., 2009), we hypothesized that epithelial Fli1 deficiency impairs thymic development and/or Aire expression in mTECs. Supporting this hypothesis, K14Cre;fl/fl mice had defective thymuses with mTECs expressing Aire at lower levels and autoreactive T cells selectively targeting the lungs. Importantly, we demonstrated that Fli1 functions as a potent transcriptional activator of the AIRE gene in epithelial cells. Accordingly, we concluded that epithelial Fli1 deficiency–dependent thymic defects with Aire suppression in mTECs largely contributes to ILD development in K14Cre;fl/fl mice and raise the possibility that such a mechanism also plays a role in SSc-ILD.
The contribution of Aire deficiency to autoimmunity has been reported in human diseases and animal models. In humans, AIRE gene mutations cause autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, a primary immunodeficiency disease characterized by various autoimmune manifestations, including ILD, via disrupted central tolerance (Mathis and Benoist, 2009). Importantly, certain AIRE gene polymorphisms are related to SSc concomitant with autoimmune thyroiditis (Ferrera et al., 2007). In mice, defective mTEC development as observed in Aire-mutated mice leads to significant autoimmune phenotypes, including ILD (Akiyama et al., 2005; Shum et al., 2009). This phenotype of Aire-mutated mice prompted us to investigate the Aire gene in K14Cre;fl/fl mice. However, infiltrating cells in ILD of the Aire-mutated mice are mainly composed of Th1/Th17 cell–polarized CD4+ T cells (Shum et al., 2009), thus suggesting that ILD of Aire-mutated mice is molecularly different from ILD in K14Cre;fl/fl mice and SSc. Importantly, mice with homozygous deletion of Ets1, a transcriptional activator of the AIRE gene (Murumägi et al., 2006) belonging to the same transcription family as Fli1, develop T cell–mediated systemic autoimmunity with Th2 cell–skewed polarization (Mouly et al., 2010), suggesting that the immune balance of autoreactive CD4+ T cells is influenced by additional factors other than Aire in Ets−/− mice. In the present study, K14Cre;fl/fl mice demonstrated ILD with Th2/Th17 cell–skewed polarization, indicating that the combination of Aire loss together with, not yet defined, additional epithelial Fli1 deficiency–dependent factors may promote Th2/Th17 immune responses. Indeed, considering that K14Cre;fl/fl mice generally exhibited thymic atrophy, which is relatively rare in Aire-knockout mice (Ramsey et al., 2002), and extensively suppressed expression of both Aire-dependent and Aire-independent genes in mTECs, Fli1 down-regulation likely suppresses some molecules regulating TSA expression, such as transcription factor Fezf2 (Takaba et al., 2015), in addition to Aire while at the same time having additional negative effects on thymic epithelial development. Similar to this, an adaptor protein Sin regulates thymic development, and its knockdown causes defective thymic development with decreased number of mTECs as well as suppressed expression of Aire, Aire-dependent genes, and Aire-independent genes, leading to autoimmune phenotype (Danzl et al., 2010). Taking these current and previous findings together, Fli1 may affect the expression of other factors as well as Aire in mTECs. Further studies are required to clarify this point.
This study identified dysregulated epithelia as a principal driver of dermal and esophageal fibrosis in K14Cre;fl/fl mice through the following findings: (a) T cell transfer from K14Cre;fl/fl mice to Rag1−/− mice failed to reproduce dermal and esophageal fibrosis and (b) Rag1−/−;K14Cre;fl/fl mice manifested dermal and esophageal fibrosis. At this moment, we can propose a couple of possible mechanisms explaining the association of dysregulated epithelia with tissue fibrosis. First, the straightforward explanation is that several humoral factors such as IL-1α and CTGF overproduced by activated epithelia directly stimulate collagen production by fibroblasts. Second, dysregulated epithelia activate innate immune cells such as macrophages and mast cells, which are present in Rag1−/− mice, resulting in fibrosis development. Indeed, there was a significantly increased number of macrophages and mast cells in parallel with fibrosis development in the skin of K14Cre;fl/fl mice, and up-regulated inflammatory cell infiltration was also observed in Rag1−/−;K14Cre;fl/fl mice. Lastly, Fli1 down-regulation might contribute to enhanced EMT. EMT has been identified as a critical driver of tissue fibrosis as well as tumor progression (Nieto et al., 2016), and previous studies have disclosed its potential roles in tissue fibrosis of SSc (Wei et al., 2011; Nikitorowicz-Buniak et al., 2015). Notably, Fli1 is involved in EMT in many tumors, contributing to their progression (Scheiber et al., 2014). In the current study, Fli1-deficient NHKs strictly recapitulated EMT-like changes of SSc keratinocytes, namely heightened SNAI1 expression, despite the unaffected E-cadherin expression (Nikitorowicz-Buniak et al., 2015). This phenotype is unique for SSc keratinocytes because SNAI1 up-regulation normally leads to decreased E-cadherin expression in keratinocytes (Nieto et al., 2016). Even though it is still unclear whether SSc keratinocytes with EMT-like changes truly provide fibroblasts through EMT, our present data indicate that Fli1 deficiency strictly induces SSc-like phenotypes in keratinocytes. Further studies are required to assess these three possibilities, but it is intriguing that dysregulated epithelia may be involved in selective organ fibrosis in the skin and esophagus of SSc.
Another critical point in the experiments with Rag1−/−;K14Cre;fl/fl mice is that the fold-increase of collagen deposition in Rag1−/−;K14Cre;fl/fl mice compared with their control mice was considerably smaller than that between K14Cre;fl/fl mice and fl/fl mice. This evidence suggests the roles of lymphocyte activation in the development of robust tissue fibrosis in K14Cre;fl/fl mice. In sum, our findings indicate that dermal and esophageal fibrosis in K14Cre;fl/fl mice is primed by epithelial activation and subsequently promoted by the activation of the innate and adaptive immune systems. These mechanisms might have possible homology in the pathogenesis of fibrosis in human SSc.
K14Cre;fl/fl mice fulfill the criteria for a new animal model of SSc by reproducing the autoimmunity and selective organ fibrosis seen in human disease. Moreover, K14Cre;fl/fl mice demonstrate constitutive activation of B cells with SSc-like phenotypes. Similar to SSc B cells, B cells of K14Cre;fl/fl mice showed augmented expression of CD19, which functions as the dominant signaling component on B cells and plays a crucial role in maintaining the balance between humoral immune response and immune tolerance (Wang et al., 2012). Also, B cells of K14Cre;fl/fl mice produced autoantibodies and a higher amount of IgG, IgM, and IL-6. Because IL-6 enhances collagen synthesis by inducing myofibroblastic differentiation and promotes Th2/Th17 cell differentiation (Rincón et al., 1997; O’Reilly et al., 2013), its excessive production from B cells may contribute to fibrosis development in K14Cre;fl/fl mice. However, K14Cre;fl/fl mice lack the vascular abnormalities of SSc (unpublished data) recapitulated in endothelial cell–specific Fli1 knockout mice (Asano et al., 2010b). Given the close analogy of dermal and esophageal fibrosis and autoimmunity, but not vasculopathy, between K14Cre;fl/fl mice and human SSc, the mechanisms driven by epithelial Fli1 deficiency might be in action in the fibrotic and immunological components of SSc.
There are several limitations to our study. First, we did not assess Fli1 protein expression in the esophagus or thymus of patients with SSc, as such specimens are largely unavailable. Therefore, we cannot exclude the possibility that dysregulation of a different set of genes causing the phenotypical alteration of epithelial cells may be contributing to SSc. Second, we did not analyze the role and significance of Aire expression in keratinocytes of human skin and its suppression in SSc. There have been only a few previous analyses on Aire expression in the skin. Among them, importantly, Clark et al. (2005) reported that human keratinocytes and fibroblasts express Aire, and co-culture system composed of these cells could induce T cell development and self-tolerance in the hematopoietic precursor cells via Aire expression in vitro. Thus, it might be possible that Fli1 deficiency leads to the decreased expression of Aire in keratinocytes, having some impacts on the peripheral immune homeostasis in the human skin. Further studies are required to clarify this point in the future.
Nonetheless, the profound similarity in autoimmunity and fibrosis between SSc and K14Cre;fl/fl mice seems to indicate the fundamental roles of Fli1 down-regulation in epithelial cells in SSc. In particular, our study might shed light on the fundamental yet unanswered questions in SSc research: why is SSc an autoimmune disease and why are the skin, esophagus, and lung the primary targets of fibrosis in SSc? Further investigations are awaited to elucidate the contribution of dysregulated epithelial cells to SSc development.
Materials and methods
Human samples
For immunohistochemical analysis of Fli1, skin samples collected from the forearm of 13 SSc patients (five patients with dcSSc with anti–topoisomerase I antibody and eight patients with lcSSc with anticentromere antibody) with skin sclerosis for <2 yr were used. For immunohistochemical analyses of Aire, skin samples collected from eight SSc patients (four patients with dcSSc with anti–topoisomerase I antibody and four patients with lcSSc with anticentromere antibody) were used. All of the patients were diagnosed with SSc based on clinical appearance and histological findings and fulfilled the new classification criteria of SSc (van den Hoogen et al., 2013). None of them had any other collagen diseases. Control specimens were obtained by skin biopsy from eight sex- and site-matched healthy donors with closely matched age. For mRNA expression analyses of the epidermis, cDNA was prepared from the epidermal sheets derived from forearm skin samples of six dcSSc patients and four healthy donors. For mRNA expression analyses of the whole skin, cDNA was prepared from the forearm skin samples of 33 SSc patients and 7 healthy donors. Institutional approval (University of Tokyo Graduate School of Medicine) and written informed consent were obtained from all subjects. The whole study was performed according to the Declaration of Helsinki.
Mice
Fli1+/− and Fli1flox/flox mice were generated as described previously (Spyropoulos et al., 2000; Asano et al., 2010b). K14Cre (strain Tg[KRT14-cre]1Amc/J), Rag1−/−, and C57BL/6 mice were purchased from The Jackson Laboratory. In the analyses of Fli1+/−, K14Cre;Fli1flox/flox, and Rag1−/−;K14Cre;Fli1flox/flox mice, their littermate Fli1+/+, Fli1flox/flox, and Rag1−/−;Fli1flox/flox mice were used as controls, respectively. All mice used in the experiments were female. All mice were bred in a specific pathogen–free facility. All studies and procedures were approved by the Committee on Animal Experimentation of the University of Tokyo Graduate School of Medicine.
Gene silencing of Fli1 by siRNA
NHKs (Kurabo) were transfected with 10 nM Fli1 siRNA (Santa Cruz Biotechnology, Inc.) or control scrambled RNA (SCR; Santa Cruz Biotechnology, Inc.) for 6 h using Lipofectamine 2000 (Thermo Fisher Scientific). Thereafter, cells were cultured in MCDB153 medium lacking bovine hypothalamus extract and used for further studies.
Histological assessment, immunohistochemistry, and immunofluorescence
6-µm–thick sections of skin and internal organs were stained with H&E and Masson-Trichrome staining. Dermal thickness was examined as previously described (Takahashi et al., 2015a). The measurement of the thickness of the subcutaneous adipose tissue in the skin and the thickness of the lamina propria and circular muscle layer of the esophagus was carried out with a similar method used in the measurement of dermal thickness as conducted in previous studies (Ishikawa et al., 2009; Marangoni et al., 2015). Evaluation of the total area and mean area per islet of the thymic medulla was conducted with the method described by Danzl et al. (2010). Immunohistochemistry in mouse specimens was performed using antibodies directed against Fli1 (sc-356; Santa Cruz Biotechnology, Inc.), K6 (sc-22481; Santa Cruz Biotechnology, Inc.), K16 (MA5-13730; Thermo Fisher Scientific), IL-1α (ab9724; Abcam), IL-1β (ab9722; Abcam), CTGF (sc-14939; Santa Cruz Biotechnology, Inc.), SNAI1 (AV33314; Sigma-Aldrich), α-SMA (ab5694; Abcam), CD3 (bd555273; BD), F4/80 (MCA497; Serotec), B220 (41-0452; eBioscience), and Aire (14-5934; eBioscience). Immunohistochemistry with human skin specimens was performed with antibodies against Fli1 (sc-356; Santa Cruz Biotechnology, Inc.) and Aire (ab13573; Abcam). In the cell count of positive cells in immunohistochemical staining, stained cells were counted in 10 random grids under high power fields (HPFs) by two independent researchers (T. Takahashi. and Y. Asano) with blinded manners. For the scoring of staining intensity of immunohistochemistry, the intensities were scored by two independent researchers (T. Takahashi and Y. Asano) with blinded manners with the following scoring system: none = 0, weak = 1, moderate = 2, and strong = 3. The higher score was adopted when there was a discrepancy between the researchers. The severity of the tissue inflammation in the skin and internal organs in the recipient mice of transfer experiments was evaluated according to the scoring system by Shum et al. (2009). For immunofluorescence staining of thymuses, rat anti-Aire antibody (14-5934; eBioscience), rabbit anti-Fli1 antibody (sc-356; Santa Cruz Biotechnology, Inc.), and rabbit anti-K5 antibody (ab24647; Abcam) were used as primary antibodies. In Aire and Fli1 double staining, FITC-conjugated goat anti–rat IgG antibody (sc-2011; Santa Cruz Biotechnology, Inc.) and Alexa Fluor 555 donkey anti–rabbit IgG antibody (A31572; Thermo Fisher Scientific) and, in K5 and Aire double staining, FITC-conjugated donkey anti–rabbit IgG antibody (sc-2090; Santa Cruz Biotechnology, Inc.) and Alexa Fluor 555 goat anti–rat IgG antibody (ab150158; Abcam) were used as secondary antibodies. For immunofluorescence staining of lungs from Rag1−/− mice, rat anti-CD3 antibody (bd555273; BD) and Alexa Fluor 555 goat anti–rat IgG antibody (ab150158; Abcam) were used. Coverslips were mounted using Vectashield with DAPI (Vector Laboratories), and staining was examined with a fluorescence microscope (Bio Zero BZ-8000; Keyence) at 495 (green), 565 (red), and 400 (blue) nm. Photomicrographs of H&E staining, Masson-Trichrome staining, toluidine blue staining, and other immunohistochemical stainings were obtained with an Olympus microscope (BX51) and Olympus camera (DP73).
Collagen content measurement
Following the instructions of QuickZyme Total Collagen Assay (QuickZyme Biosciences), tissues were hydrolyzed with 6N HCl, and hydroxyproline content was quantified. 6-mm punch biopsy skin samples and total left lungs were used for the measurement of skin and lung tissue. The esophagus was marked at 2 and 5 mm from the gastroesophageal junction and cut out, and the 3-mm–length specimens were subject to the assay.
Transmission electron microscopy
Samples were collected from the back skin of 3-mo-old mice of each strain, fixed, postfixed, rinsed, dehydrated, transferred to propylene oxide, and embedded in epoxy resin. Ultrathin sections were stained with tannate, uranyl acetate, and lead citrate and examined with a microscope (JEM-1200EX; JEOL Ltd.). Multiple micrographs of cross sections of dermal collagen fibrils were chosen randomly and used for morphometry.
RNA isolation and qRT-PCR
RNA was isolated, and qRT-PCR was performed as described previously (Takahashi et al., 2015a). In the analyses for mRNA expression in the human epidermal sheets, the biopsy skin specimens were incubated in medium containing 2 U/ml Dispase II (Roche) overnight. Then, the epidermal sheets were peeled off with forceps and used for RNA isolation. In the analyses for mice epidermal sheets, ears of 3-mo-old mice were obtained. The skin was mechanically detached from the cartilage and floated on the medium containing 2 U/ml Dispase II overnight. The epidermal sheet was peeled off with forceps and used for analyses as well. The sequences of the primers used are summarized in Table 1. The relative change in the levels of genes was determined by the 2−ΔΔCT method.
Table 1. Primer sequences.
| Species | Gene | Forward | Reverse |
|---|---|---|---|
| Human | FLI1 | GGATGGCAAGGAACTGTGTAA | GGTTGTATAGGCCAGCAG |
| AIRE | GAGAGTGCTGAGAAGGACA | GTTTAATTTCCAGGCACATGA | |
| IL1A | ATCAGTACCTCACGGCTGCT | TGGGTATCTCAGGCATCTCC | |
| K6 | CTGAATGGCGAAGGCGTT | CCACTGCCGACACCACT | |
| K16 | GACCGGCGGAGATGTGAAC | CTGCTCGTACTGGTCACGC | |
| CTGF | TTGCGAAGCTGACCTGGAAGAGAA | AGCTCGGTATGTCTTCATGCTGGT | |
| SNAI1 | ACCCCAATCGGAAGCCTAACT | GGTCGTAGGGCTGCTGGAA | |
| CDH1 | ACAACAAGCCCGAATTCACCCA | TCACAGCTGTTGCTGTTGTGCT | |
| GAPDH | ACCCACTCCTCCACCTTTGA | CATACCAGGAAATGAGCTTGACAA | |
| Mouse | Il1a | ATGATCTGGAAGAGACCATCC | GGCAACTCCTTCAGCAACA |
| Il1b | TTGACGGACCCCAAAAGAT | GAAGCTGGATGCTCTCATCTG | |
| Il6 | GATGGATGCTACCAAACTGGAT | CCAGGTAGCTATGGTACTCCAGA | |
| Il8 | CTGGCCGTGGCTCTCTTG | CCTTGGCAAAACTGCACCTT | |
| Il13 | GGATATTGCATGGCCTCTGTAAC | AACAGTTGCTTTGTGTAGCTGA | |
| Il17a | CTCCAGAAGGCCCTCAGACTAC | AGCTTTCCCTCCGCATTGACACAG | |
| Tnfa | ACCCTCACACTCAGATCATCTTC | TGGTGGTTTGCTACGACGT | |
| Ccl2 | CATCCACGTGTTGGCTCA | GATCATCTTGCTGGTGAATGAGT | |
| Ifng | TCAAGTGGCATAGATGTGGAAGAA | TGGCTCTGCAGGATTTTCATG | |
| Ctgf | GTGCCAGAACGCACACTG | CCCCGGTTACACTCCAAA | |
| Tgfb1 | GCAACATGTGGAACTCTACCAGAA | GACGTCAAAAGACAGCCACTCA | |
| Col1a1 | GCCAAGAAGACATCCCTGAAG | TGTGGCAGATACAGATCAAGC | |
| Col1a2 | GGAGGGAACGGTCCACGAT | GAGTCCGCGTATCCACAA | |
| Adamts2 | AGTGGGCCCTGAAGAAGTG | CAGAAGGCTCGGTGTACCAT | |
| Lum | AGATGCTTGATCTTGGAGTAAGA | CAATGAACTTGAAAAGTTTGATG | |
| Dcn | TGAGCTTCAACAGCATCACC | AAGTCATTTTGCCCAACTGC | |
| K6 | TTCTCTACTTCCCAGCCTTCTCA | GCCACGGTGGCTGGTTT | |
| K16 | GACCTCAGCCGCATTCTGA | GCGAAGCTGAGCCAGCTGCT | |
| Snai1 | CAACTATAGCGAGCTGCAGG | ACTTGGGGTACCAGGAGAGAGT | |
| Ins2 | AGACCATCAGCAAGCAGGTC | CTGGTGCAGCACTGATCCAC | |
| Csna | TGACTGGACCCTCCATTCTC | CCTTGATTCTCTCCGCTCAG | |
| Chrna1 | GTGCTGGGCTCTTTCATCTC | TTCTGTGCGCGTTCTCATAC | |
| s100a8 | GGAAATCACCATGCCCTCTA | GCTGTCTTTGTGAGATGCCA | |
| Tg | CTGTGGTGTTCTCAGCCTCAACTCC | TTGGCCTGAGTAGCAGAGGT | |
| Spt1 | TTGTGTTGCTTGGTGTTT | TCGACTGAATCAGAGGAATCAACT | |
| Gad67 | CACAAACTCAGCGGCATAGA | CTGGAAGAGGTAGCCTGCAC | |
| Col2a1 | ACTGGTAAGTGGGGCAAGAC | CCACACCAAATTCCTGTTCA | |
| K10 | AACAGATTCGGGCTGAGACC | TTCTAGCAGGCTGCGGTAGG | |
| Crp | TACTCTGGTGCCTTCTGATCATGA | GGCTTCTTTGACTCTGCTTCCA | |
| Aire | GTACAGCCGCCTGCATAGC | CCCTTTCCGGGACTGGTTTA | |
| Fli1 | ACTTGGCCAAATGGACGGGACTAT | CCCGTAGTCAGGACTCCCG | |
| Gapdh | CGTGTTCCTACCCCCAATGT | TGTCATCATACTTGGCAGGTTTCT | |
| SNAI1 ChIP primer | Amplified Region | ||
| –715/–472 | AGAAGCTACCCTTCGGGAGA | GCATTGACGAGGGAAACG | |
| AIRE ChIP primer | Amplified Region | ||
| 1 | –403/–235 | CAGGACAGGGCCACATTC | AGGGACACTTCCCTGTGATG |
| 2 | –587/–389 | TAGGGGCTCTCAGCTTGTGT | GAATGTGGCCCTGTCCTG |
| 3 | –807/–634 | CTTTGCTCTTTGCGTGGTC | CCTCAGAAGCCGGCGTAG |
| 4 | –1038/–759 | CACTGTCCCCACTCACGTC | CTTTAAAAGGGGCACACTCG |
| 5 | –1229/–1036 | GACCTCTCGTGGTTCTCTGC | TGCCCCCAGTAAAACAAGAC |
The sequences of the primers used for qRT-PCR and ChIP of SNAI1 and AIRE gene promoter regions are shown. The nucleotide sequences of the primers are listed in the 5′ to 3′ direction.
Measurement of immunoglobulins, IL-6, and ANA levels in the sera and/or culture supernatant
Sera from 3- and 8-mo-old mice were analyzed with ELISA kits for the measurement of total IgG, IgM, and IgA (88-50400, 88-50470, and 88-50450; all from eBioscience). For IL-6 measurement, we used a high-sensitivity ELISA kit for the measurement of sera from 1-, 2-, and 3-mo-old mice (BMS603HS; eBioscience) and otherwise used an IL-6 ELISA kit (M600B; R&D Systems). The titers of ANA in the sera of 8-mo-old mice were analyzed with a specific ELISA kit (MBS731183; MyBioSource).
ANA detection by indirect immunofluorescence
ANA was detected by indirect immunofluorescence using human Hep-2 cells (Medical & Biological Laboratories). Sera at a 1:20 dilution from 1-, 2-, 3-, and 8-mo-old mice were incubated on the slides, followed by detection with FITC-labeled anti–mouse IgG antibody (bd553605; BD) at a 1:100 dilution using a fluorescence microscope (Bio Zero BZ-8000) at 495 nm (green).
Immunoblotting
Whole cell lysates of NHKs were subjected to sodium dodecyl sulfate–PAGE (Thermo Fisher Scientific) and immunoblotting. Bands were detected using enhanced chemiluminescent techniques (Thermo Fisher Scientific). Antibodies used were against β-actin (A1978; Sigma-Aldrich), Fli1 (sc-356; Santa Cruz Biotechnology, Inc.), K6 and K16 (MA5-14127 and MA5-13730; Thermo Fisher Scientific), IL-1α (ab9614; Abcam), CTGF (sc-14939; Santa Cruz Biotechnology, Inc.), SNAI1 (AV33314; Sigma-Aldrich), and E-cadherin (ab15148; Abcam). In experiments with lung tissue lysates from Rag1−/− mice, the homogenized lysates were incubated with sera (1:100) from fl/fl and K14Cre;fl/fl mice, followed by horseradish-peroxidase–conjugated anti–mouse IgG secondary antibody (sc-2371; Santa Cruz Biotechnology, Inc.). The protein bands were visualized with chemiluminescence. The density of each band was quantified with ImageJ software (National Institutes of Health).
ChIP assay
The ChIP assay was performed using an EpiQuik ChIP kit (Epigentek). In brief, cells were first treated with 1% formaldehyde, and cross-linked chromatin was prepared and sonicated to a mean size of 300 bp. DNA fragments were immunoprecipitated with anti-Fli1 antibody (sc-356; Santa Cruz Biotechnology, Inc.). As a negative control, normal rabbit IgG (sc-2027; Santa Cruz Biotechnology, Inc.) was used. Input corresponds to the extracted DNA from the sample before immunoprecipitation. After reversing the cross-link, the immunoprecipitated chromatin was analyzed by agarose gel electrophoresis and qRT-PCR. Primers amplifying the specific promoter regions of the SNAI1 and AIRE genes were used. The sequences of the PCR primers are summarized in Table 1.
Plasmids
A human AIRE gene promoter luciferase construct (−1235 to 1) cloned into the pUC18 basic vector was provided by P. Peterson (University of Tartu, Tartu, Estonia; Murumägi et al., 2003, 2006). The Fli1 expression vector pSG5-Fli1 was described previously (Shirasaki et al., 1999). Site-directed mutant plasmids were generated with a QuikChange II Site-Directed Mutagenesis kit (Agilent Technologies) following the manufacturer’s instructions.
Reporter gene assay
NHKs were transfected with plasmids using Lipofectamine 2000 (Thermo Fisher Scientific). Cells were harvested after 48 h, and the activities of both firefly and Renilla luciferase were measured with a luminometer (AB-2200; ATTO) using a dual luciferase reporter system (Promega). Firefly luciferase activity was normalized to that of Renilla luciferase.
Oligonucleotide pull-down assay
The oligonucleotides containing biotin on the 5′ nucleotide of the sense strand were used. The sequences of these oligonucleotides were as follows: (a) AIRE EBS-A oligonucleotides 5′-GCAGCGCCTCCATCACAGGGAAGTGTCCCTGCGGGAGGCC-3′ and 5′-GGCCTCCCGCAGGGACACTTCCCTGTGATGGAGGCGCTGC-3′, which correspond to base pairs −259 to −219 of AIRE promoter containing EBS-A and (ii) AIRE-mutated EBS-A oligonucleotides 5′-GCAGCGCCTCCATCACAGTTAAGTGTCCCTGCGGGAGGCC-3′ and 5′-GGCCTCCCGCAGGGACACTTAACTGTGATGGAGGCGCTGC-3′, which have mutated EBS-A (the underlined portions of sequences are the naive and mutated EBS-A). These oligonucleotides were annealed to their respective complementary oligonucleotides at 95°C for 1 hr. Nuclear extracts prepared from NHKs were incubated with streptavidin-coupled agarose beads and 500 pmol of each double-stranded oligonucleotide for 2 h at room temperature with gentle rocking. The protein–DNA–streptavidin–agarose complex was washed four times with PBS containing protease inhibitors. The precipitates were subjected to immunoblotting using the indicated antibodies.
Flow cytometric analysis
In the intracellular cytokine staining experiments, cells isolated from inguinal lymph nodes were stimulated with 10 ng/ml PMA and 1 µg/ml ionomycin (Sigma-Aldrich) in the presence of 1 µg/ml brefeldin A (BD) for 4 h. Cells were washed and stained for CD3 and CD4 (100319 and 100515; BioLegend). Samples were treated with fixative/permeabilization buffer (BD) and then stained with anti–IL-4, anti–IL-17A, and anti–IFN-γ antibodies (504123, 517007, and 505805; all from BioLegend). In the analysis of B cells, anti-B220 and anti-CD19 antibodies were used (103205 and 115507; BioLegend). Cells were analyzed on a FACSVerse flow cytometer (BD). The positive and negative populations of cells were determined using unreactive isotype-matched antibodies as controls for background staining. Flow data were analyzed using FlowJo software (Tree Star).
T cell transfer to Rag1−/− mice and splenocyte transfer to Rag1−/−;K14Cre;fl/fl mice
In T cell transfer experiments, single-cell suspensions from inguinal lymph nodes and spleen were stained for NK1.1 (14-5941; eBioscience) and CD3 (bd100205; BioLegend), and NK1.1−CD3+ cells were sorted with a FACS Aria flow cytometer (BD). Sorted cells were suspended in normal saline solution in a concentration of 5 × 106 cells/ml, and 0.2 ml of cell suspension was intravenously injected into each recipient Rag1−/− mouse. 2 or 6 mo later, recipient mice were sacrificed and used for further analyses. In splenocyte transfer experiments from fl/fl mice to Rag1−/−;K14Cre;fl/fl mice, splenocytes were isolated from 3-mo-old fl/fl mice, and 107 cells were transferred to 1-mo-old Rag1−/−;K14Cre;fl/fl mice. The recipient mice were sacrificed 3 mo after transfer and used for analyses.
Isolation of B cells, culture, and measurement of supernatant IL-6 concentration
Splenic B cells were isolated by CD19-positive selection using magnetic-activated cell-sorting beads (130-052-201; Miltenyi Biotec) as described previously (Noda et al., 2014). 105 isolated B cells were cultured in 0.1 ml RPMI medium. Some cells were stimulated with 10 µg/ml anti-CD40 antibody (102810; BioLegend). The supernatants were harvested after 48-h incubation and subjected to measurement of IL-6 concentration by ELISA.
Isolation of mTECs
Thymuses from 8-wk-old fl/fl and K14Cre;fl/fl mice were excised, mashed with slide glasses, and then digested in the medium containing 0.15% collagenase D (Roche) and 0.1% DNase (Sigma-Aldrich) at 37°C for 30 min. Cells were filtered, centrifuged, washed in the medium, counted, and stained with anti-CD45, anti-EpCAM, and anti-Ly51 antibodies (103115, 118207, and 108307; all from BioLegend). CD45−EpCAM+Ly51− cells were sorted using a flow cytometer (FACS Aria; BD). The number of mTECs per lobe was calculated with the number of the total cells and the proportion in the population.
Statistical analysis
Statistical analysis was performed with two-tailed unpaired Student’s t test for two-group comparison. For comparing two group values that did not follow Gaussian distribution, a two-tailed Mann-Whitney U test was used. In the comparison between more than two groups (i.e., healthy controls and dcSSc/lcSSc patients), Kruskal-Wallis test with Dunn’s posthoc test was used. For correlation analysis, Spearman’s correlation analysis was used. P < 0.05 was considered statistically significant. Within-group distributions are expressed as mean ± SEM.
Acknowledgments
We thank Prof. Pärt Peterson for providing us the AIRE gene promoter construct. We also thank Dr. Kaoru Morita, Dr. Keishi Fujio, and Prof. Kazuhiko Yamamoto for the technical support in flow cytometry. In addition, we appreciate the technical assistance from Natsumi Toda, Yumi Hasegawa, and Tamami Kaga for histochemical analyses.
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant numbers JP16H05366 (to Y. Asano) and JP25293242 (to S. Sato). This study was also supported by grants from the Japan Intractable Diseases Research Foundation, the Rohto Dermatology Prize, the Japanese Society for Investigative Dermatology Fellowship Shiseido Award, the Mochida Memorial Foundation for Medical and Pharmaceutical Research, and from the Takeda Science Foundation to Y. Asano. M. Trojanowska was supported by the National Institutes of Health (grant AR042334).
The authors declare no competing financial interests.
Author contributions: T. Takahashi contributed to data collection, analysis, and manuscript writing for all experiments. Y. Asano contributed to experimental design, data analysis, and manuscript writing for all experiments. K. Nakamura, T. Yamashita, R. Saigusa, Y. Ichimura, T. Toyama, T. Taniguchi, K. Akamata, S. Noda, and A. Yoshizaki contributed to sample collection from human subjects and data analysis for all experiments. K. Sugawara and D. Tsuruta contributed to electron microscopic analysis. M. Trojanowska produced plasmids and contributed to manuscript writing. S. Sato contributed to experimental design, data analysis, and manuscript writing for all experiments.
Footnotes
Abbreviations used:
- Aire
- autoimmune regulator
- ANA
- antinuclear antibody
- ChIP
- chromatin immunoprecipitation
- CTGF
- connective tissue growth factor
- dcSSc
- diffuse cutaneous SSc
- EBS
- Ets consensus binding site
- EMT
- epithelial–mesenchymal transition
- EpCAM
- epithelial cell adhesion molecule
- Fli1
- Friend leukemia virus integration 1
- H&E
- hematoxylin and eosin
- HPF
- high power field
- ILD
- interstitial lung disease
- lcSSc
- limited cutaneous SSc
- mTEC
- medullary TEC
- NHK
- normal human keratinocyte
- qRT-PCR
- quantitative RT-PCR
- SCR
- scrambled RNA
- SSc
- systemic sclerosis
- TEC
- thymic epithelial cell
- TSA
- tissue-specific self-antigen
References
- Aden N., Nuttall A., Shiwen X., de Winter P., Leask A., Black C.M., Denton C.P., Abraham D.J., and Stratton R.J.. 2010. Epithelial cells promote fibroblast activation via IL-1α in systemic sclerosis. J. Invest. Dermatol. 130:2191–2200. 10.1038/jid.2010.120 [DOI] [PubMed] [Google Scholar]
- Agarwal S.K., and Reveille J.D.. 2010. The genetics of scleroderma (systemic sclerosis). Curr. Opin. Rheumatol. 22:133–138. 10.1097/BOR.0b013e3283367c17 [DOI] [PubMed] [Google Scholar]
- Akamata K., Asano Y., Taniguchi T., Yamashita T., Saigusa R., Nakamura K., Noda S., Aozasa N., Toyama T., Takahashi T., et al. 2015a Increased expression of chemerin in endothelial cells due to Fli1 deficiency may contribute to the development of digital ulcers in systemic sclerosis. Rheumatology (Oxford). 54:1308–1316. 10.1093/rheumatology/keu479 [DOI] [PubMed] [Google Scholar]
- Akamata K., Asano Y., Yamashita T., Noda S., Taniguchi T., Takahashi T., Ichimura Y., Toyama T., Trojanowska M., and Sato S.. 2015b Endothelin receptor blockade ameliorates vascular fragility in endothelial cell-specific Fli-1–knockout mice by increasing Fli-1 DNA binding ability. Arthritis Rheumatol. 67:1335–1344. 10.1002/art.39062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T., Maeda S., Yamane S., Ogino K., Kasai M., Kajiura F., Matsumoto M., and Inoue J.. 2005. Dependence of self-tolerance on TRAF6-directed development of thymic stroma. Science. 308:248–251. 10.1126/science.1105677 [DOI] [PubMed] [Google Scholar]
- Anderson M.S., Venanzi E.S., Klein L., Chen Z., Berzins S.P., Turley S.J., von Boehmer H., Bronson R., Dierich A., Benoist C., and Mathis D.. 2002. Projection of an immunological self shadow within the thymus by the Aire protein. Science. 298:1395–1401. 10.1126/science.1075958 [DOI] [PubMed] [Google Scholar]
- Asano Y. 2010. Future treatments in systemic sclerosis. J. Dermatol. 37:54–70. 10.1111/j.1346-8138.2009.00758.x [DOI] [PubMed] [Google Scholar]
- Asano Y. 2015. Epigenetic suppression of Fli1, a potential predisposing factor in the pathogenesis of systemic sclerosis. Int. J. Biochem. Cell Biol. 67:86–91. 10.1016/j.biocel.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Asano Y., and Sato S.. 2015. Vasculopathy in scleroderma. Semin. Immunopathol. 37:489–500. 10.1007/s00281-015-0505-5 [DOI] [PubMed] [Google Scholar]
- Asano Y., Markiewicz M., Kubo M., Szalai G., Watson D.K., and Trojanowska M.. 2009. Transcription factor Fli1 regulates collagen fibrillogenesis in mouse skin. Mol. Cell. Biol. 29:425–434. 10.1128/MCB.01278-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano Y., Bujor A.M., and Trojanowska M.. 2010a The impact of Fli1 deficiency on the pathogenesis of systemic sclerosis. J. Dermatol. Sci. 59:153–162. 10.1016/j.jdermsci.2010.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano Y., Stawski L., Hant F., Highland K., Silver R., Szalai G., Watson D.K., and Trojanowska M.. 2010b Endothelial Fli1 deficiency impairs vascular homeostasis: a role in scleroderma vasculopathy. Am. J. Pathol. 176:1983–1998. 10.2353/ajpath.2010.090593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assassi S., Swindell W.R., Wu M., Tan F.D., Khanna D., Furst D.E., Tashkin D.P., Jahan-Tigh R.R., Mayes M.D., Gudjonsson J.E., and Chang J.T.. 2015. Dissecting the heterogeneity of skin gene expression patterns in systemic sclerosis. Arthritis Rheumatol. 67:3016–3026. 10.1002/art.39289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boin F., and Rosen A.. 2007. Autoimmunity in systemic sclerosis: current concepts. Curr. Rheumatol. Rep. 9:165–172. 10.1007/s11926-007-0012-3 [DOI] [PubMed] [Google Scholar]
- Clark R.A., Yamanaka K., Bai M., Dowgiert R., and Kupper T.S.. 2005. Human skin cells support thymus-independent T cell development. J. Clin. Invest. 115:3239–3249. 10.1172/JCI24731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzl N.M., Donlin L.T., and Alexandropoulos K.. 2010. Regulation of medullary thymic epithelial cell differentiation and function by the signaling protein Sin. J. Exp. Med. 207:999–1013. 10.1084/jem.20092384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrera F., Rizzi M., Sprecacenere B., Balestra P., Sessarego M., Di Carlo A., Filaci G., Gabrielli A., Ravazzolo R., and Indiveri F.. 2007. AIRE gene polymorphisms in systemic sclerosis associated with autoimmune thyroiditis. Clin. Immunol. 122:13–17. 10.1016/j.clim.2006.09.013 [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J.S., and Reeves J.R.. 1977. Cellular infiltrates in scleroderma skin. Arthritis Rheum. 20:975–984. 10.1002/art.1780200410 [DOI] [PubMed] [Google Scholar]
- Gabrielli A., Avvedimento E.V., and Krieg T.. 2009. Scleroderma. N. Engl. J. Med. 360:1989–2003. 10.1056/NEJMra0806188 [DOI] [PubMed] [Google Scholar]
- Hamaguchi Y. 2010. Autoantibody profiles in systemic sclerosis: predictive value for clinical evaluation and prognosis. J. Dermatol. 37:42–53. 10.1111/j.1346-8138.2009.00762.x [DOI] [PubMed] [Google Scholar]
- Harris M.L., and Rosen A.. 2003. Autoimmunity in scleroderma: the origin, pathogenetic role, and clinical significance of autoantibodies. Curr. Opin. Rheumatol. 15:778–784. 10.1097/00002281-200311000-00016 [DOI] [PubMed] [Google Scholar]
- Hobbs R.P., DePianto D.J., Jacob J.T., Han M.C., Chung B.M., Batazzi A.S., Poll B.G., Guo Y., Han J., Ong S., et al. 2015. Keratin-dependent regulation of Aire and gene expression in skin tumor keratinocytes. Nat. Genet. 47:933–938. 10.1038/ng.3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y., Asano Y., Akamata K., Noda S., Taniguchi T., Takahashi T., Toyama T., Tada Y., Sugaya M., Sato S., and Kadono T.. 2015. Progranulin overproduction due to Fli-1 deficiency contributes to the resistance of dermal fibroblasts to tumor necrosis factor in systemic sclerosis. Arthritis Rheumatol. 67:3245–3255. 10.1002/art.39312 [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Takeda K., Okamoto A., Matsuo S., and Isobe K.. 2009. Induction of autoimmunity in a bleomycin-induced murine model of experimental systemic sclerosis: an important role for CD4+ T cells. J. Invest. Dermatol. 129:1688–1695. 10.1038/jid.2008.431 [DOI] [PubMed] [Google Scholar]
- Jordan S., Distler J.H.W., Maurer B., Huscher D., van Laar J.M., Allanore Y., and Distler O.. EUSTAR Rituximab study group . 2015. Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann. Rheum. Dis. 74:1188–1194. 10.1136/annrheumdis-2013-204522 [DOI] [PubMed] [Google Scholar]
- Joseph C.G., Darrah E., Shah A.A., Skora A.D., Casciola-Rosen L.A., Wigley F.M., Boin F., Fava A., Thoburn C., Kinde I., et al. 2014. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science. 343:152–157. 10.1126/science.1246886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y., McCarthy S.A., Watkins S.C., and Wright T.M.. 2004. Autocrine activation by interleukin 1alpha induces the fibrogenic phenotype of systemic sclerosis fibroblasts. J. Rheumatol. 31:1946–1954. [PubMed] [Google Scholar]
- Khan K., Xu S., Nihtyanova S., Derrett-Smith E., Abraham D., Denton C.P., and Ong V.H.. 2012. Clinical and pathological significance of interleukin 6 overexpression in systemic sclerosis. Ann. Rheum. Dis. 71:1235–1242. 10.1136/annrheumdis-2011-200955 [DOI] [PubMed] [Google Scholar]
- Kont V., Laan M., Kisand K., Merits A., Scott H.S., and Peterson P.. 2008. Modulation of Aire regulates the expression of tissue-restricted antigens. Mol. Immunol. 45:25–33. 10.1016/j.molimm.2007.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M., Czuwara-Ladykowska J., Moussa O., Markiewicz M., Smith E., Silver R.M., Jablonska S., Blaszczyk M., Watson D.K., and Trojanowska M.. 2003. Persistent down-regulation of Fli1, a suppressor of collagen transcription, in fibrotic scleroderma skin. Am. J. Pathol. 163:571–581. 10.1016/S0002-9440(10)63685-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Pedroza L.A., Mace E.M., Seeholzer S., Cotsarelis G., Condino-Neto A., Payne A.S., and Orange J.S.. 2011. The autoimmune regulator (AIRE), which is defective in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy patients, is expressed in human epidermal and follicular keratinocytes and associates with the intermediate filament protein cytokeratin 17. Am. J. Pathol. 178:983–988. 10.1016/j.ajpath.2010.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafyatis R., O’Hara C., Feghali-Bostwick C.A., and Matteson E.. 2007. B cell infiltration in systemic sclerosis-associated interstitial lung disease. Arthritis Rheum. 56:3167–3168. 10.1002/art.22847 [DOI] [PubMed] [Google Scholar]
- Lafyatis R., Kissin E., York M., Farina G., Viger K., Fritzler M.J., Merkel P.A., and Simms R.W.. 2009. B cell depletion with rituximab in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 60:578–583. 10.1002/art.24249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A. 2009. Signaling in fibrosis: targeting the TGF beta, endothelin-1 and CCN2 axis in scleroderma. Front. Biosci. (Elite Ed.). 1:115–122. [DOI] [PubMed] [Google Scholar]
- LeRoy E.C., Black C., Fleischmajer R., Jablonska S., Krieg T., Medsger T.A. Jr., Rowell N., and Wollheim F.. 1988. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J. Rheumatol. 15:202–205. [PubMed] [Google Scholar]
- Liston A., Gray D.H., Lesage S., Fletcher A.L., Wilson J., Webster K.E., Scott H.S., Boyd R.L., Peltonen L., and Goodnow C.C.. 2004. Gene dosage–limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J. Exp. Med. 200:1015–1026. 10.1084/jem.20040581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetti M., Neumann E., Milia A.F., Tarner I.H., Bechi P., Matucci-Cerinic M., Ibba-Manneschi L., and Müller-Ladner U.. 2007. Severe fibrosis and increased expression of fibrogenic cytokines in the gastric wall of systemic sclerosis patients. Arthritis Rheum. 56:3442–3447. 10.1002/art.22940 [DOI] [PubMed] [Google Scholar]
- Marangoni R.G., Korman B.D., Wei J., Wood T.A., Graham L.V., Whitfield M.L., Scherer P.E., Tourtellotte W.G., and Varga J.. 2015. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 67:1062–1073. 10.1002/art.38990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D., and Benoist C.. 2009. Aire. Annu. Rev. Immunol. 27:287–312. 10.1146/annurev.immunol.25.022106.141532 [DOI] [PubMed] [Google Scholar]
- Matsumoto M. 2011. Contrasting models for the roles of Aire in the differentiation program of epithelial cells in the thymic medulla. Eur. J. Immunol. 41:12–17. 10.1002/eji.201041024 [DOI] [PubMed] [Google Scholar]
- Mélet F., Motro B., Rossi D.J., Zhang L., and Bernstein A.. 1996. Generation of a novel Fli-1 protein by gene targeting leads to a defect in thymus development and a delay in Friend virus-induced erythroleukemia. Mol. Cell. Biol. 16:2708–2718. 10.1128/MCB.16.6.2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouly E., Chemin K., Nguyen H.V., Chopin M., Mesnard L., Leite-de-Moraes M., Burlen-defranoux O., Bandeira A., and Bories J.C.. 2010. The Ets-1 transcription factor controls the development and function of natural regulatory T cells. J. Exp. Med. 207:2113–2125. 10.1084/jem.20092153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murumägi A., Vähämurto P., and Peterson P.. 2003. Characterization of regulatory elements and methylation pattern of the autoimmune regulator (AIRE) promoter. J. Biol. Chem. 278:19784–19790. 10.1074/jbc.M210437200 [DOI] [PubMed] [Google Scholar]
- Murumägi A., Silvennoinen O., and Peterson P.. 2006. Ets transcription factors regulate AIRE gene promoter. Biochem. Biophys. Res. Commun. 348:768–774. 10.1016/j.bbrc.2006.07.135 [DOI] [PubMed] [Google Scholar]
- Nakamura M., and Tokura Y.. 2011. Expression of SNAI1 and TWIST1 in the eccrine glands of patients with systemic sclerosis: possible involvement of epithelial-mesenchymal transition in the pathogenesis. Br. J. Dermatol. 164:204–205. 10.1111/j.1365-2133.2010.10021.x [DOI] [PubMed] [Google Scholar]
- Nieto M.A., Huang R.Y., Jackson R.A., and Thiery J.P.. 2016. Emt: 2016. Cell. 166:21–45. 10.1016/j.cell.2016.06.028 [DOI] [PubMed] [Google Scholar]
- Nikitorowicz-Buniak J., Shiwen X., Denton C.P., Abraham D., and Stratton R.. 2014. Abnormally differentiating keratinocytes in the epidermis of systemic sclerosis patients show enhanced secretion of CCN2 and S100A9. J. Invest. Dermatol. 134:2693–2702. 10.1038/jid.2014.253 [DOI] [PubMed] [Google Scholar]
- Nikitorowicz-Buniak J., Denton C.P., Abraham D., and Stratton R.. 2015. Partially evoked epithelial-mesenchymal transition (EMT) is associated with increased TGFβ signaling within lesional scleroderma skin. PLoS One. 10:e0134092 10.1371/journal.pone.0134092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda S., Asano Y., Akamata K., Aozasa N., Taniguchi T., Takahashi T., Ichimura Y., Toyama T., Sumida H., Yanaba K., et al. 2012. A possible contribution of altered cathepsin B expression to the development of skin sclerosis and vasculopathy in systemic sclerosis. PLoS One. 7:e32272 10.1371/journal.pone.0032272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda S., Asano Y., Takahashi T., Akamata K., Aozasa N., Taniguchi T., Ichimura Y., Toyama T., Sumida H., Kuwano Y., et al. 2013. Decreased cathepsin V expression due to Fli1 deficiency contributes to the development of dermal fibrosis and proliferative vasculopathy in systemic sclerosis. Rheumatology (Oxford). 52:790–799. 10.1093/rheumatology/kes379 [DOI] [PubMed] [Google Scholar]
- Noda S., Asano Y., Nishimura S., Taniguchi T., Fujiu K., Manabe I., Nakamura K., Yamashita T., Saigusa R., Akamata K., et al. 2014. Simultaneous downregulation of KLF5 and Fli1 is a key feature underlying systemic sclerosis. Nat. Commun. 5:5797 10.1038/ncomms6797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly S., Hügle T., and van Laar J.M.. 2012. T cells in systemic sclerosis: a reappraisal. Rheumatology (Oxford). 51:1540–1549. 10.1093/rheumatology/kes090 [DOI] [PubMed] [Google Scholar]
- O’Reilly S., Cant R., Ciechomska M., and van Laar J.M.. 2013. Interleukin-6: a new therapeutic target in systemic sclerosis? Clin. Transl. Immunology. 2:e4 10.1038/cti.2013.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlish J.S., Lemlich G., and Fleischmajer R.. 1988. Identification of collagen fibrils in scleroderma skin. J. Invest. Dermatol. 90:48–54. 10.1111/1523-1747.ep12462561 [DOI] [PubMed] [Google Scholar]
- Qiu W., Li X., Tang H., Huang A.S., Panteleyev A.A., Owens D.M., and Su G.H.. 2011. Conditional activin receptor type 1B (Acvr1b) knockout mice reveal hair loss abnormality. J. Invest. Dermatol. 131:1067–1076. 10.1038/jid.2010.400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey C., Winqvist O., Puhakka L., Halonen M., Moro A., Kämpe O., Eskelin P., Pelto-Huikko M., and Peltonen L.. 2002. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum. Mol. Genet. 11:397–409. 10.1093/hmg/11.4.397 [DOI] [PubMed] [Google Scholar]
- Rangel-Moreno J., Hartson L., Navarro C., Gaxiola M., Selman M., and Randall T.D.. 2006. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J. Clin. Invest. 116:3183–3194. 10.1172/JCI28756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder F., Biancani P., Harnett K., Yerian L., and Falk G.W.. 2010. Inflammatory mediators in gastroesophageal reflux disease: impact on esophageal motility, fibrosis, and carcinogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 298:G571–G581. 10.1152/ajpgi.00454.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón M., Anguita J., Nakamura T., Fikrig E., and Flavell R.A.. 1997. Interleukin (IL)-6 directs the differentiation of IL-4–producing CD4+ T cells. J. Exp. Med. 185:461–470. 10.1084/jem.185.3.461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C.G., Hummers L.K., Ravich W.J., Wigley F.M., and Hutchins G.M.. 2006. A case-control study of the pathology of oesophageal disease in systemic sclerosis (scleroderma). Gut. 55:1697–1703. 10.1136/gut.2005.086074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano E., Chora I., Manetti M., Mazzotta C., Rosa I., Bellando-Randone S., Blagojevic J., Soares R., Avouac J., Allanore Y., et al. 2016. Decreased expression of neuropilin-1 as a novel key factor contributing to peripheral microvasculopathy and defective angiogenesis in systemic sclerosis. Ann. Rheum. Dis. 75:1541–1549. 10.1136/annrheumdis-2015-207483 [DOI] [PubMed] [Google Scholar]
- Saigusa R., Asano Y., Taniguchi T., Yamashita T., Takahashi T., Ichimura Y., Toyama T., Tamaki Z., Tada Y., Sugaya M., et al. 2015. A possible contribution of endothelial CCN1 downregulation due to Fli1 deficiency to the development of digital ulcers in systemic sclerosis. Exp. Dermatol. 24:127–132. 10.1111/exd.12602 [DOI] [PubMed] [Google Scholar]
- Saito E., Fujimoto M., Hasegawa M., Komura K., Hamaguchi Y., Kaburagi Y., Nagaoka T., Takehara K., Tedder T.F., and Sato S.. 2002. CD19-dependent B lymphocyte signaling thresholds influence skin fibrosis and autoimmunity in the tight-skin mouse. J. Clin. Invest. 109:1453–1462. 10.1172/JCI0215078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano S., Takahama Y., Sugawara T., Kosaka H., Itami S., Yoshikawa K., Miyazaki J., van Ewijk W., and Takeda J.. 2001. Stat3 in thymic epithelial cells is essential for postnatal maintenance of thymic architecture and thymocyte survival. Immunity. 15:261–273. 10.1016/S1074-7613(01)00180-7 [DOI] [PubMed] [Google Scholar]
- Sato S., Hasegawa M., Fujimoto M., Tedder T.F., and Takehara K.. 2000. Quantitative genetic variation in CD19 expression correlates with autoimmunity. J. Immunol. 165:6635–6643. 10.4049/jimmunol.165.11.6635 [DOI] [PubMed] [Google Scholar]
- Sato S., Fujimoto M., Hasegawa M., Takehara K., and Tedder T.F.. 2004. Altered B lymphocyte function induces systemic autoimmunity in systemic sclerosis. Mol. Immunol. 41:1123–1133. 10.1016/j.molimm.2004.06.025 [DOI] [PubMed] [Google Scholar]
- Scheiber M.N., Watson P.M., Rumboldt T., Stanley C., Wilson R.C., Findlay V.J., Anderson P.E., and Watson D.K.. 2014. FLI1 expression is correlated with breast cancer cellular growth, migration, and invasion and altered gene expression. Neoplasia. 16:801–813. 10.1016/j.neo.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki F., Makhluf H.A., LeRoy C., Watson D.K., and Trojanowska M.. 1999. Ets transcription factors cooperate with Sp1 to activate the human tenascin-C promoter. Oncogene. 18:7755–7764. 10.1038/sj.onc.1203360 [DOI] [PubMed] [Google Scholar]
- Shum A.K., DeVoss J., Tan C.L., Hou Y., Johannes K., O’Gorman C.S., Jones K.D., Sochett E.B., Fong L., and Anderson M.S.. 2009. Identification of an autoantigen demonstrates a link between interstitial lung disease and a defect in central tolerance. Sci. Transl. Med. 1:9ra20 10.1126/scitranslmed.3000284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyropoulos D.D., Pharr P.N., Lavenburg K.R., Jackers P., Papas T.S., Ogawa M., and Watson D.K.. 2000. Hemorrhage, impaired hematopoiesis, and lethality in mouse embryos carrying a targeted disruption of the Fli1 transcription factor. Mol. Cell. Biol. 20:5643–5652. 10.1128/MCB.20.15.5643-5652.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukseree S., Mildner M., Rossiter H., Pammer J., Zhang C.F., Watanapokasin R., Tschachler E., and Eckhart L.. 2012. Autophagy in the thymic epithelium is dispensable for the development of self-tolerance in a novel mouse model. PLoS One. 7:e38933 10.1371/journal.pone.0038933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwara M.I., Green N.J., Borthwick L.A., Mann J., Mayer-Barber K.D., Barron L., Corris P.A., Farrow S.N., Wynn T.A., Fisher A.J., and Mann D.A.. 2014. IL-1α released from damaged epithelial cells is sufficient and essential to trigger inflammatory responses in human lung fibroblasts. Mucosal Immunol. 7:684–693. 10.1038/mi.2013.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaba H., Morishita Y., Tomofuji Y., Danks L., Nitta T., Komatsu N., Kodama T., and Takayanagi H.. 2015. Fezf2 orchestrates a thymic program of self-antigen expression for immune tolerance. Cell. 163:975–987. 10.1016/j.cell.2015.10.013 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Asano Y., Ichimura Y., Toyama T., Taniguchi T., Noda S., Akamata K., Tada Y., Sugaya M., Kadono T., and Sato S.. 2015a Amelioration of tissue fibrosis by toll-like receptor 4 knockout in murine models of systemic sclerosis. Arthritis Rheumatol. 67:254–265. 10.1002/art.38901 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Asano Y., Noda S., Aozasa N., Akamata K., Taniguchi T., Ichimura Y., Toyama T., Sumida H., Kuwano Y., et al. 2015b A possible contribution of lipocalin-2 to the development of dermal fibrosis, pulmonary vascular involvement and renal dysfunction in systemic sclerosis. Br. J. Dermatol. 173:681–689. 10.1111/bjd.13779 [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Asano Y., Akamata K., Noda S., Takahashi T., Ichimura Y., Toyama T., Trojanowska M., and Sato S.. 2015. Fibrosis, vascular activation, and immune abnormalities resembling systemic sclerosis in bleomycin-treated Fli-1–haploinsufficient mice. Arthritis Rheumatol. 67:517–526. 10.1002/art.38948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taroni J.N., Martyanov V., Huang C.C., Mahoney J.M., Hirano I., Shetuni B., Yang G.Y., Brenner D., Jung B., Wood T.A., et al. 2015. Molecular characterization of systemic sclerosis esophageal pathology identifies inflammatory and proliferative signatures. Arthritis Res. Ther. 17:194 10.1186/s13075-015-0695-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bon L., Affandi A.J., Broen J., Christmann R.B., Marijnissen R.J., Stawski L., Farina G.A., Stifano G., Mathes A.L., Cossu M., et al. 2014. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N. Engl. J. Med. 370:433–443. 10.1056/NEJMoa1114576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoogen F., Khanna D., Fransen J., Johnson S.R., Baron M., Tyndall A., Matucci-Cerinic M., Naden R.P., Medsger T.A. Jr., Carreira P.E., et al. 2013. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 65:2737–2747. 10.1002/art.38098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Wei G., and Liu D.. 2012. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp. Hematol. Oncol. 1:36 10.1186/2162-3619-1-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Fan P.S., and Kahaleh B.. 2006. Association between enhanced type I collagen expression and epigenetic repression of the FLI1 gene in scleroderma fibroblasts. Arthritis Rheum. 54:2271–2279. 10.1002/art.21948 [DOI] [PubMed] [Google Scholar]
- Wei J., Bhattacharyya S., Tourtellotte W.G., and Varga J.. 2011. Fibrosis in systemic sclerosis: emerging concepts and implications for targeted therapy. Autoimmun. Rev. 10:267–275. 10.1016/j.autrev.2010.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield M.L., Finlay D.R., Murray J.I., Troyanskaya O.G., Chi J.T., Pergamenschikov A., McCalmont T.H., Brown P.O., Botstein D., and Connolly M.K.. 2003. Systemic and cell type-specific gene expression patterns in scleroderma skin. Proc. Natl. Acad. Sci. USA. 100:12319–12324. 10.1073/pnas.1635114100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.S., and Wynn T.A.. 2009. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2:103–121. 10.1038/mi.2008.85 [DOI] [PMC free article] [PubMed] [Google Scholar]