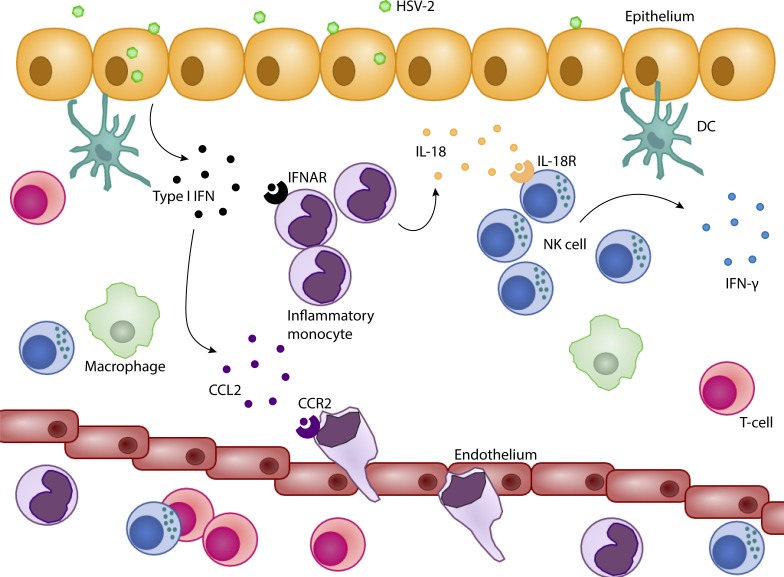
Although type I interferon is critical for NK cell activation, the underlying mechanism is under debate and is unknown during a mucosal infection. Lee et al. have determined that type I interferon induces inflammatory monocytes to produce IL-18 to directly activate NK cells to combat viral infections.
Abstract
The requirement of type I interferon (IFN) for natural killer (NK) cell activation in response to viral infection is known, but the underlying mechanism remains unclear. Here, we demonstrate that type I IFN signaling in inflammatory monocytes, but not in dendritic cells (DCs) or NK cells, is essential for NK cell function in response to a mucosal herpes simplex virus type 2 (HSV-2) infection. Mice deficient in type I IFN signaling, Ifnar−/− and Irf9−/− mice, had significantly lower levels of inflammatory monocytes, were deficient in IL-18 production, and lacked NK cell–derived IFN-γ. Depletion of inflammatory monocytes, but not DCs or other myeloid cells, resulted in lower levels of IL-18 and a complete abrogation of NK cell function in HSV-2 infection. Moreover, this resulted in higher susceptibility to HSV-2 infection. Although Il18−/− mice had normal levels of inflammatory monocytes, their NK cells were unresponsive to HSV-2 challenge. This study highlights the importance of type I IFN signaling in inflammatory monocytes and the induction of the early innate antiviral response.
Introduction
NK cells are an important component of the innate immune response, as they are rapidly activated upon viral infection and can directly recognize infected cells and eliminate them (Vivier et al., 2008). Additionally, they can release proinflammatory cytokines, which activate other immune cells and facilitate the initiation of the adaptive immune response (Vivier et al., 2008). In particular, their ability to produce IFN-γ during the early stages of an infection has been shown to be critical for the defense against viral infections (Orange et al., 1995; Thapa et al., 2007; Gill et al., 2011). Indeed, the absence of NK cells, in Il15−/− mice or through NK cell depletion, results in significantly increased susceptibility to HSV-2 infection (Ashkar and Rosenthal, 2003; Thapa et al., 2007). Depletion of NK cells in mice led to increased HSV-2 viral titers found in the vaginal tract, spinal cord, and brain stem (Thapa et al., 2007). Further, Ifng−/− mice have increased mortality rates when infected with HSV-2 (Ashkar and Rosenthal, 2003). As a critical component of the innate immune response, it is important to understand how NK cells are activated, particularly to produce IFN-γ early in the response.
The functional state of NK cells is greatly influenced by their microenvironment. An overwhelming increase in activation signals over inhibitory signals will cause activation of their antiviral functions (Pegram et al., 2011). In contrast, a plethora of inhibitory signals will prevent NK cell activation (Pegram et al., 2011). Cytokines, including type I IFN, IL-15, IL-12, IL-18, and ISG15, have all been shown to activate NK cell function, particularly IFN-γ production (Pegram et al., 2011). Alternatively, inhibitory receptor recognition of MHC class I on target cells inhibits NK cell activation (Pegram et al., 2011).
Type I IFNs are central to the activation of NK cells during viral infections, including mouse CMV (MCMV), adenovirus, vaccinia virus, and HSV infections (Lucas et al., 2007; Martinez et al., 2008; Zhu et al., 2008; Gill et al., 2011; Baranek et al., 2012). Type I IFNs comprise a family of cytokines that includes IFN-β and numerous subtypes of IFN-α (Platanias, 2005). These cytokines signal through their specific receptors, IFN α/β receptor 1 (IFNAR1) and IFNAR2, which together form the type I IFN receptor (Platanias, 2005). Type I IFNs are rapidly produced upon viral infection and play an essential role in the antiviral innate immune response (Platanias, 2005). Although type I IFNs are required for NK cell activation, the underlying mechanism is still controversial. Evidence in the literature suggests that type I IFNs directly activate NK cells during vaccinia virus, adenovirus, and lymphocytic choriomeningitis virus infection (Martinez et al., 2008; Zhu et al., 2008; Mack et al., 2011). However, it has also been reported that type I IFNs act on DCs to produce and trans-present IL-15, which leads to NK cell activation in response to TLR ligand stimulation and MCMV infection (Lucas et al., 2007; Baranek et al., 2012). This suggests that type I IFN signaling is required for IL-15 induction after viral infection. However, the majority of studies examining NK cell activation have used i.v., i.p., or subcutaneous routes of viral infection. The mechanism underlying NK cell activation during a mucosal infection has yet to be explored.
Inflammatory monocytes (defined by the phenotype CCR2+, Ly6Chi) are rapidly recruited to sites of inflammation and produce a plethora of inflammatory cytokines to combat infection. Once in the inflammatory environment, these inflammatory monocytes can differentiate into DCs, which can aid in the development of adaptive immunity against infection. However, their role in stimulating innate antiviral immunity, particularly NK cell antiviral responses, has been relatively unexplored. Depletion of inflammatory monocytes from human PBMCs during an in vitro hepatitis C virus (HCV) infection suppressed NK cell responses, suggesting that these cells are capable of activating NK cells (Zhang et al., 2013; Serti et al., 2014).
Here, we describe a thorough mechanism by which NK cells are activated during a mucosal viral infection in vivo. We clearly show that type I IFN does not directly act on NK cells or DCs to activate NK cells. Instead, during vaginal HSV-2 infection, type I IFNs signal through inflammatory monocytes to produce IL-18, which then activates NK cells to produce IFN-γ and augment host defense.
Results
IFNAR and IRF9 are essential for NK cell IFN-γ production during HSV-2 infection
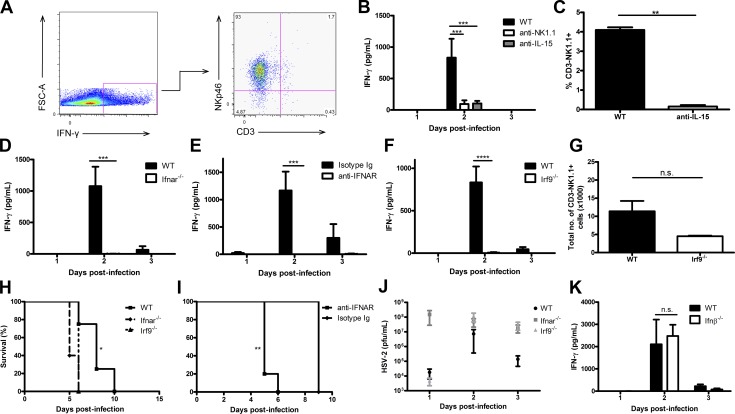
Production of IFN-γ by NK cells is the hallmark of the host innate immune response to genital HSV-2 infection (Ashkar and Rosenthal, 2003; Gill et al., 2011), Indeed, NK cells have been shown to be the main producers of IFN-γ during viral infections, as in many cases the absence or depletion of these cells diminishes IFN-γ production (Krug et al., 2004; Martinez et al., 2008; Zhu et al., 2008; Mack et al., 2011). We first confirmed that IFN-γ is produced directly by NK cells in response to HSV-2 infection (Fig. 1 A; Gill et al., 2011). Depletion of NK cells using anti-NK1.1 or anti–IL-15 antibodies significantly diminished IFN-γ levels in vaginal lavages of HSV-2–infected mice (Fig. 1, B and C). We have previously shown that IFNAR is required for NK cell IFN-γ production during HSV-2 infection (Gill et al., 2011). Production of IFN-γ was completely abrogated in Ifnar−/− mice during vaginal infection with HSV-2 (Fig. 1 D). This deficiency in vaginal IFN-γ level was not a result of decreased NK cell recruitment to the vaginal tract (Gill et al., 2011). Additionally, blocking type I IFN receptor in C57BL/6 (B6) mice before infection resulted in a complete abrogation of IFN-γ production, similar to that observed in genetically deficient type I IFN receptor mice (Fig. 1 E). We determined whether type I IFNs’ downstream canonical signaling cascade, which involves IRF9, is also required for NK cell function during infection. There was a complete abrogation of NK cell IFN-γ production in the vaginal lavages of HSV-2–infected Irf9−/− mice, similar to that observed in Ifnar−/− mice (Fig. 1 F). Additionally, the total number of NK cells within the vaginal mucosa between WT and Irf9−/− B6 mice was not significantly different (Fig. 1 G). This suggests that the deficiency in IFN-γ production was not caused by a reduction in vaginal NK cell numbers. We also determined that both Ifnar−/− and Irf9−/− mice have decreased survival after infection compared with WT mice (Fig. 1, H and I). It has been reported that levels of vaginal HSV-2 viral replication are proportional to the amount of IFN-γ released from NK cells (Gillgrass et al., 2003). At odds with this finding, we detected similar, if not higher, levels of HSV-2 viral replication within the vaginal mucosa in Ifnar−/− and Irf9−/− mice compared with WT mice, despite a lack of NK cell IFN-γ production in Ifnar−/− and Irf9−/− mice (Fig. 1 J). Overall, our data suggest that type I IFN signaling is essential for NK cell IFN-γ production. It has been shown that IFN-β is rapidly produced in the vaginal tract in response to HSV-2 infection or TLR challenge (Gill et al., 2006, 2011). IFN-β production occurs at 6 h post-infection (p.i.) and is then down-regulated at 12 and 24 h p.i., which would suggest the involvement of IFN-β in activating NK cell IFN-γ production. However, when Ifnb−/− mice were infected with HSV-2, they produced similar amounts of IFN-γ compared with WT mice (Fig. 1 K; Gill et al., 2006, 2011). This would suggest that IFN-β is not essential for activating NK cells during HSV-2 infection.
Figure 1.
Type I IFN receptor and its respective signaling through IRF9 is required for NK cell IFN-γ production during HSV-2 infection. (A) WT B6 mice were infected with 104 pfu HSV-2 intravaginally (ivag). On day 2 p.i., vaginal tracts were processed and examined for NKp46, CD3, and IFN-γ expression. (B) WT B6 mice were depleted of NK cells using anti-NK1.1 antibody or IL-15 using anti–IL-15 antibody and then infected with 104 pfu HSV-2 ivag. Day 1–3 p.i. vaginal lavages were examined for IFN-γ levels (n = 5). (C) Peripheral blood was examined for NK cells in mice given anti–IL-15 antibody (n = 5). (D) WT and Ifnar−/− mice were infected with 104 pfu HSV-2 ivag, and vaginal lavages were examined for IFN-γ production on days 1–3 p.i. (n = 3; repeated twice with similar results). (E) WT B6 mice were administered anti-IFNAR antibody or the respective isotype-matched control Ig on days −1 through 2 i.p. and then infected with 104 pfu HSV-2 ivag. Their vaginal lavages were examined for IFN-γ content (n = 4; repeated once with similar results). (F) WT and Irf9−/− mice were infected with 104 pfu HSV-2 ivag, and vaginal lavages were examined for IFN-γ levels on days 1–3 p.i. (n = 3; repeated once with similar results). (G) WT and Irf9−/− mice were infected with 104 pfu HSV-2 ivag. At day 3 p.i., the vaginal mucosa was examined for CD3-NK1.1+ NK cells (n = 3; repeated once with similar results). (H) WT, Ifnar−/−, and Irf9−/− mice were infected with 104 pfu HSV-2 ivag and followed for survival (n = 5; repeated once with similar results). (I) WT mice were administered anti-IFNAR antibody or the respective isotype control Ig on days −1 through 2 and infected with 104 pfu HSV-2 ivag. Mice were followed for survival (n = 4). (J) After infection with HSV-2, vaginal lavages were collected from WT, Ifnar−/−, and Irf9−/− mice and assessed for HSV-2 level via plaque assay (n = 5). (K) WT and Ifnb−/− mice were infected with 104 pfu HSV-2 ivag. Day 1-3 vaginal washes were collected and examined for IFN-γ amount (n = 4). Data in B, D–F, and K are displayed as mean ± SEM and were analyzed using two-way ANOVA: n.s., not significant; ***, P < 0.001; ****, P < 0.0001. Data in C and G are displayed as mean ± SEM and were analyzed using an unpaired Student’s t test and a Mann–Whitney test (for nonparametric data), respectively: n.s., not significant; **, P < 0.01. Data in J are displayed as mean ± SEM and were analyzed using one-way ANOVA. Data in H and I were analyzed using a log-rank test: *, P < 0.05; **, P < 0.01.
Expression of IFNAR on NK cells is not required for their activation during HSV-2 infection
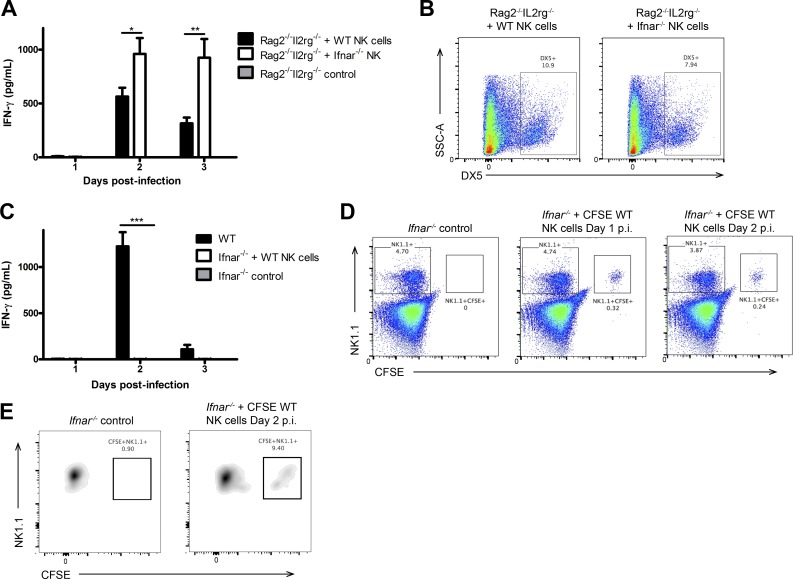
After establishing the requirement of type I IFN signaling for NK cell function, we inquired whether type I IFN activates NK cell IFN-γ production through a direct or indirect mechanism. To address this question, we adoptively transferred either WT or Ifnar−/− NK cells into Rag2−/−Il2rg−/− mice, which lack all lymphocytes including NK cells. In transferring Ifnar−/− NK cells into these mice, type I IFN signaling in the environment is retained, but the adoptively transferred NK cells are unable to respond to type I IFN. Upon infection, Ifnar−/− NK cells produced IFN-γ in response to HSV-2 infection when transferred to an environment with functional type I IFN signaling (Fig. 2 A). In fact, Ifnar−/− NK cells produced a significantly greater amount of IFN-γ than their WT controls, suggesting that IFNAR on NK cells is not required for NK cell activation and IFN-γ production (Fig. 2 A). Additionally, we were able to detect these adoptively transferred cells at day 3 p.i. (Fig. 2 B). As further evidence, we adoptively transferred WT NK cells (able to respond to type I IFNs) into Ifnar−/− mice (unable to respond to type I IFNs) and observed that WT NK cells in an Ifnar−/− environment were unable to produce IFN-γ in response to HSV-2 infection (Fig. 2 C). The presence of these adoptively transferred cells persisted in the Ifnar−/− mice to day 2 p.i. in both the spleen and vaginal mucosa (Fig. 2, D and E).
Figure 2.
IFNAR is not required directly on NK cells to activate their IFN-γ production. (A) NK cells were isolated from WT or Ifnar−/− spleens and adoptively transferred into Rag2−/−Il2rg−/− mice i.v. 24 h after transfer, mice were infected with 104 pfu HSV-2 ivag, and on days 1–3, vaginal lavages were examined for IFN-γ levels (n = 3; repeated twice with similar results). (B) Spleens were collected on day 3 p.i. and analyzed for DX5 expression. (C) NK cells were isolated from WT spleens, CFSE-labeled, and then adoptively transferred into Ifnar−/− mice. 24 h after transfer, Ifnar−/− mice given WT NK cells, and WT controls were infected with 104 pfu HSV-2 ivag. Day 1–3 p.i. vaginal lavages were examined for IFN-γ content (n = 3; repeated once with similar results). (D) Spleens were examined for CFSE+NK1.1+ adoptively transferred cells on days 1 and 2 p.i. (representative of two independent experiments). (E) Vaginal tissue from Ifnar−/− mice with or without adoptive transfer of CFSE-labeled NK cells was examined on day 2 p.i. for CFSE+NK1.1+ cells (representative of two independent experiments). Vaginal cells were first gated on the CD45+CD3−NK1.1+ population and then examined for CFSE expression. Data in A and C are displayed as mean ± SEM and were analyzed using two-way ANOVA: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
NK cell activation does not require type I IFN activation of DCs or IL-15 trans-presentation
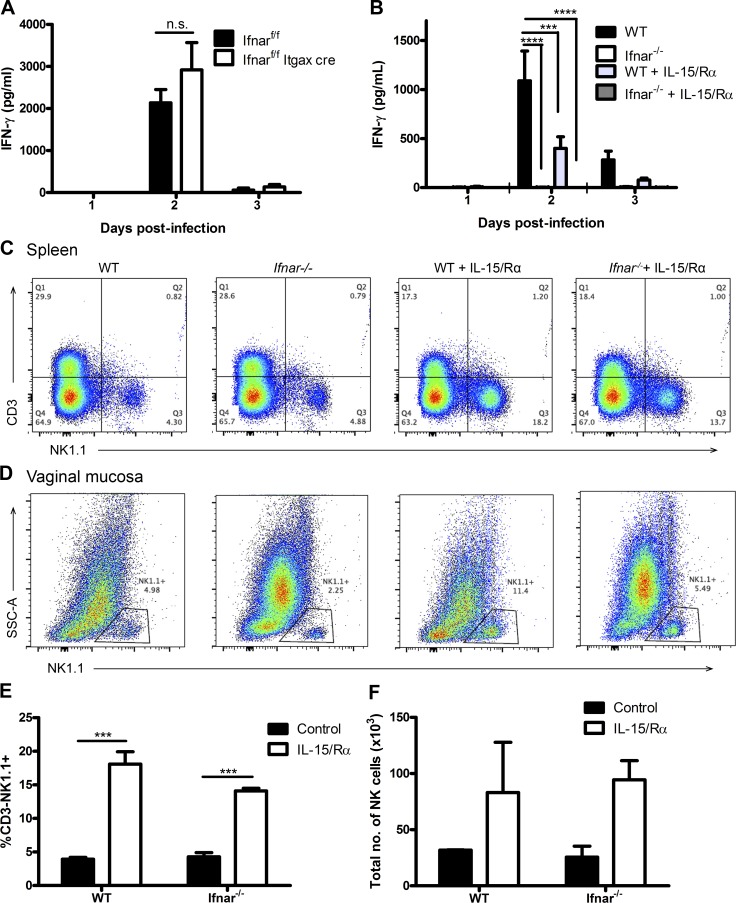
Previously, Lucas et al. (2007) and Baranek et al. (2012) reported that during TLR stimulation and MCMV infection, type I IFN stimulates DCs to trans-present IL-15 to NK cells and induce their production of IFN-γ. To determine whether IFNAR is required on DCs for the subsequent activation of NK cells during a mucosal vaginal infection, we used a mouse model wherein only DCs are deficient in IFNAR. As detailed in Diamond et al. (2011), we crossed Ifnarf/f mice with CD11c-Cre+ transgenic mice to generate offspring that are selectively IFNAR deficient in DCs. No IFNAR-expressing CD11c+ DCs could be identified by flow cytometry in these mice (not depicted). Infection of these mice yielded no significant difference in vaginal NK cell IFN-γ production in comparison to controls, suggesting that DCs are not required to induce NK cell IFN-γ production (Fig. 3 A). To determine whether IL-15 trans-presentation is involved in the activation of NK cells during HSV-2 infection, we provided Ifnar−/− and WT mice with a trans-presentation mimic, IL-15 in complex with its specific receptor α (Rα), in an attempt to rescue NK cell IFN-γ production in Ifnar−/− mice. IL-15/IL-15Rα complexes (IL-15/Rα) have been shown to induce rapid proliferation as well as induction of IFN-γ production in NK cells (Elpek et al., 2010). However, we were unable to rescue IFN-γ production in Ifnar−/− mice, despite finding NK cell proliferation in both the spleen and vaginal tract (Fig. 3, B–F). Significant increases in proportion of NK cells were observed in the spleen. Thus, IL-15/Ra complexes were sufficient to stimulate NK cell proliferation, but not IFN-γ production.
Figure 3.
CD11c+ cells are not required to respond to type I IFN or trans-present IL-15 during the activation of NK cell IFN-γ production during infection. (A) Ifnarf/f and Ifnarf/f Itgax-cre mice were infected with 104 pfu HSV-2 ivag and examined for IFN-γ in the vaginal lavages collected days 1–3 p.i. (n = 5; repeated once with similar results). (B) WT and Ifnar−/− mice were given 0.5 µg IL-15 in complex with 1 µg IL-15Rα i.p. and subsequently infected with 5 × 104 pfu HSV-2 ivag on the same day. Vaginal washes were collected on days 1–3 p.i. and examined for IFN-γ levels (n = 4; repeated once with similar results). (C and D) Spleen (C; n = 3) and vaginal (D; n = 2) tissue were collected on day 3 p.i. and examined for CD45+CD3−NK1.1+ NK cells as shown in the representative flow plots. (E and F) Flow data are quantitatively shown for spleen (E) and vaginal mucosa (F). Data in A, B, E, and F are displayed as mean ± SEM and were analyzed using two-way ANOVA: n.s., not significant; ***, P < 0.001; ****, P < 0.0001.
IL-18 is required for NK cell IFN-γ production during HSV-2 infection
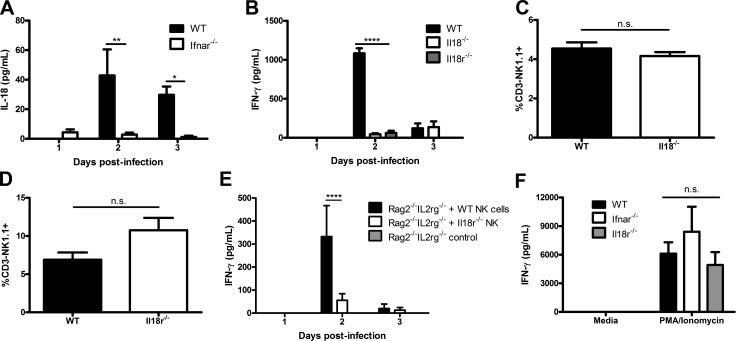
Several factors, including IL-15, IL-12, ISG15, and IL-18, have been shown to induce NK cell IFN-γ production, particularly during viral infection (Pegram et al., 2011). We have determined that IL-15 is not sufficient to activate NK cells during genital HSV-2 infection (Fig. 3 B). Further, we previously reported that Ifnar−/− mice can produce IL-15 in the vaginal mucosa after genital HSV-2 infection (Gill et al., 2011). IL-12 is a well-known activator of IFN-γ production, but we have previously shown that HSV-2 infection of IL-12−/− mice had no impact on IFN-γ production by NK cells (Gill et al., 2011). Here, we examined the role of ISG15 in the activation of NK cells, as human ISG15 deficiency has been observed to lead to decreased NK cell IFN-γ production during mycobacterial infection (Bogunovic et al., 2012). HSV-2 infection of Isg15−/− mice yielded no difference in NK cell IFN-γ production in comparison to WT mice (not depicted). We investigated whether IL-18 is required for NK cell activation in response to HSV-2 infection. We first compared IL-18 content in the vaginal lavages of Ifnar−/− and WT B6 mice and detected significantly lower levels of IL-18 in Ifnar−/− vaginal lavages, particularly at day 2 p.i. (Fig. 4 A). To further investigate the role of IL-18 in the induction of IFN-γ production by NK cells, we infected mice that lacked IL-18 or IL-18R. There was a significant abrogation in the production of IFN-γ by NK cells in both Il18−/− and Il18r1−/− mice infected with HSV-2 compared with WT mice (Fig. 4 B). This decrease was not caused by a lack of NK cell recruitment to the vaginal mucosa in Il18−/− or Il18ra−/− mice (Fig. 4, C and D). These data demonstrate that IL-18 is required for the activation of NK cell IFN-γ production during HSV-2 infection.
Figure 4.
IL-18 and IL-18R are both required for NK cell IFN-γ production during HSV-2 infection. (A) WT and Ifnar−/− mice were infected with 104 pfu HSV-2 ivag, and day 1–3 vaginal lavages were examined for IL-18. Data were normalized to Il18−/− data (n = 5; repeated once with similar results). (B) WT, Il18−/−, and Il18r1−/− B6 mice were infected with 104 pfu HSV-2 ivag, and on day 1–3 p.i. vaginal lavages were examined for IFN-γ content (n = 5; repeated once with similar results). (C and D) Vaginal tissue was isolated on day 3 p.i. and examined for CD45+CD3−NK1.1+ cells in Il18−/− (C; n = 3) and Il18r1−/− (D; n = 3) mice. (E) WT and Il18r1−/− NK cells were isolated from the spleen and adoptively transferred into Rag2−/−Il2rg−/− mice i.v. 24 h p.i. The mice were infected with 104 pfu HSV-2 ivag. On days 1–3 p.i., vaginal lavages were collected and examined for IFN-γ (n = 6). (F) NK cells were isolated from WT, Ifnar−/−, and Il18r−/− spleens and stimulated with 50 ng/ml PMA and 500 ng/ml ionomycin for 24 h ex vivo. Supernatants were collected and assayed for IFN-γ (n = 3). Data in A, B, and E are displayed as mean ± SEM and were analyzed using two-way ANOVA: *, P < 0.05; **, P < 0.01; ****, P < 0.0001. Data in C and D are displayed as mean ± SEM and were analyzed using an unpaired Student’s t test: n.s., not significant. Data in F are displayed as mean ± SEM and were analyzed using one-way ANOVA: n.s., not significant.
NK cells respond to IL-18 directly to produce IFN-γ
To investigate whether IL-18 directly activates NK cells, we adoptively transferred either Il18ra−/− or WT B6 NK cells into Rag2−/−Il2rg−/− mice before infection with HSV-2. After infection, Il18r1−/− NK cells adoptively transferred to Rag2−/−Il2rg−/− mice had significantly decreased IFN-γ production compared with WT NK cells (Fig. 4 E). However, Il18r1−/− NK cells produced copious amounts of IFN-γ when stimulated with PMA and ionomycin (Fig. 4 F). These findings suggest that Il18r1−/− NK cells are not inherently incapable of producing IFN-γ but lack the required signaling via IL-18R to do so during HSV-2 infection.
Hematopoietic cells respond to type I IFN and produce IL-18 to activate NK cells
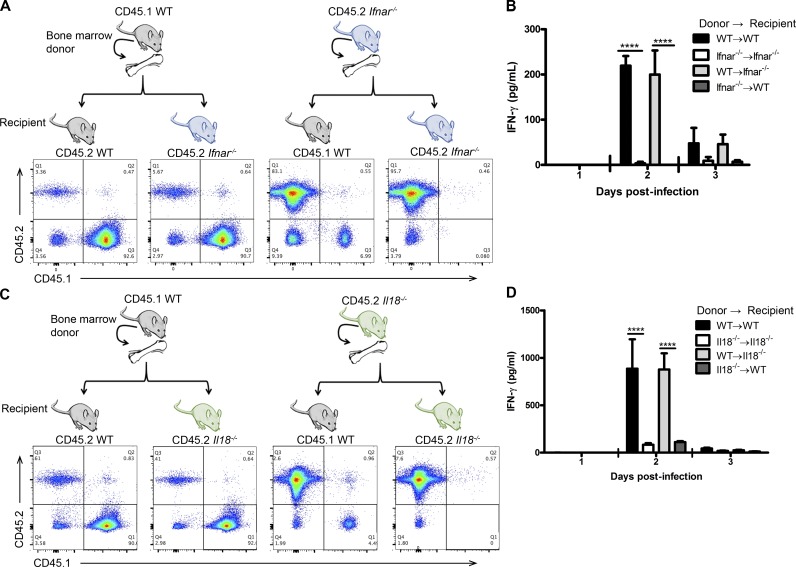
Because our data have clearly indicated that type I IFNs are required but do not act directly on NK cells, we investigated for the cell types that produce IL-18 in response to type I IFN. We first used bone marrow chimeric mice to determine whether hematopoietic or nonhematopoietic cells were required. We created chimeric mice by reconstituting WT and Ifnar−/− mice with either WT or Ifnar−/− bone marrow and examined reconstitution in the peripheral blood (Fig. 5 A). After HSV-2 infection, WT recipients reconstituted with Ifnar−/− bone marrow were unable to produce IFN-γ at days 2 and 3 p.i., whereas Ifnar−/− recipients reconstituted with WT bone marrow did produce IFN-γ (Fig. 5 B). To determine whether the hematopoietic cell compartment is also required to produce IL-18, we created bone marrow chimeric mice between WT and Il18−/− mice and examined reconstitution in the peripheral blood (Fig. 5 C). Upon infection with HSV-2, NK cells from Il18−/− mice that received WT bone marrow produced IFN-γ, whereas WT mice reconstituted with Il18−/− bone marrow did not (Fig. 5 D). These data suggest that the cells responding to type I IFN, as well as producing IL-18, belong to the hematopoietic cell compartment. Furthermore, this intermediary cell type is likely of myeloid lineage, as Rag2−/−Il2rg−/− mice are alymphoid, yet the transfer of WT or Ifnar−/− NK cells into these mice yielded IFN-γ production (Fig. 2 A).
Figure 5.
The hematopoietic cell compartment is required to respond to type I IFN and produce IL-18 to activate IFN-γ production during HSV-2 infection. (A) WT and Ifnar−/− mice were lethally irradiated and reconstituted with either WT or Ifnar−/− bone marrow. Mice were allowed to reconstitute for 6–8 wk, and peripheral blood was assessed for reconstitution by examining the frequency of cells expressing CD45.1 or CD45.2. (B) Mice were then infected with 5 × 104 pfu HSV-2 ivag. On days 1–3 p.i., vaginal lavages were examined for IFN-γ content (n = 3; repeated once with similar results). (C) WT and Il18−/− mice were lethally irradiated and reconstituted with either WT or Il18−/− bone marrow. 6–8 wk after reconstitution, mice were examined for CD45.1 and CD45.2 expression. (D) Mice were then infected with 104 pfu HSV-2 ivag. On days 1–3 p.i., vaginal lavages were examined for IFN-γ (n = 7). Data in B and D are displayed as mean ± SEM and were analyzed using two-way ANOVA: ****, P < 0.0001.
Decreased vaginal recruitment of inflammatory monocytes in Ifnar−/− mice
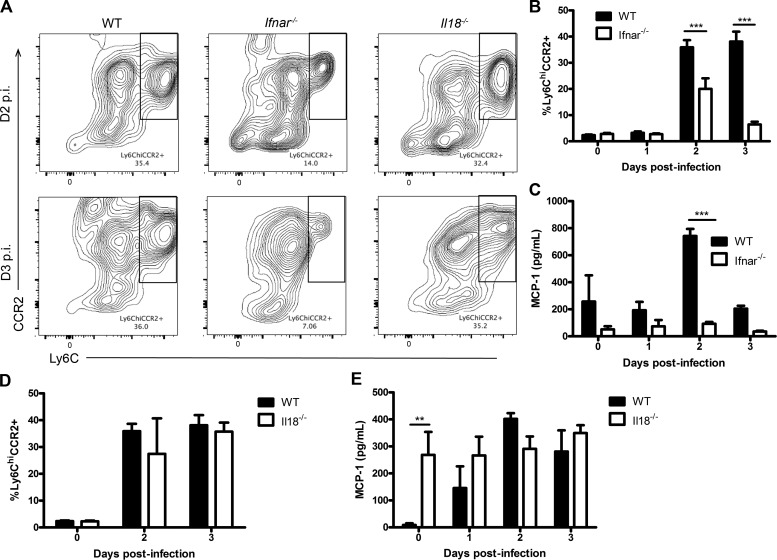
It has been documented that macrophages can activate NK cells during infection (Hamerman et al., 2004; Sirén et al., 2004). However, we found that depletion of macrophages by clodronate administration had no impact on NK cell IFN-γ production during HSV-2 infection (not depicted). Eosinophils are a cell type prominent within the reproductive tract and may play a role against infection (Robertson et al., 2000). However, similar to macrophages, we found no difference in vaginal NK cell production of IFN-γ between Gata1−/− mice, which lack eosinophils, and WT controls (not depicted). When we compared the composition of myeloid immune cells within the vaginal mucosa between WT and Ifnar−/− mice during infection, we observed significantly decreased recruitment of inflammatory monocytes in Ifnar−/− mice on both days 2 and 3 p.i. (Fig. 6, A and B). In agreement with this, we also found that Ifnar−/− mice had significantly decreased vaginal content of MCP-1, the chemokine responsible for recruiting inflammatory monocytes, during infection (Fig. 6 C). In contrast, there was no significant difference in MCP-1 levels and inflammatory monocyte recruitment in Il18−/− mice compared with WT mice on days 2 and 3 p.i. (Fig. 6, A, D, and E).
Figure 6.
Decreased vaginal inflammatory monocyte infiltration in Ifnar−/− mice during HSV-2 infection. (A) WT, Ifnar−/−, and Il18−/− mice were infected with 104 pfu HSV-2, and vaginal tissue was collected at baseline and on days 0–3 p.i. and examined for inflammatory monocytes, defined as CD45+CD11c−CD11b+Ly6G−Ly6ChiCCR2+ cells. Day 2 and 3 p.i. data are shown in representative flow plots. (B) Flow cytometry data were quantified and graphically represented (n = 3; repeated once with similar results). (C) Vaginal lavages were collected on days 0–3 p.i. and examined for MCP-1 (n = 3; repeated once with similar results). (D) WT and Il18−/− mice were infected with HSV-2 ivag, and vaginal tissue was collected at baseline and on days 2 and 3 p.i. and examined for inflammatory monocytes (n = 3). (E) WT and Il18−/− mice were infected with 104 pfu HSV-2 ivag, and on days 0–3 p.i., vaginal lavages were examined for MCP-1 (n = 4). Data in B–E are displayed as mean ± SEM and were analyzed using two-way ANOVA: **, P < 0.01; ***, P < 0.001.
Inflammatory monocytes, but not neutrophils, are essential for NK cell IFN-γ production
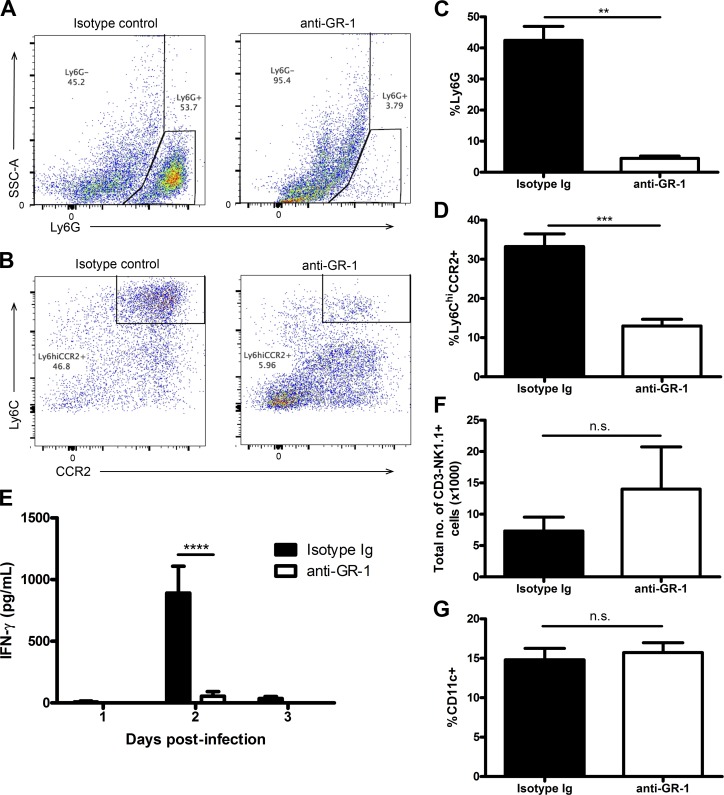
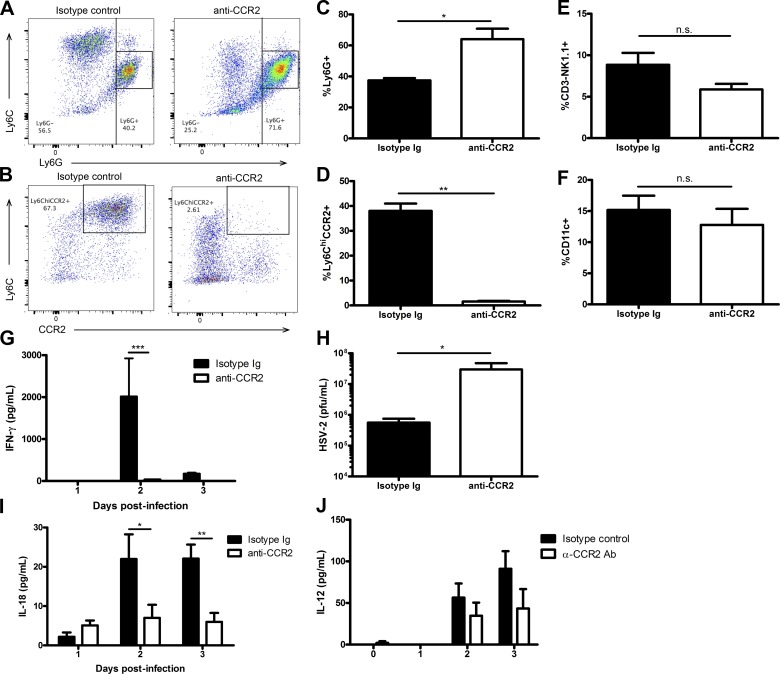
Because the recruitment of inflammatory monocytes is significantly reduced in Ifnar−/− mice, we examined whether these cells were involved in activating NK cells during infection. Initially, we depleted both inflammatory monocytes and neutrophils using the anti–GR-1 depletion antibody RB6-8C5 in WT mice before infection with HSV-2 (Fig. 7, A–D; Daley et al., 2008). This led to a drastic reduction in NK cell IFN-γ production, with no impact on NK cell recruitment to the vaginal tissue or proportion of vaginal CD11c+ cells during infection (Fig. 7, E–G). To assess the role of inflammatory monocytes, we used a recently developed anti-CCR2 antibody that has been shown to successfully deplete inflammatory monocytes while leaving the neutrophil cell population intact (Mack et al., 2001; Schumak et al., 2015). This antibody depleted the inflammatory monocyte population in the vaginal mucosa without impacting the neutrophil population during infection (Fig. 8, A–D). In the absence of inflammatory monocytes, there was an almost complete abrogation of IFN-γ production by NK cells within the vaginal mucosa, while the percentage of NK cells and CD11c+ cells in the vaginal mucosa during infection were similar (Fig. 8, E–G). Furthermore, when we examined vaginal HSV-2 viral titers in the absence of inflammatory monocytes, we found that there was a significant increase in HSV-2 viral titer level compared with mice given an isotype-matched control Ig (Fig. 8 H). This suggests that inflammatory monocytes are essential in activating NK cell IFN-γ production, which is necessary for controlling HSV-2 infection in the innate immune response.
Figure 7.
Inflammatory monocytes are required for activating NK cell IFN-γ production during HSV-2 infection. (A and B) WT B6 mice were given an anti–GR-1 antibody or its respective isotype-matched control Ig and infected with 104 pfu HSV-2 ivag. Vaginal tissue was examined for neutrophil (CD45+CD11c−CD11b+Ly6G+) and inflammatory monocyte (CD45+CD11c−CD11b+Ly6G−Ly6ChiCCR2+) depletion on day 3 p.i. Representative flow plots of vaginal neutrophil and inflammatory monocyte populations are shown (respectively in A and B). (C and D) Vaginal neutrophil (C) and inflammatory monocyte (D) populations are displayed graphically (n = 5; repeated once with similar results). (E) On days 1–3 p.i., vaginal washes were examined for IFN-γ (n = 5; repeated twice with similar results). (F and G) Vaginal tissue collected on day 3 p.i. was also examined for total vaginal NK cell number (F; CD45+CD3−NK1.1+; n = 5) and proportion of CD11c+ cells (G; CD45+CD11c+; n = 5; repeated once with similar results). Data in C, D, F, and G are displayed as mean ± SEM and were analyzed using an unpaired Student’s t test in D and G and a Mann–Whitney test (for nonparametric data) in C and F: n.s., not significant; **, P < 0.01; ***, P < 0.001. Data in E are displayed as mean ± SEM and were analyzed using two-way ANOVA: ****, P < 0.0001.
Figure 8.
Inflammatory monocytes are required for activation of NK cell IFN-γ production during infection. (A–D) WT B6 mice were given an anti-CCR2 antibody or the respective isotype-matched control Ig to deplete inflammatory monocytes and then infected with 104 pfu HSV-2 ivag. Vaginal tissue was collected on day 3 p.i. and examined for neutrophil and inflammatory monocyte populations. Representative flow plots are respectively shown in A and B and graphically in C and D (n = 5). (E and F) On day 3 p.i., vaginal cells were also examined for NK cells (E; n = 5) and CD11c+ cells (F; n = 5). (G) On days 0–3 p.i., vaginal lavages were examined for IFN-γ levels (n = 4; repeated once with similar results). (H) On day 2 p.i., vaginal washes were examined for HSV-2 viral titers using a plaque assay method (n = 4; repeated once with similar results). (I and J) Day 0–3 p.i. vaginal lavages were also examined for IL-18 levels (I; n = 4; repeated once with similar results) and IL-12 levels (J; n = 5). Data in C–F and H are displayed as mean ± SEM and were analyzed using an unpaired Student’s t test in F and a Mann-Whitney test (for nonparametric data) in C–E and H: n.s., not significant; *, P < 0.05; **, P < 0.01. Data in G, I, and J are displayed as mean ± SEM and were analyzed using two-way ANOVA: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Depletion of inflammatory monocytes abrogates IL-18 production during HSV-2 infection
To determine whether inflammatory monocytes are responsible for producing IL-18 during vaginal HSV-2 infection, we examined IL-18 levels in the vaginal lavages of mice depleted of inflammatory monocytes. Compared with mice given an isotype-matched control Ig, mice given the anti-CCR2 antibody had significantly decreased IL-18 content in their vaginal lavages, particularly on days 2 and 3 p.i. (Fig. 8 I). We have previously published that Ifnar−/− mice have significantly lower levels of vaginal IL-12 at days 2 and 3 p.i. compared with WT mice (Gill et al., 2011). We examined whether depletion of inflammatory monocytes impacted induction of IL-12 during HSV-2 infection. Upon depletion of inflammatory monocytes, however, we observed no significant difference in levels of IL-12 (Fig. 8 J). Overall, these data suggest that inflammatory monocytes are responsible for IL-18, but not IL-12, production in the vaginal mucosa during HSV-2 infection.
Discussion
Early IFN-γ production from NK cells is the hallmark of the innate immune response and is required for protection during vaginal HSV-2 infection (Ashkar and Rosenthal, 2003; Gill et al., 2011). We and others have found that the presence of IFNAR is absolutely required for the activation of IFN-γ production by NK cells (Lucas et al., 2007; Martinez et al., 2008; Zhu et al., 2008; Gill et al., 2011; Baranek et al., 2012). Here, we demonstrate a mechanism by which type I IFN activates IFN-γ production by NK cells during a mucosal viral infection. Although IFNAR is not required on NK cells for their activation, we have also determined that type I IFN does not go through the canonical pathway of DC activation and IL-15 trans-presentation in our model (Lucas et al., 2007; Baranek et al., 2012). Instead, type I IFN is necessary to recruit inflammatory monocytes, as well as signal through these cells, to release IL-18, which then acts directly on NK cells through their IL-18R to induce their production of IFN-γ (Fig. 9).
Figure 9.
Type I IFN activates NK cell IFN-γ production through the stimulation of IL-18 production in inflammatory monocytes during vaginal HSV-2 infection. Rapidly after HSV-2 infection, type I IFN is produced in the vaginal mucosa. Type I IFN induces CCL2 production from a cell type or types and recruits inflammatory monocytes to the site of infection. Type I IFN binds IFNAR on inflammatory monocytes and signals through IRF9 to induce their release of IL-18. IL-18 then ligates to IL-18R on NK cells to induce their production of IFN-γ.
Previously, several studies have suggested that type I IFN signals directly on NK cells to induce their activation (Martinez et al., 2008; Zhu et al., 2008; Mack et al., 2011). Through our adoptive transfer system, we found that NK cells do not require IFNAR expression to become activated to produce IFN-γ during HSV-2 infection. This is similar to previously published results by Guan et al. (2014) in which they demonstrated that IFNAR was not required on NK cells for their activation in the context of MCMV infection. Surprisingly, our results showed that Ifnar−/− NK cells have increased expression of IFN-γ when adoptively transferred to Rag2−/−Il2rg−/− mice compared with WT NK cells. Type I IFN has been shown to negatively regulate IFN-γ production from NK cells, explaining the increase in IFN-γ expression from Ifnar−/− NK cells (Nguyen et al., 2000; Teles et al., 2013). On the other hand, Lucas et al. (2007) and Baranek et al. (2012) reported that type I IFN stimulates DCs to trans-present IL-15, which in turn activates NK cells (Lucas et al., 2007; Baranek et al., 2012). However, we found the mucosal route involves inflammatory monocytes and IL-18, rather than DCs and IL-15 trans-presentation. This is supported by previous evidence showing that IL-15 production in the vaginal mucosa of Ifnar−/− mice is similar to, if not higher than, that of WT mice during HSV-2 infection (Gill et al., 2011). Furthermore, we found similar amounts of NK cell IFN-γ production in mice that had a specific absence of IFNAR on CD11c+ cells versus WT controls, indicating that DCs were not responsible for responding to type I IFN. Additionally, administration of IL-15–IL-15 receptor complexes, although leading to significant NK cell proliferation, did not rescue NK cell IFN-γ production in Ifnar−/− mice. The difference in findings may lie in the route of viral infection, which involves infecting through a mucosal surface, the vaginal mucosa. The majority of articles examining the relationship between type I IFN and NK cell activation administer viral infection or TLR stimulants through in vitro or systemic, i.p., or subcutaneous routes of infection and found contradicting outcomes (Lucas et al., 2007; Martinez et al., 2008; Zhu et al., 2008; Baranek et al., 2012). Although we observed a decrease in IFN-γ production in WT mice after administration of IL-15/IL-15Rα, it is known that IL-15 is able to reduce HSV-2 virus replication both in vivo and in vitro independently of its effects on NK cells. Because NK cell IFN-γ production is correlated with HSV-2 replication, IL-15’s ability to reduce HSV-2 viral titers subsequently reduces IFN-γ expression from NK cells (Gill et al., 2005).
IFN-β and numerous subtypes of IFN-α have both been shown to signal through the type I IFN receptor and are produced upon virus infection (Perry et al., 2005). Indeed, Gill et al. (2011) have previously shown that vaginal HSV-2 infection rapidly induces IFN-β production at 6 h p.i. but is rapidly controlled at 12 and 24 h p.i. Because IFN-β is produced early during HSV-2 infection, we were interested in determining the role of IFN-β in activating NK cells. However, we found that IFNβ−/− mice had comparable levels of IFN-β production to WT mice. It is likely that IFN-α is able to compensate for the absence of IFN-β.
During vaginal HSV-2 infection, we observed that DCs were not required to respond to IFNAR, but instead, inflammatory monocytes were necessary for the activation of NK cell IFN-γ production. In agreement with Iijima et al. (2011), we found that Ifnar−/− mice had decreased MCP-1 production and a reduction in inflammatory monocyte recruitment during HSV-2 infection. They also showed that type I IFN induced CCR2 ligand production (MCP-1, CCL7, CCL8, and CCL12), which was ultimately responsible for inflammatory monocyte recruitment (Iijima et al., 2011). When we depleted inflammatory monocytes using an anti-CCR2 antibody, we observed a complete abrogation of NK cell IFN-γ production. However, Iijima et al. (2011) also observed that Ccr2−/− mice, which have significant deficiencies in inflammatory monocyte recruitment, also had decreased NK cell IFN-γ production. Although they found that CCR2 was required for NK cell recruitment to the vaginal mucosa, our depletion of inflammatory monocytes did not impact NK cell recruitment to the vaginal mucosa (Iijima et al., 2011). Furthermore, we did not observe any difference in proportion of CD11c+ cells in the vaginal mucosa when depleting inflammatory monocytes. We also found that depletion of inflammatory monocytes increased vaginal HSV-2 viral titer levels at day 2 p.i., suggesting that inflammatory monocyte activation of NK cell IFN-γ production is critical for limiting HSV-2 virus replication during the early stages of infection. In humans, depletion of monocytes from PBMCs in an in vitro model of HCV infection reduced NK cell IFN-γ production (Zhang et al., 2013; Serti et al., 2014). In particular, Serti et al. (2014) observed that monocyte production of IL-18 was responsible for activating the NK cells.
IL-18 is a well-known cytokine activator of NK cell IFN-γ production (Okamura et al., 1995). Interestingly, like IL-1β, IL-18 is first produced intracellularly as an immature protein without a signal peptide and, in this form, is constitutively expressed (Dinarello et al., 2013). To be secreted, the IL-18 precursor needs to be cleaved into a mature and bioactive protein, usually by caspase-1 (Dinarello et al., 2013). Once secreted from the cell, IL-18 can bind to its two specific receptors, IL-18α and IL-18β, and cause signal transduction through MyD88 within the receiving cell (Dinarello et al., 2013). During mucosal HSV-2 infection, Il18−/− mice have significantly increased shedding of HSV-2 in their vaginal washes and decreased survival when compared with control mice (Harandi et al., 2001). Accordingly, we found that type I IFN–induced IL-18 production was a critical factor for NK cell activation. Similar to our findings, others have shown that a deficiency in IL-18 production leads to decreased NK cell IFN-γ production in vivo during vaccinia virus, Francisella tularensis, and Chlamydia muridarum infections (Nagarajan et al., 2011; Pierini et al., 2013; Brandstadter et al., 2014). Even when using a natural mouse pathogen, orthopoxvirus ECTV, Il18−/− mice produced significantly less NK cell IFN-γ (Wang et al., 2009). Using an i.v. HSV-1 infection model, Barr et al. (2007) observed that IL-18 production by CD11c+ cells was responsible for IFN-γ production by NK cells in an ex vivo splenocyte culture.
Much of the data regarding the relationship between type I IFN and IL-18 suggests that type I IFN activates cleaving of pro–IL-18 into active IL-18 (Fernandes-Alnemri et al., 2010; Fang et al., 2014). Recently, Fang et al. (2014) observed that type I IFN produced during Streptococcus pneumoniae infection led to IL-18 production through the activation of caspase-1. During F. tularensis infection, Fernandes-Alnemri et al. (2010) observed that Ifnar−/− mice were defective in their ability to induce caspase-1 activation, which in turn inhibited their ability to cleave pro–IL-18 into active IL-18 (Fernandes-Alnemri et al., 2010). They attributed this to the ability of type I IFN to activate the absent in melanoma 2 (AIM2) inflammasome complex, which is responsible for activating caspase-1 (Fernandes-Alnemri et al., 2010). Further studies will be required to determine whether a similar mechanism occurs during viral infection and NK cell activation.
Our findings provide a complete mechanism detailing the steps that type I IFN takes to activate NK cells. Type I IFN produced during a viral infection stimulates MCP-1 production, which is responsible for inflammatory monocyte migration to the site of inflammation. Once recruited, type I IFN stimulates inflammatory monocytes to produce IL-18, which then signals through the IL-18R expressed by NK cells to induce their production of IFN-γ. In understanding the mechanism of NK cell activation during mucosal vaginal HSV-2 infection, this information can be applied to other infections, cancers, and ailments that involve IL-18 and the activation of NK cells. Moreover, this information furthers our understanding of the innate immune response to mucosal virus infections.
Materials and methods
Mice
6–8-wk-old C57BL/6 (B6) mice were purchased from Charles River. Il18−/− and Il18r1−/− mice on a B6 background were purchased from The Jackson Laboratory. Breeding pairs of Ifnar−/− mice on a B6 background were provided by L. Lenz (University of Colorado, Boulder, CO) and then bred at McMaster University’s Central Animal Facility (CAF). Breeding pairs of Rag2−/−Il2rg−/− mice on a BALB/c background were provided by M. Ito (Central Institute for Experimental Animals, Kawasaki, Japan) and then bred in our CAF. Breeding pairs of Irf9−/− mice were provided by T. Taniguchi (University of Tokyo, Tokyo, Japan) to K.L. Mossman, and a colony was established at McMaster’s CAF. Breeding pairs of Gata1−/− mice on a B6 background were established in M. Jordana’s laboratory at McMaster’s CAF. Ifnb−/− mice on a B6 background were generously provided by E.N. Fish (University of Toronto, Toronto, ON, Canada). Isg15−/− mice were generously provided by P. Gros (McGill University, Montreal, QC, Canada). Ifnarf/f Itgax-cre+ and Ifnarf/f control mice were provided by R.D. Schreiber and bred in our CAF. All mice were housed in specific pathogen–free conditions with a 12-h day and 12-h night cycle. All experiments were performed in accordance with Canadian Council on Animal Care guidelines and approved by the Animal Research Ethics Board at McMaster University.
Genital HSV-2 infection
Mice were injected subcutaneously with 2 mg Depo-Provera (medroxyprogesterone acetate) 5 d before HSV-2 infection. Mice were then infected with HSV-2, 333 strain, and assessed for genital pathology and survival. Genital pathology was scored on a scale of 5 according to severity of redness, swelling, lesion development, hair loss, ulceration, and lower limb paralysis. Ulceration of a lesion and/or lower limb paralysis was considered endpoint.
Vaginal viral titration
Vero cells were grown in a monolayer to confluence in 12-well plates in α-MEM supplemented with 1% (vol/vol) each of l-glutamine, penicillin and streptomycin, and Hepes. Vaginal lavages were serially diluted and incubated with the monolayer for 2 h. The vero cells were then overlaid with human immune serum containing α-MEM and subsequently incubated for 48 h. After incubation, the cells were fixed and stained with crystal violet, and plaques were quantified using an inverted microscope.
Vaginal lavages
During days 0–3 p.i., vaginal lavages were performed by flushing the vaginal cavity with 30 µl PBS. Two 30-µl washes were taken per mouse per day. Samples were subsequently centrifuged at 800 g for 5 min, and supernatants were removed for IFN-γ or IL-18 measurement by ELISA.
In vivo treatments
0.5 µg recombinant mouse IL-15 was incubated with 1 µg recombinant mouse IL-15Rα in PBS at 37°C for 30 min. Each mouse was administered 0.5 µg IL-15 with 1 µg IL-15 Rα complex i.p. on day 0. To deplete neutrophils and inflammatory monocytes, anti–GR-1 antibody or isotype-matched control antibodies were administered i.p. on days −2, −1, and 1 p.i. To deplete inflammatory monocytes, 10–20 µg anti-CCR2 antibody or isotype control antibodies were administered i.p. on days −1, 0, 1, and 2 p.i. To block IFNAR in vivo, B6 mice were administered 500 µg anti-IFNAR antibody or isotype-matched control antibody on days −1, 0, and 1 and 250 µg of each antibody on days 3 and 5. To deplete NK cells, 200 µg anti-NK1.1 antibody was given i.p. on days −2 and −1 before infection. Anti–IL-15 was generated by E. Butz and provided by Amgen. 40 µg anti–IL-15 antibody was then given i.p. to mice on day −1 before infection.
Cell isolation from tissues
Blood and splenocytes were obtained and processed to a single-cell suspension. Red blood cells were removed using ACK lysis buffer. Vaginal tissue was isolated, minced, and incubated in a digestion mixture of RPMI-1640 with 10% FBS, 1% penicillin and streptomycin, 1% l-glutamine, 1% Hepes, and 0.030 mg/ml collagenase A for two separate 1-h incubations. Cells were collected after each incubation and passed through a 40-µm filter.
NK cell adoptive transfer
Mouse splenocytes were obtained and processed into a single-cell suspension in 2% BSA in PBS before isolation of NK cells. NK cells were isolated using Stem Cell EasySep pan-NK cell isolation or EasySep PE selection (using PE-conjugated anti-NK1.1) kits with magnetic separation. 2 × 106 to 3 × 106 B6, Ifnar−/−, or Il18r1−/− NK cells were adoptively transferred i.v. into Rag2−/−Il2rg−/− or Ifnar−/− mice.
Ex vivo stimulation
WT, Ifnar−/−, and Il18r−/− NK cells were isolated as previously described. NK cells were stimulated with 50 ng/ml PMA and 500 ng/ml ionomycin for 24 h ex vivo. Supernatants were collected and assayed for IFN-γ levels.
Cytokine detection
IFN-γ in either supernatants or vaginal lavages was detected by using DuoSet ELISA kits (R&D Systems). IL-18 in vaginal lavages was detected by using the IL-18 ELISA from MBL. CCL2 in vaginal lavages was detected by using the CCL2 and MCP-1 DuoSet ELISA kit.
Flow cytometry staining
In brief, cells were plated at 106 cells/well in 0.2% BSA in PBS. Nonspecific antibody binding was blocked with anti–CD16+CD32 antibody 2.4G2. Extracellular cell staining was conducted by using combinations of Alexa Fluor 700– or PE-Texas Red–conjugated anti-CD45; Alexa Fluor 700– or PE Texas Red–conjugated anti-CD3; PE- or Brilliant Violet 421–conjugated anti-NK1.1; APC-conjugated DX5 mAb; PerCp-conjugated anti-NKp46; PE-Cy-7– or PerCp-Cy5.5–conjugated anti-CD11b; FITC-, Alexa Fluor 700–, or APC-conjugated anti-F4/80; Alexa Fluor 700–, APC-, PE-, or Pe-Cy-7–conjugated anti-CD11c; Brilliant Violet 421– or Alexa Fluor 700–conjugated anti-GR1; FITC- or BV510-conjugated anti-Ly6G; Pacific Blue–conjugated anti-Ly6C; APC-conjugated anti-CCR2; and PE-conjugated anti-IFNAR. For intracellular staining, cells were permeabilized using Cytofix/Cytoperm (BD) and then stained with APC-conjugated anti–IFN-γ. Cells were fixed in 1% PFA in PBS and run on the FACSCanto or LSRII (BD). Data were analyzed using FlowJo software.
Generation of bone marrow chimeras
Bone marrow was collected from WT, Ifnar−/−, and IL18−/− B6 mice and T cell depleted using anti-CD4 (GK1.5, anti-CD8 [2.43], and anti-Thy1.2 [BD] and low-tox guinea pig complement [Cedarlane]). Recipient mice were irradiated with two doses of 550 rad with a 3-h interval between irradiations and reconstituted with 107 T cell–depleted bone marrow cells after the second dose of irradiation.
Statistical analyses
Differences were assessed using Student’s t test (parametric data), Mann–Whitney test (nonparametric data), one-way ANOVA (if more than two groups were being analyzed), or two-way ANOVA (if more than two groups were being analyzed with more than one independent variable). If post-statistical analysis was required, a Bonferroni post-test was applied. Outliers were determined using the extreme studentized deviate (ESD) method and removed if deemed a significant outlier (0.05). All statistical analyses were completed using GraphPad Prism 4.0. Statistical significance is indicated as ****, P < 0.0001; ***, P < 0.001; **, P < 0.01; *, P < 0.05; or n.s. (not significant).
Acknowledgments
We thank Dr. Eleanor N. Fish for providing us with Ifnb−/− mice and Dr. Philippe Gros for providing us with breeding pairs of Isg15−/− mice.
This work was supported by a grant from the Canadian Institutes of Health Research (CIHR) awarded to A.A. Ashkar (CIHR-123401). A.A. Ashkar is also a recipient of a CIHR Tier 1 Canada Research Chair. A.J. Lee is a recipient of a CIHR Vanier Scholarship.
R.D. Schreiber is a cofounder, stock holder, and scientific advisory board member of Jounce Therapeutics and Neon Therapeutics and a member of the scientific advisory boards of BioLegend, Constellation, Lytix, and NGM. He also received research funding from Janssen and Agios. The authors declare no other competing financial interests.
Footnotes
Abbreviations used:
- IFNAR
- IFN α/β receptor
- MCMV
- mouse CMV
- p.i.
- post-infection
References
- Ashkar A.A., and Rosenthal K.L.. 2003. Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J. Virol. 77:10168–10171. 10.1128/JVI.77.18.10168-10171.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek T., Manh T.P., Alexandre Y., Maqbool M.A., Cabeza J.Z., Tomasello E., Crozat K., Bessou G., Zucchini N., Robbins S.H., et al. 2012. Differential responses of immune cells to type I interferon contribute to host resistance to viral infection. Cell Host Microbe. 12:571–584 (published erratum appears in Cell Host Microbe 2013. 13:372) . 10.1016/j.chom.2012.09.002 [DOI] [PubMed] [Google Scholar]
- Barr D.P., Belz G.T., Reading P.C., Wojtasiak M., Whitney P.G., Heath W.R., Carbone F.R., and Brooks A.G.. 2007. A role for plasmacytoid dendritic cells in the rapid IL-18-dependent activation of NK cells following HSV-1 infection. Eur. J. Immunol. 37:1334–1342. 10.1002/eji.200636362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic D., Byun M., Durfee L.A., Abhyankar A., Sanal O., Mansouri D., Salem S., Radovanovic I., Grant A.V., Adimi P., et al. 2012. Mycobacterial disease and impaired IFN-γ immunity in humans with inherited ISG15 deficiency. Science. 337:1684–1688. 10.1126/science.1224026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstadter J.D., Huang X., and Yang Y.. 2014. NK cell-extrinsic IL-18 signaling is required for efficient NK-cell activation by vaccinia virus. Eur. J. Immunol. 44:2659–2666. 10.1002/eji.201344134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley J.M., Thomay A.A., Connolly M.D., Reichner J.S., and Albina J.E.. 2008. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83:64–70. 10.1189/jlb.0407247 [DOI] [PubMed] [Google Scholar]
- Diamond M.S., Kinder M., Matsushita H., Mashayekhi M., Dunn G.P., Archambault J.M., Lee H., Arthur C.D., White J.M., Kalinke U., et al. 2011. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 208:1989–2003. 10.1084/jem.20101158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C.A., Novick D., Kim S., and Kaplanski G.. 2013. Interleukin-18 and IL-18 binding protein. Front. Immunol. 4:289 10.3389/fimmu.2013.00289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elpek K.G., Rubinstein M.P., Bellemare-Pelletier A., Goldrath A.W., and Turley S.J.. 2010. Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15Rα complexes. Proc. Natl. Acad. Sci. USA. 107:21647–21652. 10.1073/pnas.1012128107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R., Hara H., Sakai S., Hernandez-Cuellar E., Mitsuyama M., Kawamura I., and Tsuchiya K.. 2014. Type I interferon signaling regulates activation of the absent in melanoma 2 inflammasome during Streptococcus pneumoniae infection. Infect. Immun. 82:2310–2317. 10.1128/IAI.01572-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T., Yu J.W., Juliana C., Solorzano L., Kang S., Wu J., Datta P., McCormick M., Huang L., McDermott E., et al. 2010. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11:385–393. 10.1038/ni.1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill N., Rosenthal K.L., and Ashkar A.A.. 2005. NK and NKT cell-independent contribution of interleukin-15 to innate protection against mucosal viral infection. J. Virol. 79:4470–4478. 10.1128/JVI.79.7.4470-4478.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill N., Deacon P.M., Lichty B., Mossman K.L., and Ashkar A.A.. 2006. Induction of innate immunity against herpes simplex virus type 2 infection via local delivery of Toll-like receptor ligands correlates with beta interferon production. J. Virol. 80:9943–9950. 10.1128/JVI.01036-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill N., Chenoweth M.J., Verdu E.F., and Ashkar A.A.. 2011. NK cells require type I IFN receptor for antiviral responses during genital HSV-2 infection. Cell. Immunol. 269:29–37. 10.1016/j.cellimm.2011.03.007 [DOI] [PubMed] [Google Scholar]
- Gillgrass A.E., Ashkar A.A., Rosenthal K.L., and Kaushic C.. 2003. Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J. Virol. 77:9845–9851. 10.1128/JVI.77.18.9845-9851.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J., Miah S.M., Wilson Z.S., Erick T.K., Banh C., and Brossay L.. 2014. Role of type I interferon receptor signaling on NK cell development and functions. PLoS One. 9:e111302 10.1371/journal.pone.0111302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamerman J.A., Ogasawara K., and Lanier L.L.. 2004. Cutting edge: Toll-like receptor signaling in macrophages induces ligands for the NKG2D receptor. J. Immunol. 172:2001–2005. 10.4049/jimmunol.172.4.2001 [DOI] [PubMed] [Google Scholar]
- Harandi A.M., Svennerholm B., Holmgren J., and Eriksson K.. 2001. Interleukin-12 (IL-12) and IL-18 are important in innate defense against genital herpes simplex virus type 2 infection in mice but are not required for the development of acquired gamma interferon-mediated protective immunity. J. Virol. 75:6705–6709. 10.1128/JVI.75.14.6705-6709.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima N., Mattei L.M., and Iwasaki A.. 2011. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proc. Natl. Acad. Sci. USA. 108:284–289. 10.1073/pnas.1005201108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug A., French A.R., Barchet W., Fischer J.A., Dzionek A., Pingel J.T., Orihuela M.M., Akira S., Yokoyama W.M., and Colonna M.. 2004. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 21:107–119. 10.1016/j.immuni.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Lucas M., Schachterle W., Oberle K., Aichele P., and Diefenbach A.. 2007. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 26:503–517. 10.1016/j.immuni.2007.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack E.A., Kallal L.E., Demers D.A., and Biron C.A.. 2011. Type 1 interferon induction of natural killer cell gamma interferon production for defense during lymphocytic choriomeningitis virus infection. MBio. 2:e00169-11 10.1128/mBio.00169-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack M., Cihak J., Simonis C., Luckow B., Proudfoot A.E., Plachý J., Brühl H., Frink M., Anders H.J., Vielhauer V., et al. 2001. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J. Immunol. 166:4697–4704. 10.4049/jimmunol.166.7.4697 [DOI] [PubMed] [Google Scholar]
- Martinez J., Huang X., and Yang Y.. 2008. Direct action of type I IFN on NK cells is required for their activation in response to vaccinia viral infection in vivo. J. Immunol. 180:1592–1597. 10.4049/jimmunol.180.3.1592 [DOI] [PubMed] [Google Scholar]
- Nagarajan U.M., Sikes J., Prantner D., Andrews C.W. Jr., Frazer L., Goodwin A., Snowden J.N., and Darville T.. 2011. MyD88 deficiency leads to decreased NK cell gamma interferon production and T cell recruitment during Chlamydia muridarum genital tract infection, but a predominant Th1 response and enhanced monocytic inflammation are associated with infection resolution. Infect. Immun. 79:486–498. 10.1128/IAI.00843-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K.B., Cousens L.P., Doughty L.A., Pien G.C., Durbin J.E., and Biron C.A.. 2000. Interferon α/β-mediated inhibition and promotion of interferon gamma: STAT1 resolves a paradox. Nat. Immunol. 1:70–76. 10.1038/76940 [DOI] [PubMed] [Google Scholar]
- Okamura H., Nagata K., Komatsu T., Tanimoto T., Nukata Y., Tanabe F., Akita K., Torigoe K., Okura T., Fukuda S., et al. 1995. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect. Immun. 63:3966–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange J.S., Wang B., Terhorst C., and Biron C.A.. 1995. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J. Exp. Med. 182:1045–1056. 10.1084/jem.182.4.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegram H.J., Andrews D.M., Smyth M.J., Darcy P.K., and Kershaw M.H.. 2011. Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol. 89:216–224. 10.1038/icb.2010.78 [DOI] [PubMed] [Google Scholar]
- Perry A.K., Chen G., Zheng D., Tang H., and Cheng G.. 2005. The host type I interferon response to viral and bacterial infections. Cell Res. 15:407–422. 10.1038/sj.cr.7290309 [DOI] [PubMed] [Google Scholar]
- Pierini R., Perret M., Djebali S., Juruj C., Michallet M.C., Förster I., Marvel J., Walzer T., and Henry T.. 2013. ASC controls IFN-γ levels in an IL-18-dependent manner in caspase-1-deficient mice infected with Francisella novicida. J. Immunol. 191:3847–3857. 10.4049/jimmunol.1203326 [DOI] [PubMed] [Google Scholar]
- Platanias L.C. 2005. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5:375–386. 10.1038/nri1604 [DOI] [PubMed] [Google Scholar]
- Robertson S.A., Mau V.J., Young I.G., and Matthaei K.I.. 2000. Uterine eosinophils and reproductive performance in interleukin 5-deficient mice. J. Reprod. Fertil. 120:423–432. 10.1530/reprod/120.2.423 [DOI] [PubMed] [Google Scholar]
- Schumak B., Klocke K., Kuepper J.M., Biswas A., Djie-Maletz A., Limmer A., van Rooijen N., Mack M., Hoerauf A., and Dunay I.R.. 2015. Specific depletion of Ly6C(hi) inflammatory monocytes prevents immunopathology in experimental cerebral malaria. PLoS One. 10:e0124080 10.1371/journal.pone.0124080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serti E., Werner J.M., Chattergoon M., Cox A.L., Lohmann V., and Rehermann B.. 2014. Monocytes activate natural killer cells via inflammasome-induced interleukin 18 in response to hepatitis C virus replication. Gastroenterology. 147:209–220.e3. 10.1053/j.gastro.2014.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirén J., Sareneva T., Pirhonen J., Strengell M., Veckman V., Julkunen I., and Matikainen S.. 2004. Cytokine and contact-dependent activation of natural killer cells by influenza A or Sendai virus-infected macrophages. J. Gen. Virol. 85:2357–2364. 10.1099/vir.0.80105-0 [DOI] [PubMed] [Google Scholar]
- Teles R.M., Graeber T.G., Krutzik S.R., Montoya D., Schenk M., Lee D.J., Komisopoulou E., Kelly-Scumpia K., Chun R., Iyer S.S., et al. 2013. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science. 339:1448–1453. 10.1126/science.1233665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa M., Kuziel W.A., and Carr D.J.. 2007. Susceptibility of CCR5-deficient mice to genital herpes simplex virus type 2 is linked to NK cell mobilization. J. Virol. 81:3704–3713. 10.1128/JVI.02626-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E., Tomasello E., Baratin M., Walzer T., and Ugolini S.. 2008. Functions of natural killer cells. Nat. Immunol. 9:503–510. 10.1038/ni1582 [DOI] [PubMed] [Google Scholar]
- Wang Y., Chaudhri G., Jackson R.J., and Karupiah G.. 2009. IL-12p40 and IL-18 play pivotal roles in orchestrating the cell-mediated immune response to a poxvirus infection. J. Immunol. 183:3324–3331. 10.4049/jimmunol.0803985 [DOI] [PubMed] [Google Scholar]
- Zhang S., Saha B., Kodys K., and Szabo G.. 2013. IFN-γ production by human natural killer cells in response to HCV-infected hepatoma cells is dependent on accessory cells. J. Hepatol. 59:442–449. 10.1016/j.jhep.2013.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Huang X., and Yang Y.. 2008. A critical role for type I IFN-dependent NK cell activation in innate immune elimination of adenoviral vectors in vivo. Mol. Ther. 16:1300–1307. 10.1038/mt.2008.88 [DOI] [PubMed] [Google Scholar]