Diny et al. report a pathogenic role for eosinophils in autoimmune myocarditis and dilated cardiomyopathy. Eosinophils are required for progression of myocarditis to dilated cardiomyopathy and drive severe disease when present in large numbers. Activated cardiac eosinophils mediate this process through IL-4.
Abstract
Inflammatory dilated cardiomyopathy (DCMi) is a major cause of heart failure in children and young adults. DCMi develops in up to 30% of myocarditis patients, but the mechanisms involved in disease progression are poorly understood. Patients with eosinophilia frequently develop cardiomyopathies. In this study, we used the experimental autoimmune myocarditis (EAM) model to determine the role of eosinophils in myocarditis and DCMi. Eosinophils were dispensable for myocarditis induction but were required for progression to DCMi. Eosinophil-deficient ΔdblGATA1 mice, in contrast to WT mice, showed no signs of heart failure by echocardiography. Induction of EAM in hypereosinophilic IL-5Tg mice resulted in eosinophilic myocarditis with severe ventricular and atrial inflammation, which progressed to severe DCMi. This was not a direct effect of IL-5, as IL-5TgΔdblGATA1 mice were protected from DCMi, whereas IL-5−/− mice exhibited DCMi comparable with WT mice. Eosinophils drove progression to DCMi through their production of IL-4. Our experiments showed eosinophils were the major IL-4–expressing cell type in the heart during EAM, IL-4−/− mice were protected from DCMi like ΔdblGATA1 mice, and eosinophil-specific IL-4 deletion resulted in improved heart function. In conclusion, eosinophils drive progression of myocarditis to DCMi, cause severe DCMi when present in large numbers, and mediate this process through IL-4.
Introduction
Eosinophils play an important role in heart disease. Cardiac complications occur in ∼20–50% of patients with prolonged elevation of eosinophil numbers, such as hypereosinophilic syndrome (HES) and eosinophilic granulomatosis with polyangiitis, and are a major cause of mortality (Parrillo et al., 1979; Weller and Bubley, 1994; Ogbogu et al., 2007; Comarmond et al., 2013; Moosig et al., 2013). Myocarditis, the inflammation of the heart muscle in the absence of an ischemic event, can cause sudden cardiac death and heart failure (Čiháková and Rose, 2008; Sagar et al., 2012). Predominant infiltration of the heart with eosinophils characterizes the clinically recognized subtype eosinophilic myocarditis. In addition to HES and eosinophilic granulomatosis with polyangiitis patients, eosinophilic myocarditis can develop in patients with parasitic infections, in response to toxins or drugs, or by unknown etiology (Ginsberg and Parrillo, 2005; Baandrup, 2012). Eosinophilic myocarditis is rarely diagnosed clinically but was found in up to 0.5% of patients in a hospital autopsy series (Hawkins et al., 1995) and ∼7% of explanted hearts from transplant patients (Gravanis et al., 1991; Hawkins et al., 1995; Takkenberg et al., 2004).
Although most myocarditis patients recover from the acute illness, a major burden lies in the sequela inflammatory dilated cardiomyopathy (DCMi). About 9–16% of patients with new onset DCMi have evidence of prior myocarditis (Herskowitz et al., 1993; Kasper et al., 1994; Felker et al., 2000); yet, the mechanisms involved in disease progression are poorly understood (Schultheiss et al., 2011). DCMi is the major cause of heart failure in patients <40 yr of age. The 5-yr survival rate is <50% (Daubeney et al., 2006; Magnani and Dec, 2006). Currently, the only option for patients with end-stage DCMi is heart transplantation. Even if a transplant is available, patients with a history of myocarditis have a particularly poor survival rate (Pietra et al., 2012). No drugs are available to arrest or delay development of DCMi or to improve long-term survival. Thus, there is an urgent need to better understand the progression of myocarditis to DCMi and to develop new therapeutic approaches to prevent it. It is not known at what rate eosinophilic myocarditis patients progress to DCMi or how this rate compares with other myocarditis subtypes.
Eosinophils infiltrate the site of inflammation and release preformed toxic granule proteins, cytokines, and growth factors, thereby contributing to tissue injury and remodeling (Rothenberg and Hogan, 2006). Eosinophils have been thought to activate other cardiac cell types, to be directly cytotoxic to the endocardium or cardiomyocytes, or to release granule proteins or pro-thrombotic factors (Tai et al., 1982; Spry et al., 1983; deMello et al., 1990; Patella et al., 1996; Corradi et al., 2004; Cugno et al., 2014). However, many of these studies are purely observational, and the proposed pathogenic mechanisms have not been thoroughly tested. Others have demonstrated eosinophilic myocarditis developing after infection with various parasites (Molina and Kierszenbaum, 1988; Cookston et al., 1990; Dimayuga et al., 1991; Monteón et al., 1996; Hokibara et al., 1998; Chisty et al., 1999) or spontaneously in DBA/2 mice (Hirasawa et al., 1998, 2003, 2007), Socs1-deficient mice (Metcalf et al., 2001), and Bcl6-deficient mice (Yoshida et al., 1999). However, none of these studies has examined the importance of eosinophils for myocarditis severity or their role in progression to DCMi or how eosinophils damage the heart. The National Institutes of Health workshop study on the Research Needs of Eosinophil-Associated Diseases points out an urgent need for preclinical models and a mechanistic understanding of eosinophil-mediated cardiac damage (Bochner et al., 2012).
To study myocarditis and DCMi, we use the animal model of experimental autoimmune myocarditis (EAM; Rose et al., 1987; Ciháková et al., 2004). Mice lacking both the key T helper 1 cytokine (Th1 cytokine) and Th17 cytokine, IFN-γ and IL-17A, developed a rapidly fatal eosinophilic myocarditis after induction of EAM. Ablation of eosinophils in these mice improved survival (Barin et al., 2013). In another model, NK cell depletion resulted in increased eosinophil infiltration in the heart, aggravating myocarditis (Ong et al., 2015). These results suggest that eosinophils are highly pathogenic in myocarditis and DCMi. In this study, we used eosinophil-deficient and hypereosinophilic mouse models to examine the impact of eosinophils in myocarditis and its sequela DCMi. Moreover, we identified the mechanisms by which eosinophils contribute to pathology.
Results
Eosinophil-deficient mice develop myocarditis but are protected from dilated cardiomyopathy
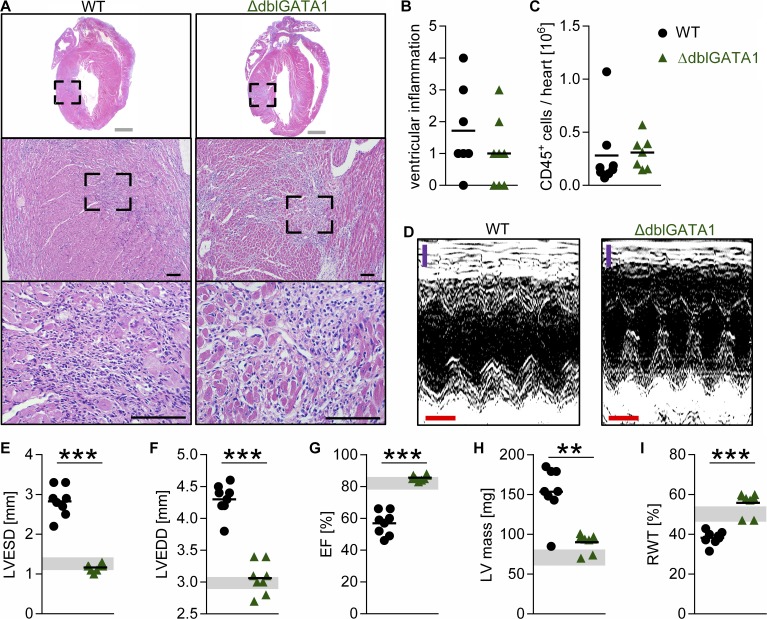
To study the mechanism of eosinophil-mediated damage in myocarditis and its sequela DCMi, we used eosinophil-deficient ΔdblGATA1 mice. In these mice, a deletion in the promoter of Gata1 blocks eosinophil development in the bone marrow (Yu et al., 2002). Myocarditis was induced in WT and ΔdblGATA1 mice, and the degree of inflammation was compared on day 21 of EAM. ΔdblGATA1 mice developed myocarditis with similar severity as WT mice (Fig. 1, A and B). Moreover, no differences were observed in the number of heart-infiltrating CD45+ cells by flow cytometry at day 21 of EAM (Fig. 1 C).
Figure 1.
Eosinophil-deficient mice develop EAM but are protected from DCMi. (A–C) Mice were analyzed at day 21 of EAM. (A) Representative images of H&E-stained heart sections of the median animal. Bars: (gray) 1 mm; (black) 100 µm. (B) The degree of ventricular inflammation was scored on H&E-stained heart sections. (C) Total heart-infiltrating CD45+ cells were determined by flow cytometry. (D–I) Heart function was assessed by echocardiography on day 45 of EAM. (D) Representative M-mode pictures of the median animal are shown. Bars: (purple) 1 mm; (red) 0.1 s. (E–I) Gray bands indicate 95% confidence interval for naive WT mice of the indicated parameters: LVESD (E), LVEDD (F), EF (G), LV mass (H), and RWT (I). Groups were compared using Student’s t test. Data are representative of three to five independent experiments with six to eight mice per group. **, P < 0.01; ***, P < 0.001.
In WT mice, EAM progresses to DCMi (Čiháková and Rose, 2008). Surprisingly, typical signs of ventricular dilation were present in WT but not ΔdblGATA1 mice examined by echocardiography (Fig. 1 D). ΔdblGATA1 mice had preserved left ventricular (LV) end-diastolic diameters (LVEDDs) and LV end-systolic diameters (LVESDs) and preserved ejection fraction (EF) at day 42 of EAM (Fig. 1, E–G). ΔdblGATA1 mice were also protected from the increase in LV mass and thinning of the LV wall (relative wall thickness [RWT]) seen in WT mice (Fig. 1, H and I). These results indicate that eosinophils are not required for EAM development but are necessary for progression of myocarditis to DCMi.
ΔdblGATA1 mice develop fibrosis but show altered tissue remodeling
Progression to DCMi is associated with changes in the connective tissue in the heart, namely fibrosis and tissue remodeling. We previously reported on EAM in IL-17A−/− and IL-17RA−/− mice. In these mice, protection from DCMi is associated with reduced fibrosis (Baldeviano et al., 2010; Wu et al., 2014). However, ΔdblGATA1 mice developed fibrosis to a similar degree as WT mice. Fibrosis was assessed by scoring of Masson’s trichrome–stained heart sections (Fig. S1 A) and quantitation of total cardiac hydroxyproline, a collagen-specific amino acid (Fig. S1 B). These results demonstrate that heart function can be preserved, despite development of cardiac fibrosis. Protection of IL-17A−/− mice from DCMi is associated with differential expression of Col1a2, Col3, Timp1, and Mmp9 (Baldeviano et al., 2010). Expression of these genes was not changed in ΔdblGATA1 mice (Fig. S1 C). However, other tissue remodeling–associated genes, Mmp2 and its regulator Timp2, were decreased in ΔdblGATA1 mice (Fig. S1 D). This indicates that eosinophils promote DCMi through pathways that are identifiably distinct from those driven by IL-17 signaling. High serum MMP2 levels are associated with a poor prognosis in acute and chronic heart failure (Thomas et al., 1998; Shirakabe et al., 2010), suggesting that similar processes may be involved in patients with heart failure.
Lack of eosinophils does not alter cardiac inflammatory infiltrate in EAM
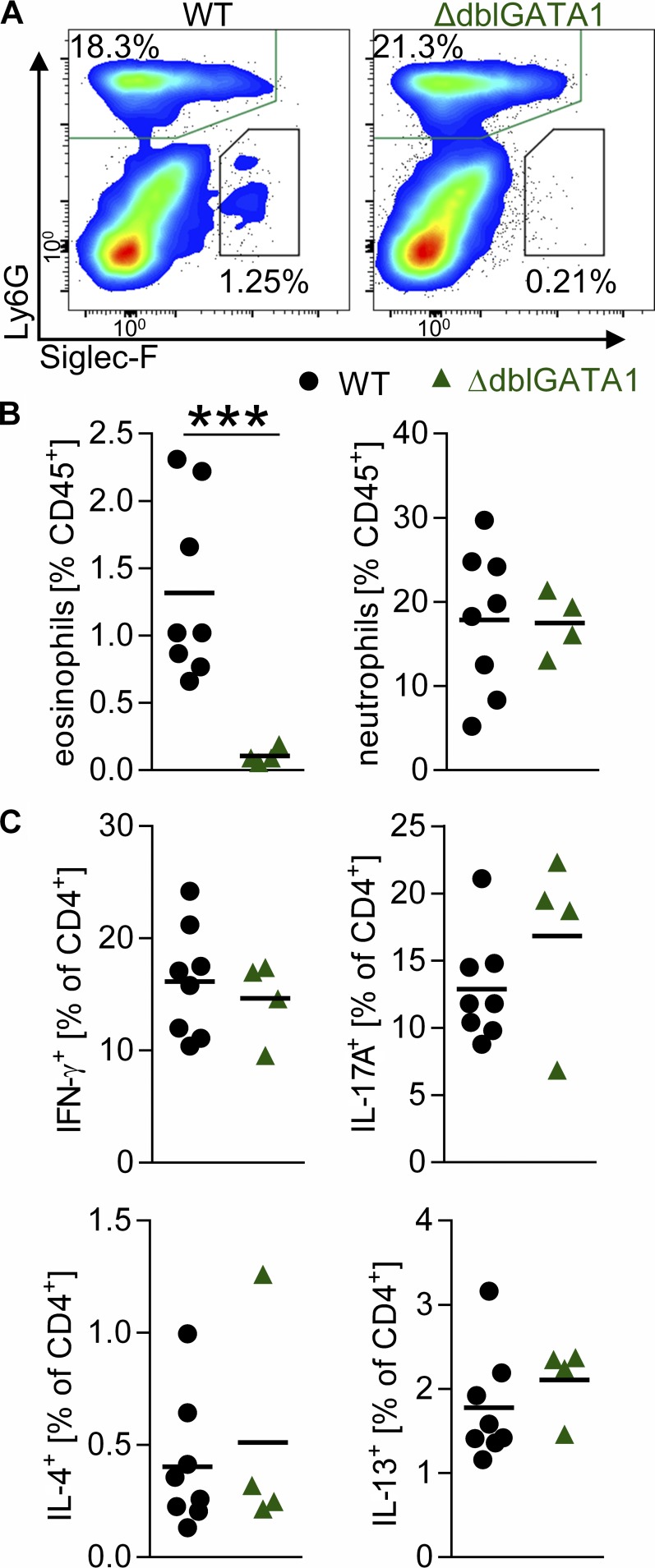
In WT mice, eosinophils accounted for 1–3% of heart-infiltrating cells at day 21, the peak of EAM (Fig. 2, A and B). As expected, eosinophils were not detected in ΔdblGATA1 mice (Fig. 2, A and B). We first hypothesized that eosinophils may affect the makeup of the cellular infiltrate in myocarditis. However, the lack of eosinophils in ΔdblGATA1 mice did not affect the composition of other heart-infiltrating cell populations (Fig. 2 B and Fig. S1 E). We did not observe any differences in the percentage of heart-infiltrating neutrophils, T cells, B cells, NK cells, monocytes/macrophages, basophils, or mast cells. Production of IL-4, IL-13, IL-17A, and IFN-γ by heart-infiltrating Th cells was comparable between WT and ΔdblGATA1 mice (Fig. 2 C and Fig. S1 F), indicating that the lack of eosinophils did not affect T cell polarization. These findings are consistent with the notion that the lack of eosinophils conferred disease protection in ΔdblGATA1 mice.
Figure 2.
Lack of eosinophils does not alter cardiac infiltrate in EAM. (A–C) Frequency of heart-infiltrating cells was determined by flow cytometry on day 21 of EAM. (A) Representative flow plots show viable CD45+ cells of the median animal. (B) Frequency of eosinophils and neutrophils out of CD45+ cells. (C) IFN-γ, IL-17A, IL-4, and IL-13 production by heart-infiltrating CD4+ T cells. Data are representative of two to five independent experiments with four to eight mice per group. Groups were compared by Student’s t test. ***, P < 0.001.
IL-5Tg mice develop eosinophilic myocarditis
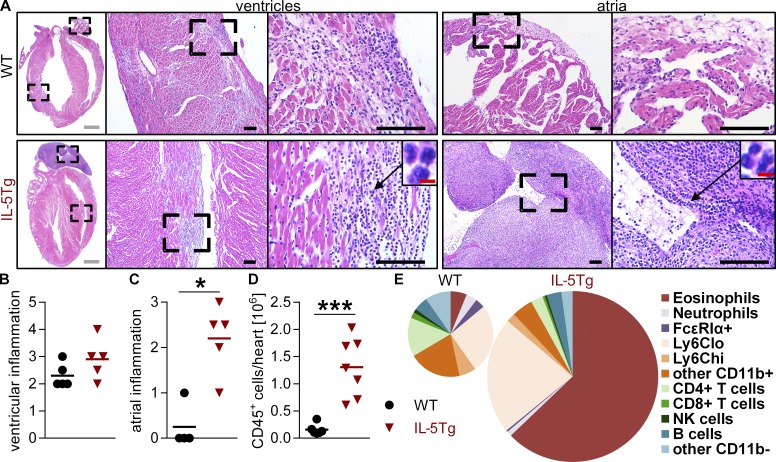
To address whether increased levels of eosinophils would aggravate myocarditis and DCMi, we used transgenic mice expressing IL-5 under the CD3 promoter (IL-5Tg). These mice express high serum levels of IL-5, a key cytokine for eosinophilopoiesis and eosinophil survival, and develop extensive peripheral eosinophilia (Lee et al., 1997). After immunization, IL-5Tg mice developed severe eosinophilic myocarditis (Fig. 3 A). Scoring of the degree of inflammation revealed a trend toward more severe inflammation in the ventricles (Fig. 3, A and B) and significantly more severe inflammation in the atria of IL-5Tg mice (Fig. 3, A and C). This resulted in an increased number of heart-infiltrating CD45+ cells in IL-5Tg compared with WT mice (Fig. 3 D). As evident from the histology, eosinophils comprised a large proportion of heart-infiltrating cells (Fig. 3 A, insets). We quantified heart-infiltrating cell types using flow cytometry. Eosinophils accounted for >60% of CD45+ cells in the whole hearts of IL-5Tg mice compared with only 3% in WT mice (Fig. 3 D). This corresponded to a dramatic difference in absolute eosinophil numbers in both atria and ventricles (Fig. S2 A). Because of this tremendous increase in heart-infiltrating eosinophils and overall increase of CD45+ cells, the absolute numbers of other cell populations were either not different or higher in IL-5Tg mice (Fig. S2 B). A possible explanation for the severe eosinophilic atrial inflammation was increased expression of eotaxins (Ccl11 and Ccl24) in the atria of IL-5Tg mice (Fig. S2 C). Eotaxins are key chemokines for eosinophil trafficking to the heart (Diny et al., 2016). Collectively, these results show that IL-5Tg mice develop severe eosinophilic myocarditis. EAM in IL-5Tg mice is a novel inducible model of eosinophilic myocarditis.
Figure 3.
IL-5 transgenic mice develop severe eosinophilic myocarditis. (A–E) Mice were analyzed at day 21 of EAM. (A) Representative images of H&E-stained heart sections showing the median animal. Bars: (gray) 1 mm; (black) 100 µm; (red) 5 µm. (B and C) Scoring of inflammation in the ventricles (B) and atria (C). (D and E) Total heart-infiltrating CD45+ cells (D) and heart-infiltrating cell populations (E) were quantified by flow cytometry. (E) Mean frequency of different cell populations is shown. The sizes of pie charts are relative to total heart-infiltrating CD45+ cells. Data are representative of two to three independent experiments with four to five mice per group. Groups were compared using Mann-Whitney test (B and C) or Student’s t test (D). *, P < 0.05; ***, P < 0.001.
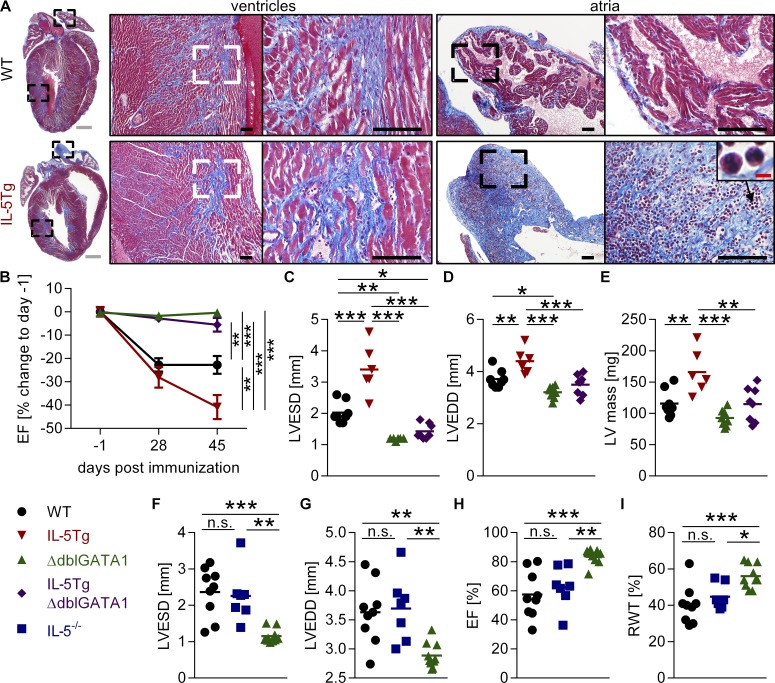
Eosinophilic atrial inflammation progresses to severe fibrosis
By day 45 of EAM, IL-5Tg mice developed ventricular fibrosis similar to WT mice. The severe atrial inflammation in IL-5Tg mice progressed into extensive fibrosis of the atria, often with ongoing eosinophilic inflammation (Fig. 4 A and Fig. S3, A–D). We wondered whether atrial inflammation/fibrosis was also a common feature of eosinophilic myocarditis in patients. We identified three patients with biopsy-confirmed eosinophilic myocarditis and cardiac magnetic resonance imaging at the Johns Hopkins Hospital Department of Cardiology. Two of these three patients showed late gadolinium enhancement (LGE) in the atria, indicating fibrosis or inflammation of the atria. Patient 1 had relatively more atrial than ventricular involvement (Fig. S2, D and E). Patient 2 had predominantly LV involvement with some LGE of the right ventricle. There was less atrial involvement with patchy LGE located near the annulus. Other studies have also described atrial involvement either alone or together with ventricular inflammation in some cases of eosinophilic myocarditis and giant cell myocarditis (Frustaci et al., 1991; Ahmad et al., 2006; Carver et al., 2010; Tian et al., 2010; Larsen et al., 2013; Xie et al., 2015). This suggests that atrial inflammation and/or fibrosis may also be a feature in some eosinophilic myocarditis patients.
Figure 4.
IL-5Tg but not IL-5TgΔdblGATA1 or IL-5−/− mice progress to severe DCMi. (A) Representative images of Masson’s trichrome–stained heart sections from day 45 of EAM showing the median animal. Bars: (gray) 1 mm; (black) 100 µm; (red) 5 µm. (B) Serial echocardiography was performed on days −1, 28, and 45 of EAM. EF for individual mice was normalized to the EF of day −1. Mean ± SEM of combined data from two independent experiments with five to eight mice per group is shown. Percent change in EF on day 45 was compared for all groups. (C–I) Echocardiography was performed on day 45 of EAM in the indicated strains to asses cardiac function: LVESD (C and F), LVEDD (D and G), LV mass (E), EF (H), and RWT (I). (A and C–I) Data are representative of two independent experiments with five to nine mice per group. Groups were compared by one-way ANOVA followed by Tukey’s multiple comparisons test for all groups against each other. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Eosinophils drive severe DCMi
To determine whether large numbers of eosinophils could contribute to DCMi severity, we analyzed heart function over time in WT, hypereosinophilic IL-5Tg, and eosinophil-deficient ΔdblGATA1 mice. As expected, WT mice developed DCMi, and ΔdblGATA1 mice displayed no change in EF over time. IL-5Tg mice showed an even further reduction in EF compared with WT mice, developing very severe DCMi by day 45 of EAM (Fig. 4 B). IL-5Tg mice also progressed to more severe dilation (LVEDD and LVESD) of the left ventricle and increased LV mass compared with WT mice (Fig. 4, C–E). Thus, the level of eosinophilia correlated with DCMi severity, indicating that eosinophils drive progression of myocarditis to DCMi and heart failure.
IL-5 is dispensable for progression to DCMi
To discriminate between the effects of IL-5 and eosinophils, we crossed IL-5Tg mice with ΔdblGATA1 mice. The resulting IL-5TgΔdblGATA1 mice had high serum levels of IL-5 but lacked eosinophils (Fig. S3, E and F). IL-5TgΔdblGATA1 mice were completely protected from the decrease in EF and LV dilation (LVEDD and LVESD) and the increase in LV mass seen in IL-5Tg mice (Fig. 4, B–E). Like ΔdblGATA1 mice, IL-5TgΔdblGATA1 mice were completely protected from DCMi. This demonstrates that high IL-5 levels were not directly responsible for progression to DCMi, but rather, eosinophils were driving this process.
We assessed IL-5−/− mice to determine whether IL-5 is necessary for the development of DCMi after myocarditis. Myocarditis severity in IL-5−/− mice was comparable with WT mice (Fig. S3, G and H), and IL-5−/− mice developed DCMi with the same severity as WT mice, showing no difference in LVEDD, LVESD, EF, or RWT (Fig. 4, F–I). Together, these data led us to conclude that IL-5 was neither necessary for DCMi development nor did it drive disease progression. In contrast, eosinophils were required for DCMi development and caused severe DCMi when present in large numbers.
Eosinophils do not cause DCMi through effects on DCs, antibody production, or cytotoxic effects
In allergic asthma, eosinophils are required for DC activation and suppress downstream Th17 cell responses (Jacobsen et al., 2011). The frequency of cardiac DCs and expression of co-stimulatory molecules on their surface was not reduced in ΔdblGATA1 compared with WT mice on day 10 of EAM (Fig. S4 A). We also did not observe differences in Th17 cells (Fig. 2 C and Fig. S1, E and F). Eosinophils have also been reported to promote plasma cell survival in the bone marrow and IgA production in the intestine (Chu et al., 2011, 2014; Jung et al., 2015). Although antibodies are neither required nor sufficient for induction of myocarditis in BALB/c mice, it is unclear whether they play a role in progression to DCMi (Guthrie et al., 1984; Malkiel et al., 1999; Čiháková and Rose, 2008), and pathogenic autoantibodies have been demonstrated in other experimental models and in patients (Jahns et al., 2004; Mascaro-Blanco et al., 2008). ΔdblGATA1 and WT mice had comparable levels of cardiac myosin-specific IgG in serum on days 21 and 45 and of plasma cells on day 21 of EAM (Fig. S4, B and C). Thus, it is unlikely that eosinophils drive DCMi through effects on antibody production. Previously, eosinophil granule proteins were shown to activate mast cells (Patella et al., 1996). We did not observe differences in mast cell numbers (Fig. S1 E) or in serum IgE (not depicted) between WT and ΔdblGATA1 mice.
Eosinophils are thought to damage the heart by releasing cytotoxic granule proteins (Tai et al., 1982; Spry et al., 1983; deMello et al., 1990; Corradi et al., 2004). We tested whether the granule protein eosinophil peroxidase, which is pathogenic in a colitis model (Forbes et al., 2004), was important for DCMi. Mice lacking functional eosinophil peroxidase (homozygous EoCretg/tg mice with recombination into the eosinophil peroxidase locus) were not protected from DCMi (Fig. S4, D and E). Using in vitro co-culture of primary cardiomyocytes from adult WT mice with eosinophils, we found no effect of eosinophils on the survival of cardiomyocytes (Fig. S4, F and G). Some cardiomyocyte necrosis was evident on the histological sections from all strains of mice during EAM. However, the extent of necrosis was not increased in strains with higher numbers of heart-infiltrating eosinophils (Figs. 1 A and 3 A). We conclude from these data that eosinophils do not drive DCMi through cytotoxic effects on cardiomyocytes.
Eosinophils in the heart are activated
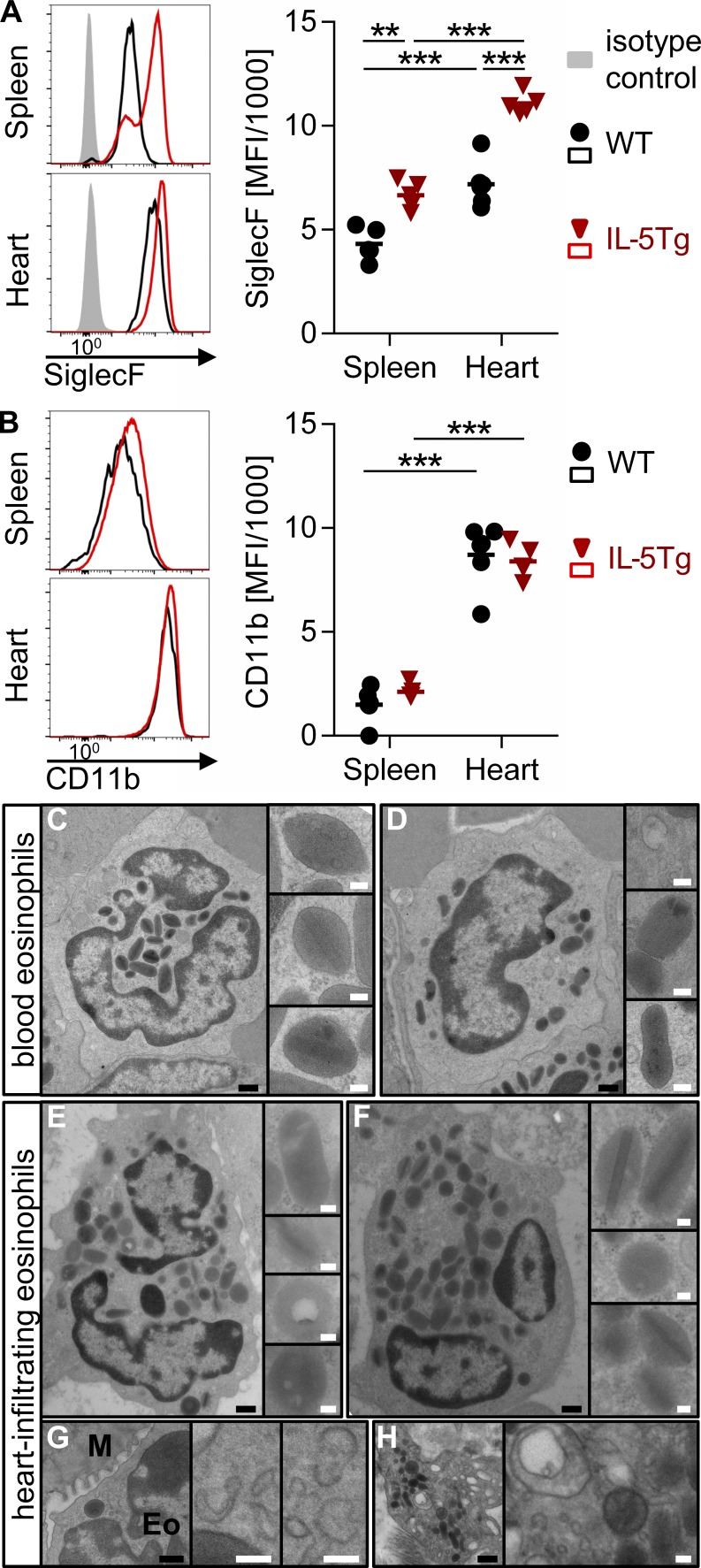
Eosinophil activation results in up-regulation of Siglec-F (Voehringer et al., 2007), a surface receptor that triggers eosinophil apoptosis as part of a negative feedback loop (Zhang et al., 2007; Zimmermann et al., 2008; Kiwamoto et al., 2013). Eosinophils in the hearts of WT and IL-5Tg mice showed increased expression of Siglec-F compared with splenic eosinophils during EAM (Fig. 5 A). Eosinophils from IL-5Tg mice had higher Siglec-F expression than WT mice in both spleen and heart. Cardiac eosinophils also expressed higher levels of CD11b (Fig. 5 B). This concurs with our previously reported findings that eosinophils in the heart of NK cell–depleted mice are activated during EAM, showing increased expression of Siglec-F and CD11b and an altered transcriptional profile as compared with splenic eosinophils from the same mice (Ong et al., 2015).
Figure 5.
Heart-infiltrating eosinophils show an activated phenotype. (A and B) Cardiac and splenic eosinophils were gated by flow cytometry as viable CD45+Ly6C−Ly6Glo/intSiglec-F+ cells on day 21 of EAM. Histograms of the median animal and mean fluorescent intensity (MFI) for Siglec-F (A) and CD11b (B) are shown. Groups were compared by one-way ANOVA followed by Tukey’s multiple comparisons test for all groups against each other. Data are representative of three independent experiments with four to six mice per group. (C–H) Electron microscopy of eosinophils in the heart and blood of an IL-5Tg mouse at day 21 of EAM. Representative images of intravascular (blood) eosinophils (C and D) and heart-infiltrating eosinophils (E–H) are depicted. The insets show individual granules (C–F) or cytoplasmic vesiculotubular structures (G and H). Eo, eosinophil; M, cardiomyocyte. Bars: (black) 500 nm; (white) 100 nm. **, P < 0.01; ***, P < 0.001.
We used electron microscopy to determine the extent of eosinophil degranulation in the heart. The ultrastructure of tissue-infiltrating eosinophils was compared with blood eosinophils on cardiac sections from an IL-5Tg mouse. Blood eosinophils showed largely regular architecture of mature granules with an electron-dense core surrounded by an electron-lucent matrix (Fig. 5, C and D). Granules in tissue eosinophils were also mostly intact without apparent signs of degranulation (Fig. 5, E and F). The number of granules was comparable in blood and tissue eosinophils (not depicted). Only rarely did we observe granules with a loss of matrix or core as described for models of allergic asthma, colitis, or in vitro degranulation (Fig. 5, E and H; Clark et al., 2004; Forbes et al., 2004; Shamri et al., 2012). Free extracellular granules or compound exocytosis of granules was not observed. Therefore, we conclude that eosinophils in the heart do not degranulate on a large scale during EAM. However, vesiculotubular organelles and ruffled membranes were very prominent in the cytoplasm of tissue-infiltrating eosinophils (Fig. 5, G and H), suggesting that eosinophils might be releasing specific granule contents through piecemeal degranulation.
Eosinophils account for the majority of IL-4–expressing cells in EAM
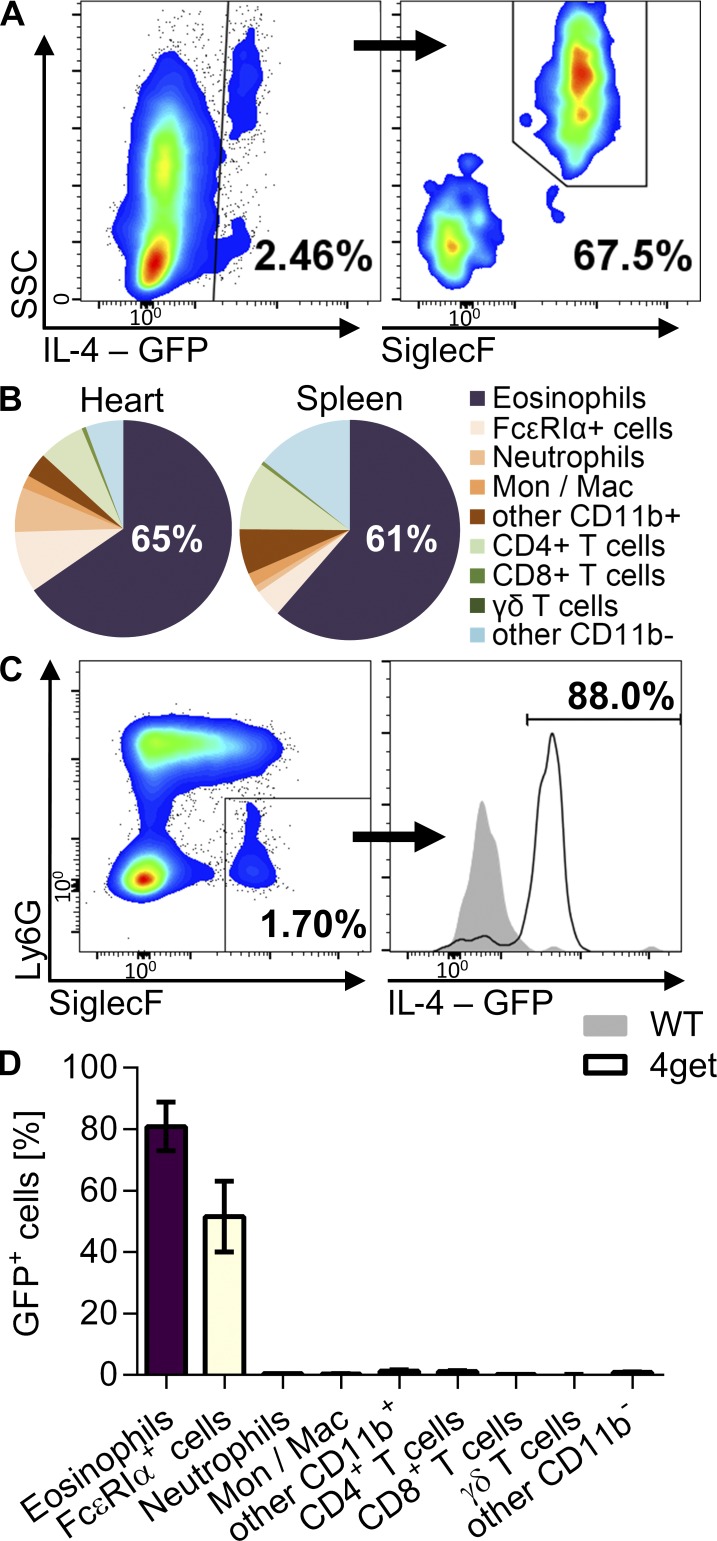
Eosinophil granules harbor numerous cytokines in addition to granule proteins and can selectively release them upon stimulation (Spencer et al., 2006; Melo and Weller, 2010). Of these, eosinophil-derived IL-4 has been shown to affect several physiological processes (Wu et al., 2011; Goh et al., 2013; Heredia et al., 2013; Qiu et al., 2014). To assess IL-4 production by eosinophils in myocarditis, we used the IL-4 reporter mouse 4get, in which IL-4–expressing cells are GFP+. Eosinophils accounted for the majority (>60%) of IL-4–expressing cells in myocarditis in 4get mice (Fig. 6, A and B). Greater than 80% of eosinophils were GFP+, indicating that the vast majority of eosinophils expressed IL-4 (Fig. 6, C and D). Hence, eosinophils are the major IL-4–producing cells in myocarditis.
Figure 6.
Eosinophils are the major producers of IL-4 in myocarditis. (A–D) Mice were analyzed at day 21 of EAM. (A) Gating of IL-4–expressing (GFP+) cells out of viable, CD45+ cells in the heart of 4get mice (IL4-GFP reporter mice) is shown for the median animal. SSC, side scatter. (B) Frequency of cell types out of heart-infiltrating or splenic GFP+ cells (mean of three mice). (C) Gating of eosinophils out of viable CD45+ cells and GFP expression in eosinophils from WT and 4get mice. (D) Frequency of GFP+ cells among different heart-infiltrating cell types (mean ± SD). Data are representative of two independent experiments with three 4get mice. Mac, macrophage; Mon, monocyte.
IL-4−/− mice are protected from DCMi
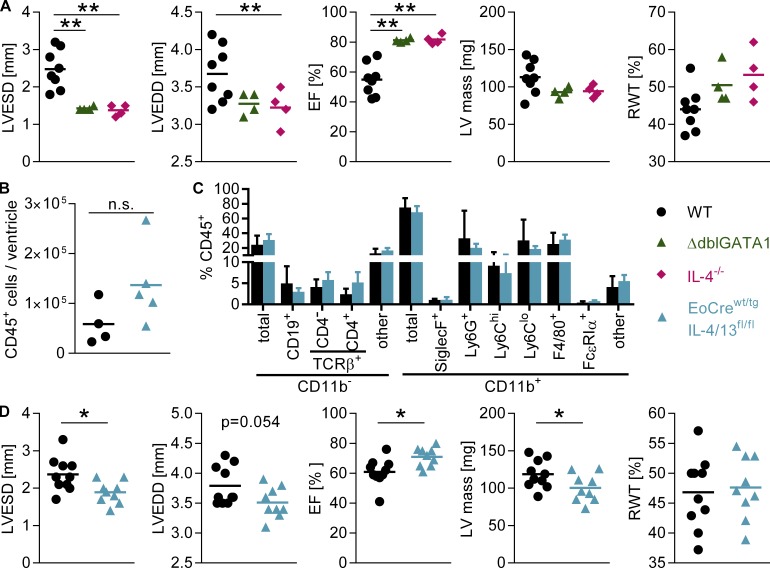
Next, we determined the role of IL-4 in myocarditis and DCMi. We previously published that IL-4−/− BALB/c mice develop myocarditis with the same severity as WT mice (Čiháková et al., 2008). When we analyzed heart function in IL-4−/− mice by echocardiography on day 45 of EAM, we found that they were completely protected from DCMi. Like ΔdblGATA1 mice, IL-4−/− mice had reduced LVESD and LVEDD and preserved EF (Fig. 7 A). There was also a trend to reduced LV mass and increased RWT (Fig. 7 A). Collectively with the data showing eosinophils comprise the majority of IL-4–expressing cells, these results strongly suggested that eosinophils drive progression of myocarditis to DCMi through their production of IL-4.
Figure 7.
Eosinophil-derived IL-4 promotes DCMi. (A and D) Heart function (LVESD, LVEDD, EF, LV mass, and RWT) was assessed by echocardiography on day 45 of EAM in the indicated strains. (A) Data are representative of three independent experiments with four to eight mice per group. (D) Data are from one experiment with 9–10 mice/group. (B and C) Infiltrating cells in the ventricle were quantified by flow cytometry on day 21 of EAM. Data are from one experiment with four to five mice per group. (C) Mean ± SD is shown. Groups were compared by one-way ANOVA followed by Tukey’s multiple comparisons test for all groups against each other (A) or by Student’s t test (B–D). *, P < 0.05; **, P < 0.01.
Eosinophil-specific deletion of IL-4 ameliorates DCMi
To assess whether IL-4 derived from eosinophils is necessary for DCMi development, we generated mice with eosinophil-specific IL-4 and IL-13 deletion. We crossed mice with eosinophil-specific Cre recombinase expression (EoCre) with IL-4/13fl/fl mice. The resulting EoCrewt/tgIL-4/13fl/fl mice developed myocarditis similar to WT mice. Absolute numbers and composition of infiltrating CD45+ cells were comparable (Fig. 7, B and C). However, at day 45 of EAM, EoCrewt/tgIL-4/13fl/fl mice showed decreased LVESD, increased EF, and decreased LV mass compared with WT controls (Fig. 7 D). LVEDD and RWT were comparable with WT mice (Fig. 7 D). Ventricular and atrial fibrosis was similar in WT, IL-4−/−, and EoCrewt/tgIL-4/13fl/fl mice (Fig. S5, A–C). These results show that eosinophils drive dilated cardiomyopathy through the production of IL-4. Eosinophil-specific IL-4 deletion resulted in limited protection from DCMi as compared with IL-4−/− mice (Fig. 7, A and D). This is likely a result of incomplete Cre-mediated recombination of the IL-4/13 locus; crossing EoCre mice to the deleter strain ROSA-DTA resulted in a reduction but not complete ablation of eosinophils during EAM (Fig. S5 D). We cannot exclude effects from residual IL-4 production by other cell types or other eosinophil-derived mediators. Expression of tissue remodeling–associated genes Mmp2 and Timp2, which was reduced in ΔdblGATA1 mice, was also diminished in EoCrewt/tgIL-4/13fl/fl mice (Fig. S5 E), suggesting that these changes in gene expression are mediated through eosinophil-derived IL-4. This demonstrates that eosinophils lead to DCMi through their production of IL-4.
Discussion
In this study, we demonstrated that eosinophils are necessary for the progression of autoimmune myocarditis to DCMi. Mice lacking eosinophils developed myocardial inflammation similar to WT mice after EAM induction but did not develop DCMi and showed no signs of heart failure. Eosinophils accounted for 1–3% of heart-infiltrating CD45+ cells in WT mice. Although their absence did not prevent myocarditis or affect its severity, it had a prominent effect on DCMi development. This shows that the severity of myocarditis does not necessarily determine the long-term disease outcome, but specific infiltrating cell types are decisive for disease progression.
IL-5Tg mice can develop spontaneous cardiac enlargement and eosinophil infiltration at an unknown rate (Lee et al., 1997). EAM induction results in myocarditis in nearly all mice, making it a much more reliable model and more feasible to study because of the defined onset. EAM in IL-5Tg mice is a model of eosinophilic myocarditis with hypereosinophilia. HES patients frequently develop endomyocardial thrombi, which are thought to result in endomyocarditis and endomyocardial fibrosis (Weller and Bubley, 1994; Ogbogu et al., 2007). We rarely observed intraatrial or intraventricular thrombi and observed no signs of endocarditis in IL-5Tg mice after EAM induction. HES patients can also develop eosinophilic myocarditis or pericarditis (Weller and Bubley, 1994; Ogbogu et al., 2007). In IL-5Tg mice, inflammation was primarily located in the ventricular myocard and atria; pericarditis was mild. Most notably, IL-5Tg mice develop extensive eosinophil infiltration of the myocard associated with a decline in cardiac function, which is also observed in HES patients.
We previously reported on acutely fatal eosinophilic myocarditis developing in IFN-γ−/−IL-17A−/− mice (Barin et al., 2013). Induction of EAM in IL-5Tg mice offers a second model of eosinophilic myocarditis with several differences: (a) IL-5Tg mice have hypereosinophilia, whereas IFN-γ−/−IL-17A−/− mice have normal peripheral blood eosinophil levels. (b) Eosinophils reach >60% of heart-infiltrating cells in IL-5Tg mice and ∼30% in IFN-γ−/−IL-17A−/− mice. (c) Eosinophil migration to the heart depends on the eotaxin–CCR3 pathway in IFN-γ−/−IL-17A−/− mice (Diny et al., 2016). In IL-5Tg mice, this pathway was not thoroughly investigated. Eotaxin expression is comparable with WT mice in the ventricles and increased in the atria. (d) The severity of myocardial inflammation is moderate in IL-5Tg mice, whereas IFN-γ−/−IL-17A−/− mice develop massive inflammation that encompasses almost the entire myocardium and likely causes fatality in these mice. IL-5Tg mice rarely died after EAM induction. Severe atrial inflammation is present in both strains. Importantly, both strains develop heart failure that is rescued by ablation of eosinophils. This confirms a crucial role for eosinophils in driving DCMi.
Severe atrial inflammation and fibrosis were striking features in IL-5Tg mice. Severe atrial fibrosis may have caused atrial fibrillation or arrhythmias in IL-5Tg mice. We noted atrial involvement in two out of three of the eosinophilic myocarditis patients described here. A recent case series found that eight of nine patients with eosinophilic myocarditis had enlargement of the atria (Xie et al., 2015). Another study characterized six patients with isolated atrial giant cell myocarditis showing atrial fibrillation, atrial wall thickening, atrial enlargement, and marked atrial inflammation (Larsen et al., 2013). Similar observations were made in case studies of giant cell myocarditis (Ahmad et al., 2006) and eosinophilic myocarditis (Parrillo et al., 1979; Frustaci et al., 1991; Carver et al., 2010). Eosinophilic myocarditis has also been identified as a cause of atrioventricular block in one patient (Tian et al., 2010). This suggests that atrial inflammation and/or fibrosis are also a feature of some eosinophilic myocarditis patients. Future studies should explore what determines atrial involvement in eosinophilic myocarditis and assess possible proarrhythmogenic effects of such inflammation.
The exacerbated heart failure in IL-5Tg mice was caused by the high numbers of eosinophils. IL-5 was not directly responsible for this phenotype because IL-5TgΔdblGATA1 mice with high serum IL-5 but no eosinophils were protected from DCMi, and IL-5−/− mice exhibited DCMi comparable with WT mice. This clearly demonstrated that the role of IL-5 was limited to promoting eosinophilia. Eosinophils, in turn, were responsible for the DCMi phenotype, and increasing numbers resulted in more severe DCMi. As a result of the size difference between atria and ventricles, most cardiac eosinophils located to the ventricles despite more severe inflammation in the atria. It is unclear whether eosinophil infiltration in the atria or ventricles was responsible for the severe DCMi observed in these mice. Because of the rarity of eosinophilic myocarditis, it is not known what percentage of eosinophilic myocarditis patients progress to DCMi. In a study on the survival of 112 biopsy-proven myocarditis patients, four out of seven patients with eosinophilic myocarditis underwent cardiac transplantation within 1 yr of diagnosis (Magnani et al., 2006), suggesting that transplant-free survival may be particularly poor among these patients. In one series of eosinophilic myocarditis patients, EF was impaired in all five patients and severely impaired in two patients (Wang et al., 2016). More studies will be necessary to determine whether eosinophilic myocarditis is associated with progression to DCMi in patients.
In previous studies, we showed that IL-17A is another driver of DCMi (Baldeviano et al., 2010; Wu et al., 2014). In the absence of IL-17A, mice are protected from DCMi and show markedly reduced levels of cardiac fibrosis. Eosinophils drive DCMi through a distinct mechanism. Lack of eosinophils or eosinophil-derived IL-4 did not affect fibrosis or collagen levels in the heart but, rather, affected expression of tissue remodeling–associated genes. In this paper, we demonstrate that mice can develop cardiac fibrosis but still retain a high EF. Therefore, the level of fibrosis is not a necessary negative regulator of cardiac function. It is possible that both increased collagen production induced by IL-17A and tissue remodeling by matrix metalloproteinases stimulated by eosinophils are necessary to drive progression of myocarditis to DCMi. Another conceivable explanation would be a direct link between Th17 cell responses and eosinophils, as it was suggested in recent studies. The Th17 effector cytokine GM-CSF enhances eosinophilopoiesis, induces cytokine secretion, and promotes survival (Griseri et al., 2015; Willebrand and Voehringer, 2016).
We tested numerous hypotheses regarding the mechanism by which eosinophils promote DCMi: direct cytotoxic effects, activation of other immune cell types (T cells or DCs), effects on antibody production, and eosinophil peroxidase-driven effects. None of these was supported by our data. However, eosinophils in the heart showed an activated phenotype with formation of vesiculotubular structures in the cytoplasm without a large extent of degranulation. We hypothesized that eosinophils may be releasing specific mediators to promote DCMi.
We showed that eosinophil-derived IL-4 is critical for progression of myocarditis to DCMi. Several previous studies have uncovered a role for eosinophils and IL-4 in physiological processes, including tissue regeneration, differentiation of beige fat, and glucose homeostasis (Wu et al., 2011; Goh et al., 2013; Heredia et al., 2013; Qiu et al., 2014). Here, we report a pathogenic role for eosinophil-derived IL-4. By specifically deleting IL-4 in eosinophils, we demonstrate that eosinophils are indeed the source of IL-4 required for DCMi development. This does not exclude the possibility of additional eosinophil-derived mediators contributing to heart failure.
Although eosinophils are not the only cells known to produce IL-4, other cell types were only a minor source of IL-4 in the heart. Basophils can produce IL-4 (Voehringer, 2013; Motomura et al., 2014) and can be affected by the ΔdblGATA1 mutation that prevents eosinophil development (Nei et al., 2013). However, basophils comprised <2% of all IL-4–producing cells in myocarditis, and there was no difference in the frequency of heart-infiltrating basophils between WT and ΔdblGATA1 mice. Another potential source of IL-4 is mast cells, but they accounted for <2% of GFP-positive IL-4–producing cells. Although T cells can also produce IL-4, they represented <10% of all IL-4–expressing heart-infiltrating cells during myocarditis.
Our studies also provide insight into the opposing effects of IL-4 and IL-13 in myocarditis. We previously published that IL-13 is protective in myocarditis and DCMi (Čiháková et al., 2008). IL-13–deficient mice develop severe myocarditis and rapidly progress to heart failure. In contrast, IL-4–deficient mice exhibit myocarditis of similar severity as WT mice (Čiháková et al., 2008) but were completely protected from DCMi. Although both are hallmark cytokines of a type 2 immune response, the sources of these cytokines are different. IL-4 is mostly expressed by eosinophils and to a lesser extent by CD4+ T cells. The major source of IL-13 is likely lymphoid: T cells and innate lymphoid cells. IL-13 protects against myocarditis through multiple effects on monocytes and macrophages (Čiháková et al., 2008). IL-4 may act on other target cells. Given these different roles of IL-4 and IL-13 in myocarditis, it is unlikely that the absence of IL-13 in EoCrewt/tgIL-4/13fl/fl mice is responsible for the observed protective effect.
Currently, no drugs are available to arrest or delay development of DCMi or to improve long-term survival of DCMi patients. Our results uncovered a new pathway critically involved in the progression of myocarditis to DCMi. Preventing eosinophilia or blocking IL-4 in patients with myocarditis may halt disease progression and thereby avert the need for heart transplants.
Materials and methods
Patients
Patients with endomyocardial biopsy–confirmed eosinophilic myocarditis for whom cardiac magnetic resonance imaging was also available from 2000–2014 were retrospectively identified at the Johns Hopkins Hospital Department of Cardiology. Endomyocardial biopsy of the right ventricular septum was performed using the Jawz Endomyocardial Bioptome (Argon Medical Devices). A cardiac pathologist examined all specimens at a minimum of four section levels with typical stains (Leone et al., 2012). Diagnosis of eosinophilic myocarditis was established using the Dallas Criteria for myocarditis and presence of eosinophilic infiltrate as detected on hematoxylin and eosin (H&E)–stained sections (Aretz et al., 1987). Patients underwent clinically indicated cardiac magnetic resonance with a 1.5-T scanner (Siemens Healthcare), including standard cine and LGE studies covering the whole heart. Analysis was performed on a dedicated workstation by an experienced observer. The study was approved by the Johns Hopkins Institutional Review Board.
Mice
IL-5Tg (founder line NJ.1638; Lee et al., 1997) and EoCre mice (Doyle et al., 2013) were provided on the BALB/c background by J. and N. Lee (Mayo Clinic, Scottsdale, AZ). IL-5−/− mice (Kopf et al., 1996) were provided by M. Rothenberg (Cincinnati Children’s Hospital, Cincinnati, OH). BALB/cJ WT (stock no. 651; The Jackson Laboratory), ΔdblGATA1 (JAX no. 5653; Yu et al., 2002), IL-4−/− (JAX no. 2496; Noben-Trauth et al., 1996), 4get (JAX no. 4190; Mohrs et al., 2001), IL-4/13flox/flox (JAX no. 15859; Voehringer et al., 2009), and ROSA-DTA (JAX no. 9670; Voehringer et al., 2008) mice were purchased from The Jackson Laboratory. All mice used in this study were on BALB/c background. Mice were housed in specific pathogen–free animal facilities at the Johns Hopkins University. Experiments were conducted on 6–10-wk-old age-matched male mice in compliance with the Animal Welfare Act and the principles set forth in the Guide for the Care and Use of Laboratory Animals. All methods and protocols were approved by the Animal Care and Use Committee of Johns Hopkins University.
Induction of EAM
To induce EAM, mice received subcutaneous immunizations on days 0 and 7 of 100 µg myosin heavy chain α (MyHCα)614–629 peptide (AcSLKLMATLFSTYASAD; Genscript; Pummerer et al., 1996) emulsified in CFA (Sigma-Aldrich) supplemented to 5 mg/ml heat-killed Mycobacterium tuberculosis strain H37Ra (Difco). On day 0, mice also received 500 ng pertussis toxin intraperitoneally (List Biologicals; Ciháková et al., 2004).
Assessment of EAM severity and fibrosis
Heart tissue was fixed in SafeFix solution (Thermo Fisher Scientific), embedded, and cut into 5-µm serial sections. Ventricular inflammation was scored by microscopic assessment of the area of infiltration with hematopoietic cells on H&E-stained sections according to the following scale: grade 0, no inflammation; grade 1, <10% of the heart section is infiltrated; grade 2, 10–30%; grade 3, 30–50%; grade 4, 50–90%; and grade 5, >90%. Ventricular fibrosis was scored on Masson’s trichrome–stained sections according to the same scale. Atrial inflammation and fibrosis were scored on the following scale: 0, none; 1, mild; 2, moderate; and 3, severe. Grading was performed by two independent, blinded investigators and averaged (Ciháková et al., 2004).
Light microscopy
Images were acquired on a microscope (BX43; Olympus) with a camera (DP72; Olympus) using cellSens Standard software (version 1.4.1; Olympus).
Electron microscopy
Mice were euthanized, the heart was removed, and the left ventricle was cut into 2–3 mm3 pieces and immediately fixed in 2.5% glutaraldehyde (EM grade; Electron Microscopy Sciences) dissolved in 0.1 M Na cacodylate, pH 7.4, for 2 h at room temperature. Samples were processed as previously described (Fölsch et al., 2001) before examination with an electron microscope (CM120; Philips) under 80 kV. Images were acquired with Image Capture Engine (V602; Advanced Microscopy Techniques).
Echocardiography
Transthoracic echocardiography was performed using an ultrasonic imaging system (Acuson Sequoia C256; Siemens) with a 13-MHz transducer. Conscious, depilated mice were held in a supine position. The heart was imaged in two-dimensional mode in the parasternal short axis view. An M-mode cursor was positioned perpendicular to the interventricular septum and the LV posterior wall at the level of the papillary muscles. The LVEDD, LVESD, interventricular septal wall thickness at end diastole, and LV posterior wall thickness at end diastole were measured three times for each mouse from a frozen M-mode tracing and averaged. EF, RWT, and LV mass were calculated from these parameters as previously described (Čiháková et al., 2008).
Flow cytometry
For flow cytometry analysis, single-cell suspensions were made from mouse spleen by mechanical dissociation followed by RBC lysis with ACK lysis buffer (Quality Biologicals). Heart-infiltrating leukocytes were isolated by perfusing mouse hearts for 3 min with PBS and 0.5% FBS and digested for 30 min at 37°C in gentleMACS C Tubes (Miltenyi Biotec) with 3,000 U/ml Collagenase II and 90 U/ml DNase I (Worthington Biochemical Corporation). For intracellular cytokine staining, cells were stimulated in vitro for 5 h with 50 ng/ml phorbol 12-myristate 13-acetate, 750 ng/ml ionomycin (Sigma-Aldrich), GolgiStop, and GolgiPlug (BD) before staining. Viability was determined by LIVE/DEAD staining according to the manufacturer’s instructions (Thermo Fisher Scientific). For intracellular cytokine staining, cells were resuspended in Cytofix/Cytoperm (BD). Cells were blocked with anti-CD16/CD32 (eBioscience) and were stained with fluorochrome-conjugated monoclonal antibodies (eBioscience, BD, and BioLegend). For absolute quantification, viable cells were counted with Trypan blue or using CountBright beads (Thermo Fisher Scientific). Samples were acquired on an LSR II cytometer running FACSDiva 6 software (BD). Data were analyzed with FlowJo 10 (Tree Star).
Isolation of primary adult mouse cardiomyocytes
To isolate cardiomyocytes, the heart was dissected from 6–8-wk-old male mice pretreated with heparin as previously described (Wu et al., 2014). The aorta was cannulated, and the heart was perfused with calcium-free perfusion buffer and digested with 3,000 U/ml Collagenase II (Worthington Biochemical Corporation) and 0.05 mg/ml Protease XIV (Sigma-Aldrich) for 15 min at 30°C followed by mechanical dissociation. Cardiomyocytes were separated from the resulting suspensions by their rapid spontaneous precipitation. Isolated cardiomyocytes were cultured in mouse laminin–coated plates. Nonadherent cells were washed off after 1 h.
Hydroxyproline assay
Heart samples were weighed, homogenized in deionized water, and hydrolyzed overnight in 6N HCl at 120°C. Lysates were transferred to and desiccated in 96-well plates and reconstituted in deionized water. After incubation with 50 mM chloramine T (Sigma-Aldrich) followed by 1 M dimethylaminobenzaldehyde (Sigma-Aldrich), the OD values were read at 570 nm. The concentration of hydroxyproline was determined by a 1–100 µg/ml standard curve of hydroxyproline (Sigma-Aldrich) and normalized to starting heart tissue mass (Wu et al., 2014).
ELISA
Sera were stored at −80°C before analysis. IL-5 was determined by quantitative sandwich ELISA according to the manufacturer’s recommended protocols (R&D Systems). For antimyosin IgG ELISA, plates were coated with 0.5 µg/ml MyHC614629 peptide (AcSLKLMATLFSTYASAD; Genscript) overnight at 4°C, washed with 1× PBS and 2% FBS, and incubated with sera for 2 h at room temperature. Plates were washed and incubated with secondary anti–mouse IgG antibody (Abcam) diluted 1:1,000 in 1× PBS for 2 h at room temperature. Plates were washed and developed with alkaline phosphatase (Bio-Rad Laboratories), and OD was read at 405 nm.
Quantitative PCR
Tissue RNA was extracted in TRIzol (Invitrogen) and quantitated. cDNA was synthesized with the High Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific), amplified with iQ SYBR Green Mastermix (Bio-Rad Laboratories), and acquired on the MyiQ2 thermocycler (Bio-Rad Laboratories) running iQ5 software (Bio-Rad Laboratories). Data were analyzed by the 2−ΔΔCt method (Livak and Schmittgen, 2001), normalizing threshold cycles first to Gapdh or Hprt expression and then to controls. Primer sequences are listed in Table S1.
Statistics
Two groups with normally distributed data were analyzed using Student’s t test. Mann-Whitney test was used for nonparametric data. Multiple group analysis was performed by one-way ANOVA followed by Tukey’s multiple comparisons test for continuous variables or by Kruskal-Wallis test followed by Dunn’s multiple comparisons test for nonparametric data. Calculations were performed in Prism 6 (GraphPad Software). P-values <0.05 were considered statistically significant and are denoted by asterisks: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Online supplemental material
Fig. S1 shows reduced expression of tissue remodeling–associated genes in ΔdblGATA1 mice and the frequency of heart-infiltrating cell types in WT and ΔdblGATA1 mice in EAM. Fig. S2 shows that the number of infiltrating eosinophils and several other types is increased in IL-5Tg mice and depicts cardiac imaging of a patient with HES and atrial involvement. Fig. S3 shows that atrial fibrosis is increased in IL-5Tg mice during the chronic stage of EAM. Fig. S4 shows that there are no differences between WT and ΔdblGATA1 mice in DC activation or antibody responses and that mice lacking eosinophil peroxidase are not protected from DCMi. Fig. S5 shows that fibrosis is similar in WT, IL-4−/−, and EoCrewt/tgIL-4/13fl/fl mice and that EoCrewt/tgIL-4/13fl/fl mice have decreased expression of tissue remodeling–associated genes. Table S1 lists sequences of quantitative PCR primers.
Supplementary Material
Acknowledgments
We thank Lei Wu, Xuezhou Hou, HeeSun Choi, Guobao Chen, and William Bracamonte-Baran for insightful discussions; Marc Halushka for taking biopsy images; the animal resources at Johns Hopkins University, Elizabeth Gebremariam, and Julie Schaub for mouse colony management; and Sean Doughty for manuscript editing.
This work was supported by grants from National Institutes of Health/National Heart, Lung, and Blood Institute (R01HL118183 and R01HL113008) to D. Čiháková, an American Heart Association Predoctoral Fellowship (15PRE25400010) and Johns Hopkins Bloomberg School of Public Health Richard J. and Margaret Conn Himelfarb Student Support fund to N. Diny, and a Gilead Sciences Research Scholars Program and American Autoimmune Related Diseases Association grant to J.G. Barin.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- DCMi
- inflammatory dilated cardiomyopathy
- EAM
- experimental autoimmune myocarditis
- EF
- ejection fraction
- H&E
- hematoxylin and eosin
- HES
- hypereosinophilic syndrome
- LGE
- late gadolinium enhancement
- LV
- left ventricular
- LVEDD
- LV end-diastolic diameter
- LVESD
- LV end-systolic diameter
- RWT
- relative wall thickness
References
- Ahmad I., Craig Miller D., Berry G.J., Hsia H.H., Wang P.J., and Al-Ahmad A.. 2006. Isolated giant cell myocarditis in the atrium: an incidental finding? Pacing Clin. Electrophysiol. 29:1179–1180. 10.1111/j.1540-8159.2006.00512.x [DOI] [PubMed] [Google Scholar]
- Aretz H.T., Billingham M.E., Edwards W.D., Factor S.M., Fallon J.T., Fenoglio J.J. Jr., Olsen E.G., and Schoen F.J.. 1987. Myocarditis. A histopathologic definition and classification. Am. J. Cardiovasc. Pathol. 1:3–14. [PubMed] [Google Scholar]
- Baandrup U. 2012. Eosinophilic myocarditis. Herz. 37:849–853. 10.1007/s00059-012-3701-2 [DOI] [PubMed] [Google Scholar]
- Baldeviano G.C., Barin J.G., Talor M.V., Srinivasan S., Bedja D., Zheng D., Gabrielson K., Iwakura Y., Rose N.R., and Čiháková D.. 2010. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ. Res. 106:1646–1655. 10.1161/CIRCRESAHA.109.213157 [DOI] [PubMed] [Google Scholar]
- Barin J.G., Baldeviano G.C., Talor M.V., Wu L., Ong S., Fairweather D., Bedja D., Stickel N.R., Fontes J.A., Cardamone A.B., et al. 2013. Fatal eosinophilic myocarditis develops in the absence of IFN-γ and IL-17A. J. Immunol. 191:4038–4047. 10.4049/jimmunol.1301282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B.S., Book W., Busse W.W., Butterfield J., Furuta G.T., Gleich G.J., Klion A.D., Lee J.J., Leiferman K.M., Minnicozzi M., et al. 2012. Workshop report from the National Institutes of Health Taskforce on the Research Needs of Eosinophil-Associated Diseases (TREAD). J. Allergy Clin. Immunol. 130:587–596. 10.1016/j.jaci.2012.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver J.R., Nasta S., Chong E.A., Stonecypher M., Wheeler J.E., Ahmadi T., and Schuster S.J.. 2010. Myocarditis during lenalidomide therapy. Ann. Pharmacother. 44:1840–1843. 10.1345/aph.1P044 [DOI] [PubMed] [Google Scholar]
- Chisty M.M., Nargis M., Sato H., Inaba T., and Kamiya H.. 1999. Occurrence of myocarditis in rodents infected with Schistosoma mansoni. Southeast Asian J. Trop. Med. Public Health. 30:556–561. [PubMed] [Google Scholar]
- Chu V.T., Fröhlich A., Steinhauser G., Scheel T., Roch T., Fillatreau S., Lee J.J., Löhning M., and Berek C.. 2011. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat. Immunol. 12:151–159. 10.1038/ni.1981 [DOI] [PubMed] [Google Scholar]
- Chu V.T., Beller A., Rausch S., Strandmark J., Zänker M., Arbach O., Kruglov A., and Berek C.. 2014. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity. 40:582–593. 10.1016/j.immuni.2014.02.014 [DOI] [PubMed] [Google Scholar]
- Ciháková D., Sharma R.B., Fairweather D., Afanasyeva M., and Rose N.R.. 2004. Animal models for autoimmune myocarditis and autoimmune thyroiditis. Methods Mol. Med. 102:175–193. [DOI] [PubMed] [Google Scholar]
- Čiháková D., and Rose N.R.. 2008. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv. Immunol. 99:95–114. 10.1016/S0065-2776(08)00604-4 [DOI] [PubMed] [Google Scholar]
- Čiháková D., Barin J.G., Afanasyeva M., Kimura M., Fairweather D., Berg M., Talor M.V., Baldeviano G.C., Frisancho S., Gabrielson K., et al. 2008. Interleukin-13 protects against experimental autoimmune myocarditis by regulating macrophage differentiation. Am. J. Pathol. 172:1195–1208. 10.2353/ajpath.2008.070207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K., Simson L., Newcombe N., Koskinen A.M.L., Mattes J., Lee N.A., Lee J.J., Dent L.A., Matthaei K.I., and Foster P.S.. 2004. Eosinophil degranulation in the allergic lung of mice primarily occurs in the airway lumen. J. Leukoc. Biol. 75:1001–1009. 10.1189/jlb.0803391 [DOI] [PubMed] [Google Scholar]
- Comarmond C., Pagnoux C., Khellaf M., Cordier J.-F., Hamidou M., Viallard J.-F., Maurier F., Jouneau S., Bienvenu B., Puéchal X., et al. French Vasculitis Study Group . 2013. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 65:270–281. 10.1002/art.37721 [DOI] [PubMed] [Google Scholar]
- Cookston M., Stober M., and Kayes S.G.. 1990. Eosinophilic myocarditis in CBA/J mice infected with Toxocara canis. Am. J. Pathol. 136:1137–1145. [PMC free article] [PubMed] [Google Scholar]
- Corradi D., Vaglio A., Maestri R., Legname V., Leonardi G., Bartoloni G., and Buzio C.. 2004. Eosinophilic myocarditis in a patient with idiopathic hypereosinophilic syndrome: insights into mechanisms of myocardial cell death. Hum. Pathol. 35:1160–1163. 10.1016/j.humpath.2004.05.008 [DOI] [PubMed] [Google Scholar]
- Cugno M., Marzano A.V., Lorini M., Carbonelli V., and Tedeschi A.. 2014. Enhanced tissue factor expression by blood eosinophils from patients with hypereosinophilia: a possible link with thrombosis. PLoS One. 9:e111862 10.1371/journal.pone.0111862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubeney P.E.F., Nugent A.W., Chondros P., Carlin J.B., Colan S.D., Cheung M., Davis A.M., Chow C.W., and Weintraub R.G.. National Australian Childhood Cardiomyopathy Study . 2006. Clinical features and outcomes of childhood dilated cardiomyopathy: results from a national population-based study. Circulation. 114:2671–2678. 10.1161/CIRCULATIONAHA.106.635128 [DOI] [PubMed] [Google Scholar]
- deMello D.E., Liapis H., Jureidini S., Nouri S., Kephart G.M., and Gleich G.J.. 1990. Cardiac localization of eosinophil-granule major basic protein in acute necrotizing myocarditis. N. Engl. J. Med. 323:1542–1545. 10.1056/NEJM199011293232207 [DOI] [PubMed] [Google Scholar]
- Dimayuga E., Stober M., and Kayes S.G.. 1991. Eosinophil peroxidase levels in hearts and lungs of mice infected with Toxocara canis. J. Parasitol. 77:461–466. 10.2307/3283136 [DOI] [PubMed] [Google Scholar]
- Diny N.L., Hou X., Barin J.G., Chen G., Talor M.V., Schaub J., Russell S.D., Klingel K., Rose N.R., and Čiháková D.. 2016. Macrophages and cardiac fibroblasts are the main producers of eotaxins and regulate eosinophil trafficking to the heart. Eur. J. Immunol. 46:2749–2760. 10.1002/eji.201646557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle A.D., Jacobsen E.A., Ochkur S.I., Willetts L., Shim K., Neely J., Kloeber J., Lesuer W.E., Pero R.S., Lacy P., et al. 2013. Homologous recombination into the eosinophil peroxidase locus generates a strain of mice expressing Cre recombinase exclusively in eosinophils. J. Leukoc. Biol. 94:17–24. 10.1189/jlb.0213089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felker G.M., Thompson R.E., Hare J.M., Hruban R.H., Clemetson D.E., Howard D.L., Baughman K.L., and Kasper E.K.. 2000. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N. Engl. J. Med. 342:1077–1084. 10.1056/NEJM200004133421502 [DOI] [PubMed] [Google Scholar]
- Fölsch H., Pypaert M., Schu P., and Mellman I.. 2001. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J. Cell Biol. 152:595–606. 10.1083/jcb.152.3.595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E., Murase T., Yang M., Matthaei K.I., Lee J.J., Lee N.A., Foster P.S., and Hogan S.P.. 2004. Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J. Immunol. 172:5664–5675. 10.4049/jimmunol.172.9.5664 [DOI] [PubMed] [Google Scholar]
- Frustaci A., Caldarulo M., Buffon A., Bellocci F., Fenici R., and Melina D.. 1991. Cardiac biopsy in patients with “primary” atrial fibrillation. Histologic evidence of occult myocardial diseases. Chest. 100:303–306. 10.1378/chest.100.2.303 [DOI] [PubMed] [Google Scholar]
- Ginsberg F., and Parrillo J.E.. 2005. Eosinophilic myocarditis. Heart Fail. Clin. 1:419–429. 10.1016/j.hfc.2005.06.013 [DOI] [PubMed] [Google Scholar]
- Goh Y.P.S., Henderson N.C., Heredia J.E., Red Eagle A., Odegaard J.I., Lehwald N., Nguyen K.D., Sheppard D., Mukundan L., Locksley R.M., and Chawla A.. 2013. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc. Natl. Acad. Sci. USA. 110:9914–9919. 10.1073/pnas.1304046110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravanis M.B., Hertzler G.L., Franch R.H., Stacy L.D., Ansari A.A., Kanter K.R., Tazelaar H.D., Rodeheffer R., and McGregor C.. 1991. Hypersensitivity myocarditis in heart transplant candidates. J. Heart Lung Transplant. 10:688–697. [PubMed] [Google Scholar]
- Griseri T., Arnold I.C., Pearson C., Krausgruber T., Schiering C., Franchini F., Schulthess J., McKenzie B.S., Crocker P.R., and Powrie F.. 2015. Granulocyte macrophage colony-stimulating factor-activated eosinophils promote interleukin-23 driven chronic colitis. Immunity. 43:187–199. 10.1016/j.immuni.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie M., Lodge P.A., and Huber S.A.. 1984. Cardiac injury in myocarditis induced by Coxsackievirus group B, type 3 in Balb/c mice is mediated by Lyt 2+ cytolytic lymphocytes. Cell. Immunol. 88:558–567. 10.1016/0008-8749(84)90188-6 [DOI] [PubMed] [Google Scholar]
- Hawkins E.T., Levine T.B., Goss S.J., Moosvi A., and Levine A.B.. 1995. Hypersensitivity myocarditis in the explanted hearts of transplant recipients. Reappraisal of pathologic criteria and their clinical implications. Pathol. Annu. 30:287–304. [PubMed] [Google Scholar]
- Heredia J.E., Mukundan L., Chen F.M., Mueller A.A., Deo R.C., Locksley R.M., Rando T.A., and Chawla A.. 2013. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 153:376–388. 10.1016/j.cell.2013.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz A., Campbell S., Deckers J., Kasper E.K., Boehmer J., Hadian D., Neumann D.A., and Baughman K.L.. 1993. Demographic features and prevalence of idiopathic myocarditis in patients undergoing endomyocardial biopsy. Am. J. Cardiol. 71:982–986. 10.1016/0002-9149(93)90918-3 [DOI] [PubMed] [Google Scholar]
- Hirasawa M., Kitaura Y., Deguchi H., Ukimura A., and Kawamura K.. 1998. Spontaneous myocarditis in DBA/2 mice. Light microscopic study with transmission and X-ray analytical electron microscopic studies. Virchows Arch. 432:461–468. 10.1007/s004280050192 [DOI] [PubMed] [Google Scholar]
- Hirasawa M., Deguchi H., Ukimura A., and Kitaura Y.. 2003. Immunologic interaction between infiltrating eosinophils and T lymphocytes in murine spontaneous eosinophilic myocarditis. Int. Arch. Allergy Immunol. 130:73–81. 10.1159/000068371 [DOI] [PubMed] [Google Scholar]
- Hirasawa M., Ito Y., Shibata M.-A., and Otsuki Y.. 2007. Mechanism of inflammation in murine eosinophilic myocarditis produced by adoptive transfer with ovalbumin challenge. Int. Arch. Allergy Immunol. 142:28–39. 10.1159/000095996 [DOI] [PubMed] [Google Scholar]
- Hokibara S., Takamoto M., Isobe M., and Sugane K.. 1998. Effects of monoclonal antibodies to adhesion molecules on eosinophilic myocarditis in Toxocara canis-infected CBA/J mice. Clin. Exp. Immunol. 114:236–244. 10.1046/j.1365-2249.1998.00661.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen E.A., Zellner K.R., Colbert D., Lee N.A., and Lee J.J.. 2011. Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation. J. Immunol. 187:6059–6068. 10.4049/jimmunol.1102299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahns R., Boivin V., Hein L., Triebel S., Angermann C.E., Ertl G., and Lohse M.J.. 2004. Direct evidence for a β1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J. Clin. Invest. 113:1419–1429. 10.1172/JCI200420149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y., Wen T., Mingler M.K., Caldwell J.M., Wang Y.H., Chaplin D.D., Lee E.H., Jang M.H., Woo S.Y., Seoh J.Y., et al. 2015. IL-1β in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol. 8:930–942. 10.1038/mi.2014.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper E.K., Agema W.R., Hutchins G.M., Deckers J.W., Hare J.M., and Baughman K.L.. 1994. The causes of dilated cardiomyopathy: a clinicopathologic review of 673 consecutive patients. J. Am. Coll. Cardiol. 23:586–590. 10.1016/0735-1097(94)90740-4 [DOI] [PubMed] [Google Scholar]
- Kiwamoto T., Katoh T., Tiemeyer M., and Bochner B.S.. 2013. The role of lung epithelial ligands for Siglec-8 and Siglec-F in eosinophilic inflammation. Curr. Opin. Allergy Clin. Immunol. 13:106–111. 10.1097/ACI.0b013e32835b594a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M., Brombacher F., Hodgkin P.D., Ramsay A.J., Milbourne E.A., Dai W.J., Ovington K.S., Behm C.A., Köhler G., Young I.G., and Matthaei K.I.. 1996. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 4:15–24. 10.1016/S1074-7613(00)80294-0 [DOI] [PubMed] [Google Scholar]
- Larsen B.T., Maleszewski J.J., Edwards W.D., Cooper L.T. Jr., Sobonya R.E., Thompson V.E., Duckett S.G., Peebles C.R., Simpson I.A., and Tazelaar H.D.. 2013. Atrial giant cell myocarditis: a distinctive clinicopathologic entity. Circulation. 127:39–47. 10.1161/CIRCULATIONAHA.112.128900 [DOI] [PubMed] [Google Scholar]
- Lee N.A., McGarry M.P., Larson K.A., Horton M.A., Kristensen A.B., and Lee J.J.. 1997. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J. Immunol. 158:1332–1344. [PubMed] [Google Scholar]
- Leone O., Veinot J.P., Angelini A., Baandrup U.T., Basso C., Berry G., Bruneval P., Burke M., Butany J., Calabrese F., et al. 2012. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc. Pathol. 21:245–274. 10.1016/j.carpath.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Livak K.J., and Schmittgen T.D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Magnani J.W., and Dec G.W.. 2006. Myocarditis: current trends in diagnosis and treatment. Circulation. 113:876–890. 10.1161/CIRCULATIONAHA.105.584532 [DOI] [PubMed] [Google Scholar]
- Magnani J.W., Danik H.J.S., Dec G.W. Jr., and DiSalvo T.G.. 2006. Survival in biopsy-proven myocarditis: a long-term retrospective analysis of the histopathologic, clinical, and hemodynamic predictors. Am. Heart J. 151:463–470. 10.1016/j.ahj.2005.03.037 [DOI] [PubMed] [Google Scholar]
- Malkiel S., Factor S., and Diamond B.. 1999. Autoimmune myocarditis does not require B cells for antigen presentation. J. Immunol. 163:5265–5268. [PubMed] [Google Scholar]
- Mascaro-Blanco A., Alvarez K., Yu X., Lindenfeld J., Olansky L., Lyons T., Duvall D., Heuser J.S., Gosmanova A., Rubenstein C.J., et al. 2008. Consequences of unlocking the cardiac myosin molecule in human myocarditis and cardiomyopathies. Autoimmunity. 41:442–453. 10.1080/08916930802031579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo R.C.N., and Weller P.F.. 2010. Piecemeal degranulation in human eosinophils: a distinct secretion mechanism underlying inflammatory responses. Histol. Histopathol. 25:1341–1354. 10.14670/HH-25.1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Alexander W.S., Ryan P.J., Mifsud S., and Di Rago L.. 2001. Production of colony-stimulating factors and IL-5 by organs from three types of mice with inflammatory disease due to loss of the suppressor of cytokine signaling-1. J. Immunol. 167:4661–4667. 10.4049/jimmunol.167.8.4661 [DOI] [PubMed] [Google Scholar]
- Mohrs M., Shinkai K., Mohrs K., and Locksley R.M.. 2001. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 15:303–311. 10.1016/S1074-7613(01)00186-8 [DOI] [PubMed] [Google Scholar]
- Molina H.A., and Kierszenbaum F.. 1988. Kinetics of development of inflammatory lesions in myocardial and skeletal muscle in experimental Trypanosoma cruzi infection. J. Parasitol. 74:370–374. 10.2307/3282040 [DOI] [PubMed] [Google Scholar]
- Monteón V.M., Furuzawa-Carballeda J., Alejandre-Aguilar R., Aranda-Fraustro A., Rosales-Encina J.L., and Reyes P.A.. 1996. American trypanosomosis: in situ and generalized features of parasitism and inflammation kinetics in a murine model. Exp. Parasitol. 83:267–274. 10.1006/expr.1996.0074 [DOI] [PubMed] [Google Scholar]
- Moosig F., Bremer J.P., Hellmich B., Holle J.U., Holl-Ulrich K., Laudien M., Matthis C., Metzler C., Nölle B., Richardt G., and Gross W.L.. 2013. A vasculitis centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg–Strauss, EGPA): monocentric experiences in 150 patients. Ann. Rheum. Dis. 72:1011–1017. 10.1136/annrheumdis-2012-201531 [DOI] [PubMed] [Google Scholar]
- Motomura Y., Morita H., Moro K., Nakae S., Artis D., Endo T.A., Kuroki Y., Ohara O., Koyasu S., and Kubo M.. 2014. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity. 40:758–771. 10.1016/j.immuni.2014.04.013 [DOI] [PubMed] [Google Scholar]
- Nei Y., Obata-Ninomiya K., Tsutsui H., Ishiwata K., Miyasaka M., Matsumoto K., Nakae S., Kanuka H., Inase N., and Karasuyama H.. 2013. GATA-1 regulates the generation and function of basophils. Proc. Natl. Acad. Sci. USA. 110:18620–18625. 10.1073/pnas.1311668110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noben-Trauth N., Köhler G., Bürki K., and Ledermann B.. 1996. Efficient targeting of the IL-4 gene in a BALB/c embryonic stem cell line. Transgenic Res. 5:487–491. 10.1007/BF01980214 [DOI] [PubMed] [Google Scholar]
- Ogbogu P.U., Rosing D.R., and Horne M.K. III. 2007. Cardiovascular manifestations of hypereosinophilic syndromes. Immunol. Allergy Clin. North Am. 27:457–475. 10.1016/j.iac.2007.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S., Ligons D.L., Barin J.G., Wu L., Talor M.V., Diny N., Fontes J.A., Gebremariam E., Kass D.A., Rose N.R., and Čiháková D.. 2015. Natural killer cells limit cardiac inflammation and fibrosis by halting eosinophil infiltration. Am. J. Pathol. 185:847–861. 10.1016/j.ajpath.2014.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrillo J.E., Borer J.S., Henry W.L., Wolff S.M., and Fauci A.S.. 1979. The cardiovascular manifestations of the hypereosinophilic syndrome: Prospective study of 26 patients, with review of the literature. Am. J. Med. 67:572–582. 10.1016/0002-9343(79)90227-4 [DOI] [PubMed] [Google Scholar]
- Patella V., de Crescenzo G., Marinò I., Genovese A., Adt M., Gleich G.J., and Marone G.. 1996. Eosinophil granule proteins activate human heart mast cells. J. Immunol. 157:1219–1225. [PubMed] [Google Scholar]
- Pietra B.A., Kantor P.F., Bartlett H.L., Chin C., Canter C.E., Larsen R.L., Edens R.E., Colan S.D., Towbin J.A., Lipshultz S.E., et al. 2012. Early predictors of survival to and after heart transplantation in children with dilated cardiomyopathy. Circulation. 126:1079–1086. 10.1161/CIRCULATIONAHA.110.011999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pummerer C.L., Luze K., Grässl G., Bachmaier K., Offner F., Burrell S.K., Lenz D.M., Zamborelli T.J., Penninger J.M., and Neu N.. 1996. Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB/c mice. J. Clin. Invest. 97:2057–2062. 10.1172/JCI118642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Nguyen K.D., Odegaard J.I., Cui X., Tian X., Locksley R.M., Palmiter R.D., and Chawla A.. 2014. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 157:1292–1308. 10.1016/j.cell.2014.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose N.R., Beisel K.W., Herskowitz A., Neu N., Wolfgram L.J., Alvarez F.L., Traystman M.D., and Craig S.W.. 1987. Cardiac myosin and autoimmune myocarditis. Ciba Found. Symp. 129:3–24. [DOI] [PubMed] [Google Scholar]
- Rothenberg M.E., and Hogan S.P.. 2006. The eosinophil. Annu. Rev. Immunol. 24:147–174. 10.1146/annurev.immunol.24.021605.090720 [DOI] [PubMed] [Google Scholar]
- Sagar S., Liu P.P., and Cooper L.T. Jr. 2012. Myocarditis. Lancet. 379:738–747. 10.1016/S0140-6736(11)60648-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss H.-P., Kühl U., and Cooper L.T.. 2011. The management of myocarditis. Eur. Heart J. 32:2616–2625. 10.1093/eurheartj/ehr165 [DOI] [PubMed] [Google Scholar]
- Shamri R., Melo R.C.N., Young K.M., Bivas-Benita M., Xenakis J.J., Spencer L.A., and Weller P.F.. 2012. CCL11 elicits secretion of RNases from mouse eosinophils and their cell-free granules. FASEB J. 26:2084–2093. 10.1096/fj.11-200246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakabe A., Asai K., Hata N., Yokoyama S., Shinada T., Kobayashi N., and Mizuno K.. 2010. Clinical significance of matrix metalloproteinase (MMP)-2 in patients with acute heart failure. Int. Heart J. 51:404–410. 10.1536/ihj.51.404 [DOI] [PubMed] [Google Scholar]
- Spencer L.A., Melo R.C.N., Perez S.A.C., Bafford S.P., Dvorak A.M., and Weller P.F.. 2006. Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc. Natl. Acad. Sci. USA. 103:3333–3338. 10.1073/pnas.0508946103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spry C.J., Tai P.C., and Davies J.. 1983. The cardiotoxicity of eosinophils. Postgrad. Med. J. 59:147–153. 10.1136/pgmj.59.689.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P.C., Hayes D.J., Clark J.B., and Spry C.J.. 1982. Toxic effects of human eosinophil products on isolated rat heart cells in vitro. Biochem. J. 204:75–80. 10.1042/bj2040075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takkenberg J.J.M., Czer L.S.C., Fishbein M.C., Luthringer D.J., Quartel A.W., Mirocha J., Queral C.A., Blanche C., and Trento A.. 2004. Eosinophilic myocarditis in patients awaiting heart transplantation. Crit. Care Med. 32:714–721. 10.1097/01.CCM.0000114818.58877.06 [DOI] [PubMed] [Google Scholar]
- Thomas C.V., Coker M.L., Zellner J.L., Handy J.R., Crumbley A.J. III, and Spinale F.G.. 1998. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation. 97:1708–1715. 10.1161/01.CIR.97.17.1708 [DOI] [PubMed] [Google Scholar]
- Tian Z., Zeng Y., Cheng K.-A., Gao P., Zhao D.-C., Cui Q.-C., Jiang X.-C., Chen L.-F., and Fang Q.. 2010. Importance of endomyocardial biopsy in unexplained cardiomyopathy in China: a report of 53 consecutive patients. Chin. Med. J. (Engl.). 123:864–870. [PubMed] [Google Scholar]
- Voehringer D. 2013. Regulation of type 2 immunity by basophils. Adv. Exp. Med. Biol. 785:37–41. 10.1007/978-1-4614-6217-0_4 [DOI] [PubMed] [Google Scholar]
- Voehringer D., van Rooijen N., and Locksley R.M.. 2007. Eosinophils develop in distinct stages and are recruited to peripheral sites by alternatively activated macrophages. J. Leukoc. Biol. 81:1434–1444. 10.1189/jlb.1106686 [DOI] [PubMed] [Google Scholar]
- Voehringer D., Liang H.-E., and Locksley R.M.. 2008. Homeostasis and effector function of lymphopenia-induced “memory-like” T cells in constitutively T cell-depleted mice. J. Immunol. 180:4742–4753. 10.4049/jimmunol.180.7.4742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voehringer D., Wu D., Liang H.-E., and Locksley R.M.. 2009. Efficient generation of long-distance conditional alleles using recombineering and a dual selection strategy in replicate plates. BMC Biotechnol. 9:69 10.1186/1472-6750-9-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.K.M., Watson T., Pemberton J., Pemberton L., Lowe B., Ellis C., Kingston N., and Ruygrok P.. 2016. Eosinophilic myocarditis: characteristics, diagnostics and outcomes of a rare condition. Intern. Med. J. 46:1104–1107. 10.1111/imj.13176 [DOI] [PubMed] [Google Scholar]
- Weller P.F., and Bubley G.J.. 1994. The idiopathic hypereosinophilic syndrome. Blood. 83:2759–2779. [PubMed] [Google Scholar]
- Willebrand R., and Voehringer D.. 2016. IL-33-induced cytokine secretion and survival of mouse eosinophils is promoted by autocrine GM-CSF. PLoS One. 11:e0163751 10.1371/journal.pone.0163751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Molofsky A.B., Liang H.-E., Ricardo-Gonzalez R.R., Jouihan H.A., Bando J.K., Chawla A., and Locksley R.M.. 2011. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 332:243–247. 10.1126/science.1201475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Ong S., Talor M.V., Barin J.G., Baldeviano G.C., Kass D.A., Bedja D., Zhang H., Sheikh A., Margolick J.B., et al. 2014. Cardiac fibroblasts mediate IL-17A–driven inflammatory dilated cardiomyopathy. J. Exp. Med. 211:1449–1464. 10.1084/jem.20132126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M., Cheng T.O., Fei H., Ren P., He Y., Wang X., Lu Q., Han W., Li K., Li L., et al. 2015. The diagnostic value of transthoracic echocardiography for eosinophilic myocarditis: A single center experience from China. Int. J. Cardiol. 201:353–357. 10.1016/j.ijcard.2015.07.104 [DOI] [PubMed] [Google Scholar]
- Yoshida T., Fukuda T., Hatano M., Koseki H., Okabe S., Ishibashi K., Kojima S., Arima M., Komuro I., Ishii G., et al. 1999. The role of Bcl6 in mature cardiac myocytes. Cardiovasc. Res. 42:670–679. 10.1016/S0008-6363(99)00007-3 [DOI] [PubMed] [Google Scholar]
- Yu C., Cantor A.B., Yang H., Browne C., Wells R.A., Fujiwara Y., and Orkin S.H.. 2002. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J. Exp. Med. 195:1387–1395. 10.1084/jem.20020656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Angata T., Cho J.Y., Miller M., Broide D.H., and Varki A.. 2007. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 109:4280–4287. 10.1182/blood-2006-08-039255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann N., McBride M.L., Yamada Y., Hudson S.A., Jones C., Cromie K.D., Crocker P.R., Rothenberg M.E., and Bochner B.S.. 2008. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 63:1156–1163. 10.1111/j.1398-9995.2008.01709.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.