Both tumor- and host-derived PD-L1 can play critical roles in immunosuppression; differences in tumor immunogenicity appear to underlie their relative contributions. Juneja et al. show that in immunogenic MC38 tumors, PD-L1 on tumor cells dominates in suppressing tumor immunity by inhibiting CD8 T cell cytotoxicity.
Abstract
It is unclear whether PD-L1 on tumor cells is sufficient for tumor immune evasion or simply correlates with an inflamed tumor microenvironment. We used three mouse tumor models sensitive to PD-1 blockade to evaluate the significance of PD-L1 on tumor versus nontumor cells. PD-L1 on nontumor cells is critical for inhibiting antitumor immunity in B16 melanoma and a genetically engineered melanoma. In contrast, PD-L1 on MC38 colorectal adenocarcinoma cells is sufficient to suppress antitumor immunity, as deletion of PD-L1 on highly immunogenic MC38 tumor cells allows effective antitumor immunity. MC38-derived PD-L1 potently inhibited CD8+ T cell cytotoxicity. Wild-type MC38 cells outcompeted PD-L1–deleted MC38 cells in vivo, demonstrating tumor PD-L1 confers a selective advantage. Thus, both tumor- and host-derived PD-L1 can play critical roles in immunosuppression. Differences in tumor immunogenicity appear to underlie their relative importance. Our findings establish reduced cytotoxicity as a key mechanism by which tumor PD-L1 suppresses antitumor immunity and demonstrate that tumor PD-L1 is not just a marker of suppressed antitumor immunity.
Introduction
The programmed cell death (PD)-1 pathway has become an attractive therapeutic target in multiple cancers (Chen and Mellman, 2013; Mahoney et al., 2015; Callahan et al., 2016). PD-1 is up-regulated on T cells upon activation and remains high on exhausted T cells. PD-1 is commonly highly expressed on tumor-infiltrating lymphocytes (TILs; Ahmadzadeh et al., 2009). Blocking the interaction of PD-1 with its ligands, PD-L1 and PD-L2, leads to impressive antitumor responses and clinical benefit in a subset of patients (Ribas, 2012; Alme et al., 2016). However, the precise cellular and molecular mechanisms underlying this efficacy are not well understood.
Early mechanistic studies of the PD-1 pathway showed that PD-1 ligation can inhibit the initial activation of T cells and suppress effector T cell generation and function, including cytokine production and cytotoxicity (Hirano et al., 2005; Francisco et al., 2009). By dampening effector T cell responses, the PD-1 pathway plays an important role in tissues where PD-1 ligands on hematopoietic and nonhematopoietic cells prevent excessive damage during an ongoing immune response, thereby controlling resolution of inflammation and tissue tolerance (Blank et al., 2004; Keir et al., 2006). This understanding, together with the finding that tumors often express PD-1 ligands, provided the rationale for targeting the PD-1 pathway in cancer (Latchman et al., 2001; Dong et al., 2002; Iwai et al., 2002; Pardoll, 2012).
Studies in animal models and clinical trials have contributed to our current understanding of mechanisms underlying the efficacy of PD-1 pathway blockade in cancer. After PD-1 blockade, TILs from mouse tumors exhibit increased polyfunctionality (characterized by production of multiple cytokines or cytotoxic molecules) compared with controls (Spranger et al., 2013; Gubin et al., 2014). In cancer patients, clinical responses to PD-1 immunotherapy positively correlate with tumor PD-L1 expression, along with other predictive biomarkers such as preexisting CD8+ T cell infiltration and mutational/neoantigen burden (Herbst et al., 2014; Tumeh et al., 2014). This has led to the speculation that PD-L1 on tumor cells may act as a molecular shield to protect PD-L1+ tumor cells from T cell lysis (Zou et al., 2016). However, in several clinical trials, some patients with tumors that do not express PD-L1 respond to PD-1 pathway blockade, albeit at a lower rate (Zou et al., 2016). PD-L1 on other cells (e.g., myeloid cells) in the tumor microenvironment also appears to have a major effect on response to therapy (Herbst et al., 2014). Therefore, the relative roles and functions of PD-L1 on tumor cells and PD-L1 expressed on other cell types in limiting antitumor immunity in the tumor microenvironment remain unclear. Here, we use mouse tumor models in which PD-1 monotherapy has a significant effect to investigate these questions. We demonstrate that PD-L1 expression on tumor cells alone can locally inhibit CD8+ T cell activities and protect PD-L1+, but not PD-L1−, tumor cells from eradication by the immune system. These findings establish a critical role for PD-L1 on the tumor cell itself in suppressing antitumor immunity.
Results and discussion
Relative contributions of PD-L1 on tumor cells and nontumor cells in the tumor microenvironment is context-dependent
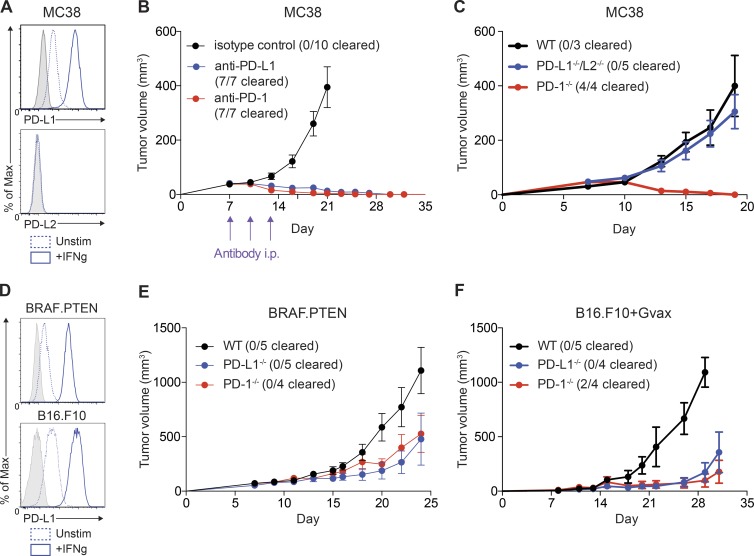
To elucidate mechanisms by which blockade of the PD-1 pathway leads to antitumor immunity, we first used MC38 colorectal adenocarcinoma, given its sensitivity to PD-1 monotherapy. MC38 cells express PD-L1, which was up-regulated by IFN-γ in vitro (Fig. 1 A). MC38 tumor cells assayed ex vivo at 24 d after implantation expressed high levels of PD-L1, supporting a potential role for PD-L1 on tumor cells themselves (unpublished data). In contrast, PD-L2 was not expressed on MC38 cells in vitro or ex vivo (Fig. 1 A and not depicted).
Figure 1.
Relative role of PD-L1 on tumor cells differs by model. (A) MC38 tumor cells were cultured in vitro and stimulated with IFN-γ (20 ng/ml) for 24 h. Expression of PD-L1 and PD-L2 was assessed by flow cytometry. FMO staining control shown in gray. (B) WT mice were given 105 MC38 tumor cells s.c. and treated on days 7, 10, and 13 with (red) anti–PD-1 (29F.1A12; 29/30 mice cleared tumors across all experiments) or (black) isotype control (rIgG2a; n = 5), or (blue) anti–PD-1 (339.6A2; 20/30 mice cleared tumors across all experiments) or isotype control (mIgG1; n = 5). Tumors were measured every 2–3 d starting on day 7. Control treated tumors had equivalent growth curves and were pooled for plotting purposes. Tumor growth is representative of five independent experiments with at least five mice per group. (C) WT, PD-1−/−, or PD-L1−/−/L2−/− mice were given 105 MC38 tumor cells s.c. Tumors were measured every 2–3 d starting on day 7. Tumor growth is representative of five independent experiments with at least five mice per group. (D) BRAF.PTEN melanoma cells and B16.F10 melanoma cells were cultured in vitro and stimulated with IFN-γ (20 ng/ml) for 24 h. Expression of PD-L1 was assessed by flow cytometry. FMO staining control is shown in gray. Expression representative of three independent experiments where n = 3. (E) WT, PD-1−/−, or PD-L1−/−/L2−/− mice were given 105 BRAF.PTEN tumor cells s.c. Tumors were measured every 2–3 d starting on day 7. Tumor growth is representative of three independent experiments with at least five mice per group. (F) WT, PD-1−/−, or PD-L1−/−/L2−/− mice were given 105 B16.F10 tumor cells s.c. and 106 irradiated B16/GM-CSF tumor cells s.c. on the contralateral side. Tumors were measured every 2–3 d starting on day 7. Tumor growth is representative of three independent experiments with at least five mice per group.
Previous work has shown that a fraction of PD-1−/− mice or WT mice given anti–PD-1 antibodies can clear large inoculums (2–5 × 106 cells) of MC38 tumor cells (Woo et al., 2012). We decreased the tumor challenge (105 cells) to slow tumor growth and allow time for a more robust adaptive immune response to develop. At this lower tumor dose, robust and durable tumor clearance was observed in the majority of mice given anti–PD-1 or anti–PD-L1 compared to controls (Fig. 1 B).
We used this lower tumor dose to investigate the relative contributions of PD-L1 expressing cell types to inhibiting the immune response to MC38. We first examined whether PD-L1 on the tumor cells could suppress the antitumor immune response by comparing MC38 tumor cell growth in mice deficient for PD-1 (PD-1−/−) or its ligands, PD-L1 and PD-L2 (PD-L1−/−/L2−/−). In the former, there is no engagement of PD-1 on T cells, whereas in the latter, PD-1 can only interact with PD-L1 expressed by MC38 tumor cells. PD-1−/− mice completely cleared MC38 tumors, with maximum tumor volume around day 10 (Fig. 1 C). In contrast, MC38 tumor growth was similarly robust in PD-L1−/−/L2−/− and WT mice (Fig. 1 C). Because PD-L1 expression on the tumor cells is the only source of ligand for PD-1 in the PD-L1−/−/L2−/− mice, this indicates that engagement of PD-1 by PD-L1 on tumor cells alone is sufficient to suppress antitumor immunity to MC38 tumors.
Within the limits of tumor biopsy, clinical studies demonstrate that PD-L1 expression on tumor cells is not a prerequisite for response to PD-1 pathway blockade (Zou et al., 2016). We hypothesized that the relative importance of PD-L1 on tumor cells versus nontumor cells may be model dependent. We speculated that the role of PD-L1 on nontumor cells may differ in models that are less sensitive to PD-1 blockade than MC38 tumors. To test this, we used two tumor models that are only moderately sensitive to PD-1 blockade, BRAF.PTEN melanoma and B16.F10 melanoma combined with GVAX (Curran et al., 2010; Cooper et al., 2014). Similar to MC38 tumors, both of these cell lines expressed PD-L1, which was increased by treatment with IFN-γ (Fig. 1 D).
We have previously shown that BRAF.PTEN tumors grow more slowly after PD-1 blockade (Cooper et al., 2014). We initiated tumors with a lower tumor cell inoculum than previously used (105 rather than 8 × 105 tumor cells) to allow a stronger T cell response to develop before tumor outgrowth. BRAF.PTEN tumor growth was slower in PD-1−/− mice than in WT mice, but these tumors still grew progressively in contrast to MC38 tumors. Also, unlike MC38 tumors, BRAF.PTEN tumor growth was delayed in PD-L1−/− mice compared with WT mice (Fig. 1 E). Because PD-L1 on BRAF.PTEN tumor cells is the only source of PD-1 engagement in PD-L1−/− mice, these data indicate that PD-L1 expression on nontumor cells in WT mice plays a significant role in inhibiting antitumor immunity to BRAF.PTEN tumors.
PD-1 blockade alone is ineffective in mice with B16.F10 tumors (Chen et al., 2015; Kleffel et al., 2015). We therefore used vaccination with a GM-CSF expressing cell line (GVAX) concurrently with tumor implantation as a method to augment the immune response (Curran et al., 2010). This approach can slow B16 tumor growth and induce a stronger T cell response compared with B16 tumor implantation without vaccination, albeit still with aggressive tumor growth. Indeed, B16 tumor growth with GVAX was robust in WT mice, but was delayed in PD-1−/− mice (Fig. 1 F). Similar to BRAF.PTEN tumors, B16 tumor growth was delayed in PD-L1−/− mice, demonstrating a role for PD-L1 on nontumor cells in suppressing antitumor immunity in the B16 tumor model. Collectively, these findings demonstrate that the relative contribution of tumor or host-derived PD-L1 is context-dependent and that both tumor and host-derived PD-L1 can play a critical role in inhibiting antitumor immunity.
PD-L1 on MC38 tumor cells is sufficient to directly suppress activated CD8 TILs
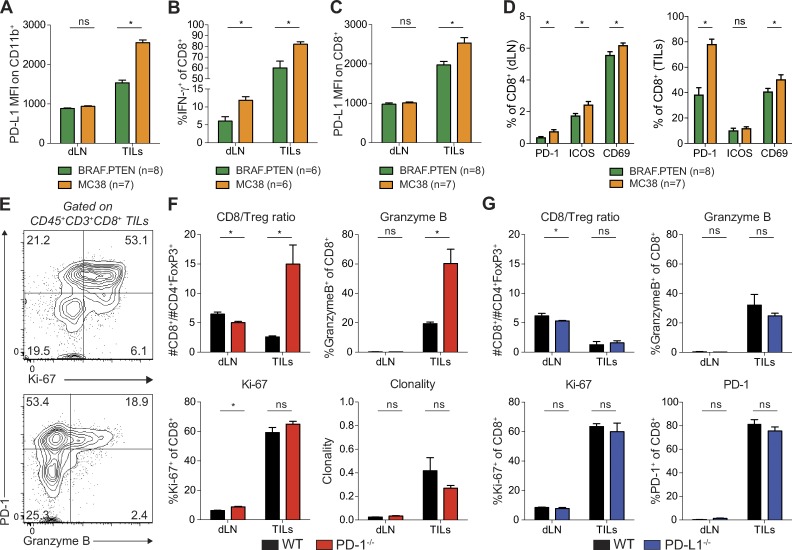
To investigate the relative contributions of PD-L1 on tumor cells and nontumor cells in different tumor models, we focused on MC38 and BRAF.PTEN tumors. We first determined whether the differential role of PD-L1 on nontumor cells in MC38 and BRAF.PTEN tumors might be due to differences in PD-L1 expression levels on cells in the tumor microenvironment in these two models. To assess this, we compared ex vivo PD-L1 expression on nontumor cells 20 d after MC38 or BRAF.PTEN implantation in WT mice. We initially evaluated PD-L1 expression on myeloid cells (CD11b+) because PD-L1 expression on myeloid cells can correlate with response to PD-1 therapy and has been implicated in driving suppression of CD8+ T cells in murine orthotropic colorectal tumors (Zhang et al., 2017). CD11b+ cells in MC38 tumors exhibited higher PD-L1 expression compared to CD11b+ cells from BRAF.PTEN tumors (Fig. 2 A). Moreover, PD-L1 expression in the MC38 tumor microenvironment was either equivalent or higher on CD45− nontumor cells, other immune cells, and tumor cells compared to expression on these cell types in BRAF.PTEN tumors (unpublished data). This is likely caused by the increased secretion of IFN-γ by CD8+ T cells in MC38 tumors, both in the percentage of cells producing IFNγ and in the amount of IFN-γ produced by each cell (Fig. 2 B), as IFN-γ increases expression of PD-L1. Indeed, PD-L1 expression is higher on CD8+ T cells themselves (Fig. 2 C). Therefore, differences in PD-L1 expression in the tumor microenvironment do not explain the distinct contributions of PD-L1 on tumor cells versus nontumor cells in suppressing the antitumor immune response.
Figure 2.
PD-L1 on MC38 tumor cells directly suppresses CD8 TILs. (A–D) Cells were isolated from the draining LN (dLN) or tumor (tumor infiltrating leukocytes; TILs) on day 20 of MC38 or BRAF.PTEN tumor growth and analyzed by flow cytometry. Analysis representative of two independent with at least six mice per group as indicated under each plot. Statistical significance determined by Student’s t test where P < 0.05 (* indicates significance; ns, not significant). (A) Expression of PD-L1 on CD45+CD11b+ myeloid cells analyzed directly ex vivo. (B) Expression of IFN-γ in CD8+ T cells after ex vivo restimulation. Gating is based on unstimulated control. (C) Expression of PD-L1 on CD8+ T cells analyzed directly ex vivo. (D) Expression of PD-1, ICOS, and CD69 on CD8+ T cells analyzed directly ex vivo. (E) CD45+CD3+CD8+ live cells were isolated from the tumor of WT mice on day 10 of tumor growth and analyzed by flow cytometry directly ex vivo. Representative plots showing expression of PD-1, and Granzyme B or Ki-67 on TILs from WT mice where the numbers within each quadrant represent the frequency of each population. (F and G) Cells were isolated from the dLN or tumor (tumor infiltrating lymphocytes; TILs) on day 10 of tumor growth and analyzed by flow cytometry directly ex vivo. Statistical significance determined by Student’s t test, where P < 0.05 (* indicates significance; ns, not significant). (F) TIL analyses from WT and PD-1−/− mice. The ratio of CD8+ T cells to CD4+FoxP3+ cells (CD8/T reg), percentage of cells expressing Ki-67 or Granzyme B, and the clonality of CD8+ T cells were compared in tumors from WT and PD-1−/− mice. (G) TIL analyses from WT and PD-L1−/− mice. The ratio of CD8+ T cells to CD4+FoxP3+ cells (CD8/T reg), percentage of cells expressing Ki-67, PD-1, or Granzyme B were compared in tumors from WT and PD-L1−/− mice. Clonality calculation is from one experiment.
In addition to increased IFN-γ in the tumor microenvironment, CD8+ T cells in the draining lymph node (dLN) of MC38 tumor-bearing mice expressed higher levels of IFN-γ relative to BRAF.PTEN tumor-bearing mice (Fig. 2 B). Therefore, MC38 tumors likely stimulate a stronger antitumor T cell response. To test this further, we compared expression of several T cell activation markers in the dLN and tumors of mice bearing MC38 or BRAF.PTEN tumors. PD-1, ICOS, and CD69 were all expressed at higher frequencies on CD8+ T cells in the dLN of MC38 tumor-bearing mice than in dLN of mice with BRAF.PTEN tumors on day 20 after implantation (Fig. 2 D). PD-1 and CD69 were also expressed at higher frequencies on TILs from MC38 tumors. Although PD-1 is clearly a potent immunoinhibitory receptor, exemplified by the complete clearance of tumors in PD-1−/− mice, PD-1 expression is rapidly up-regulated upon T cell activation. CD8+ TILs from WT mice 10 d after MC38 tumor injection (a time point before the tumor clearance observed in PD-1−/− mice) also expressed high levels of PD-1 (Fig. 2 E). PD-1+ CD8+ TILs from WT mice expressed higher levels of the proliferation marker, Ki-67, and the cytotoxic molecule, Granzyme B, than PD-1− T cells on day 10 (Fig. 2 E). Therefore, high PD-1 expression does not only signify dysfunctional T cells, but can also indicate activated T cells infiltrating the tumor. Collectively, these data demonstrate that CD8+ T cells are more activated in response to MC38 tumors relative to BRAF.PTEN tumors, both in the dLN and in the tumor itself. PD-1 may exert its suppressive effects in both the draining lymph node during initial T cell priming and in the tumor microenvironment during the effector T cell response. Our data demonstrate that CD8+ T cells are more highly activated in response to MC38 tumors relative to BRAF.PTEN tumors. This enhanced activation of CD8+ T cells in response to MC38 tumors may underlie the decreased sensitivity to suppression by PD-L1 on nontumor cells, either in the dLN or tumor.
To understand how PD-1 ligation by PD-L1 on MC38 tumor cells suppresses antitumor immunity, we further analyzed the T cells from the dLN and TILs of mice with MC38 tumors. At the time point when PD-1−/− mice began to clear MC38 tumors (day 10), the ratio of CD8+ T cells to regulatory T cells in the tumor (CD8/T reg ratio) was significantly increased in PD-1−/− mice relative to WT controls (Fig. 2 F). However, the percentage of dividing CD8+ T cells (Ki-67+) was equally high in WT and PD-1−/− mice (Fig. 2 F). These findings suggest that the increased CD8/T reg ratio may reflect increased CD8+ T cell infiltration into tumors or survival within the tumor rather than increased CD8+ T cell proliferation.
To compare the specificities of WT and PD-1−/− CD8+ T cells, we assessed the clonality of these T cells to determine if they were specific for one or a small number of tumor antigens. We hypothesized that an increased number of T cells responding to one or a small number of specific antigens would lead to increased clonality of CD8+ TILs. We did find increased clonality of CD8+ T cells in TILs relative to dLN in both WT and PD-1−/− mice, yet T cell clonality in TILs was similar from WT and PD-1−/− mice (Fig. 2 F). Despite the lack of change in overall clonality or frequency of tumor antigen–specific T cells, there was a significant increase in the percentage of CD8+ T cells producing the cytotoxic molecule, Granzyme B, in the TILs of PD-1−/− mice relative to WT controls (Fig. 2 F). An increase in the expression of Granzyme B in the tumor microenvironment is similarly observed in patients who respond to PD-1 blockade, but not in nonresponders indicating that the decrease in cytotoxicity mediated by PD-1 engagement is critical for suppression of CD8+ T cells (Herbst et al., 2014; Tumeh et al., 2014; Das et al., 2015). These results illustrate that although PD-1 is up-regulated on functional, activated cells it also decreases effector molecule expression in CD8+ T cells upon ligation.
Because MC38 tumors are cleared in PD-1−/− mice but not PD-L1−/− mice, we hypothesized that the differences in cellular infiltrate observed in PD-1−/− mice compared with WT mice would be absent in PD-L1−/− mice. Indeed, the CD8/T reg ratio and percentages of cells expressing Granzyme B, Ki-67, and PD-1 are all unchanged in TILs from PD-L1−/− mice relative to WT mice (Fig. 2 G). Together, these data indicate that PD-L1 on MC38 tumor cells can potently suppress the cytotoxic potential of CD8+ T cells expressing PD-1 in the tumor microenvironment.
PD-L1 on MC38 tumor cells is dominant in suppression of antitumor immunity
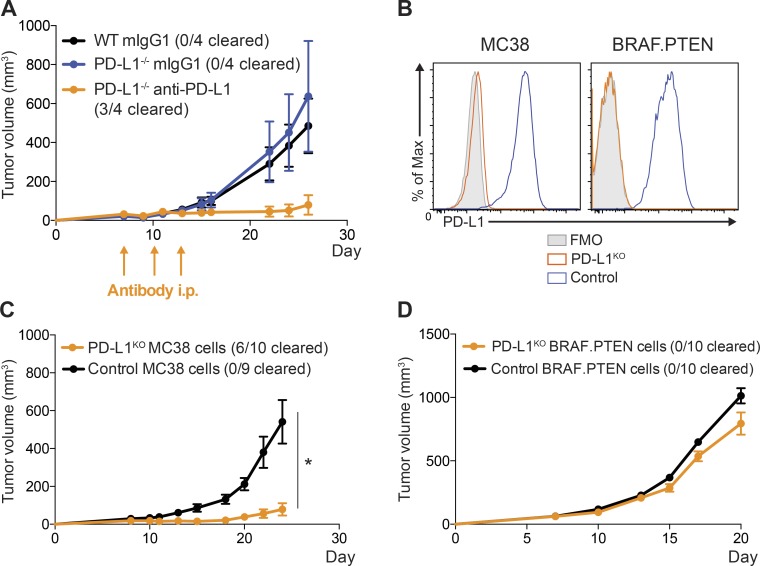
To further investigate the functional importance of PD-L1 on MC38 tumor cells in suppressing antitumor immunity, we administered PD-L1-blocking antibody to PD-L1−/− mice with MC38 tumors. In this scenario, where PD-L1 is only present on the MC38 tumor cells, anti–PD-L1 potently induced antitumor immunity, resulting in tumor clearance in the majority of mice (Fig. 3 A). We next tested the functional significance of tumor PD-L1 to immunosuppression by deleting PD-L1 on MC38 or BRAF.PTEN tumor cells. We used CRISPR/Cas9 to generate PD-L1–deficient cell lines (PD-L1KO MC38 and PD-L1KO BRAF.PTEN), along with appropriate controls, to determine whether tumor-derived PD-L1 is required for tumor progression. Even with strong IFN-γ stimulation, PD-L1KO MC38 cells did not express PD-L1, confirming loss of PD-L1 (Fig. 3 B). When injected into WT mice, which express PD-L1, PD-L1KO MC38 and control tumors grew at similar rates until around day 10, after which PD-L1KO tumors either grew more slowly than WT tumors or were completely cleared, suggesting PD-L1 on MC38 tumor cells is required to suppress the antitumor immune response (Fig. 3 C). In contrast, when we performed the analogous experiment with PD-L1KO BRAF.PTEN cells, tumor growth was only slightly slowed in WT mice, suggesting PD-L1 expression on BRAF.PTEN tumor cells is not a predominate contributor to inhibit antitumor immunity (Fig. 3 D).
Figure 3.
PD-L1 on MC38 tumor cells is critical for suppression of antitumor immunity. (A) WT or PD-L1−/− mice were given 105 MC38 tumor cells s.c. and treated on days 7, 10, and 13 with anti–PD-1 (339.6A2) or isotype control (mIgG1). Tumors were measured every 2–3 d starting on day 7. (B) Control MC38 and MC38 PD-L1KO (left) or control BRAF.PTEN and BRAF.PTEN PD-L1KO (right) cells were cultured in vitro and stimulated with IFN-γ (20 ng/ml) for 24 h. Expression of PD-L1 was assessed by flow cytometry. (C) WT mice were given either 105 control MC38 or MC38 PD-L1KO tumor cells s.c. and tumors measured every 2–3 d, starting on day 7. (D) WT mice were given either 105 control BRAF.PTEN or PD-L1KO BRAF.PTEN tumor cells s.c., and tumors were measured every 2–3 d starting on day 7. Tumor growth experiments are representative of at least two independent experiments (n = 4–10 mice per group, as indicated). Statistical significance determined by Student’s t test, where P < 0.05 (* indicates significance).
The clearance of the majority of PD-L1KO MC38 tumors in WT mice highlights the relative dominance of PD-L1 suppression mediated by tumor cells themselves in the MC38 model. However, not all MC38 tumors were cleared in WT mice, unlike PD-1−/− mice, highlighting that there is still a role for PD-L1 on nontumor cells in the MC38 model, albeit a modest one. The role for PD-L1 on nontumor cells in BRAF.PTEN tumors is greater, as only a small increase in antitumor immunity was observed in the absence of PD-L1 on tumor cells. These models, therefore, provide valuable tools for understanding the relative roles of PD-L1 on different cell types.
PD-L1 on MC38 tumor cells directly suppresses CD8+ T cell cytotoxicity
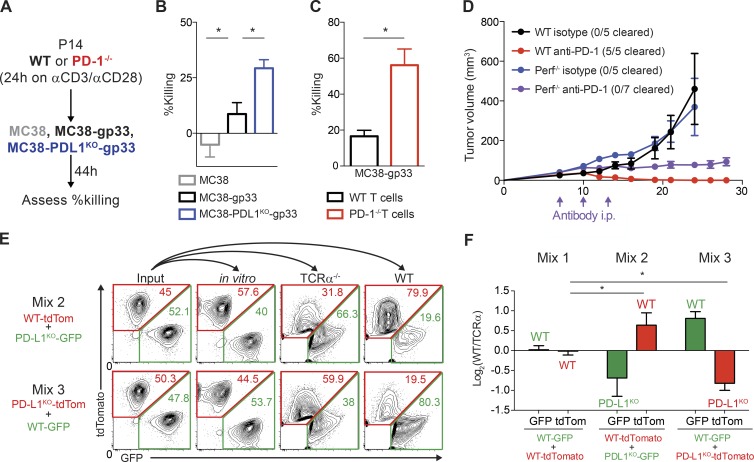
Because our data suggest that PD-L1 on MC38 cells may attenuate CD8+ T cell cytotoxicity, we next directly tested whether PD-L1 on MC38 cells can suppress CD8+ T cell cytotoxicity. To do so, we developed an in vitro cytotoxicity assay in which we stimulated CD8+ T cells specific for the gp33 peptide (P14 TCR transgenic) for 24 h with anti-CD3 and anti-CD28, and then coincubated these stimulated CD8+ T cells with MC38 tumor cells expressing the gp33 peptide (Fig. 4 A). WT P14 CD8+ T cells killed MC38 tumor cells expressing gp33, but not control MC38 cells, demonstrating the antigen specificity of this assay (Fig. 4 B). To test the role of PD-1:PD-L1 interactions, we compared killing of tumor cells without PD-L1 or T cells without PD-1 to controls (Fig. 4 A). In the absence of PD-L1 on tumor cells, the level of tumor cell killing by WT P14 CD8+ T cells increased, suggesting that tumor PD-L1 suppresses T cell killing (Fig. 4 B). Similarly, when T cells themselves lack PD-1, tumor cell killing was higher compared tokilling by WT T cells (Fig. 4 C). Therefore, PD-L1 on MC38 tumor cells suppresses T cell cytotoxicity in vitro by engaging PD-1 on CD8+ T cells.
Figure 4.
PD-L1 on MC38 tumor cells directly suppresses cytotoxicity of CD8+ T cells. (A) Experimental schema for B and C. P14 TCR Tg T cells were isolated and stimulated with anti-CD3/CD28 for 24 h, and then cultured with the indicated MC38 cells for 44 h. Tumor cell killing represents percentage reduction in number of tumor cells with T cells relative to control cultures without T cells. All experiments were performed with four biological replicates per group and are representative of at least two independent experiments. (B) Relative killing by WT T cells of MC38 control cells, MC38 cells expressing the gp33 antigen (MC38-gp33), and MC38-gp33 cells deficient for PD-L1. Statistical significance determined by Student’s t test where P < 0.05 (* indicates significance). (C) Relative killing of MC38-gp33 by WT versus PD-1–deficient T cells. Statistical significance determined by Student’s t test where P < 0.05 (* indicates significance). (D) WT or Perforin−/− mice were given 105 MC38 tumor cells s.c. and treated on days 7, 10, and 13 with anti–PD-1 (339.6A2) or isotype control (mIgG1). Tumors were measured every 2–3 d starting on day 7. Tumor growth is representative of two independent experiments with at least five mice per group. (E and F) Control MC38 and MC38 PD-L1KO tumor cells that stably express GFP or tdTomato were mixed at a 1:1 ratio (input) and either cultured in vitro for 15 d or 105 total cells administered s.c. to TCRα−/− mice or WT mice. Tumor cell composition was analyzed 20 d later. (E) Representative plots were gated on live CD45− fluorescent cells where the numbers within the red and green gates represent the frequency of TdTomato or GFP+ cells, respectively, of live CD45− fluorescent cells. (F) Ratio of frequency of GFP+ and tdTomato+ tumor cells from WT mice and TCRα−/− (Log2(WT/TCRα) mice after 15 d of growth in vivo representative of three independent experiments (n ≥ 4 mice per group). Statistical significance was determined by Student’s t test, where P < 0.05.
To investigate whether PD-1 suppression of CD8+ T cell granule–mediated cytotoxicity in vivo was critical for the beneficial effects of PD-1 blockade, we injected MC38 tumors into WT mice or mice lacking Perforin (Perf−/−), a key cytolytic protein that allows Granzymes to enter target cells, and treated with anti–PD-1 mAb. In the absence of Perforin, PD-1 blockade still slowed tumor growth, but did not lead to tumor clearance in contrast to WT mice (Fig. 4 C). These data demonstrate that CD8+ T cell cytotoxicity is required for tumor clearance mediated by PD-1 pathway blockade. Because tumor growth is controlled, but not cleared by PD-1 blockade in the Perf−/− mice, these findings further indicate that PD-1 suppresses antitumor immunity in several ways including inhibition of CD8+ T cell cytotoxicity. Inhibition of cytokine production by CD4+ and CD8+ TILs is another important mechanism by which PD-1 inhibits antitumor immunity. For example, cytokine production by TILs is increased in PD-1−/− mice with MC38 tumors (Woo et al., 2012). Together, these findings show PD-1 blockade can promote antitumor immunity by increasing CD8+ T cell cytotoxicity, as well as by increasing production of cytokines. A recent study identified β-2-microglobulin and JAK1 and JAK2 mutations as acquired resistance mechanisms that develop during PD-1 blockade (Zaretsky et al., 2016). These tumor escape mechanisms lead to resistance to CD8+ T cell recognition and cytokine sensing that can promote tumor cell growth (Zaretsky et al., 2016). Although these findings were made in the context of resistance to PD-1 blockade, whereas our study focuses on the initial response to PD-1 blockade, these mutations underscore the functional significance of PD-1–mediated inhibition of CD8+ T cell cytotoxicity.
To directly evaluate whether PD-L1 on MC38 cells is required to suppress CD8+ T cell cytotoxicity, we used a mixed competition assay in which we injected WT mice with mixtures of equivalent numbers of control MC38 and MC38 PD-L1KO tumor cells stably expressing different fluorescent markers (GFP or tdTomato) and measured cellular composition of tumors by assessing GFP and tdTomato fluorescence ex vivo 15 d after injection. We hypothesized that if PD-L1 is critical for direct suppression of CD8+ T cell cytotoxicity, then the MC38 PD-L1KO cells would be selectively depleted. Indeed, in both mixtures, regardless of the fluorescent label, MC38 PD-L1KO cells were selectively reduced in frequency in the tumor (Mixes 2 and 3, Fig. 4 E). To control for T cell–independent differences in antitumor immunity or engraftment, we normalized the ratios of each MC38 mixture after growth in WT mice to the ratios of these mixtures after growth in TCRα−/− mice (Fig. 4 F). Importantly, WT mice given mixtures of both GFP- and TdTomato-expressing PD-L1+ control tumor cell lines showed no significant change in the proportion of either population, indicating that there is no selective advantage in immunogenicity against either fluorescent protein (Mix 1, Fig. 4 E). Small differences in growth rate of the various cell lines did not explain the selective growth advantage of WT PD-L1+ MC38 cell lines in vivo because the ratios stayed close to 50:50 after 15 d of in vitro propagation (Fig. 4 E). Collectively, these data demonstrate that PD-L1 on tumor cells can directly inhibit the cytotoxicity of CD8+ T cells in the tumor microenvironment and that expression of PD-L1 on tumor cells provides a selective growth advantage by suppressing the immune response directed against PD-L1+ tumor cells. Our results are consistent with previous in vitro studies showing that retrovirally infected cells expressing high levels of PD-L1 outcompeted cells that expressed little or no PD-L1 due to a decrease in CD8+ T cell cytotoxicity (Akhmetzyanova et al., 2015). Our work extends this finding into the tumor microenvironment, and supports the concept of PD-L1 acting as a molecular shield on tumor cells to prevent cytolysis mediated by T cells (Iwai et al., 2002; Rodig et al., 2003; Hirano et al., 2005; Azuma et al., 2008).
The role of PD-L1 on tumor cells versus nontumor cells in immune suppression has been controversial. Whereas PD-L1 expression on tumor cells can correlate with a higher frequency of response to PD-1 blockade in the clinic, it has not been clear whether PD-L1 on tumor cells is functionally important in suppression of T cell responses or simply correlates with an inflamed tumor microenvironment that lends itself to increased response to immunotherapy. By comparing three tumor models with varying sensitivity to PD-1 pathway blockade, we demonstrate that the relative contribution of tumor or host-derived PD-L1 is context dependent and that both tumor and host-derived PD-L1 can play a critical role in immunosuppression. PD-L1 on nontumor cells can clearly play a role in limiting antitumor immunity, as is the case with BRAF.PTEN and B16.GVAX tumors. However, PD-L1 on tumor cells plays a dominant role in suppression of tumor immunity in the MC38 model. Our findings suggest that tumor immunogenicity may impact the contribution of host or tumor-derived PD-L1.
Furthermore, we used the MC38 model to elucidate mechanisms by which PD-L1 on tumor cells suppresses antitumor immunity. We show that PD-L1 on MC38 tumor cells can directly inhibit CD8+ T cell responses. PD-L1 on MC38 cells reduces CD8+ T cell cytotoxicity and the CD8+ ratio relative to T reg cells. PD-L1 can protect tumor cells on a single-cell basis, indicating a direct but locally restricted interaction with PD-1 expressing CD8+ T cells, which can still kill tumor cells that do not express PD-L1. This results in outgrowth of PD-L1+ tumor cells, and likely explains why patients whose tumors express high levels and percentages of PD-L1 are more sensitive to PD-1 blockade: tumor PD-L1 may render CD8+ TILs sensitive to PD-1 signaling, which can then be blocked therapeutically. Together, our work demonstrates that PD-L1 on tumor cells can exert functionally significant suppressive effects that inhibit antitumor immunity, and is far more than a marker of an ineffective immune response.
Materials and methods
Mice
6–10-wk-old mice were used for all experiments. WT C57BL/6 mice, C57BL/6-Prf1tm1Sdz/J, and B6.129S2-Tcrαtm1Mom/J mice were originally purchased from The Jackson Laboratory. P14 TCR Tg (Pircher et al., 1989) mice have been previously described. Pdcd1−/−, Cd274−/−, and Pdcd1lg2−/− mice on the C57BL/6 background were generated in the Sharpe laboratory (Latchman et al., 2004; Keir et al., 2006, 2007). Colonies for each strain of mice were maintained in the same animal facility at Harvard Medical School. All experimental mice were housed in specific pathogen–free conditions and used in accordance with animal care guidelines from the Harvard Medical School Standing Committee on Animals and the National Institutes of Health. Animal protocols were approved by the Harvard Medical School Standing Committee on Animals.
Tumor cell lines
MC38 adenocarcinoma (a gift from D. Vignali, University of Pittsburgh School of Medicine, Pittsburgh, PA), D4M.3A.3 (a gift from D. Fisher, Massachusetts General Hospital, Boston, MA), B16.F10, and B16/GM-CSF cells (both gifts from G. Dranoff, Novartis Institutes for Biomedical Research, Cambridge, MA) and BRAF.PTEN (Cooper et al., 2014) were cultured in vitro in DMEM supplemented with 10% FBS. D4M.3A.3 melanoma was generated by single-cell cloning of the D4M.3A cell line, which was derived analogously to the original BRAF.PTEN cell line (Jenkins et al., 2014). D4M.3A.3 melanoma and BRAF.PTEN melanoma were used interchangeably as BRAF mutant PTEN-deficient melanoma lines. To assess PD-L1 and PD-L2 expression in vitro, tumor cell lines were treated with IFN-γ (20 ng/ml) for 24 h, and expression was determined by flow cytometry. To generate PD-L1 KO tumor cells, MC38 cells or BRAF.PTEN cells were transiently transfected with a Cas9-single guide RNA (sgRNA) expression vector (pX459; Addgene) targeting PD-L1 or control nontargeting guide RNAs (PD-L1 sgRNA, 5′-GGTCCAGCTCCCGTTCTACA-3′; control sgRNA, 5′-GCTTTCACGGAGGTTCGACG-3′). 10 d after transfection, PD-L1 KO cells were purified by flow cytometry by sorting on PD-L1 negative cells after 48 h of IFN-γ stimulation (20 ng/ml). To generate gp33 or control expressing cell lines, the MSCV-PIG (Addgene) retroviral vector was modified to replace PuroR with the gp33 peptide. Unmanipulated MSCV-PIG was used to generate control cells. To generate TdTomato or GFP fluorescently labeled MC38 cell lines, HEK293T cells were transfected with the lentiviral expression vector pLX304 containing TdTomato or GFP along with Md2G and psPAX2 plasmids and supernatants from these transfections were used to transduce MC38 cells. TdTomato or GFP fluorescent MC38 control cells, which contained scrambled gRNAs or PD-L1KO cells were sorted by flow cytometry and maintained in vitro under standard culture conditions. For the TdTomato and GFP mixing experiments, equal numbers of cells were seeded together and maintained in vitro over the course of each tumor experiment.
Tumor models
Mice were injected in the flank subcutaneously with 105 tumor cells. Mice injected with B16.F10 tumor cells were also injected on the contralateral side with irradiated 106 B16/GM-CSF cells (16,000 rads) on the same day. Tumors were measured every 2–3 d (length × width) with a caliper. Tumor volume was determined using the formula: 1/2 × D × d2 where D is the major axis and d is the minor axis. Mice were sacrificed when tumors reached 2 cm3 or upon ulceration. Where indicated, mice were given 200 µg antibody i.p. at days 7, 10, and 13 after injection using the following antibodies: anti–PD-L1 (339.6A2; blocks PD-L1 interaction with PD-1 and B7-1; Reardon et al., 2016) and anti–PD-1 (clone 29F.1A12). All isotype control antibodies were purchased from BioXCell. Upon collection, tumor cells were dissected and mechanically disaggregated before digestion with collagenase type I (400 U/ml; Worthington Biochemical) for 30 min at 37°C. After digestion, tumors were passed through 70-µm filters and mononuclear cells isolated by centrifugation through a Percoll gradient (40 and 70%).
Intracellular cytokine staining
After isolation from dLN or tumor, cells were stimulated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) and 500 ng/ml ionomycin (Sigma-Aldrich) in the presence of GolgiStop (BD) for 3.5 h, and then stained and analyzed by flow cytometry as described in the following section.
Flow cytometry
Cells from the tumor or draining inguinal lymph nodes were isolated and resuspended in buffer (PBS with 1% FCS and 2 mM EDTA), and stained with the following fluorescently conjugated antibodies (BioLegend): anti-CD45.2 (104), anti-CD8α (53–6.7), anti-CD4 (RM4-5), anti–PD-L1 (10F.9G2), and anti–PD-1 (RMP1-30). For intracellular staining (anti-FoxP3 [FJK-16s; eBioscience]; anti-Ki67 [B56; BD]; anti-Granzyme B [GB11; BioLegend]; anti–IFN-γ [XMG1.2; BioLegend]), cells were fixed and permeabilized using the FoxP3/Transcription Factor Staining kit (eBioscience) after surface staining. Flow cytometry data were acquired on the LSRII flow cytometer (BD) and analyzed using FlowJo software (Tree Star).
CDR3 sequencing
CD8+ T cells were sorted from tumors or the dLN, and CDR3 sequencing of the TCRβ chain was performed and analyzed using the Adaptive Biotechnologies ImmunoSeq platform (Adaptive Biotechnologies). Clonality scores were calculated as 1-(entropy)/log2(# of productive unique sequences), where the entropy term takes into account the varying clone frequency.
In vitro cytotoxicity assay
CD8+ T cells from the spleens of P14 TCR Tg mice were isolated using MACS beads and columns (Miltenyi Biotec) and stimulated for 24 h in 24-well plates (∼2 × 106 cells/well) coated with anti-CD3 (4 µg/ml) and anti-CD28 (4 µg/ml) in R10 media. R10 media is RPMI-1640 supplemented with 10% FCS (Sigma-Aldrich), penicillin-streptomycin (100 U penicillin and 100 µg streptomycin; Invitrogen), 12 mM Hepes (Invitrogen), and 50 µM β-mercaptoethanol (Sigma-Aldrich). Tumor cells were seeded into 96-well plates (104 cells/well) in DMEM supplemented with 10% FBS, 1% Penicillin/Streptomycin, and 20 ng/ml IFN-γ for 24 h for activation. 104 stimulated T cells were seeded onto activated tumor cells for 44 h before counting.
Statistics
All statistical analysis was performed with Prism software (GraphPad Software) and statistical significance was determined where the p-value was <0.05 (*).
Acknowledgments
The authors thank Peter Sage for helpful discussions; Xia Bu for providing reagents; Justin Trombley and Seth Maleri for technical assistance; Baolin Chang, Xiaohui He, and HuiPing Zhang for assistance with mouse genotyping and colony maintenance; and Sarah Hillman for administrative support.
This work was supported by grants from the National Institutes of Health (U54 CA163125 and P50 CA101942 to A.H. Sharpe and G.J. Freeman, and AI40614 to A.H. Sharpe) and the Novartis–Drug Discovery Program to A.H. Sharpe, graduate fellowships from the National Science Foundation and the Department of Defense to V.R. Juneja, and an Irvington Postdoctoral Fellowship from the Cancer Research Institute to K.A. McGuire.
G.J. Freeman and A.H. Sharpe have patents on the PD-1 pathway licensed by Bristol-Myers-Squibb, Roche, Merck, EMD-Serono, Boehringer-Ingelheim, AstraZeneca, and Novartis. The authors declare no additional competing financial interests.
Footnotes
Abbreviations used:
- dLN
- draining LN
- PD
- programmed cell death
- TIL
- tumor-infiltrating lymphocytes
References
- Ahmadzadeh M., Johnson L.A., Heemskerk B., Wunderlich J.R., Dudley M.E., White D.E., and Rosenberg S.A.. 2009. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 114:1537–1544. 10.1182/blood-2008-12-195792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmetzyanova I., Drabczyk M., Neff C.P., Gibbert K., Dietze K.K., Werner T., Liu J., Chen L., Lang K.S., Palmer B.E., et al. 2015. PD-L1 expression on retrovirus-infected cells mediates immune escape from CD8+ T cell killing. PLoS Pathog. 11:e1005224 10.1371/journal.ppat.1005224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alme A.K., Karir B.S., Faltas B.M., and Drake C.G.. 2016. Blocking immune checkpoints in prostate, kidney, and urothelial cancer: An overview. Urol. Oncol. 34:171–181. 10.1016/j.urolonc.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma T., Yao S., Zhu G., Flies A.S., Flies S.J., and Chen L.. 2008. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 111:3635–3643. 10.1182/blood-2007-11-123141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank C., Brown I., Peterson A.C., Spiotto M., Iwai Y., Honjo T., and Gajewski T.F.. 2004. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 64:1140–1145. 10.1158/0008-5472.CAN-03-3259 [DOI] [PubMed] [Google Scholar]
- Callahan M.K., Postow M.A., and Wolchok J.D.. 2016. Targeting T cell co-receptors for cancer therapy. Immunity. 44:1069–1078. 10.1016/j.immuni.2016.04.023 [DOI] [PubMed] [Google Scholar]
- Chen D.S., and Mellman I.. 2013. Oncology meets immunology: the cancer-immunity cycle. Immunity. 39:1–10. 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- Chen S., Lee L.F., Fisher T.S., Jessen B., Elliott M., Evering W., Logronio K., Tu G.H., Tsaparikos K., Li X., et al. 2015. Combination of 4-1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol. Res. 3:149–160. 10.1158/2326-6066.CIR-14-0118 [DOI] [PubMed] [Google Scholar]
- Cooper Z.A., Juneja V.R., Sage P.T., Frederick D.T., Piris A., Mitra D., Lo J.A., Hodi F.S., Freeman G.J., Bosenberg M.W., et al. 2014. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer Immunol. Res. 2:643–654. 10.1158/2326-6066.CIR-13-0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran M.A., Montalvo W., Yagita H., and Allison J.P.. 2010. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA. 107:4275–4280. 10.1073/pnas.0915174107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R., Verma R., Sznol M., Boddupalli C.S., Gettinger S.N., Kluger H., Callahan M., Wolchok J.D., Halaban R., Dhodapkar M.V., and Dhodapkar K.M.. 2015. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J. Immunol. 194:950–959. 10.4049/jimmunol.1401686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., Roche P.C., Lu J., Zhu G., Tamada K., et al. 2002. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8:793–800. 10.1038/nm0902-1039c [DOI] [PubMed] [Google Scholar]
- Francisco L.M., Salinas V.H., Brown K.E., Vanguri V.K., Freeman G.J., Kuchroo V.K., and Sharpe A.H.. 2009. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206:3015–3029. 10.1084/jem.20090847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubin M.M., Zhang X., Schuster H., Caron E., Ward J.P., Noguchi T., Ivanova Y., Hundal J., Arthur C.D., Krebber W.J., et al. 2014. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 515:577–581. 10.1038/nature13988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst R.S., Soria J.C., Kowanetz M., Fine G.D., Hamid O., Gordon M.S., Sosman J.A., McDermott D.F., Powderly J.D., Gettinger S.N., et al. 2014. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 515:563–567. 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano F., Kaneko K., Tamura H., Dong H., Wang S., Ichikawa M., Rietz C., Flies D.B., Lau J.S., Zhu G., et al. 2005. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 65:1089–1096. [PubMed] [Google Scholar]
- Iwai Y., Ishida M., Tanaka Y., Okazaki T., Honjo T., and Minato N.. 2002. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA. 99:12293–12297. 10.1073/pnas.192461099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M.H., Steinberg S.M., Alexander M.P., Fisher J.L., Ernstoff M.S., Turk M.J., Mullins D.W., and Brinckerhoff C.E.. 2014. Multiple murine BRafV600E melanoma cell lines with sensitivity to PLX4032. Pigment Cell Melanoma Res. 27:495–501. 10.1111/pcmr.12220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir M.E., Liang S.C., Guleria I., Latchman Y.E., Qipo A., Albacker L.A., Koulmanda M., Freeman G.J., Sayegh M.H., and Sharpe A.H.. 2006. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 203:883–895. 10.1084/jem.20051776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir M.E., Freeman G.J., and Sharpe A.H.. 2007. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J. Immunol. 179:5064–5070. 10.4049/jimmunol.179.8.5064 [DOI] [PubMed] [Google Scholar]
- Kleffel S., Posch C., Barthel S.R., Mueller H., Schlapbach C., Guenova E., Elco C.P., Lee N., Juneja V.R., Zhan Q., et al. 2015. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell. 162:1242–1256. 10.1016/j.cell.2015.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchman Y., Wood C.R., Chernova T., Chaudhary D., Borde M., Chernova I., Iwai Y., Long A.J., Brown J.A., Nunes R., et al. 2001. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2:261–268. 10.1038/85330 [DOI] [PubMed] [Google Scholar]
- Latchman Y.E., Liang S.C., Wu Y., Chernova T., Sobel R.A., Klemm M., Kuchroo V.K., Freeman G.J., and Sharpe A.H.. 2004. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc. Natl. Acad. Sci. USA. 101:10691–10696. 10.1073/pnas.0307252101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney K.M., Rennert P.D., and Freeman G.J.. 2015. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 14:561–584. 10.1038/nrd4591 [DOI] [PubMed] [Google Scholar]
- Pardoll D.M. 2012. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 12:252–264. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H., Bürki K., Lang R., Hengartner H., and Zinkernagel R.M.. 1989. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 342:559–561. 10.1038/342559a0 [DOI] [PubMed] [Google Scholar]
- Reardon D.A., Gokhale P.C., Klein S.R., Ligon K.L., Rodig S.J., Ramkissoon S.H., Jones K.L., Conway A.S., Liao X., Zhou J., et al. 2016. Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol. Res. 4:124–135. 10.1158/2326-6066.CIR-15-0151 [DOI] [PubMed] [Google Scholar]
- Ribas A. 2012. Tumor immunotherapy directed at PD-1. N. Engl. J. Med. 366:2517–2519. 10.1056/NEJMe1205943 [DOI] [PubMed] [Google Scholar]
- Rodig N., Ryan T., Allen J.A., Pang H., Grabie N., Chernova T., Greenfield E.A., Liang S.C., Sharpe A.H., Lichtman A.H., and Freeman G.J.. 2003. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur. J. Immunol. 33:3117–3126. 10.1002/eji.200324270 [DOI] [PubMed] [Google Scholar]
- Spranger S., Spaapen R.M., Zha Y., Williams J., Meng Y., Ha T.T., and Gajewski T.F.. 2013. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci. Transl. Med. 5:200ra116 10.1126/scitranslmed.3006504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumeh P.C., Harview C.L., Yearley J.H., Shintaku I.P., Taylor E.J., Robert L., Chmielowski B., Spasic M., Henry G., Ciobanu V., et al. 2014. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 515:568–571. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S.R., Turnis M.E., Goldberg M.V., Bankoti J., Selby M., Nirschl C.J., Bettini M.L., Gravano D.M., Vogel P., Liu C.L., et al. 2012. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 72:917–927. 10.1158/0008-5472.CAN-11-1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky J.M., Garcia-Diaz A., Shin D.S., Escuin-Ordinas H., Hugo W., Hu-Lieskovan S., Torrejon D.Y., Abril-Rodriguez G., Sandoval S., Barthly L., et al. 2016. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375:819–829. 10.1056/NEJMoa1604958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Velez-Delgado A., Mathew E., Li D., Mendez F.M., Flannagan K., Rhim A.D., Simeone D.M., Beatty G.L., and Pasca di Magliano M.. 2017. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut. 66:124–136. 10.1136/gutjnl-2016-312078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W., Wolchok J.D., and Chen L.. 2016. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 8:328rv4 10.1126/scitranslmed.aad7118 [DOI] [PMC free article] [PubMed] [Google Scholar]